Summary
Isolation of ribonucleoprotein particles from living cells and cell lysates has allowed the identification of both simple bimolecular interactions and the members of large, extended complexes. A number of different strategies have been devised to isolate these complexes by using affinity purification methods that are specific for the RNA rather than the protein components of these complexes. We describe the use of two such RNA affinity tags: small RNAs that bind with high affinity and specificity to either Sephadex beads or streptavidin affinity resins and can be eluted under mild, native conditions that retain intact complexes. The tags can be inserted into appropriate locations in genes encoding the RNA components, and ribonucleoproteins can be assembled either in vivo or in vitro before affinity isolation. Strategies toward the design and production of these tagged RNA sequences are discussed, and the purification procedure is outlined.
Keywords: Aptamer, ribonucleoprotein, RNA, RNP isolation, SELEX, Sephadex, streptavidin
1. Introduction
The use of small, genetically introduced affinity tags for the recombinant production and purification of proteins as well as for the isolation of defined protein complexes has greatly facilitated the study of individual protein function and chemistry in a variety of research fields (1). These protein affinity tags include polyhistidine (2), the hemagglutinin epitope (3), myc epitope (4), the tandem affinity protein (TAP) tag (5), protein A (6), glutathione S-transferase (7), Strep-tag (8), and the FLAG epitope (9). These affinity tags all bind with high affinity to a ligand that can either be immobilized on a chromatography resin or be conjugated to an antibody detection system. In most cases, the isolated complexes can either be released from the resin by competitive elution or cleaved off by a protease with a recognition site that is incorporated along with the affinity tag as part of the fusion protein. The widespread use of protein affinity tags has led to the development of similar tags for nucleic acids. Particular emphasis has been placed on developing affinity tags for RNA molecules since this versatile nucleic acid is involved in a wide variety of cellular processes.
There are four known methods in use for tagging an RNA: (1) chemical tagging during in vitro transcription, (2) incorporation of a well-characterized protein-binding RNA sequence during in vitro or in vivo transcription, (3) hybridization of affinity-tagged oligonucleotides (biotinylated), and (4) incorporation of an artificially selected RNA motif during in vivo or in vitro transcription. Each of these methods for the tagging and affinity isolation of RNA molecules has particular advantages and disadvantages, discussed here.
There are several ways to chemically tag an in vitro transcribed RNA; these include the incorporation of modified ribonucleotide triphosphates (rNTPs) containing biotin, fluorescent dyes, digoxeginin, or other compounds. RNAs chemically modified with biotin and purified using streptavidin beads have been used to isolate RNA–protein complexes in which the chemically modified RNA is incubated in vitro with cellular extracts or recombinant proteins (10). However, there are two major drawbacks to this technique. In some cases, the chemical modifications can lead to structural perturbations that can inhibit complex formation. Furthermore, this method can only be used for in vitro studies, and the complexes formed may not reflect the true nature of the complexes formed in vivo.
The second method of tagging is the incorporation of an RNA sequence from a well-characterized RNA–protein interaction. The most widely used RNA–protein interaction is that of the MS2 coat protein and its cognate RNA (11). The U1A protein and its interaction with its cognate RNA have also been used to a lesser extent. Generally, the limiting step when using a known RNA–protein interaction as part of the affinity purification is the inability to efficiently elute or release the purified complex under native conditions since the binding affinity of the known RNA–protein interaction is usually high. One way to circumvent this challenge is to fuse an additional peptide such as the maltose-binding peptide (MBP) to the known protein-binding partner. In this case, there is no requirement for the MS2 coat protein to be immobilized, and the isolation can be performed using the MBP and not the MS2 coat protein. The complex can be released from the affinity resin by elution with maltose (12). Alternatively, a protease cleavage site can be engineered between the MS2 coat protein site and the protein that binds directly to the affinity resin. In this case, purified complexes are released from the resin on specific protease cleavage (13). A bipartite affinity tag has been used to purify RNAs under nondenaturing conditions from in vitro transcription reactions (14). The tag consists of a variant of the hepatitis delta virus (HδV) ribozyme that is activated by imidazole and the tandem stem-loop motifs from the Thermotoga maritima signal recognition particle (SRP) RNA that specifically binds the SRP protein Ffh. On the induced ribozyme cleavage, the target RNA is released from the column, while the ribozyme remains bound through its fused SRP stem-loop motifs.
The use of biotinylated oligonucleotides that are complementary to accessible single-stranded regions of a particular ribonucleoprotein (RNP) complex has also been a successful purification strategy for the isolation of these complexes. Elution of the complex can be achieved under denaturing conditions or through the use of a competitor oligonucleotide to release the RNP under native conditions. This technique has been used to isolate the U4/U6 small nuclear ribonucleoprotein (snRNP) and telomerase (15,16). In both cases, an unstructured and accessible region of RNA sequence was available to facilitate the technique. While this method can be difficult for highly structured RNAs and RNPs, it has several advantages over methods that incorporate a foreign RNA sequence into the target. (1) No misfolding of the RNA occurs in vivo due to the foreign sequence. (2) It can be used when genetic manipulations of the organism to introduce a foreign tag are not possible. (3) Several different sites in the RNA target can be quickly tested for accessibility to synthetic oligonucleotide probes.
