Abstract
Recent studies have revealed that human infants process female faces differently from male faces. To test whether a similar preference for female faces exists in other primates, we presented nursery-reared infant rhesus macaques with photographs of macaque faces and human faces. At less than 1 month old, infant macaques preferentially oriented towards female macaque faces when faces were presented upright. No preference for female human faces was found. At 9 months old, infants failed to show a visual preference for female macaque faces or female human faces, although they showed significantly more lipsmacking responses at female human faces. Compared to human infants, macaques appear to have stronger predispositions early in life but this preference may nonetheless be amendable to experience. Understanding how innate predispositions and the social rearing environment shape infants’ understanding of faces remain important issues to be explored in order to understand facial processing abilities in humans and other primates.
Keywords: Macaca mulatta, visual preferences, face perception
Human infants are experts in processing facial information. Newborns favor face-like visual stimuli over non-face-like visual stimuli (Maccia Cassia, Turati & Simion, 2004) and prefer their mother’s face over the face of a female stranger within a few days after birth (Bushnell, Sai & Mullin, 1989). Recent studies have revealed that not all faces are processed equally by infants. For example, at 3–4 months of age, infants discriminate and recognize female faces more easily than male faces (Quinn, Yahr, Kuhn, Salter & Pascalis, 2002), and at 6 months old infants more readily form a prototype of female faces than of male faces (Ramsey, Langlois & Marti, 2005). Moreover, when presented with a male face and a female face simultaneously, infants look longer at the female face (Quinn et al., 2002). Two hypotheses have been put forward as to why young infants show this female face bias. One idea proposes an evolutionary root, suggesting that infants might be predisposed towards female faces and features because mothers are biologically inclined to be the primary care giver (Ramsey-Rennels & Langlois, 2006). On the other hand, the female bias in face processing appears amendable to experience: infants whose primary care giver is male look longer at male faces than at female faces (Quinn et al., 2002). Since infants generally have more exposure to their mothers than to their fathers and also are more likely to interact with female strangers than with male strangers (Ramsey-Rennels & Langlois, 2006), an alternative hypothesis suggests that a preference for female faces is related to greater familiarity with female faces without assuming any innate preference (Quinn et al., 2002).
Like human infants, macaque infants show quite sophisticated social processing abilities at a young age. For example, infant macaques prefer faces and face-like stimuli over non-face stimuli (Lutz, Lockard, Gunderson & Grant, 1998; Kuwahata, Adachi, Fujita, Tomonaga & Matsuzawa, 2004; Sugita, 2008), and they prefer their mothers over unfamiliar adult females (Rosenblum & Alpert, 1974). Previous studies have also explored rhesus infants’ reactions to unfamiliar male and female adult macaques. For example, Suomi, Sackett and Harlow (1970) presented socially naïve rhesus infants with an unfamiliar male and female adult macaque in a self-selection circus, and found that in the first two years of life, infants preferentially orient towards the female adult; however, since live models were used, it is possible that this preference was influenced by the adult monkeys’ behavior. Plimpton, Swartz and Rosenblum (1981) presented young bonnet macaques with video clips of male and female macaques, and found more frequent approaches to female rather than male stimuli. Whilst controlling for model behavior, it is still unclear from this study whether infants used facial cues or other cues such as body size or posture to discriminate male from female macaques. It therefore remains unclear whether similar to human infants, other primate infants can discriminate males from females based on facial cues alone.
In the present study, we investigated visual responses to male and female faces in infant rhesus macaques. We tested macaque infants who were being reared in a neonatal primate nursery for ongoing, unrelated experiments. These infants were typically born during the night, separated from their mothers early the next morning (ca. 8am) and reared by human care takers thereafter without exposure to adult macaques. Given that rhesus’ eye sight is not fully mature at birth (Mendelson, 1982) and that infants spent much of their time asleep, we would argue that these infants are minimally influenced by their experiences with macaque faces. We specifically addressed infant’ perception of faces by presenting them with photographs of male and female faces of both macaques and humans. We sought to determine: (i) whether infant rhesus macaques show a preference for female macaque faces, (ii) whether a preference for female faces is species-specific, and (iii) whether a preference for female faces endures across development.
