Abstract
The present study explored the ability to control familiarity-based information in a memory exclusion paradigm in healthy young, older adults, and early-stage DAT individuals. We compared the predictive power of memory exclusion performance to standard psychometric performance in discriminating between healthy aging and the earliest detectable form of DAT and between APOe4-present and APOe4-absent genotype in healthy control individuals. Participants responded “yes” to words that were previously semantically encoded, and “no” to other words. The number of targets and distractors on the read “distractor” list was manipulated to investigate the degree to which aging and DAT influence the ability to recollect specific details of study episodes in the face of distractor familiarity due to repetition. Memory exclusion performance (as reflected by d′) decreased across participant groups (young > healthy old control > very mild DAT). Logistic regression analyses showed that d′ increased the discriminative power for healthy older adults vs. very mild DAT individuals above and beyond standard psychometric measures. Memory exclusion d′ was also lower for healthy control individuals with APOe4 allele, compared to those without the APOe4 allele after partialing out baseline psychometric performance. Discussion focuses on the importance of attentional control systems in memory retrieval and the utility of the opposition paradigm for early discrimination between healthy and pathological aging.
Keywords: AGING, APOE, ATTENTIONAL CONTROL, ALZHEIMER’S DISEASE, MEMORY EXCLUSION
A breakdown in episodic memory performance is one of the hallmark findings for individuals with Dementia of the Alzheimer’s Type (DAT, see Bäckman et al., 2001; Balota et al., 2000, Hodges, 2000). Although mildly demented individuals show decreases in both veridical and false memory performance for related items (e.g., Budson et al., 2000, 2002, 2003), individuals in the earliest stage of DAT (hereafter very mild DAT individuals) show a decrease in their veridical memory performance but an increase in their false memory performance (e.g., Balota et al., 1999, 2002), compared to age-matched healthy older adult controls. This is an extension of a pattern observed in healthy aging (e.g., Norman & Schacter, 1997; Tun et al., 1998). At first glance, one might argue that this pattern simply reflects the widespread memory impairment in very mild DAT individuals. Relative to healthy older adults, these individuals may use poorer strategies to encode items, forget items more rapidly over the retention interval, and/or be more susceptible to interference during retrieval. This hereafter is called the memory deficit hypothesis. However, in addition to the memory deficits observed in these individuals, previous studies have also found that very mild DAT individuals produce relatively poor performance in tasks with high attentional demands (see Balota & Faust, 2001; Perry & Hodges, 1999, for reviews), such as Stroop (e.g., Bondi et al., 2002; Spieler et al., 1996) and Simon tasks (e.g., Castel et al., 2007). In fact, Sommers and Huff (2003) have demonstrated a direct link between age-related changes in false memory performance and age-related changes in Stroop performance. Also, the role of attention in memory encoding and retrieval has long been recognized as being central to memory performance (e.g., Craik & Lockhart, 1972; Duchek, 1984; Jacoby, 1991). Thus, apart from the failure to form and retrieve declarative memories, memory breakdowns in DAT may also be modulated by a deficit in attentional control (e.g., Balota et al., 2000; Parasuraman & Haxby, 1993; Perry & Hodges, 1999, see Hasher & Zacks, 1988, for a similar explanation for age-related changes in healthy older adults). This is hereafter called the attentional control hypothesis.
Due to accumulating evidence regarding the interplay between the attentional control system and episodic memory in healthy aging and early-stage DAT, in the present study we explored a paradigm that maximizes the role of attention by pitting recollection for specific targets against a high degree of familiarity for distractors across groups of healthy young, older adults and individuals in the earliest stage of DAT. The present study had three major goals. First, we compared the memory deficit hypothesis vs. attentional control hypothesis. Because memory for both targets and distractors may worsen across participant groups, a simple memory deficit hypothesis predicts that very mild DAT individuals should show similar error rates in rejecting highly familiar but incorrect distractors, compared to the healthy older adults. In contrast, the attentional control hypothesis predicts that relative to healthy older adults, very mild DAT individuals who have attentional control problems may have more difficulty discriminating the targets and distractors during retrieval and hence produce more errors in rejecting the familiar distractors. Of course, it is unlikely that any study will provide an “either-or” answer, because both attention and memory deficits likely contribute to any observed changes, an issue we will return to in the General Discussion section. Second, by using a large sample of participants, we assessed the extent to which memory exclusion performance may be useful in discriminating healthy older adults from those in the earliest stage of DAT, as compared to standard psychometric declarative memory measures and other general cognitive measures. Finally, we tested whether healthy older adults who possess the apolipoprotein ε4 (APOe4) allele, which is a genetic marker for the early development of Alzheimer’s disease, would show differential memory exclusion performance than those who do not possess that allele.