The development of in vitro selection or SELEX (systematic evolution of ligands by exponential enrichment) technology has led to the discovery of several RNA or DNA sequences (aptamers) with the ability to bind specifically and with high affinity to small molecules as well as more complex macromolecules (reviewed in ref. 17). One application of the RNA aptamer sequences is their use as tags for RNA affinity purification. RNA aptamers to antibiotics have been used to study RNA–protein interactions in vitro with crude extracts or recombinant proteins (18,19).
To further extend the application of aptamers as RNA affinity tags, our lab has identified two artificially selected RNA aptamers that have compact, defined structures and are particularly useful for the study of RNA–protein complexes formed in vivo and isolated under native conditions (20,21). Their applications to the study of RNA–protein complexes as well as the strengths and weaknesses of each aptamer are described here. Several factors influenced the selection of the target molecule: (1) availability and price of the potential affinity resins, (2) low background affinity (i.e., the resin did not have a high affinity for nonspecific RNAs), and (3) the complexes could be eluted under native conditions. The two target molecules that met these criteria were streptavidin and dextran B512 (in the insoluble form of Sephadex beads), and aptamers to each of these resins were successfully produced (20,21). The consensus sequence for each of these aptamers is shown in Fig. 1. The distinguishing property that makes these aptamers particularly useful for the isolation of active RNP complexes is that both can be eluted from their affinity resins under native conditions. Further advantages and disadvantages of using each aptamer are summarized in Table 1.
Fig. 1.
The minimal binding motifs of both the Sephadex and streptavidin-binding RNA affinity tags (25). These sequences were originally identified using a SELEX (systematic evolution of ligands by exponential enrichment) procedure designed to isolate sequences that displayed binding to the affinity matrix under approximately physiological conditions (50 mM HEPES, pH 7.4, 100 mM MgCl2, 10 mM NaCl). The consensus structures of the two families of aptamers isolated in SELEX are also shown, where X indicates a nonconserved nucleotide identity and ■ indicates basepairing.
Table 1.
Advantage-Disadvantage Comparison Between Two RNA Affinity Tags
| D8 Sephadex RNA motif | S1 Streptavidin RNA motif | |
|---|---|---|
| Advantages | Sephadex (G-200 is best choice) is cheap, and the concentration of ligand on the beads is nearly infinite; purification from large starting quantities of cell extract is practical; elution can be either with denaturants (such as urea) or by competition with soluble dextran B512 (average MW 6,000 or 10,000) | High affinity for streptavidin (Kd ~ 70 nM), but not for egg white avidin (allows blocking of cellular biotin and biotinylated proteins with avidin); avidin and streptavidin reagents for affinity purification and detection are readily available from multiple commercial sources; elutes cleanly and quickly with biotin under native conditions; binding stable to high salt (400 mM NaCl) |
| Disadvantages | Affinity of RNA for the ligand is not as high as with the streptavidin tag, so extensive washing of the resin after binding leads to slow loss of bound RNA; native elution by competition with dextran leaves dextran in the eluate, which is harder to remove than biotin | Resin more expensive; number of binding sites per bead much lower than with Sephadex; egg white avidin is usually needed to block biotin in crude cellular lysates |
The streptavidin aptamer has been used successfully in the affinity purification of ribonuclease P (RNase P) from Saccharomyces cerevisiae (20) and human S3 cells (22) as well as complexes as large as the ribosome (13). The Sephadex aptamer has been used in an extensive study of S. cerevisiae RNase P in which the differential protein composition of the precursor and mature forms of the RNP complex were analyzed (21).
In this chapter, we describe the use of the streptavidin and Sephadex aptamers. We have employed these within the yeast system and have previously isolated active eukaryotic RNase P holoenzyme from S. cerevisiae using either of the two aptamer tags (20,21,23); these are used as illustrative examples within this chapter. Specific examples of the use of the streptavidin aptamer to facilitate isolations from Escherichia coli and Homo sapiens can be found elsewhere (13,22).
2. Materials
2.1. Design of Hybrid RNAs
Computer with a secondary structure prediction program or access to Web-based servers for RNA-folding prediction. Downloadable programs can be found at the following addresses, links to Web-based RNA folding servers can also be found there: for the PC, RNAstructure at http://rna.urmc.rochester. edu/rnastructure.html and RNAdraw at http://www.rnadraw.com; for the Mac, Mulfold at http://iubio.bio.indiana.edu/soft/molbio/mac/.
2.2. Construction of the Hybrid RNA Sequence
2.2.1. Recursive Polymerase Chain Reaction
Template DNA for polymerase chain reaction (PCR), either genomic DNA or plasmid-borne sequence.
Oligonucleotides.
Taq DNA polymerase, supplied with commercial buffer (New England Biolabs).
Agarose gel electrophoresis equipment.
Gel purification miniprep kit (Qiagen).
Restriction enzymes, supplied with commercial buffer (New England Biolabs).
T4 DNA ligase, supplied with commercial buffer (New England Biolabs).
Competent E. coli cells DH5α or XL1-Blue.