Experiment 1
In Experiment 1, we asked whether infant macaques would prefer female macaque faces over male macaque faces. To insure that any preference was not due to low-level image differences between male and female faces, we presented faces in both upright and inverted orientations.
Methods
Subjects
Subjects were 23 infant rhesus macaques (Macaca mulatta), 13 males and 10 females. All infants had been carried to term and had been born without further complications; birth weight for all infants fell within normal parameters. All infants were born during the night, separated from their mothers early the next morning (ca. 8am), and reared in a nursery facility for ongoing, unrelated research studies (details of rearing practices described in Shannon, Champoux, & Suomi, 1998). Infants were housed individually in incubators (51 × 38 × 43 cm) for the first two weeks and in metal cages (74 × 66 × 76 cm) thereafter. Both housings arrangements contained an inanimate “surrogate mother” covered with fleece fabric, loose pieces of fleece fabric and various rubber toys. Infants could see and hear, but not physically contact, other infants of similar age. All animals were provided with a 50:50 mixture of Similac (Ross Laboratories, Columbus, Ohio, United States) and Rimilac (Bio-Serv, Frenchtown, New Jersey, United States) formulas. Formula was administered until 4 mo of age. Purina High Protein Monkey Chow (#5038) (Purina, St. Louis, Missouri, United States) was available when infants reached 2 weeks of age. Infants had no exposure to adult macaques while in the nursery. Testing began when infants were 10 days old.
Stimuli
A digital video camera was used to capture images of five adult male and five adult female rhesus macaques unrelated to any of the infants. All images were frontal, full-face with neutral expressions and standardized for interpupilar distance and background. Since hair length is not a sexually dimorphic trait in macaques, we retained the external features on all images.
Procedure
Stimuli were printed onto color photo paper (20 × 25 cm) and displayed 40 cm apart on a white background. A video camera was placed between the stimuli to record the monkey’s behavior. At the start of each trial, an experimenter secured the infant in soft fleece fabric in front of her chest facing outwards. Each trial started with the experimenter facing away from the stimuli and slowly turning toward them until the infant was positioned equidistant between the stimuli, ca. 12 inches in front of the camera. As soon as the infant looked at one of the stimuli, gaze preferences were measured for 15 continuous seconds. The experimenter holding the infant remained passive and looked straight ahead so as to not influence the monkeys’ gaze behavior. Subjects were tested on 10 trials per day, 5 times a week. Each male face was paired with each female face twice, counterbalancing left-right position. All combinations were displayed both upright and inverted, and trial order of upright and inverted stimuli was randomized (100 trials total).
Analysis
All trials were digitally analyzed (30 frames per second) by an experimenter who was aware of the purpose of the experiment but did not know left/right or upright/inverted position of male/female faces. Data from 3 monkeys (13% of total data set) were coded a second time by another experimenter for reliability analysis. Pearson correlations were run on the durations of gaze at left and right stimuli on each trial for each monkey, yielding significant correlation coefficients between the two codings (range 0.71–0.99, median 0.86, all p<0.05).
Results
Since we were interested in relative preferences for either male or female faces, we discarded all trials from the analysis in which monkeys looked at only one face. For the remaining trials (average per monkey: 53 trials), we first averaged the looking duration at male/female stimuli for identical trials, and then averaged the looking durations at each individual male/female face. Since there were no differences between looking durations at individual faces (for males: F(4, 88)=0.26, p=0.9; for females: F(4, 88)=1.62, p=0.18), we created an average looking time at male faces and an average looking time at female faces for each individual infant. Due to non-normal distribution, all data were log-transformed prior to analysis.
We analyzed the data with a 2 × 2 repeated measures ANOVA with orientation (upright, inverted) and sex of stimulus (male, female) as within-subject factors. This analysis showed no main effects (orientation: F (1,22) = 3.73, p=0.10; sex of face: F (1,22) = 3.00, p=0.07), and no interaction (F (1,22) = 0.24, p=0.63). Since our main interest was to compare looking time at male and female faces within each orientation, we ran two related-samples t-tests, comparing looking times at male and female faces in upright and inverted orientation. This analysis yielded a significant difference for upright faces (t(22)=−2.16, p=0.042), but not inverted faces (t(22)=−0.94, p=0.36). Infants looked significantly longer at upright female faces than at upright male faces (see Table 1).