Pitting Recollection Against Familiarity in Memory Exclusion Paradigm
According to the dual-process theory of recognition memory (Atkinson & Juola, 1974; Jacoby, 1991; Mandler 1980; Yonelinas, 1997), the ability to episodically recognize a past event is supported by both recollection-based and familiarity-based retrieval processes. Whereas recollection-based retrieval is proposed to be a relatively slow, attention-demanding process based on remembering contextual information such as where and when the event occurred, familiarity-based retrieval is proposed to be a relatively fast, automatic process based on the general strength of a memory trace for events. In most situations, both recollection and familiarity act in concert to drive a response, however, in the memory exclusion paradigm, participants are instructed to exclude items from one of multiple study lists. For example, participants may be asked to respond “yes” to items presented on one list (i.e., target list) and “no” to items presented on a different list (i.e., distractor list). Because the recent presentation makes items on the distractor list become more familiar, memory exclusion errors (i.e., incorrectly recognizing the distractors as targets) occur when participants are unable to use the recollection-based retrieval strategy to reject the familiar distractors. Healthy older adults are expected to make more exclusion errors than young adults. Indeed, previous studies have shown that healthy older adults are impaired in recollection-based retrieval yet show relatively intact performance in familiarity-based retrieval (e.g., Hay & Jacoby, 1999; Jacoby, 1999; Jennings & Jacoby, 1997, Prull et al., 2006, but see also Light et al., 2000, for the evidence of age-related deficit in familiarity).
The current study extends the exclusion paradigm used by Jacoby (1999). In the Jacoby study, participants read aloud a visually presented distractor list in which some of the distractors were presented up to five times and then heard an auditorily presented target list in which all targets were presented only once. Repeating the distractors on the study list presumably increased both recollection- and familiarity-based retrieval in the subsequent memory test. In the study phase, participants were told to remember all items for an unspecified memory test. However, in the memory exclusion test, they were instructed to respond “yes” only for the items heard earlier (i.e., items from the target list). Three groups of participants, young adults under speed stress, young adults under no speed stress and older adults under no speed stress, were tested. Young adults under speed stress were instructed to make their recognition decision within a very short response deadline, and hence could use minimal recollection to guide retrieval. They made a higher proportion of exclusion errors than did young adults who were not under speed stress. Older adults who were not under speed stress produced a very similar pattern as the young adults under speed stress; that is, an increase in exclusion errors due to distractor repetition. Jacoby coined this as the ironic effect of distractor repetition.
Attentional Control Hypothesis and Memory Exclusion
The ironic effect of distractor repetition can be considered within an attentional control framework (e.g., Balota & Faust, 2001), which postulates that an intact control system is able to discriminate the source of information from one list from that on another list. This memory discrimination process is analogous to the memory monitoring process that was proposed in source memory literature (e.g., Johnson et al., 1993). Relative to young adults, older adults are less likely to exert control over the activated items on the distractor list, especially when these items have been presented multiple times. Because older adults may have greater difficulty selecting the most relevant and appropriate pathway (targets) in the face of conflict from a partially activated competitor (repeated distractors), they may be more likely to produce exclusion errors. The dissociative effect of distractor presentation frequency for young and older adults suggests an age-related deficit in the use of recollection-based retrieval to oppose enhanced distractor familiarity. When young adults were told to speed up their recognition decisions, they showed an increase in memory exclusion errors as a function of distractor presentation frequency, similar to older adults when not under speed stress (e.g., Jacoby, 1999). Thus, the dissociative effect of distractor presentation frequency on young and older adults’ memory exclusion performance cannot be solely explained by older adults’ memory deficit. Rather, a breakdown in attentional control during retrieval also appears to contribute to the ironic effect of distractor repetition.