Access to an automated DNA sequencing facility.
2.2.2. Site-Directed Mutagenesis
Suitable vector bearing the target RNA sequence.
QuikChange® mutagenesis kit (Stratagene™).
Oligonucleotides.
Restriction enzymes, supplied with commercial buffer (New England Biolabs).
T4 DNA ligase, supplied with commercial buffer (New England Biolabs).
Competent E. coli cells DH5α or XL1-Blue.
Access to an automated DNA sequencing facility.
2.3. Preparing Extracts for Binding
Transformed or transfected cell line suitable for biological system under study.
Appropriate growth media and incubator system for above.
1X lysis buffer: 50 mM HEPES, pH 7.4, 10 mM MgCl2, 100 mM NaCl, 1 mM dithiothreitol (DTT), 0.1% Triton X-100, 10% glycerol, Complete® protease inhibitors (Roche).
Acid-washed glass beads 425–600 μm (Sigma).
Protein content assay, Micro Bicinchoninic acid assay (Pierce).
2.4. Affinity Purification Using Streptavidin
Streptavidin agarose (Sigma).
Avidin from egg white (Sigma).
1X lysis buffer, as in Subheading 2.3., step 3, but without Complete.
Ultrafree-MC centrifugal filter device (Millipore).
D-Biotin (Sigma).
2.5. Affinity Purification Using Sephadex G-200
Sephadex G-200 (bead size 40–120 μm) (Pharmacia).
1X lysis buffer, as in Subheading 2.3., step 3, but without Complete.
Ultrafree-MC centrifugal filter device (Millipore).
Dextran produced by Leuconostoc mesenteroides strain B-512 (approx molecular weight 6,000 or 10,000 Da) (Fluka) (Sigma).
3. Methods
The choice of aptamer to use can be guided by their individual properties, which are summarized in the Heading 1. and Table 1. The methods described next outline (1) the design of the hybrid aptamer-target RNA molecule, (2) the construction of the expression plasmid and strains (in yeast), and (3) the affinity purification of the tagged RNA/complex from cell extracts.
3.1. Guidelines for Designing Aptamer-Tagged RNAs
The most significant step in the use of aptamer tags for the isolation of RNAs and their complexes is in the successful introduction of an aptamer into the target RNA; there are three main considerations. First, the affinity tag must be inserted such that both the aptamer and the target RNA are able to maintain their correct folds (Subheadings 3.1.1.–3.1.4.). Second, it is important to consider the steric effects of using such tags and the potential for interference with the normal function or interactions of the target particle under study (Subheading 3.1.5.). Finally, the aptamer tag may introduce a sensitive target for nuclease degradation, directly affecting the purification and potentially the overall integrity of the particle under study (Subheading 3.1.6.).
3.1.1. Maintaining the Correct Fold of the RNA
Aptamer insertion is most successful where some structural information regarding the target RNA is known or can be obtained. A predicted, and preferably experimentally verified, secondary structure provides an essential starting point for design. It can be very difficult to predict the best position for the aptamer tag, and it is best to simultaneously test several hybrid RNA designs. The aptamer sequence can be inserted internally within the RNA structure at an appropriate position or at either the 5′ or 3′ end.
Not only the position of the aptamer tag should be varied, but also various linkers and spacers that may affect the folding of the RNA or how the tag is presented to the affinity matrix. In addition to testing whether a particular tagged RNA can be used to facilitate isolation, wherever possible, the experiment should be designed to test whether the tagged RNA functions normally in the cell (see Note 1).
3.1.2. Internal Tagging of the RNA
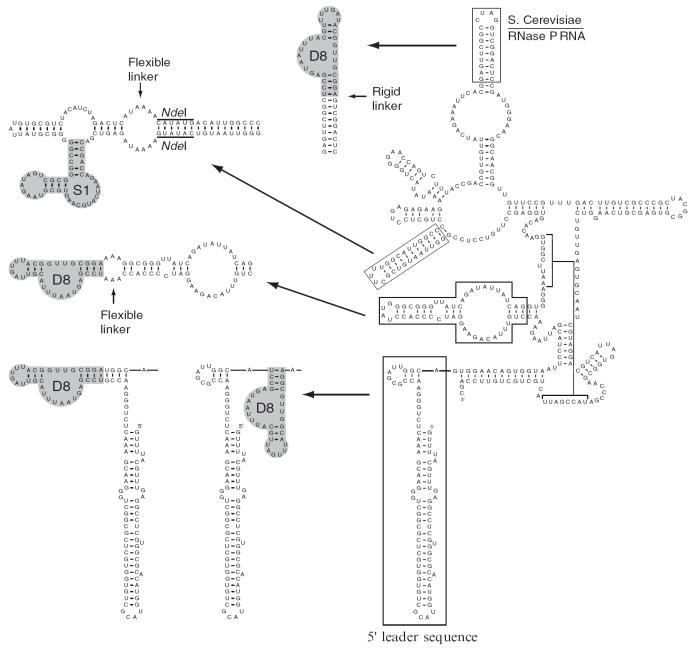
The use of internal tags has been quite successful, and it is recommended that the aptamer be positioned in the terminal loop of a long, nonconserved stem that is known to protrude into solution. When such structural information is available, the design of tagged RNAs is easier, but it is still prudent to design and test several tagged RNAs to increase the chance of success. In the case of the RPR1 RNA from the yeast RNase P, chemical probing has shown that the terminal loops of several helices protrude into solution (24), and aptamer tags at some of these positions have proven to be useful (20,25) (Fig. 2).