Table 1.
Summary of average looking times at male and female faces in Experiments 1–3. Values represent average looking time in seconds, standard deviations are given in parentheses.
| Experiment 1 | Male faces | Female faces | p |
|---|---|---|---|
| Macaque upright | 1.80 (0.43) | 1.99 (0.48) | 0.04 |
| Macaque inverted | 2.02 (0.68) | 2.17 (0.76) | 0.36 |
| Experiment 2 | |||
| Human upright | 1.61 (0.57) | 1.57 (0.55) | 0.83 |
| Human inverted | 1.58 (0.53) | 1.47 (0.46) | 0.53 |
| Experiment 3 | |||
| Macaque upright | 1.08 (0.26) | 1.30 (0.27) | 0.17 |
| Human upright | 1.19 (0.32) | 1.21 (0.26) | 0.80 |
To determine whether the observed difference in relative looking times at upright male and female macaque faces was acquired during the experiment purely by being exposed to macaque faces, we calculated an average looking time at male and female faces for the first and the last 3 trials for each monkey. We then computed a preferential looking index, representing the relative preference for female faces over male faces, as follows:
This index ranges from −1 to +1, and indicates a preference for female faces if positive and a preference for male faces if negative. A paired t-test indicated that the preferential looking index did not significantly change from the first three trials to the last three trials (first trials: mean=0.02, last trials: mean=0.12, t(22)=−0.85, p=0.41).
Discussion
Infant rhesus macaques spontaneously looked longer at female macaque faces than at male macaque faces when faces were displayed upright. This preference was not acquired during the experiment itself, but more likely it was already present in infants before the start of the experiment. No preference was found when faces were inverted. Since infants’ experience with macaque faces was minimal, it seems likely that infants have an innate predisposition to discriminate male from female faces.
These results indicate that infant macaques discriminate between male and female macaque faces when displayed upright, however it is difficult to interpret the meaning of the difference in looking time. While looking time is generally assumed to reflect interest, some have argued that it can also reflect lack of expertise and efficiency (Ramsey-Rennels & Langlois, 2006). Other studies have shown that longer gaze durations are also associated with novelty (e.g. Pascalis & Bachevalier, 1998), or attractiveness (e.g. Langlois, Ritter, Roggmann & Vaughn, 1991). The present data cannot accurately discriminate between these different interpretations, however it seems reasonable to exclude novelty as a cause for longer gaze durations since infants were not exposed to any adult macaque faces prior to the experiment, making male and female faces equally novel. Other behavioral data, such as approaches to the stimulus, might be a useful confirmatory indicator of interest, but it was not possible to collect such data under the present paradigm. Despite these reservations, the present results indicate a clear discrimination of male and female macaque faces (when displayed upright), and resemble the pattern of preference for female faces found in human infants, which is commonly interpreted as interest.
Experiment 2
The results of Experiment 1 indicate that rhesus infants are sensitive to differences between male and female macaque faces, and that infants preferentially attend to female macaque faces. In order to distinguish male and female faces, infants may use specific algorithms to extract sexually dimorphic relations between facial features. If this hypothesis is correct, then infants might use the same algorithms to also differentiate male and female faces of other species. Human faces, for example, share many of the sexually dimorphic characteristics that are displayed by macaque faces (Little et al., 2008). If macaque infants were to preferentially attend to female human faces rather than male human faces, it would suggest that infant macaques use broad processing algorithms that might be amendable to an individual’s experience with faces.
In Experiment 2, we assessed rhesus infants’ spontaneous gaze responses to male and female human faces. As in Experiment 1, we displayed faces in both upright and inverted orientations to rule out that any found preference would not be due to low-level image differences.
