Abstract
Ischemia reperfusion injury (IRI) is pivotal for renal fibrosis development via peritubular capillaries injury. Coagulation represents a key mechanism involved in this process. Melagatran® (M), a thrombin inhibitor, was evaluated in an autotransplanted kidney model, using Large White pigs. To mimic deceased after cardiac death donor conditions, kidneys underwent warm ischemia (WI) for 60 min before cold preservation for 24 hours in University of Wisconsin solution. Treatment with M before WI and/or in the preservation solution drastically improved survival at 3 months, reduced renal dysfunction related to a critical reduction in interstitial fibrosis, measured by Sirius Red staining. Tissue analysis revealed reduced expression of transforming growth factor-β (TGF-β) and activation level of its effectors phospho-Smad3, Smad 4 and connective tissue growth factor (CTGF) after M treatment. Fibrinolysis activation was also observed, evidenced by downregulation of PAI-1 protein and gene expression. In addition, M reduced S100A4 expression and vimentin staining, which are markers for epithelial mesenchymal transition, a major pathway to chronic kidney fibrosis. Finally, expression of oxidative stress markers Nox2 and iNOS was reduced. We conclude that inhibition of thrombin is an effective therapy against IRI which reduces chronic graft fibrosis, with a significantly positive effect on survival.
Keywords: Direct thrombin inhibitor, Kidney preservation, Graft function, Ischemia Reperfusion Injury, Interstitial Fibrosis/Tubular Atrophy
INTRODUCTION
Kidney transplantation remains the therapy of choice for end stage renal diseases. Due to the shortage of organs of deceased donors available for transplantation, organs from older and more ‘marginal/extended’ donors are collected. Uncontrolled deceased after cardiac death donors (DCD) are becoming an increasing source of organs. Kidneys from such donors are exposed to much greater ischemia reperfusion (IR) damage before recovery and show reduced chances for proper early as well as long-term function.
Organ transplantation raises a number of important issues regarding microcirculation conditions, hemorheology and coagulation (1, 2). In such a situation, stasis induces microthrombosis and further deterioration of capillary integrity which contributes to the impairment of capillary perfusion (2). Regional alterations in kidney blood flow persist after ischemic injury and play a central role in the extension of ischemic injury during reperfusion (3–5). When blood flow is restored during reperfusion, the critical phenomenon of “no reflow” can arise and a large number of capillaries fail to reperfuse. Several hypotheses are plausible: 1. IR induced microvascular thrombus production; 2. IR induced neutrophils activation and their adhesion on vascular endothelium. Both phenomena can induce endothelial swelling and “no reflow” via reduction of the capillary diameter (6–8). Thus, altered coagulation and inflammation contribute to IRI. Kidney fibrosis following transplantation has become recognized as a main contributor of chronic allograft nephropathy (9, 10).
This dynamic and complex process is classically defined as a reparative mechanism in which tissue growth is counterbalanced by a high rate of extracellular matrix (ECM) turnover. Several mechanisms and fibrogenic factors are implicated, such as TGF-β, CTGF, or plasminogen activator inhibitor-1 (PAI-1) (11–14). Epithelial-mesenchymal transition (EMT) is also an important mechanism characterized by a tubular cell differentiation to mesenchymal cell able to produce ECM. Our team demonstrated that in a large white pig autotransplantation model, severe IRI could lead to chronic fibrosis and subsequent renal failure (15, 16). IRI thus appears to be strongly linked to chronic deleterious events in the graft, validating the renewed interest in the study of this event and the search for therapeutic strategies to alleviate it.
Melagatran® (M) is a low molecular weight molecule, specific inhibitor of thrombin generation (17–19) and potent inhibitor of platelet activation (20, 21). We have previously demonstrated that: 1. M decreases tissue factor (TF) expression in platelet/monocyte heterotypic complexes from healthy human donors and 2. M was demonstrated to be directly protective against endothelial cell activation and inflammation in vitro and 3. M administration in the peri-preservation period improved graft outcome and ameliorated the early consequences of IRI in a model of pig kidney autotransplantation (22, 23), however the chronic effects of this treatment remained unknown. We thus designed the present study to assess the effect of M treatment on the development of chronic graft fibrosis, through its potential action on injury mechanisms of hypoxia, oxidative stress, and EMT.
MATERIAL AND METHODS
Surgical procedures and Experimental groups
Large white male pigs (INRA, GEPA, Le Magneraud, Surgères, France) weighting 30 to 35 kg were prepared as previously described (24–26). Animal protocol was in accordance with INRA ethical guidelines. Surgical teams were blinded regarding the treatment applied to each graft. To mimic conditions found in DCD donors, renal warm ischemia (WI) was induced by clamping the right renal pedicle for 60 min with a vascular nontraumatic clamp. These conditions has been already described as a model inducing consistent damage (26) in conditions reproducing DCD (27). Then, the kidney was collected, cold flushed, and preserved for 24 hours before transplantation. In each experimental group, the left kidney was removed during transplantation to mimic the nephron mass in transplanted situation. Time for vascular anastomosis was 30±5 min. Six groups were studied: 1-UW: kidneys preserved in University of Wisconsin (UW) solution; 2-UW+Hc: kidney preserved in UW to which is added Heparin (5,000 IU/L); 3-UW+Hc+Hiv: kidneys from pigs treated with Heparin (500 IU/Kg) IV 10 min before WI and preserved in UW to which was added Heparin (5,000 IU/L); 4-UW+Mc: kidney preserved in UW to which was added 0.3 mg/L M; 5-UW+Mc+Miv: kidneys from pigs treated with M (3mg/kg/10min intravenously) 30 min before WI and preserved in UW to which was added 0.3 mg/L M; 6- Normal animals (Control; age, and weight mass matched group). Seven animals were studied in groups UW and UW+Hc, ten animals were studied in M groups as well as in Control and eleven animals were studied in the UW+Hc+Hiv group. High mortality in groups 1 and 2 lead us to ethically limit the number of animals used.
Functional parameters
Pigs were placed in a metabolic cage to allow specific 24 h urine collections. Plasma creatinine and urinary proteins were measured using an automatic analyzer (Modular automatic analyzer, Roche Diagnostic, Meylan, France).
Histopathological studies
Pigs were euthanasied at 3 month, date at which we previously demonstrated development of chronic fibrosis in these grafts (26). All sections, covering both cortex and medulla, were examined under blinded conditions by a pathologist and a nephrologist. As described previously, a standard procedure was used to estimate the level of tubulointerstitial fibrosis using Picro Sirius staining (28). Briefly, on 5 non-overlapping sections the percentage of the fibrotic area was measured by a image analysis software, and mean±SEM was calculated. Vimentin (1:100, Dakopatts, Stockolm, Sweden) and α-smooth muscle actin (α-SMA; 1:50; Sigma, St Louis, MO, USA) were employed as marker of EMT. Immunoreactivity was expressed in a semiquantitative score as percentage of stained cells per high power field (x200). Five fields were surveyed for each graft.