Fig. 2.
Affinity tag insertions into both the precursor and the mature RPR1 RNA subunit of RNase P. Individual insertions of both the streptavidin (S1) and Sephadex (D8) aptamers into the RPR1 and pre-RPR1 RNA are shown. The minimal tag sequences are shown with a gray background. The streptavidin (S1) aptamer insertion was carried out using the full, originally identified aptamer sequence, prior to the identification of the minimal sequence. The minimal S1 aptamer sequence has also been used within the RPR1 RNA with equivalent results. The use of both flexible and rigid linking regions of various lengths is illustrated here (refer to Subheading 3.1. for discussion). Constructs were generated by either of the techniques discussed in Subheading 3.2., and an NdeI restriction site used to insert the streptavidin (S1) aptamer is indicated. Each position shown has been used to isolate either the mature RNase P or pre-RNase P from cellular extracts using a singly tagged RNA (see Note 8).
3.1.3. Tagging the Regions Flanking the Target RNA
An alternative strategy is to insert the tag at either the 5′ or 3′ end of a target RNA. This approach has been successful for the isolation of the pre-RNase P holoenzyme through the tagging of its known 5′ leader sequence (23) (Fig. 2). However, it should be noted that the use of these aptamers for the isolation of messenger RNA (mRNA)–protein complexes has not been as successful (see Note 2).
3.1.4. Testing the Design of Hybrid RNAs
After designing the hybrid RNA, it is useful to predict the folding of these sequences in silico. Secondary structure prediction programs based on the Mfold algorithm (26), such as RNAstructure/Mulfold, or the Vienna algorithm (27), such as RNAdraw, are freely available, and there are several Web-based RNA-folding servers available (Subheading 2.1.). The predictions generated by such programs are not always reliable and should be used as a guide only (see Note 3). Ultimately, the best way to test the designed hybrid sequences is to attempt to isolate an RNP from a strain that displays no detectable phenotypic defects. If such attempts fail, it may be due to factors such as steric effects or nuclease degradation, discussed in the next two subheadings. To investigate this possibility, attempting to bind the sequence to the affinity matrix as an in vitro transcribed RNA will confirm whether the tag itself is able to fold correctly within the context of the target RNA sequence.
3.1.5. The Steric Effects of Tagging an RNA
It is difficult to predict whether an aptamer will sterically hinder RNP formation or whether RNP formation will hinder the aptamer function. We reiterate that designing and testing several tagged RNAs is recommended. It can be useful to insert a spacer region between the tag and the RNA, whether the tag is positioned at the end of the RNA or internally. By varying the length of a spacer region or by introducing either a rigid or flexible linker, it is possible to affect how the aptamer tag interacts with both the target complex and the affinity matrix. Figure 2 shows examples of tags in the RPR1 RNA sequence that have been used to successfully isolate RNase P or its precursor complexes from S. cerevisiae.
3.1.6. Maintaining the Tag on the RNA: Considering Nucleases
In general, RNA-based tags are less stable than protein-based tags as they can be susceptible to nuclease degradation. The potential for nuclease degradation is specific to the target RNA, the positioning of the tag, and the system from which purification is being attempted (see Note 4). Here, we discuss some general strategies toward reducing potential nuclease problems. In our experience, we have found that internal tags appear to be less susceptible to nucleases than flanking tags. Degradation by 3′ to 5′ exonucleases can be especially rapid, and a tag on the 3′ end of the RNA may be prone to this. However, flanking tags can be protected from degradation through the use of a strong “closing” stem. Placing a GC-rich stem, with a tetraloop, immediately downstream from the tag will increase the resistance of the RNA to single-strand-specific 3′ to 5′ exonucleases. In addition, any spacers and flexible linkers could potentially introduce targets for endonucleases, and this should be considered.
3.2. Constructing the Hybrid Aptamer Target RNA Sequence
Once a set of hybrid RNAs has been designed, the specific sequences must be prepared and cloned into an appropriate expression vector for transcription within a chosen system. We have used the pRS315 (LEU2-marked) vector for our own work in yeast. The recombinant clones contain the RPR1 RNA sequence along with its natural upstream promoter regions and downstream terminator regions. There are at least two methods available for the construction of a particular hybrid RNA sequence. The first involves the construction of a synthetic gene via recursive, or nested, PCR. The second method involves the introduction of a restriction site via site-directed mutagenesis; the site can be used to allow the direct insertion of any designed aptamer and spacer/linker at this position (see Note 5). The methods are outlined in Subheadings 3.2.1. and 3.2.2. and shown schematically in Fig. 3. DNA manipulations such as performing a PCR reaction, gel isolation of DNA, restriction digests, ligations, transformation of plasmid DNA, or the isolation of plasmid DNA can be performed by any standard protocols.