Methods
Subjects
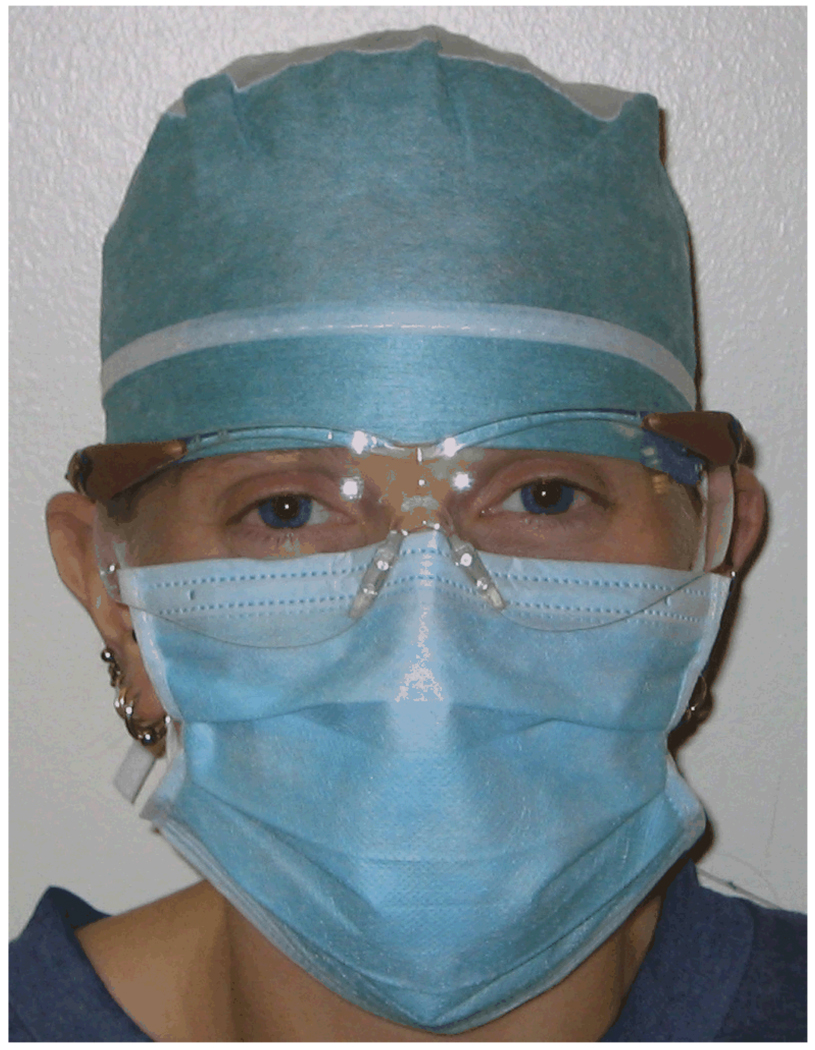
The same subjects as in Experiment 1 were tested. Infants were 24 days old at the start of testing. As part of an unrelated investigation, infants saw several female human experimenters who directed facial gestures at each infant when infants were 1, 3, 5 and 7 days old. In total, infants were exposed to full female human faces for 10 minutes on 4 different days (maximum 40 minutes). Otherwise, infants’ exposure to human faces was minimal. As a safety precaution for both caretakers and rhesus infants, caretakers wore face masks, hair covers and safety glasses at all times, covering the entire face with the exception of a small area around the eyes (see Fig. 1).
Figure 1.
Personal protection equipment worn by human care takers. Only a small area around the eyes remained visible to infants.
Stimuli
We used five male and five female human face stimuli printed onto photo paper (20 × 25 cm). All faces were frontal, full-face with neutral expressions and standardized backgrounds. Since the women in the images had long hair but the men did not, hair length may have served as a potential discriminative cue unrelated to facial features. We therefore cropped all pictures in a standard oval (cropped at the chin, hairline, and ears).
Procedure
The same procedure as in Experiment 1 was used with the only difference that male and female human faces were used as stimuli.
Analysis
As in Experiment 1, all trials were coded by an experimenter blind to left/right position and upright/inverted orientation of faces. Data from 3 monkeys (13% of total data set) were coded for a second time by another experimenter. Pearson correlations showed significant correlation coefficients between the two codings (range 0.79–0.93, median 0.87; all p<0.05).