Western blotting procedure
A standard Western blotting protocol was used, as described previously (26, 29). Antibodies against TGFβ (1:600), pSmad3 (1:1000), Smad3 (1:200), Smad4 (1:200), Smad7 (1:200) and tPa (1:120) were purchased from Santa Cruz Biotechnology (Santa Cruz, USA); antibodies against CTGF were from Biovision (1:500, Mountain View, CA, USA), against PAI-1 was from BD Bioscience (1:200, San Jose, CA, USA), and loading control β actin antibody was purchased from Sigma (1:3000, Sigma, Lyon, France). Proper secondary antibody coupled with horseradish peroxydase (HRP) was used (all from GE Healthcare, Bordeaux, France) and bands were revealed using standard procedures. Intensities of the protein bands were determined and quantified using AlphaEase FC software (AlphaInnotech Corporation, San Leandro, CA,USA).
Determination of mRNA expression by quantitative Real Time PCR
Tissues obtained at three month were snap frozen in liquid nitrogen and processed for RNA extraction using Trizol (Fisher Scientific, Illkirch, France) according to the manufacturer’s recommandations. Genomic DNA was removed using DNA-free kit (PE Applied Biosystems, Foster City, CA, USA). Each cDNA template for RT-PCR was prepared by first-strand reverse transcription using random primers (Applied Biosystems). Real Time PCR assays were performed in du/triplicate with 10ng total cDNA templates in 96-well optical plates on an ABI Prism7300 Sequence Detection System following the manufacturer’s recommendations (Applied Biosystems). Porcine primers were designed using OligoPerfect ™ (Invitrogen, Carlsbad, CA, USA), with the sequences detailed in Table 1. Finally, mRNA expression level in the sample relative to expression in normal tissue was obtained with the 2(-Delta Delta C(T)) Method (30).
Table 1.
Primer sequences for RT-PCR analysis in Pig Kidneys.
| Gene | Forward | Reverse |
|---|---|---|
| 18S | AGCCTGCGGCTTAATTTGAC | AACCAGACAAATCGCTCCAC |
| PAI-1 | TTGAGGAGAAGGGCATGG | CATCGGCCGTGCTG |
| Nox2 | GTGCACCATGATGAGGAGAA | AGTTAGGCCGTCCGTACAAG |
| iNOS | CCCCAAATACGAGTGGTTCC | CCACCTCGAGCAGCATGT |
| S100A4 | GGAAAAGGACGGATGAAGC | GAAGACGCAGTACTCCTGGAA |
Statistical methods
Results are shown as mean ± SEM. For the statistical analysis among groups, we used an ANOVA analysis with Newman Keuls test for multiple comparisons. Student T-test as well as Unpaired t test with Welch correction was used for Real Time PCR data. Statistical significance was accepted for P <0.05.
RESULTS
Melagatran® preserved graft function and promoted survival
Survival at 3 month post transplant is presented in Table 2. Primary non function defined by absence of urine production at day 7 after reperfusion was observed in all animals in groups UW and UW+Hc with a plasma creatinine level ranging between 2100 and 2850 μmol/L; these were not included in the remainder of the study. Survivals of pigs with kidneys preserved in UW+Hc+Hiv was 27.3%, whereas pigs with grafts preserved in M-added solution had 90% survival (UW+Mc, p<0.05 to UW+Hc+Hiv). Addition of M IV prior to WI achieved the same survival (90%, UW+Miv+Mc, p<0.05 to UW+Hc+Hiv). Animals lost to surgical complications were excluded from the analysis. Animal loss was due to graft failure, showing necrosis and extensive tubular atrophy, fibrosis and absence of efficient (or adapted) regenerative process. No sign of graft thrombosis was detected.
Table 2.
Survival and renal function evaluation in transplanted animals at 3 month.
| Control | UW+Mc+Miv | UW+Mc | UW+Hc+Hiv | |
|---|---|---|---|---|
| Survival (%) | N/A | 90* | 90* | 27.3 |
| Plasma creatinine μmol/L | 104.8 ± 4.9 | 130 ± 6.5* £ | 167 ± 8.5* | 435 ± 47.5 |
| Proteinuria/Creatinuria ratio (mg/mmol) | 0.3± 0.1 | 11.3 ± 0.8* | 42.8± 3.5* | 176.7± 8.8 |
Shown are mean±SEM,
p<0.05 vs. UW+Hc+Hiv,
p < 0.05 versus UW+Mc. n=10–11.
This striking survival difference was reflected by plasma creatinine measurements: whereas UW+Hc+Hiv grafts showed high levels of creatinine (435.0 ± 47.5 μmol/L), grafts in the UW+Mc group displayed significantly improved filtration capabilities (167 ± 8.5 μmol/L, p<0.05 to UW+Hc+Hiv), which were significantly further ameliorated in UW+Mc+Miv grafts (130 ± 6.5 μmol/L, p<0.05 vs. UW+Hc+Hiv, p<0.05 vs. UW+Mc). Similar findings were found regarding proteinuria: while UW+Hc+Hiv grafts presented high levels of proteins in urine (3.76 ± 0.47 g/24h), UW+Mc and UW+Mc+Miv grafts showed reduced levels of proteins excretion (0.48 ± 0.05 g/24h and 0.40 ± 0.04 g/24h respectively, p<0.05 vs. UW+Hc+Hiv).
Therefore, M IV prior to WI as well as in the cold storage solution appeared to further protect the graft regarding long term outcome.