Fig. 3.
Strategies for the construction of aptamer-tagged RNA sequences. (A) The insertion of the aptamer at the required position using a strategy based on the polymerase chain reaction (PCR). Individual PCR fragments are produced, (i) and (ii), where the aptamer sequence or a required restriction site (RS) is introduced at the desired location by incorporation into the 5′ regions of the primers. After the two aptamer-tagged sequence fragments have been produced and isolated, they are used in trace amounts as template for a further round of PCR. The outlying primers (2 and 3) can be used to amplify the entire region and produce the final product bearing an accurately inserted aptamer within the target RNA sequence. (B) The insertion of an aptamer at a required position using a cloning-based strategy. First, site-directed mutagenesis is used to introduce a suitable restriction site into the target RNA sequence (plasmid borne). The restriction site is used to facilitate the introduction of a synthetic insert bearing the required aptamer sequence. The presence of a correctly oriented insert can be confirmed by PCR screening colonies prior to sequencing.
3.2.1. Generation of a Hybrid RNA Sequence Using Recursive PCR
Once a particular site for the introduction of the aptamer sequence has been designed, it is useful to split the sequence into upstream and downstream sections between which the aptamer sequence is to be inserted (refer to Fig. 3). To design primers that amplify the downstream fragment: For primer 1, there must be sufficient complementarity to the 5′ end of the fragment sequence (20–25 nt), and the aptamer sequence, which does not anneal to the template, is added to the 5′ end of this oligonucleotide. Primer 2 must have sufficient complementarity to the 3′ end of the fragment sequence (20–25 nt), and a suitable restriction site can be added at the 5′ end of the oligonucleotide to facilitate cloning. For the upstream fragment, the aptamer is added at the 3′end of the fragment (primer 4) and the restriction site, or other required sequence (see Note 6), at the 5′ end of the fragment (primer 3). The entire construction can be carried out in three PCR reactions using these four designed primers as outlined next.
The downstream fragment (Fig. 3A(i)) is produced by PCR using primers 1 and 2 using a suitable template DNA encoding the target RNA sequence.
The upstream fragment [Fig. 3A(ii)] is produced by PCR using primers 3 and 4 using a suitable template DNA encoding the target RNA sequence.
The PCR inserts are checked on an agarose gel next to size markers and are gel purified.
The two PCR fragments are used as a template (1–5 ng each fragment) in PCR with primers 2 and 3 to amplify the entire fragment bearing the tagged RNA sequence and introduced 5′ and 3′ restriction sites. The first round of the PCR creates a full-length gene fragment due to the overlapping half-gene fragments, which will be extended by the DNA polymerase.
The correct size of the PCR fragment is checked on an agarose gel next to size markers, and the DNA is gel isolated.
The fragment is cut with the appropriate restriction enzymes at the termini and ligated into a suitable plasmid at the correct restriction sites.
The ligation reaction is transformed to competent DH5α or XL1-Blue cells and plated onto selective media.
Plasmid is isolated from individual colonies, and the PCR insert is verified by appropriate restriction digests and automated DNA sequencing.
3.2.2. Site-Directed Mutagenesis and Aptamer Cloning at the Introduced Restriction Sites
For the specific introduction of restriction sites into a particular sequence, we routinely use the QuikChange kit (Stratagene). This kit uses two user-designed oligonucleotides bearing the mutated sequence to generate two mutant strands from a plasmid template. The parental (methylated) DNA is digested, using DpnI, and the resulting annealed double-stranded, nicked complementary DNA (cDNA) molecules are transformed into E. coli, in which the nicked cDNA is repaired to generate a plasmid bearing the required mutations. For more exact details of the technique, including oligonucleotide design, refer to the product manual. For insertion of an aptamer sequence, within a stem internal to the target RNA, it is possible to mutate the terminal loop of the stem to a single-hexameric restriction site. The site can then be used to introduce the tag by the insertion of synthetic DNA into this restriction site. The result is that the introduced tag is flanked by two copies of the initial restriction site, which could potentially be used for future insertion of altered tags. The overall cloning strategy is represented in Fig. 3B. When using a single restriction site, the direction of the insert must be screened by PCR or by sequencing. In some cases, the sequential introduction of two restrictions sites, to facilitate the insertion of the aptamer sequence, may be necessary.
Restriction site(s) are introduced into the plasmid sequence by site-directed mutagenesis using the QuikChange kit (Stratagene).
The plasmid is cut with the appropriate restriction enzyme. After digestion, the cut vector is treated with alkaline phosphatase to remove terminal phosphates and prevent religation to itself. The DNA is then purified from an agarose gel in preparation for ligation with the insert.
The designed aptamer for insertion is purchased as two individual DNA oligonucleotides bearing the correct overhangs for ligation. To facilitate ligation, these oligonucleotides are individually phosphorylated with polynucleotide kinase. The enzyme is removed by phenol/chloroform extraction, and the two oligonucleotides are annealed in equal concentration by heating to 65 °C and rapid cooling on ice to create a double-stranded insert.
The insert is ligated into the vector at the restriction site.
The ligation reaction is transformed to competent DH5α or XL1-Blue cells and plated onto selective media.