Results
Similar to Experiment 1, we discarded all trials in which monkeys only looked at one of the two faces. We averaged first across identical trials, and then within each individual face. Using a repeated measure ANOVA, we found that monkeys did not look equally at all male faces (F(4,84)=3.06, p=0.042). Post-hoc comparisons indicated that infants tended to look at one particular male face more than at the other male faces. While the reason for this preference is not clear, we discarded all trials with this particular male face from further analysis, leaving on average 40 trials per monkey for analysis. No preference was found for any female human face (F(4,88)=2.03, p=0.1). We created an average looking score for male and a score for female faces from all remaining stimuli for each infant.
A 2 × 2 repeated measures ANOVA with orientation (upright, inverted) and sex of stimulus (male, female) failed to show any main effects (orientation: F(1,22) = 0.63, p=0.44; sex of stimulus: F(1,22)=0.45, p=0.51) or interaction (F (1,22) = 0.07, p=0.79). Similarly, related samples t-tests comparing looking times within each orientation failed to show any significant differences between male and female faces (upright: t(22)=0.22, p=0.83; inverted: t(22)=0.65, p=0.53; see Table 1.).
Discussion
Rhesus infants did not discriminate between male and female human faces regardless of whether faces were displayed in upright or inverted orientation. These findings confirm that human faces were indeed processed differently from macaque faces despite equally low exposure to both human and macaque faces. One difference between Experiment 1 and 2 is that infants were 14 days older at the start of Experiment 2; however, it seems unlikely that this age difference significantly affected the results. If at all, at 24 days old infants had more experience with human faces than at 10 days old, and should have been more likely to be able to discriminate male and female human faces. Infant macaques’ preference for female faces found in Experiment 1 therefore appears to be specific to their own species, and is in line with the suggestion that the female face preference is innate.
Experiment 3
The results of Experiment 1 and Experiment 2 demonstrate that young rhesus infants (aged < 1 month) show a significant visual preference for female macaque faces, which does not extend to female faces of another species. In order to test whether facial preferences persist across development, in Experiment 3 we measured facial preferences in 9-month old rhesus infants, who were similarly reared without visual exposure to adult macaques. Exposure to human faces was equally low as human care givers wore face masks, hair covers and safety glasses at all times (see Fig. 2). We evaluated infants’ reactions to both monkey faces and human faces at this later age.
Methods
Subjects
Subjects were 8 infant rhesus macaques (Macaca mulatta), 4 male and 4 female. At the beginning of the study, infants were between 8.2–9.5 months old. All infants had been separated from their mothers on the day they were born (with the exception of one female who was separated when 11 days old) and nursery reared according to procedures described by Shannon et al. (1998). Infants were singly housed and in visual and auditory contact with other infants of similar age. Once a day for 2 hours, infants were socialized in a play cage with other infants of similar age. Home cages were enriched with a hanging surrogate mother, various plastic manipulanda, rubber toys and soft fleece fabric. Infants were not food deprived for this experiment and received a diet of commercial monkey chow and daily enrichments of fruits, seeds and nuts. Water was available ad libitum.
Stimuli
The same stimuli as in Experiment 1 and Experiment 2 were used.
Procedure
The same procedure as in Experiment 1 was used with the only difference that we only displayed upright faces. We first tested monkeys with monkey faces (10 trials a day, 50 trials total) followed by the same procedure using human faces (10 trials a day, 50 trials total).
Analysis
All trials were digitally analyzed (30 frames per second) by an experimenter who was aware of the purpose of the experiment but did not know left/right position of male/female faces. One monkey (12.5% of total data set) was coded a second time for interobserver reliability. Pearson correlations run on the durations of gaze at left and right stimuli on each trial revealed significant correlation coefficients for the two codings (monkey faces: r= 0.84 and r=0.93, human faces: r=0.82 and r=0.86, all p<0.001).
Results
As in Experiments 1 and 2, we discarded all trials in which monkeys looked at only one face, then averaged across identical trial and within individual faces. There were no differences between looking durations at individual faces for all male/female monkey/human faces (all p>0.05), and we created an average looking time at male/female faces for both monkey/human faces for each infant (based on average on a total of 50 trials per monkey). Due to non-normal distribution, all data were log-transformed prior to analysis.