Treatment with Melagatran® prevented interstitial fibrosis
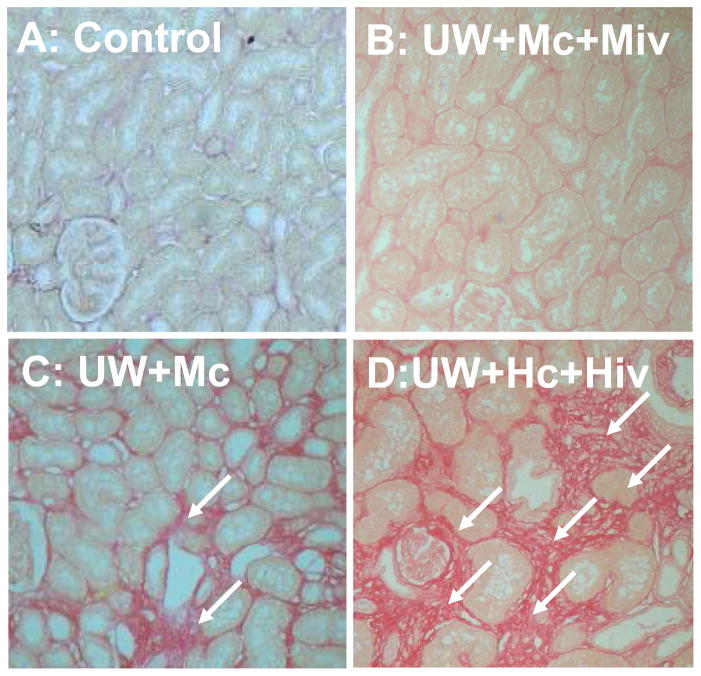
In order to determine the impact of graft treatment with M on chronic fibrosis, we evaluated the degree of interstitial collagen deposition using Picro sirius staining (Fig 1, Table 3). We found that control pigs display less than 5% of renal fibrosis. Kidneys preserved with UW+Hc+Hiv showed extensive interstitial fibrosis (47.09 ± 4.26%, Fig 1A), while kidneys preserved with UW+Mc displayed more moderate staining (18.71 ± 2.97 %, Fig 1B) and kidneys preserved with UW+Mc and Melagatran IV injection showed little fibrosis of the parenchyma, close to levels found in controls (7.43 ± 0.23 %, Fig 1C, p<0.05 versus UW+Hc+Hiv). Similarly to the serum creatinine, fibrosis was inferior in UW+Mc+Miv compared to UW+Mc (p<0.05), indicating a possible additive effect of M. Therefore, treatment with M in the peri-IRI period seemed to protect efficiently against the development of chronic interstitial fibrosis three months post transplant.
Figure 1. Histological evaluation of tissue Fibrosis at 3 months.
Tissue sections were stained with Picro Sirius. Shown are representative staining of grafts from Control (A), UW+Mc+Miv (B), UW+Mc (C) and UW+Hc+Hiv (D) groups. Arrows designate areas with intense interstitial fibrosis. Magnification 100X.
Table 3.
Semi quantitative evaluation of the extend of fibrosis in transplanted kidney grafts at 3 month
| Control | UW+Mc+Miv | UW+Mc | UW+Hc+Hiv | |
|---|---|---|---|---|
| Fibrosis (%, Sirius Red) | <5 | 7.43 ± 0.23* £ | 18.71 ± 2.97* | 47.09 ± 4.26 |
Shown are mean±SEM,
p<0.05 vs. UW+Hc+Hiv,
p < 0.05 versus UW+Mc. n=3–5.
Melagatran® impacted TGF-β signaling in the graft
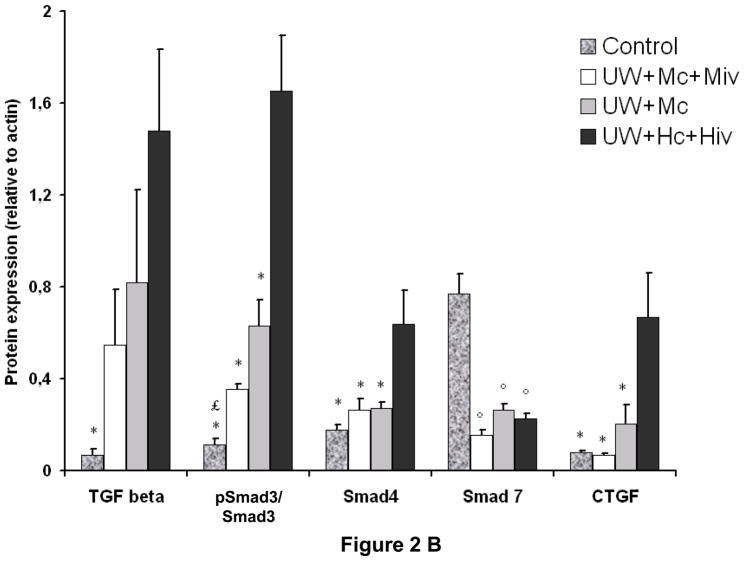
To further define the impact of treatment with M on tissue fibrosis, we analyzed TGF-β signaling, a critical pathway involved in graft chronic fibrosis (Fig 2). We demonstrated that the high degree of collagen deposition in grafts from the UW+Hc+Hiv group was paralleled with high TGF-β expression compared to control (1.48±0.36, p<0.05 versus 0.07±0.03 in Control). Grafts from the two M-treated groups displayed lower levels of TGF-β expression. The intensity of the decrease seemed to correlate with the intensity of the treatment: M in the preservation solution appeared to decrease TGF-β expression to a lesser degree than M both in the solution and IV injection.
Figure 2. Western Blot analysis of the TGFβ signaling pathway in kidney graft, 3 months post transplantation.
A: representative blots for each antibody used in the four groups of survivors. B: Quantitative analysis by densitometry of blots. Psmad3 is expressed related to Smad3 expression. Shown are mean±SEM, * p < 0.05 versus UW+Hc+Hiv; ° p < 0.05 versus Control, £ p < 0.05 versus UW+Mc. n=3–5.
Furthermore, while in UW+Hc+Hiv group there was intense phosphorylation of Smad 3 (1.65±0.24, p<0.05 versus all other groups), the detected levels of phospho-Smad3 in UW+Mc was lower (0.63±0.11, p<0.05 versus control) and moreover the activation of the protein was almost suppressed in the UW+Mc+Miv group (0.35±0.02, NS versus control). The protective effect of M treatment was even more obvious on the downstream effectors and targets of TGF-β signaling: indeed both the expression of Smad4 and connective tissue growth factor (CTGF) was suppressed in UW+Mc and UW+Mc+Miv groups, while it was found to be elevated in the UW+Hc+Hiv group (0.64±0.14 for Smad4, p<0.05 to all other groups, 0.67±0.19 for CTGF, p<0.05 to all other groups). Finally, expression of the protein level of Smad 7, inhibitor of the TGF-β signaling pathway, was decreased in all groups (p<0.05 to control). Therefore, treatment with M significantly reduced the activation of the TGF-β signaling pathway in the transplanted kidney 3 months later, with a further protection offered to the graft by the injection of M IV prior to WI..