Plasmid is isolated from individual colonies, and the presence and correct orientation of the inserted aptamer are verified by appropriate restriction digests and automated DNA sequencing.
3.3. Preparing Extracts for Binding
Recombinant DNA clones that express the aptamer-tagged RNA sequence should be transformed or transfected into a suitable cell line, dependant on the biological system under study. We have used a yeast strain bearing a chromosomal knockout of the essential RPR1 gene, allowing us to introduce the pRS315-RPR1 plasmid bearing the modified RNA sequence (23). This has allowed us to directly assess the impact of the tagging modifications on the phenotype of the transformed strains. In other systems in which such genetic manipulation may not be possible, the tagged RNA can be introduced into cells containing the chromosomal wild-type copy and will result in the isolation of only the introduced, aptamer-tagged, RNA and its complexes. In these cases, efforts to assess the integrity of the isolated complexes through an appropriate activity assay are required. An example is the successful processing of pre-transfer RNA (tRNA) substrates that was shown by the human RNase P holoenzyme, isolated using the streptavidin aptamer (22). In contrast, cotranscription of the tagged RNA alongside the chromosomal wild-type sequence has been exploited to specifically isolate known lethal mutants of the 23S ribosomal RNA sequence for further study (13).
The particular method used to generate crude extracts will vary depending on the system in which the tagged RNA is being expressed. In general, any protocol for the preparation of crude extracts should focus on first maintaining the temperature at 4 °C throughout and second producing the extract and binding it to beads as quickly as possible to minimize ribonuclease (and protease) degradation of the RNP complexes. For the preparation of extracts from yeast cultures, we use the following protocol:
A single colony of the appropriate yeast strain is grown to saturation in YPD media at 30 °C and used to inoculate 1 L YPD media.
The culture is grown at 30 °C until an OD600 (optical density at 600 nm) of around 1–2, and the cells are harvested by centrifugation at 4000 g for 15 min at 4 °C.
The pellet is washed and resuspended in 10 pellet volumes of ice-cold sterile water, then repelleted by centrifugation at 4000 g for 5 min at 4 °C.
The pellet is resuspended in 3 mL of lysis buffer containing Complete protease inhibitors (Roche).
The cells are lysed by vortexing with one-third volume (~1 mL) of acid-washed beads, 425–600 μm (Sigma) for 20–30 min. Efforts to keep the mixture cool during the lysis procedure are strongly recommended. Periodic cooling on crushed dry ice can be used to keep the sample cool, taking care to avoid freezing the sample. For larger-scale preparations (>20 L culture), we have carried out lysis by passing the resuspended cells (step 4) through a microfluidizer three to four times (model 110Y, Microfluidics). Again, efforts to keep the mixture well cooled throughout the lysis process are strongly recommended.
The lysate is cleared by centrifugation at 14,000g for 20 min at 4 °C. A second ultracentrifugation step is optional.
The protein content in the cleared lysate is determined using a Micro BCA assay (Pierce).
3.4. Isolation of Aptamer-Tagged RNA Complexes From Lysates Using Streptavidin
In the case of the streptavidin aptamer, a background of free biotin and biotinylated cellular material can be blocked by the addition of egg white avidin (Sigma) to the lysate prior to binding to the streptavidin affinity resin (the RNA affinity tag does not bind to egg white avidin). For optimal yields from different lysates, it can be useful to experimentally determine the correct amount of avidin required to block biotin in the lysate. In our hands, lysates produced from yeast cultures can be sufficiently blocked by the addition of 10 μg avidin per milligram protein in the lysate. The streptavidin aptamer can stably bind to the affinity resin at up to 400 mM NaCl, allowing purification under different salt conditions to be attempted if desired.
Block the lysate with 5–20 μg of avidin per milligram of protein in the extract. Incubate at 4 °C for 10 min prior to binding to the affinity resin.
Incubate the lysate with 10–20 μL of streptavidin beads per milligram of protein in the extract. Binding should be carried out at 4 °C for 1 h in 1X lysis buffer at an appropriate volume (5–10 times the bed volume). The beads are separated by centrifugation at 4000 g for 5 min at 4 °C.
Wash the beads with 20 bed volumes of 1X lysis buffer five times for 5 min each at 4 °C.
Transfer the beads to an Ultrafree-MC centrifugal filter unit (0.45-μm pore size Millipore) and wash twice with 5 bed volumes of 1X lysis buffer at 4 °C.
Elute the beads by incubating for 0.5–1 h at 4 °C with 2 bed volumes of 1X lysis buffer containing 5 mM D-biotin.
3.5. Isolation of Aptamer-Tagged RNA From Lysates Using Sephadex G-200
The Sephadex G-200 beads are prepared by swelling 1 g in 50–100 mL of lysis buffer (without Complete) overnight at room temperature, resulting in approx 20 mL of resin. The beads are then washed several times with lysis buffer, and a 50% suspension is prepared.
Incubate the lysate with 10 μL of Sephadex beads per milligram of protein in the extract. Binding should be carried out at 4 °C for 1 h in 1X lysis buffer at an appropriate volume (5–10 times the bed volume). The beads are separated by centrifugation at 4,000g for 5 min at 4 °C.