As a group, monkeys did not prefer female macaque faces over male macaque faces (t(7)=−1.53, p=0.17). Similarly, monkeys did not prefer to look at female human faces (t(7)=−0.26, p=0.80; see Table 1.).
We noted that several monkeys spontaneously lipsmacked at face stimuli. Lipsmacking is a communicative gesture between macaques, conveying affiliation and/or appeasement in slightly tense situations. Six monkeys lipsmacked at at least one monkey face (total 43 trials) and five monkeys lipsmacked at at least one human face (total 14 trials). We measured durations of these lipsmacking responses (starting from the first mouth opening to the last mouth closing) and averaged them in the same way as looking durations. For monkey faces, five out of six monkeys directed more lipsmacking at male monkey faces. For human faces, all five monkeys directed more lipsmacking at female human faces. Wilcoxon signed ranks tests (two-tailed) revealed that this difference in lipsmacking is statistically significant in human faces (average male faces: 0.68 sec, average female faces: 1.30 sec, z=−2.02, p=0.043) but not monkey faces (average male faces: 0. 83 sec, average female faces: 0.35, z=−1.48, p=0.14).
Discussion
Unlike younger infants in Experiment 1, rhesus infants aged 9 months old did not significantly prefer female macaque faces over male macaque faces. Infants in Experiment 3 also failed to show a significant preference for female human faces. These results may suggest that these older infants failed to discriminate male and female faces, however other factors might have had a significant impact on the results. Our stimuli (neutral, face-on photographs of monkeys or humans, gazing directly ahead) might potentially have posed an exceptionally salient stimulus to older macaque infants. Direct gaze in particular is a low-level threat signal between macaques, but infants do not openly react to threats until they are ca. 2.5 months old (Sackett, 1966). As such, gaze durations in older infants were likely to be affected by the perceived level of threat from photographs, and do not represent an unbiased measure of preference. Using lipsmacking as a second behavioral measure of discrimination, it appears that infants discriminated male and female human faces, but not macaque faces. These results should be interpreted with caution, however, since not all infants showed lipsmacking responses, and lipsmacking only occurred in some of the trials. While these results are clearly in need of replication using a larger sample size, they nonetheless seem to suggest that older infants are less sensitive to the differences between male and female faces, particularly macaque faces, than younger infants (< 1 month old).
General Discussion
There is no doubt that human infants are sensitive to facial information and that from an early age, human infants process male and female human faces differently. To compare facial processing abilities of human infants with those of other non-human primate infants, we examined rhesus macaque infants’ reactions to male and female macaque and human faces. The results of Experiment 1 indicate that young rhesus infants preferentially look at female macaque faces but only when faces are presented upright. Furthermore, results of Experiment 2 show that this preference is specific to macaque faces; the same infants did not discriminate between male and female human faces regardless of whether faces were displayed upright or inverted. Together these results suggest that rhesus infants from a very early age are differentially sensitive to male and female macaque faces. Since infants had very limited experience with macaque faces prior to testing, it seems likely that similar to other reactions to social stimuli (Sackett, 1966), this preference is an innate predisposition. It is worth remembering, however, that the observed preference for female macaque faces was found in socially-naïve macaque infants, and that these data do not necessarily represent the behavior of infants who have formed a strong attachment bond with a care giver. In other words, mother-reared infants might focus their attention exclusively on their mother’s face rather than on all encountered female faces. Future studies examining reactions of mother-reared infants to macaque faces, thereby studying the interaction between innate and environmental factors, would undoubtedly represent a valuable contribution to the field.
Our third experiment offered a glimpse into macaque social processing capabilities in later life. At 9 months old, infants failed to show a difference in attention to male and female macaque faces and human faces. This difference could potentially represent a developmental difference to <1 month old infants, who discriminated male from female macaque faces. While 1 month old infants are dependent on their mothers for food, warmth and comfort, infants enter the weaning process at ca. 6 months old when their mothers start to conceive again. Given that rhesus macaques are seasonal breeders (at least in the wild), infants are generally considered weaned when their mothers give birth to a sibling when infants are ca. 1 year old. It may therefore be beneficial for young infants to be able to quickly discriminate male from female faces due to their dependence on female caregivers, but perhaps less so for infants at weaning age. However, a failure to differentiate could also have been caused by other factors, such as potential perceived threats from the facial stimuli (see discussion, above). Alternatively, 9 months old infants were reared without contact to adult monkeys so that lack of experience with adult macaque faces might also explain the lack of discrimination. Data on mother-reared infant macaques’ reaction to male and female faces at a similar age could help to clarify these issues.