Melagatran® protected against tPA/PAI-1 imbalance
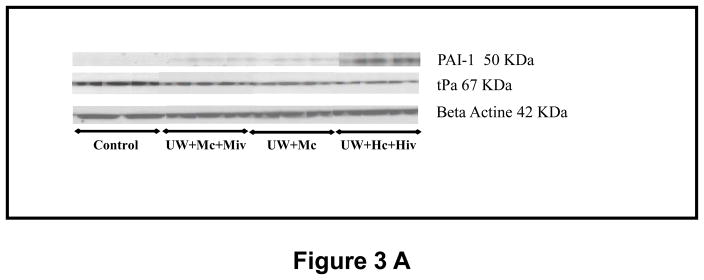
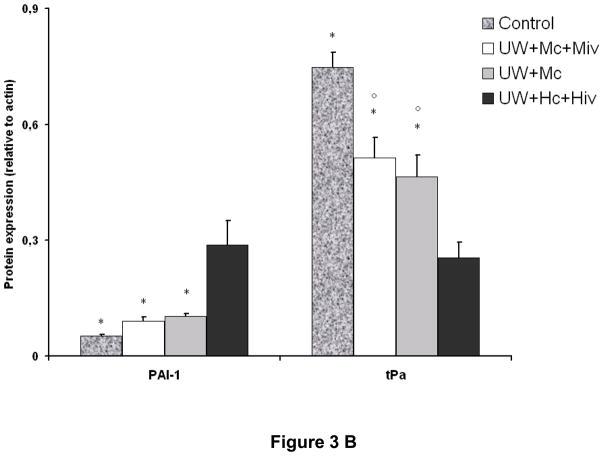
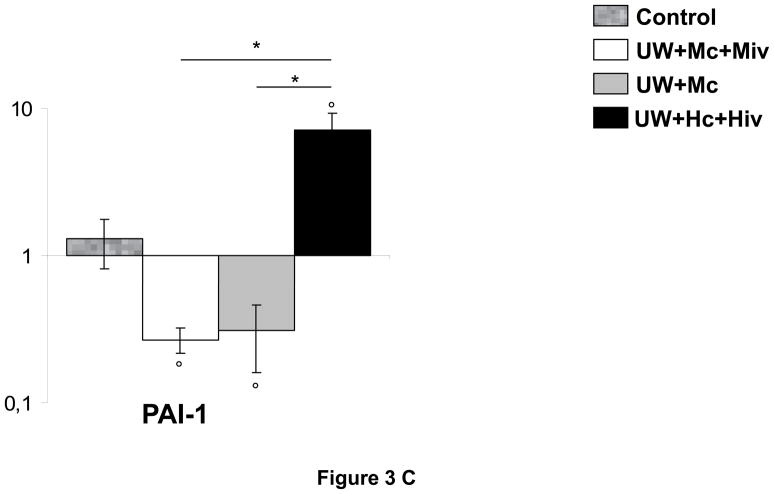
The deposition of fibrin in the small vessels and capillaries of the graft is a critical element in the development of chronic kidney fibrosis (Fig 3, A and B). Analysis of the expression of tissue plasminogen activator (tPA), an enhancer of fibrinolysis and thus protective of the graft, revealed that the protein was critically suppressed in grafts of the UW+Hc+Hiv group (0.25±0.04, p<0.05 to all other groups), whereas downregulation was less extensive in the M-treated groups (0.46±0.06 in UW+Mc and 0.51±0.05 in UW+Mc+Miv, versus 0.75±0.04 in Control, p<0.05 for both). Supporting this observation, we found that plasminogen activator inhibitor 1 (PAI-1), inhibitor of tPA, was overexpressed in UW+Hc+Hiv group (p<0.05 to all other groups), whereas it was not altered significantly in grafts treated with M. Modulation of PAI-1 expression was confirmed by real time PCR: in UW+Hc+Hiv grafts, expression of PAI-1 mRNA was significantly increased (5.48±1.83 folds to Control, p<0.05 to Control), whereas it was decreased in M treated groups: UW+Mc was 0.31±0.15 folds (p<0.05 to Control) and UW+Mc+Miv was 0.27±0.05 folds (p<0.05 to Control), with both groups being significantly different from the UW+Hc+Hiv group (p<0.05 in both cases). We thus demonstrate that M exposure in the peri-preservation period has a long term protective effect on the graft against the inhibition of fibrinolysis in the vessels, underlined by tPA downregulation and PAI-1 upregulation, also known to promote fibrosis.
Figure 3. mRNA and protein expression of tPA and PAI-1 in transplanted kidneys, 3 months post transplantation.
A: representative blots for each antibody used in the four surviving groups. B: Quantitative analysis by densitometry of blots, shown are mean±SEM, Statistics: * p<0.05 versus UW+Hc+Hiv; ° p<0.05 versus Control; n=2–5. C: Quantitative Real Time PCR analysis of PAI-1 expression normalized to Control. Shown are mean±SEM, Statistics to Control: °:p<0.05. Statistics inter group: *:p<0.05. n=3–5.
Melagatran® limited EMT within the graft
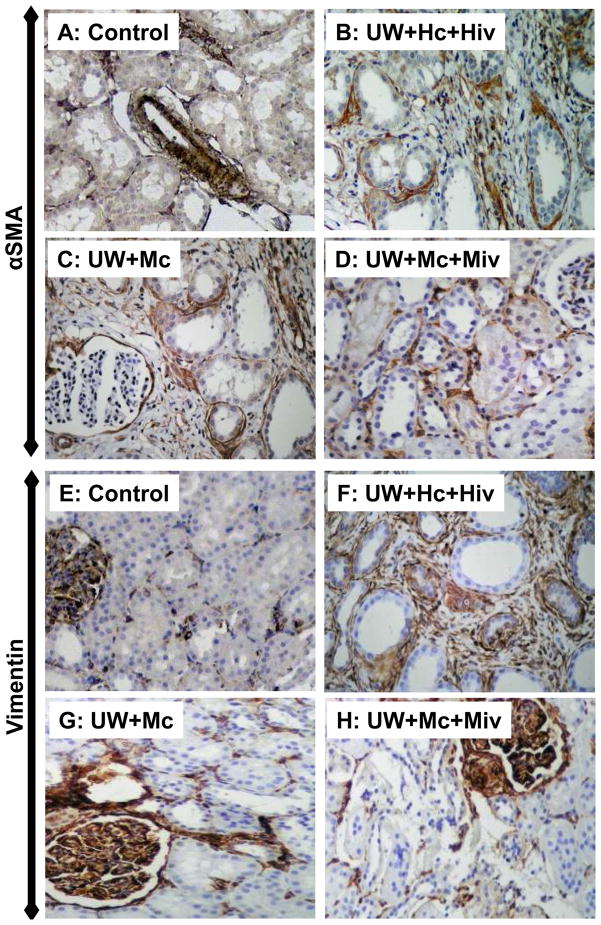
To investigate the effect of the treatment on epithelial to mesenchymal transition, a critical injury mechanism in chronic fibrosis, we measured the expression of αSMA and Vimentin in kidney grafts 3 months after transplantation (Fig 4). As shown in Table 4, UW+Hc+Hiv grafts showed extensive staining for αSMA (23%) and Vimentin (Vim, 50%), indicating EMT. On the other hand, UW+Mc kidneys showed significantly reduced staining for αSMA and Vimentin (14% and 30% respectively, p<0.05 vs. UW+Hc+Hiv for both). Further staining reduction was observed in UW+Mc+Miv grafts (αSMA=8% and Vim=15%, p<0.05 vs. UW+Hc+Hiv for both). Moreover, αSMA staining was reduced in UW+Mc+Miv compared to UW+Mc (p<0.05).