Present Study
As noted earlier there are clear breakdowns in attentional control systems in healthy aging and early-stage DAT and these breakdowns have been shown to contribute to the memory changes observed in these two groups. In the present experiment we used a modified version of Jacoby’s (1999) memory exclusion paradigm to increase the tension between recollection and familiarity in memory retrieval. We are interested in replicating the ironic effect of distractor repetition for healthy young and older adults and comparing the latter group’s memory exclusion abilities with those of very mild DAT individuals. To our knowledge, no studies have used this paradigm to examine the memory exclusion abilities of very mild DAT individuals. Prior studies using the Deese/Roediger-McDermott (DRM) false memory paradigm (Roediger & McDermott, 1995) and involving mild DAT individuals showed that they had lower levels of both veridical and false memories than healthy older adults (e.g., Budson et al., 2000, 2002, 2003). This pattern could suggest that individuals with DAT have difficulty encoding the study items and DAT has a detrimental effect on both recollection- and familiarity-based retrieval, thereby consistent with the memory deficit hypothesis. Hence, this hypothesis predicts that very mild DAT individuals should show lower hit rates and fewer exclusion errors than healthy older adults because (a) the distractors are likely to produce weaker memory traces in the DAT individuals and (b) hence they are influenced by distractor familiarity to a lesser extent than healthy older adults. Nevertheless, it is also possible that the earliest stages of DAT may be more on a continuum with healthy aging and so one might predict a larger influence of distractor familiarity.
Individuals in their earliest stage of DAT (i.e., very mild DAT individuals) have however been shown to have a decrease in their veridical memory performance but an increase in their false memory performance (e.g., Balota et al., 1999; Watson et al., 2001). This suggests that their decrements in the use of recollection-based retrieval strategy are more severe than their declines in the use of familiarity-based retrieval strategy. It should be noted that these very mild DAT individuals still showed a higher level of false memory than healthy older adults even when levels of veridical memory were taken into account, indicating that a memory deficit per se could not solely account for the decline of memory recall performance for very mild DAT individuals, consistent with attentional control hypothesis. The discrepancy in the levels of false memory in Balota et al. and in Budson et al. (2000, 2002, 2003) can be attributed to possible differences in DAT severity in their samples (i.e., very mild DAT in Balota et al. vs. mild DAT in Budson et al.). Indeed, the mean MMSE scores of Balota et al.’s very mild DAT samples, which were from the same pool as those in the present study (i.e., about 27–28), were higher than the mean scores reported in Budson et al. (2002; 2003) (i.e., 22–23).1 Because the current study, as in Balota et al., involved very mild DAT individuals, the attentional control hypothesis might expect these individuals to show more memory exclusion errors than healthy older adults.
As shown in Figure 1, the present experiment consisted of two sessions. In both sessions, participants were presented two lists and given different incidental encoding instructions. When they were presented a distractor list prior to a target list in one session they would receive the target list prior to a distractor list in the other session. For the distractor list, participants were asked to read words aloud and for the target list they were asked to semantically encode the items (by rating the words for self-relevance or for pleasantness). We decided to constrain deep encoding strategies because with an unspecified intentional encoding strategy (as used in Jacoby, 1999), it is possible that the differences across participant groups may be due to adoption of different encoding strategies (e.g., Kirchhoff et al., 2007; Thomas & Sommers, 2005). Hence, it is not clear whether the age difference in memory exclusion can be attributed to differential use of encoding strategies or older adults’ deficit in recollection-based retrieval. We expected that our use of an incidental learning instruction would decrease differences across participants in the use of encoding strategies and thus increase the likelihood that the effects may be due to group differences in memory retrieval. Finally, in the memory exclusion task given at the end of each session, participants responded “yes” only to the items previously rated and were told to make their decisions as quickly and as accurately as possible. The speeded nature of this task made it more difficult for the participants, especially those who have problems in attentional control system, to use the recollection-based retrieval to pit against the rise of distractor familiarity, thus providing a more sensitive measure of memory exclusion abilities in the three participant groups.
Figure 1.