The found increase in lipsmacking at female human faces in 9 month old infants should be interpreted with caution on the basis of the small sample size and its relatively infrequent occurrence. It is possible, however, that this differential reaction to male and female human faces was affected by the infants’ daily exposure and interaction with human care takers. In human infants, expertise in processing facial information is often considered an acquired skill (Quinn et al., 2002). For example, Pascalis, de Haan and Nelson (2002) showed that 6 months old infants can recognize and differentiate individual monkey and human faces, but 9 months old infants only recognize and differentiate human faces. This finding has prompted the suggestion that during the first year of infants’ lives, their perceptual window narrows and tunes to a human face template (Pascalis et al., 2002). At the basis of this narrowing processes may lay an individual’s experience with faces. In other words, individuals should most fluently process those faces that they experience most frequently (Pascalis & Bachevalier, 1998), whether they are distinguished by sexual or species-typical characteristics. There is some evidence that the same principles may also apply to the face processing abilities of non-human primates. For example, Neiworth, Hassett and Sylvester (2007) showed that cotton-top tamarins (Saguinus oedipus) recognize images of both humans and tamarins, and suggested that the expertise for human faces in tamarins is due to their daily exposure to humans. On the other hand, monkeys tested by Pascalis and Bachevalier (1998) failed to show any superior processing capabilities of human faces despite daily exposure to human care takers. Even though care takers in the present study were required to wear face masks, which only leave a small area around the eyes visible, it is possible that the human eye region is sufficiently sexually dimorphic for infants to be able to discriminate male from female human faces. Sugita (2008) showed that when completely deprived of facial information, infant macaques nonetheless show a preference for faces (both human and monkey) over other objects, and more importantly, when then exposed to facial information of one particular species, they then retain this preference for an extended period of time. Since human infants still show superior recognition skills for monkey faces at 6 months old (Pascalis et al., 2002), it is conceivable that the time frame of the perceptual window is larger in humans than it is in monkeys. Moreover, the narrowing of the perceptual window may not depend on chronological age but rather on exposure to and experience with faces. That is, while there is an initial preference for female macaque faces in young infants, macaques might need continuing experience with macaque faces to become experts in processing macaque faces. Therefore, while infant macaques may show innate predispositions, the early rearing environment may nonetheless have a profound effect on their facial processing capacities.
In conclusion, similar to human infants’ reactions to human faces, macaque infants in the present study preferentially oriented towards female macaque faces within the first month of life but they did not discriminate between male and female human faces. Rhesus infants in the present study had little if any experience with macaque faces, indicating an innate predisposition to preferentially orient towards female macaque faces. Such a behavioral strategy might have significant adaptive value as it can potentially initiate care giving responses from adult females and especially the mother who takes on the role of sole care giver for macaque infants. Nine months old infants did not show a clear preference for female macaque faces, however taking into account other behavioral measures, it appears that older infants might have discriminated between male and female human faces. Compared to human infants, macaques appear to have stronger predispositions early in life, which may then be affected by the early rearing environment. Understanding how innate predispositions shape infants’ understanding of faces and social partners in general and to what extent the early social rearing environment affects the processing of facial information remain important factors to be explored in order to understand facial processing abilities in humans and other primates.
Acknowledgments
We thank Sarah Unbehagen, Lisa Darcey and Angela Ruggiero for help in collecting the data. Human face stimuli came from the PICS image archive, Stirling University, UK (http://pics.stir.ac.uk). This study was supported by the Division of Intramural Research, NICHD.