Figure 4. Histological evaluation of αSMA and Vimentin staining.
Shown are representative stainings of αSMA (A–D) and Vimentin (E–H) of kidneys from Control (A,E), UW+Hc+Hiv (B, F), UW+Mc (C, G) and UW+Mc+Miv (D, H) groups. Magnification 200X
Table 4.
Semi quantitative evaluation of αSMA and Vimentin immunostaining in transplanted kidney grafts at 3 month
Shown are % of stained cells per field as explained in methods.
p<0.05 vs. UW+Hc+Hiv,
p < 0.05 versus UW+Mc. n=3–5.
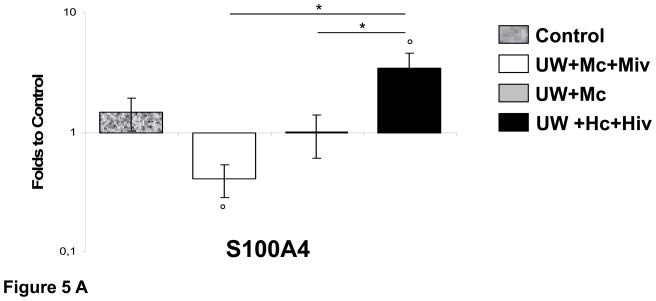
Furthermore, we performed Real Time PCR analysis for S100A4, a well described marker of EMT, in grafts preserved with our three different conditions (Fig 5A). We determined that whereas S100A4 was over expressed in grafts preserved with UW+Hc+Hiv (3.43±0.84 folds, p<0.05 vs. control), presence of M in preservation solution allowed this expression to remain at control levels (1.01±0.40 folds, p<0.05 vs. UW+Hc+Hiv) and further exposure to M through IV injection before WI allowed downregulation of this marker (0.41±0.13 folds, p<0.05 vs. UW+Hc+Hiv). Use of M in the peri-transplant period is thus able to prevent the onset of EMT in the graft and fibrosis development.
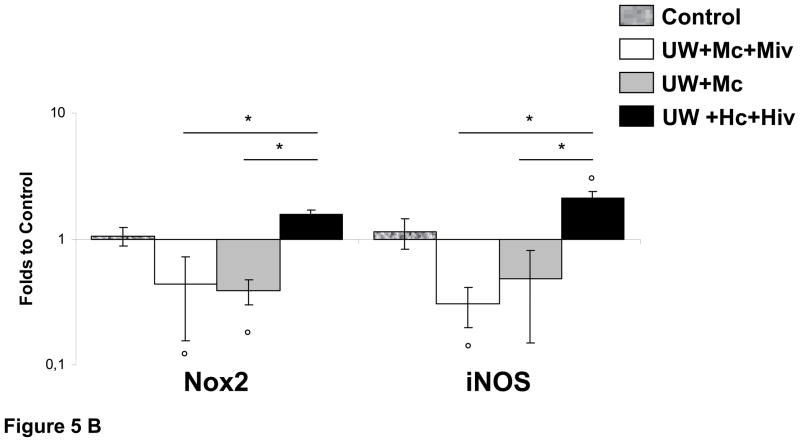
Figure 5. Quantitative Real Time analysis of Nox2, iNOS and S100A4 in transplanted kidneys 3 month after reperfusion.
A: S100A4 expression normalized to Control. B: Analysis of Nox2 and iNOS expression, normalized to Control. Shown are mean±SEM, Statistics to Control: °:p<0.05. Statistics inter group: *:p<0.05. n=3–5.
Melagatran® reduced oxidative stress markers expression within the graft
To explore possible changes in oxidative stress, another critical injury pathway, we measured the changes in gene expression of Nox2, an element of NADPH oxidase, responsible for the production of reactive oxygen species (ROS); and the inducible form of nitric oxide synthase (iNOS), responsible for the production of nitric oxide (NO) under inflammatory conditions (Fig 5B). Expression of Nox2 was elevated in grafts preserved by UW+Hc+Hiv (1.58±0.11 folds), whereas in grafts exposed to M the expression was lower than in control (0.39±0.09 folds in UW+Mc, p<0.05 to Control; and 0.43±0.28 folds in UW+Mc+Miv, p<0.05 to Control); these levels were significantly inferior compared to the UW+Hc+Hiv group (p<0.05 for both). A similar pattern was found for iNOS gene expression: grafts of the UW+Hc+Hiv group exhibited a significant overexpression (2.11±0.25 folds, p<0.05 vs. Control), while treatment with M was significantly protective against such overexpression: UW+Mc: 0.48±0.33 folds; and UW+Mc+Miv: 0.30±0.11 folds (p<0.05 vs. UW+Hc+Hiv for both). In conclusion, we detected a moderate increase expression of oxidative stress markers in UW+Hc+Hiv grafts, while M treatment reduced this expression.
DISCUSSION
This study demonstrates that in a pig model mimicking the conditions found in DCD, anticoagulant therapy by antithrombin added to the standard UW flush and preservation medium substantially improved survival and renal functional recovery, with a decrease in interstitial fibrosis at 3 months. This work is one of the few studies shedding light, on the mechanistic level, on the long term consequences of IRI: we show that several pathways are implicated such as the TGF-β, PAI1/tPA pathways as well as EMT, and possibly oxidative stress, and determined that M treatment reduced their activation. Moreover, this study is conducted in a large mammal model, and presents an efficient therapeutic strategy to alleviate IRI.
This is particularly relevant when considering that mechanistic results obtained at 3 month on grafts preserved with UW+Hc+Hiv were obtained only from the remaining pigs (27.3% survival rate) and thus represent the situation in the grafts of the survivors. This implies that the processes we observed in these grafts were likely even more intense in the other grafts of this group, leading to the early death of the animals and outlines the weak impact of heparin when compared to M. Our results appear in conflict with a study on the effect of M treatment in a rat model of IRI in which it was observed that treatment did not have any effect on injury development (31). However, the use of 35 min WI in rats does not induces as extensive an injury as 60min WI and 24h cold storage in pig kidneys, moreover there are important anatomical differences between rat and pig kidney. These considerations render difficult the comparison between the two studies.