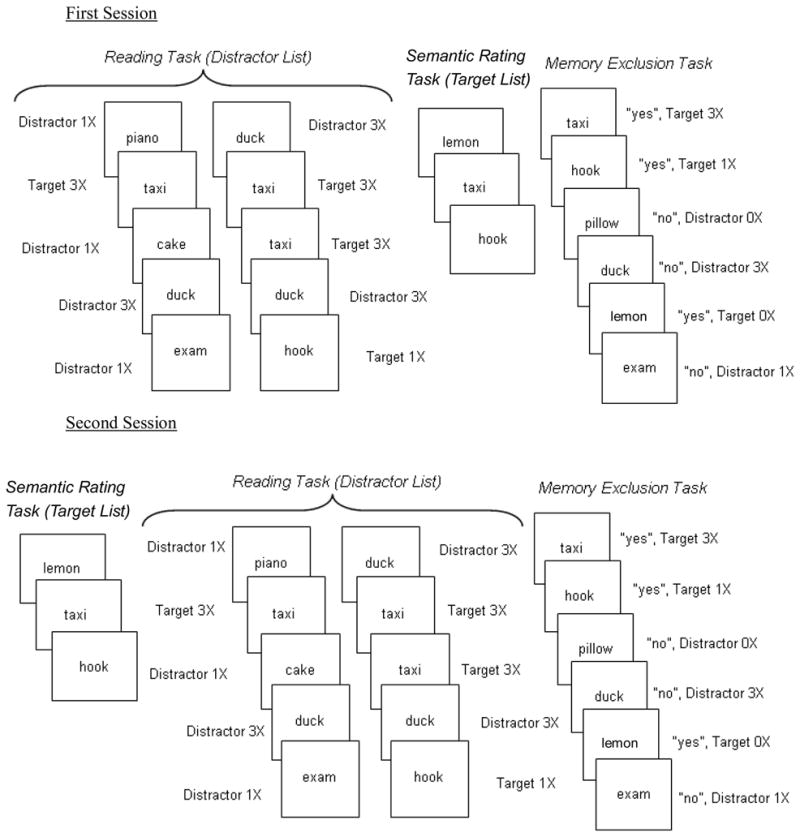
Illustration of procedures used in the first session and second session of the experiment
Unlike Jacoby (1999), as shown in Figure 1, we manipulated the presentation frequency of both distractors and targets (zero, once or thrice) on the distractor list. Each target was presented once on the target list and participants were asked to respond “yes” to these items in the memory exclusion task. However on the distractor list, one-fourth of the targets were presented once and one-fourth were presented thrice. (The remaining half of the targets were never presented on the distractor list.) This procedure allowed us to compute d′, in addition to the hit and false alarm rates to quantify memory accuracy for each level of target and distractor presentation frequency (zero, once or thrice) in the present experiment.2 We were interested in whether familiarity for the targets could also be boosted when presented on the distractor list, a list that participants were supposed to exclude in the memory exclusion task. We expected hit rates to steadily increase as a function of target presentation frequency for all participant groups. Importantly, regarding the ironic effect of distractor repetition, we expected to replicate Jacoby’s findings that healthy older adults would produce greater increases in memory exclusion errors over young adults as a function of distractor presentation frequency. As mentioned above, we also expected the very mild DAT individuals to show even higher memory exclusion errors than healthy older adults due to more severe breakdowns in their attentional control systems.
Several standard psychometric measures have proven to be useful in discriminating healthy aging and early-stage DAT (e.g., Brown & Storandt, 2000, see Table 1) in our sample, especially those that tap declarative memory (e.g., logical memory) and working memory (e.g., computational span). It should be noted that these single-point estimates may a priori be regarded to be more powerful discriminators than the measures in difference scores, such as d′, due to their higher reliability (e.g., Lord, 1963; but see Rogosa & Willett, 1983). Hence, it is particularly important to test whether memory exclusion abilities, as estimated by d′, add discriminative power above and beyond these standard psychometric measures. We conducted logistic regression analyses to determine whether participants’ memory exclusion abilities could be used to discriminate very mild DAT individuals from healthy older adults even after the scores from the psychometric measures were entered into the regression equation.
Table 1.