References
- Bushnell IWR, Sai F, Mullin JT. Neonatal recognition of the mother’s face. British Journal of Developmental Psychology. 1989;7:3–15. [Google Scholar]
- Kuwahata H, Adachi I, Fujita F, Tomonaga M, Matsuzawa T. Development of schematic face preference in macaque monkeys. Behavioural Processes. 2004;66:17–21. doi: 10.1016/j.beproc.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Langlois JH, Ritter JM, Roggmann LA, Vaughn LS. Facial diversity and infant preference for attractive faces. Developmental Psychology. 1991;27:79–84. [Google Scholar]
- Little AC, Jones BC, Waitt C, Tiddeman BP, Feinberg DR, Perrett DI, Apicella CL, Marlowe FW. Symmetry is related to sexual dimorphism in faces: data across culture and species. PLoS One. 2008;3:e2106. doi: 10.1371/journal.pone.0002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz CK, Lockard JS, Gunderson VM, Grant KS. Infant monkeys’ visual responses to drawings of normal and distorted faces. American Journal of Primatology. 1998;44:169–174. doi: 10.1002/(SICI)1098-2345(1998)44:2<169::AID-AJP7>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Maccia Cassia V, Turati C, Simion F. Can a nonspecific bias toward top-heavy patterns explain newborns’ face preferences? Psychological Science. 2004;15:379–383. doi: 10.1111/j.0956-7976.2004.00688.x. [DOI] [PubMed] [Google Scholar]
- Mendelson MJ. Visual and social responses in infant rhesus monkeys. American Journal of Primatology. 1982;3:333–340. doi: 10.1002/ajp.1350030134. [DOI] [PubMed] [Google Scholar]
- Neiworth JJ, Hassett JM, Sylvester CJ. Face processing in humans and new world monkeys: the influence of experiential and ecological factors. Animal Cognition. 2007;10:125–134. doi: 10.1007/s10071-006-0045-4. [DOI] [PubMed] [Google Scholar]
- Pascalis O, Bachevalier J. Face recognition in primates: a cross-species study. Behavioural Processes. 1998;43:87–96. doi: 10.1016/s0376-6357(97)00090-9. [DOI] [PubMed] [Google Scholar]
- Pascalis O, de Haan M, Nelson CA. Is face processing species-specific during the first year of life? Science. 2002;296:1321–1323. doi: 10.1126/science.1070223. [DOI] [PubMed] [Google Scholar]
- Plimpton EH, Swartz KB, Rosenblum LA. Responses of juvenile bonnet macaques to social stimuli presented through color videotapes. Developmental Psychobiology. 1981;14:109–115. doi: 10.1002/dev.420140204. [DOI] [PubMed] [Google Scholar]
- Quinn PC, Yahr J, Kuhn A, Slater AM, Pascalis O. Representation of the gender of human faces by infants: a preference for female. Perception. 2002;31:1109–1121. doi: 10.1068/p3331. [DOI] [PubMed] [Google Scholar]
- Ramsey LJ, Langlois JH, Marti NC. Infant categorization of faces: ladies first. Developmental Review. 2005;25:212–246. [Google Scholar]
- Ramsey-Rennels JL, Langlois JH. Infants’ differential processing of female and male faces. Current Directions in Psychological Science. 2006;15:59–62. [Google Scholar]
- Rosenblum LA, Alpert S. Fear of strangers and specificity of attachment in monkeys. In: Lewis M, Rosenblum LA, editors. The origins of fear. New York: John Wiley & Sons; 1974. pp. 165–193. [Google Scholar]
- Sackett GP. Monkeys reared in isolation with pictures as visual input: evidence for an innate releasing mechanism. Science. 1966;154:1468–1473. doi: 10.1126/science.154.3755.1468. [DOI] [PubMed] [Google Scholar]
- Shannon C, Champoux M, Suomi SJ. Rearing condition and plasma cortisol in rhesus monkey infants. American Journal of Primatology. 1998;46:311–321. doi: 10.1002/(SICI)1098-2345(1998)46:4<311::AID-AJP3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Sugita Y. Face perception in monkeys reared with no exposure to faces. Proceedings of the National Academy of Sciences USA. 2008;105:394–398. doi: 10.1073/pnas.0706079105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomi SJ, Sackett GP, Harlow HF. Development of sex preference in rhesus monkeys. Developmental Psychology. 1970;3:326–336. [Google Scholar]