The current study demonstrates that relative increases of TGF-β and expression in the kidney was accompanied by upregulation and activation of its Smad effectors. TGF-β receptors dimerize and change their conformation upon binding TGF-β then receptor-regulated Smad-2 and Smad-3 are phosphorylated by the TGF-β receptor, a process that induces linking to Smad-4 and therefore initiates recruitment of transcriptional cofactors involved in cell proliferation, tissue growth, and matrix remodeling by enhanced collagen synthesis (12). In this study, we show that concomitantly with increased TGF-β expression, there was increased Smad 3 phosphorylation and Smad 4 expression in graft with intense interstitial fibrosis. Furthermore, we show upregulation of CTGF, an important downstream effector of TGF-β involved in fibrosis (32, 33) and EMT (11). Smad 7, inhibitor of the TGF-β pathway, was downregulated in all groups. The regulation of the TGF-β pathway during chronic kidney injury is complex and still unclear, and no direct link is drawn between M and TGF-β. The correlation between intensity of IRI and chronic injury has been demonstrated (34, 35), however the mechanisms through which such correlation is possible remain unclear. In the present study, we show that decreasing the intensity of IRI by peri-preservation treatment with a thrombin inhibitor plays an active role in limiting the chronic activation of TGF-β; this provides a possible area of study to link IRI and chronic fibrosis mechanistically, and reinforce the pivotal role of the coagulation cascade in the mechanisms of IRI.
Fibrin deposition induces an acute flow blockade and ultimately vasculo-sclerosis, hence chronically limiting blood flow to the tissue. Plasmin, the protease that catalyzes fibrin degradation, is activated by a tightly regulated balance between plasminogen activators (tPA or uPA) and plasminogen activator inhibitors (36, 37). Among the latter, PAI-1 drew the interest of several investigators as the culprit of persistent fibrin deposition in different animal models of chronic rejection (13, 14). Interestingly, plasmin can degrade not only fibrin but also many extracellular matrix components, indirectly linking plasmin inhibition by PAI-1 to ECM accumulation. Moreover, PAI-1 complexes tPA and other targeted proteinases to inactivate them, hence promoting fibrin deposition and fibrosis. We presently show that IRI induced a chronic upregulation of PAI-1, correlated with a downregulation of tPA, and further that the mitigation of IRI through inhibition of thrombin had a chronic positive effect by reversing the pro-injury alterations on the expression of PAI-1 and tPA.
During the development of chronic injury in the kidney, tubular cells are driven to de-differenciate and alter their phenotype towards that of a mesenchymal cell, thus going from a polarized, anchored and non dividing cell to an unpolarized, mobile and fast proliferating cell. This process is named EMT (38) and is thought to be a repair mechanism that can be deregulated during injury and promote interstitial fibrosis (39–41). We demonstrate herein that EMT, detected by the increase in S100A4 expression, is present in grafts preserved with UW+Hc+Hiv, which show a high degree of fibrosis and low survival rate. This increase was prevented by treatment of the graft by M. Therefore, treatment of IRI with M preserved the graft outcome against a major pathway towards injury. Since TGF-β is a major inducer of EMT (42, 43), the observed protection may be related to the reduced importance of the TGB-β pathway demonstrated in M-treated grafts.
After IR, there is overproduction of reactive oxygen species (ROS) and nitric oxide (NO), which leads to permanent damage to the cell and the tissue (44). A well known producer of ROS is NADPH oxidase, a protein complex located in the plasma membrane as well as in the membrane of phagosome, which variation in expression have been linked to the level of oxidative stress in the tissue and to chronic injury (44). We presently observed that Nox2, one of the subunit of NADPH oxidase, and the inducible form of nitric oxide synthase (iNOS), an important producer of NO, tend to be overexpressed in grafts of the UW+Hc+Hiv group, and also demonstrate the mitigating effect of M therapy: indeed, grafts of the UW+MC and UW+Mc+Miv groups displayed a lowering of both pro-deleterious stress markers. Although expression levels of these genes does not relate directly to protein activity, decreased expression could indicate a lowering of oxidative stress in the graft.
This supports the assumption that an anti thrombin therapy during preservation has wide spread effects on graft outcome, by preventing the activation of a large array of pro-injury mechanisms. Interestingly, PAI-1 expression has been shown to be strikingly upregulated by TGF-β, linked to EMT and participate in fibrosis development, but PAI-1 can also induce interstitial fibrosis even in the absence of TGF-β activation (32, 33, 45). This illustrates the multicomponent nature of chronic kidney fibrosis and the importance of focusing therapeutical efforts on strategies with wide repercussions rather than concentrating the attention on one specific pathway.
This study confirms a novel concept for therapeutic strategies aimed at the better use of DCD donor grafts for transplantation. Such therapeutic option should be useful for all different categories of the Maastricht classification, particularly uncontrolled donors (category 1 or 2). We also report further protection when M is injected prior to the onset of WI, possibly affecting coagulation mechanisms put in place during WI, indicating potential use in donor pretreatment strategies. In addition, M is more efficient than heparin treatment which is not capable to counteract IRI as previously published, probably linked to a longer half life and a better efficiency at 4°C (1, 46).
In conclusion, we demonstrate that peri-preservation use of M as anti-IRI therapy has long range beneficial effects on graft outcome. This work complements our previous study on the short term beneficial effect of such therapy on kidney graft (23), supporting the clinical use of antithrombotic drugs during preservation of kidney graft in DCD conditions.
Acknowledgments
This study was supported in part by a research grant from Astra Zeneca.
Other funding source: Conseil Général de la Vienne, Région Poitou Charentes, the Banque Tarneaud, Poitiers, CHU de Poitiers and Inserm, the Société Francophone de Transplantation, the French Foundation of Transplantation and grants numbers HL085307, DK73608, DK77013, and HL77131 from the National Institutes of Health.
We are grateful to Pr Michel Eugene and Pr Jean Michel Goujon for their guidance and support in the conduction of this study.