Wash the beads with 5 volumes of 1X lysis buffer five times for 3 min each at 4 °C.
Transfer the beads to an Ultrafree-MC centrifugal filter unit (0.45-μm pore size Millipore) and wash twice with 2 bed volumes of 1X lysis buffer at 4 °C.
Elute the beads by incubating for 0.5–1 h at 4 °C with 2 bed volumes of 1X lysis buffer containing 50 mg/mL dextran B512 (molecular weight 6,000 or 10,000 Da) (see Note 7).
4. Notes
If an essential RNA is the target of aptamer tagging, then if an appropriate genetic system is available, a knockout strain can be used such that the only copy of the RNA present is the tagged version. This will enable a thorough assessment of the growth phenotype to determine the impact of the tagged RNA on normal functions. In the case of the RPR1 RNA from the S. cerevisiae RNase P, the growth phenotype has been assessed at various temperatures. Northern blot analysis has been carried out to determine that the relative levels of precursor and product RPR1 RNA are normal, and the pre-tRNA processing profile was also shown to be the same as wild type (20,25).
Attempts to purify mRNA and pre-mRNA complexes have not worked well, even though the purified tagged mRNAs themselves were able to bind the affinity resins. However, their complexes did not isolate well from the cellular extracts. It is not clear whether this is because the tags are obscured by the protein, the aptamer structure is antagonized by RNP formation, or both. When the RNA structure is not constrained, we recommend that the investigator consider of the use of biotinylated oligonucleotide tagging (see Heading 1.) as an alternative approach should the use of aptamers fail.
When testing a hybrid RNA design by in silico folding, it can be useful to analyze both the full sequence and also smaller fragments, such as the aptamer and 50 nt of upstream and downstream sequence. Smaller sequence fragments of RNA and their interactions with local sequence elements are often more reliably predicted by such programs.
In the yeast system, it is possible to reduce the overall nuclease activity by constructing a PEP4 deletion strain. The PEP4 gene encodes a protease that is responsible for the maturation of many cellular nucleases; when PEP4 is deleted, these nucleases are not processed and no longer function.
The use of restriction sites to introduce aptamer sequences can place some constraints on the sequence design in these areas. However, these can also be used for future manipulations, allowing alternative tags and spacer/linker designs to be introduced via these sites. Although not necessary for the construction of a gene via PCR, it can be useful to include such sites in the design for this future purpose.
For the rapid production of templates to be used for in vitro transcription of the target RNA, the T7 RNA polymerase promoter sequence (5′-TAATACGACTCACTATAGG-3′) can be encoded by the outlying primer and thus incorporated into the final PCR product. In vitro RNA transcripts produced from such templates can be used to test the binding of the tagged RNA to the affinity matrix in the absence of cellular components.
It has also been possible to elute the aptamer using lower molecular weight dextran, such as enzymatically synthesized dextran (Mr ~ 1500). However, higher concentrations are required (100 mg/mL) as the minimal binding unit for the D8 aptamer is estimated as dextran with 9–10 glucose subunits (molecular weight ~1600–1800). These elution conditions can be used to help facilitate concentration or dialysis.
We have successfully performed dual-affinity steps on a doubly tagged RPR1 RNA construct (both Sephadex and streptavidin aptamers). The doubly tagged strains show no phenotypic defects, and the isolation of active RNase P can be successfully carried out from either of the two inserted tags. The second affinity tag can suffer nuclease degradation during the first affinity step (when it is not being used). We have been able to overcome this by taking great care to keep our extracts cold and working quickly. The use of the Sephadex tag as the initial step is recommended since the low cost allows a larger volume of resin to be used. Employing the streptavidin aptamer second allows the eluted sample to be more concentrated and avoids potential problems associated with removing dextran from the sample. Although successful, the use of dual aptamer tags to purify yeast nuclear RNase P resulted in significantly lower yields when compared to a dual protein affinity tag (TAP-tag).
Acknowledgments
This work was supported by a National Institute of Health grant (R01 6M34869) to D.R.E. We thank both Shaohua Xiao and Rebecca Haeusler for their helpful comments and review of the text.