Footnotes
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
References
- 1.Cicco G, Panzera PC, Catalano G, Memeo V. Microcirculation and reperfusion injury in organ transplantation. Advances in experimental medicine and biology. 2005;566:363–373. doi: 10.1007/0-387-26206-7_48. [DOI] [PubMed] [Google Scholar]
- 2.Yan SF, Mackman N, Kisiel W, Stern DM, Pinsky DJ. Hypoxia/Hypoxemia-Induced activation of the procoagulant pathways and the pathogenesis of ischemia-associated thrombosis. Arteriosclerosis, thrombosis, and vascular biology. 1999;19(9):2029–2035. doi: 10.1161/01.atv.19.9.2029. [DOI] [PubMed] [Google Scholar]
- 3.Mason J, Joeris B, Welsch J, Kriz W. Vascular congestion in ischemic renal failure: the role of cell swelling. Mineral and electrolyte metabolism. 1989;15(3):114–124. [PubMed] [Google Scholar]
- 4.Molitoris BA, Sutton TA. Endothelial injury and dysfunction: role in the extension phase of acute renal failure. Kidney international. 2004;66(2):496–499. doi: 10.1111/j.1523-1755.2004.761_5.x. [DOI] [PubMed] [Google Scholar]
- 5.Sutton TA, Mang HE, Campos SB, Sandoval RM, Yoder MC, Molitoris BA. Injury of the renal microvascular endothelium alters barrier function after ischemia. American journal of physiology. 2003;285(2):F191–198. doi: 10.1152/ajprenal.00042.2003. [DOI] [PubMed] [Google Scholar]
- 6.Brodsky SV, Yamamoto T, Tada T, Kim B, Chen J, Kajiya F, et al. Endothelial dysfunction in ischemic acute renal failure: rescue by transplanted endothelial cells. American journal of physiology. 2002;282(6):F1140–1149. doi: 10.1152/ajprenal.00329.2001. [DOI] [PubMed] [Google Scholar]
- 7.Mazzoni MC, Borgstrom P, Intaglietta M, Arfors KE. Lumenal narrowing and endothelial cell swelling in skeletal muscle capillaries during hemorrhagic shock. Circulatory shock. 1989;29(1):27–39. [PubMed] [Google Scholar]
- 8.Mazzoni MC, Intaglietta M, Cragoe EJ, Jr, Arfors KE. Amiloride-sensitive Na+ pathways in capillary endothelial cell swelling during hemorrhagic shock. J Appl Physiol. 1992;73(4):1467–1473. doi: 10.1152/jappl.1992.73.4.1467. [DOI] [PubMed] [Google Scholar]
- 9.Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. American journal of physiology. 2001;281(5):F887–899. doi: 10.1152/ajprenal.2001.281.5.F887. [DOI] [PubMed] [Google Scholar]
- 10.Forbes JM, Hewitson TD, Becker GJ, Jones CL. Ischemic acute renal failure: long-term histology of cell and matrix changes in the rat. Kidney international. 2000;57(6):2375–2385. doi: 10.1046/j.1523-1755.2000.00097.x. [DOI] [PubMed] [Google Scholar]
- 11.Cheng O, Thuillier R, Sampson E, Schultz G, Ruiz P, Zhang X, et al. Connective tissue growth factor is a biomarker and mediator of kidney allograft fibrosis. Am J Transplant. 2006;6(10):2292–2306. doi: 10.1111/j.1600-6143.2006.01493.x. [DOI] [PubMed] [Google Scholar]
- 12.Wang W, Koka V, Lan HY. Transforming growth factor-beta and Smad signalling in kidney diseases. Nephrology (Carlton, Vic. 2005;10(1):48–56. doi: 10.1111/j.1440-1797.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Pratt JR, Hartley B, Evans B, Zhang L, Sacks SH. Expression of tissue type plasminogen activator and type 1 plasminogen activator inhibitor, and persistent fibrin deposition in chronic renal allograft failure. Kidney international. 1997;52(2):371–377. doi: 10.1038/ki.1997.343. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Pratt JR, Tam FW, Hartley B, Wolff JA, Olavesen MG, et al. Up-regulation of type 1 plasminogen activator inhibitor messenger RNA with thrombotic changes in renal grafts. Transplantation. 1996;61(5):684–689. doi: 10.1097/00007890-199603150-00002. [DOI] [PubMed] [Google Scholar]
- 15.Faure JP, Petit I, Zhang K, Dutheil D, Doucet C, Favreau F, et al. Protective roles of polyethylene glycol and trimetazidine against cold ischemia and reperfusion injuries of pig kidney graft. Am J Transplant. 2004;4(4):495–504. doi: 10.1111/j.1600-6143.2004.00365.x. [DOI] [PubMed] [Google Scholar]
- 16.Hauet T, Goujon JM, Baumert H, Petit I, Carretier M, Eugene M, et al. Polyethylene glycol reduces the inflammatory injury due to cold ischemia/reperfusion in autotransplanted pig kidneys. Kidney international. 2002;62(2):654–667. doi: 10.1046/j.1523-1755.2002.00473.x. [DOI] [PubMed] [Google Scholar]
- 17.Mattsson C, Sarich TC, Carlsson SC. Mechanism of action of the oral direct thrombin inhibitor ximelagatran. Semin Vasc Med. 2005;5(3):235–244. doi: 10.1055/s-2005-916162. [DOI] [PubMed] [Google Scholar]
- 18.Sarich TC, Osende JI, Eriksson UG, Fager GB, Eriksson-Lepkowska M, Ohlsson L, et al. Acute antithrombotic effects of ximelagatran, an oral direct thrombin inhibitor, and r-hirudin in a human ex vivo model of arterial thrombosis. J Thromb Haemost. 2003;1(5):999–1004. doi: 10.1046/j.1538-7836.2003.00201.x. [DOI] [PubMed] [Google Scholar]
- 19.Sorensen B, Ingerslev J. A direct thrombin inhibitor studied by dynamic whole blood clot formation. Haemostatic response to ex-vivo addition of recombinant factor VIIa or activated prothrombin complex concentrate. Thrombosis and haemostasis. 2006;96(4):446–453. [PubMed] [Google Scholar]
- 20.Nylander S, Mattsson C. Thrombin-induced platelet activation and its inhibition by anticoagulants with different modes of action. Blood Coagul Fibrinolysis. 2003;14(2):159–167. doi: 10.1097/00001721-200302000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Sarich TC, Wolzt M, Eriksson UG, Mattsson C, Schmidt A, Elg S, et al. Effects of ximelagatran, an oral direct thrombin inhibitor, r-hirudin and enoxaparin on thrombin generation and platelet activation in healthy male subjects. J Am Coll Cardiol. 2003;41(4):557–564. doi: 10.1016/s0735-1097(02)02868-1. [DOI] [PubMed] [Google Scholar]
- 22.Belliard A, Mauco G, Bonnet ML, Hauet T, Macchi L. Melagatran prevents tissue factor expression in human platelet-monocyte heterotypic complexes. Cellular and molecular biology (Noisy-le-Grand, France) 2008;54(Suppl):OL1077–1082. [PubMed] [Google Scholar]