References
- 1.Nygren PA, Stahl S, Uhlen M. Engineering proteins to facilitate bioprocessing. Trends Biotechnol. 1994;12:184–188. doi: 10.1016/0167-7799(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 2.Porath J. Immobilized metal ion affinity chromatography. Protein Expr Purif. 1992;3:263–281. doi: 10.1016/1046-5928(92)90001-d. [DOI] [PubMed] [Google Scholar]
- 3.Field J, Nikawa J, Broek D, et al. Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol Cell Biol. 1988;8:2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evan GI, Lewis GK, Ramsay G, Bishop JM. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rigaut G, Shevchenko A, Rutz B, Wilm M, Mann M, Séraphin B. A generic protein purification method for protein complex characterization and proteome exploration. Nat Biotechnol. 1999;17:1030–1032. doi: 10.1038/13732. [DOI] [PubMed] [Google Scholar]
- 6.Uhlen M, et al. Gene fusion vectors based on the gene for staphylococcal protein A. Gene. 1983;23:369–378. doi: 10.1016/0378-1119(83)90025-2. [DOI] [PubMed] [Google Scholar]
- 7.Simons PC, Vander Jagt DL. Purification of glutathione S-transferases by glutathione-affinity chromatography. Methods Enzymol. 1981;77:235–237. doi: 10.1016/s0076-6879(81)77031-9. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt TG, Koepke J, Frank R, Skerra A. Molecular interaction between the Strep-tag affinity peptide and its cognate target, streptavidin. J Mol Biol. 1996;255:753–766. doi: 10.1006/jmbi.1996.0061. [DOI] [PubMed] [Google Scholar]
- 9.Prickett KS, Amberg DC, Hopp TP. A calcium-dependent antibody for identification and purification of recombinant proteins. Biotechniques. 1989;7:580–589. [PubMed] [Google Scholar]
- 10.Rouault TA, Hentze MW, Haile DJ, Harford JB, Klausner RD. The iron-responsive element binding protein: a method for the affinity purification of a regulatory RNA-binding protein. Proc Natl Acad Sci U S A. 1989;86:5768–5772. doi: 10.1073/pnas.86.15.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bardwell VJ, Wickens M. Purification of RNA and RNA-protein complexes by an R17 coat protein affinity method. Nucleic Acids Res. 1990;18:6587–6594. doi: 10.1093/nar/18.22.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das R, Zhou Z, Reed R. Functional association of U2 snRNP with the ATP-independent spliceosomal complex E. Mol Cell. 2000;5:779–787. doi: 10.1016/s1097-2765(00)80318-4. [DOI] [PubMed] [Google Scholar]
- 13.Leonov AA, Sergiev PV, Bogdanov AA, Brimacombe R, Dontsova OA. Affinity purification of ribosomes with a lethal G2655C mutation in 23 S rRNA affects the translocation. J Biol Chem. 2003;278:25664–25670. doi: 10.1074/jbc.M302873200. [DOI] [PubMed] [Google Scholar]
- 14.Kieft JS, Batey RT. A general method for rapid and nondenaturing purification of RNAs. RNA. 2004;10:988–995. doi: 10.1261/rna.7040604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blencowe BJ, Sproat BS, Ryder U, Barabino S, Lamond AI. Antisense probing of the human U4/U6 snRNP with biotinylated 2′-OMe RNA oligonucleotides. Cell. 1989;59:531–539. doi: 10.1016/0092-8674(89)90036-6. [DOI] [PubMed] [Google Scholar]
- 16.Lingner J, Cech TR. Purification of telomerase from Euplotes aediculatus: requirement of a primer 3′ overhang. Proc Natl Acad Sci U S A. 1996;93:10712–10717. doi: 10.1073/pnas.93.20.10712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson DS, Szostak JW. In vitro selection of functional nucleic acids. Annu Rev Biochem. 1999;68:611–647. doi: 10.1146/annurev.biochem.68.1.611. [DOI] [PubMed] [Google Scholar]
- 18.Bachler M, Schroeder R, von Ahsen U. StreptoTag: a novel method for the isolation of RNA-binding proteins. RNA. 1999;5:1509–1516. doi: 10.1017/s1355838299991574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartmurth K, Vornlocher HP, Luhrmann R. Tobramycin affinity tag purification of spliceosomes. Methods Mol Biol. 2004;257:47–64. doi: 10.1385/1-59259-750-5:047. [DOI] [PubMed] [Google Scholar]
- 20.Srisawat C, Engelke DR. Streptavidin aptamers: affinity tags for the study of RNAs and ribonucleoproteins. RNA. 2001;7:632–641. doi: 10.1017/s135583820100245x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srisawat C, Goldstein IJ, Engelke DR. Sephadex-binding RNA ligands: rapid affinity purification of RNA from complex RNA mixtures. Nucleic Acids Res. 2001;29:e4. doi: 10.1093/nar/29.2.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Altman S. Partial reconstitution of human RNase P in HeLa cells between its RNA subunit with an affinity tag and the intact protein components. Nucleic Acids Res. 2002;30:3706–3711. doi: 10.1093/nar/gkf499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srisawat C, Houser-Scott F, Bertrand E, Xiao S, Singer RH, Engelke DR. An active precursor in assembly of yeast nuclear ribonuclease P. RNA. 2002;8:1348–1360. doi: 10.1017/s1355838202027048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tranguch AJ, Kindelberger DW, Rohlman CE, Lee JY, Engelke DR. Structure-sensitive RNA footprinting of yeast nuclear ribonuclease P. Biochemistry. 1994;33:1778–1787. doi: 10.1021/bi00173a022. [DOI] [PubMed] [Google Scholar]
- 25.Srisawat C, Engelke DR. RNA affinity tags for purification of RNAs and ribonucleoprotein complexes. Methods. 2002;26:156–161. doi: 10.1016/S1046-2023(02)00018-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuker M. Mfold Web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hofacker IL. Vienna RNA secondary structure server. Nucleic Acids Res. 2003;31:3429–3431. doi: 10.1093/nar/gkg599. [DOI] [PMC free article] [PubMed] [Google Scholar]