- 23.Giraud S, Thuillier R, Belliard A, Hebrard W, Nadeau C, Milin S, et al. Direct thrombin inhibitor prevents delayed graft function in a porcine model of renal transplantation. Transplantation. 2009;87(11):1636–1644. doi: 10.1097/TP.0b013e3181a5b154. [DOI] [PubMed] [Google Scholar]
- 24.Faure JP, Baumert H, Han Z, Goujon JM, Favreau F, Dutheil D, et al. Evidence for a protective role of trimetazidine during cold ischemia: targeting inflammation and nephron mass. Biochemical pharmacology. 2003;66(11):2241–2250. doi: 10.1016/j.bcp.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Hauet T, Goujon JM, Vandewalle A, Baumert H, Lacoste L, Tillement JP, et al. Trimetazidine reduces renal dysfunction by limiting the cold ischemia/reperfusion injury in autotransplanted pig kidneys. J Am Soc Nephrol. 2000;11(1):138–148. doi: 10.1681/ASN.V111138. [DOI] [PubMed] [Google Scholar]
- 26.Jayle C, Favreau F, Zhang K, Doucet C, Goujon JM, Hebrard W, et al. Comparison of protective effects of trimetazidine against experimental warm ischemia of different durations: early and long-term effects in a pig kidney model. American journal of physiology. 2007;292(3):F1082–1093. doi: 10.1152/ajprenal.00338.2006. [DOI] [PubMed] [Google Scholar]
- 27.Doucet C, Milin S, Favreau F, Desurmont T, Manguy E, Hebrard W, et al. A p38 mitogen-activated protein kinase inhibitor protects against renal damage in a non-heart-beating donor model. American journal of physiology. 2008;295(1):F179–191. doi: 10.1152/ajprenal.00252.2007. [DOI] [PubMed] [Google Scholar]
- 28.Grimm PC, Nickerson P, Gough J, McKenna R, Stern E, Jeffery J, et al. Computerized image analysis of Sirius Red-stained renal allograft biopsies as a surrogate marker to predict long-term allograft function. J Am Soc Nephrol. 2003;14(6):1662–1668. doi: 10.1097/01.asn.0000066143.02832.5e. [DOI] [PubMed] [Google Scholar]
- 29.Louapre P, Grongnet JF, Tanguay RM, David JC. Effects of hypoxia on stress proteins in the piglet heart at birth. Cell stress & chaperones. 2005;10(1):17–23. doi: 10.1379/CSC-74R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Nitescu N, Grimberg E, Ricksten SE, Marcussen N, Guron G. Thrombin inhibition with melagatran does not attenuate renal ischaemia-reperfusion injury in rats. Nephrol Dial Transplant. 2007;22(8):2149–2155. doi: 10.1093/ndt/gfm158. [DOI] [PubMed] [Google Scholar]
- 32.Burns WC, Kantharidis P, Thomas MC. The role of tubular epithelial-mesenchymal transition in progressive kidney disease. Cells, tissues, organs. 2007;185(1–3):222–231. doi: 10.1159/000101323. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney international. 2006;69(2):213–217. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- 34.Banasik M, Klinger M. Chronic allograft nephropathy--immunologic and nonimmunologic factors. Ann Transplant. 2006;11(1):7–10. [PubMed] [Google Scholar]
- 35.Kim J, Seok YM, Jung KJ, Park KM. Reactive oxygen species/oxidative stress contributes to progression of kidney fibrosis following transient ischemic injury in mice. American journal of physiology. 2009 doi: 10.1152/ajprenal.90735.2008. [DOI] [PubMed] [Google Scholar]
- 36.Grandaliano G, Di Paolo S, Monno R, Stallone G, Ranieri E, Pontrelli P, et al. Protease-activated receptor 1 and plasminogen activator inhibitor 1 expression in chronic allograft nephropathy: the role of coagulation and fibrinolysis in renal graft fibrosis. Transplantation. 2001;72(8):1437–1443. doi: 10.1097/00007890-200110270-00018. [DOI] [PubMed] [Google Scholar]
- 37.Ranby M, Brandstrom A. Biological control of tissue plasminogen activator-mediated fibrinolysis. Enzyme. 1988;40(2–3):130–143. doi: 10.1159/000469155. [DOI] [PubMed] [Google Scholar]
- 38.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. The Journal of clinical investigation. 2003;112(12):1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Djamali A, Reese S, Yracheta J, Oberley T, Hullett D, Becker B. Epithelial-to-mesenchymal transition and oxidative stress in chronic allograft nephropathy. Am J Transplant. 2005;5(3):500–509. doi: 10.1111/j.1600-6143.2004.00713.x. [DOI] [PubMed] [Google Scholar]
- 40.Vongwiwatana A, Tasanarong A, Rayner DC, Melk A, Halloran PF. Epithelial to mesenchymal transition during late deterioration of human kidney transplants: the role of tubular cells in fibrogenesis. Am J Transplant. 2005;5(6):1367–1374. doi: 10.1111/j.1600-6143.2005.00843.x. [DOI] [PubMed] [Google Scholar]
- 41.Bedi S, Vidyasagar A, Djamali A. Epithelial-to-mesenchymal transition and chronic allograft tubulointerstitial fibrosis. Transplantation reviews (Orlando, Fla. 2008;22(1):1–5. doi: 10.1016/j.trre.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeisberg M, Kalluri R. The role of epithelial-to-mesenchymal transition in renal fibrosis. Journal of molecular medicine (Berlin, Germany) 2004;82(3):175–181. doi: 10.1007/s00109-003-0517-9. [DOI] [PubMed] [Google Scholar]
- 43.Zeisberg M, Maeshima Y, Mosterman B, Kalluri R. Renal fibrosis. Extracellular matrix microenvironment regulates migratory behavior of activated tubular epithelial cells. The American journal of pathology. 2002;160(6):2001–2008. doi: 10.1016/S0002-9440(10)61150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Djamali A. Oxidative stress as a common pathway to chronic tubulointerstitial injury in kidney allografts. American journal of physiology. 2007;293(2):F445–455. doi: 10.1152/ajprenal.00037.2007. [DOI] [PubMed] [Google Scholar]
- 45.Rerolle JP, Hertig A, Nguyen G, Sraer JD, Rondeau EP. Plasminogen activator inhibitor type 1 is a potential target in renal fibrogenesis. Kidney international. 2000;58(5):1841–1850. doi: 10.1111/j.1523-1755.2000.00355.x. [DOI] [PubMed] [Google Scholar]
- 46.Strock PE, Majno G. Vascular responses to experimental tourniquet ischemia. Surgery, gynecology & obstetrics. 1969;129(2):309–318. [PubMed] [Google Scholar]