Demographic characteristics and performance in psychometric measures as a function of older adults and very mild DAT individuals
| Healthy Older Adults | Very Mild DAT Individuals | p | |||||
|---|---|---|---|---|---|---|---|
| Mean | N | SD | Mean | N | SD | ||
| Age | 74.65 | 105 | 8.59 | 74.31 | 48 | 8.61 | 0.86 |
| Years of Education | 15.10 | 102 | 2.86 | 15.33 | 46 | 2.67 | 0.75 |
| Mini-Mental State Exam | 29.12 | 100 | 1.20 | 27.41 | 46 | 2.01 | <.01 |
| WMS Logical Memory | 11.70 | 98 | 3.66 | 8.77 | 44 | 4.51 | <.01 |
| WMS Digit Forward | 6.65 | 98 | 1.36 | 6.36 | 44 | 1.28 | 0.19 |
| WMS Digit Backward | 4.92 | 98 | 1.40 | 4.50 | 44 | 1.32 | 0.07 |
| WMS Associate Memory | 14.82 | 100 | 3.59 | 10.22 | 45 | 3.98 | <.01 |
| Selective Reminding Free Recall | 30.81 | 100 | 5.95 | 20.16 | 44 | 9.12 | <.01 |
| WAIS-R Information | 21.86 | 100 | 4.22 | 19.40 | 45 | 4.39 | <.01 |
| WAIS-R Similarities | 25.38 | 100 | 4.22 | 23.59 | 46 | 4.66 | 0.02 |
| Crossing Off | 177.75 | 100 | 81.28 | 154.23 | 44 | 35.76 | 0.07 |
| Trail Making A | 34.66 | 98 | 14.36 | 39.47 | 45 | 24.40 | 0.18 |
| Trail Making B | 87.67 | 97 | 36.30 | 121.57 | 44 | 58.00 | <.01 |
| Reading Span | 7.22 | 104 | 1.64 | 5.81 | 48 | 1.84 | <.01 |
| Rotation Span | 8.43 | 98 | 3.26 | 6.57 | 44 | 3.98 | <.01 |
| Computational Span | 8.44 | 105 | 3.62 | 6.33 | 46 | 3.20 | <.01 |
| Animal Naming | 19.54 | 98 | 6.23 | 15.52 | 44 | 5.86 | <.01 |
| Word Fluency S-P | 33.42 | 100 | 11.53 | 27.78 | 45 | 10.47 | <.01 |
Note. The p column indicates the p-value associated with the independent t statistics for the comparison between healthy older adults and very mild DAT individuals.
Finally, we examined the relationship between APOe4 genotype status and memory exclusion performance in our healthy older adults. Recently, several studies have attempted to identify early cognitive markers for DAT occurring in the absence of deficits in more global measures of cognition, by comparing nondemented individuals who are at risk for DAT with nondemented individuals who are not at risk for DAT. The presence of the ε4 allele of APOe4 genotype is a well-established risk factor for DAT (e.g., Blacker, 1997; Corder et al., 1993). Individuals homozygous for the ε4 allele are 15 times more likely to develop the DAT and heterozygotes have 3 times increased risk over noncarriers of being diagnosed with this disease (e.g., Farrer et al., 1997). Because APOe4 is a quite reliable biomarker for the development of DAT, we examined whether memory exclusion performance would be worse for those who are at greater risk for developing DAT (i.e., ε4 carriers) than for those who are less likely to develop DAT (i.e., ε4 noncarriers). With respect to the present study, there has recently been increasing evidence suggesting that ε4 carriers, compared to ε4 noncarriers, produce some deficits in spatial attention and executive control systems (e.g., Parasuraman et al., 2000; 2002 and Rosen et al., 2002, 2005). However, it should also be noted that other studies have failed to find APOe4 difference in standard psychometric tests (e.g., Caselli et al., 2004; Wilson et al., 2002). In a meta-analysis, Small et al. (2004) reported that non-demented individuals with ε4 allele show deficits in executive function, but the authors also stressed that this finding requires further verification with more empirical evidence. Because of the relation between attentional control and memory exclusion performance described above (also see Daniels et al., 2006), we were particularly interested in whether ε4 carriers and ε4 noncarriers would show differential memory exclusion performance. This finding could provide more evidence that some subtle aspects of attentional control system may be deficient in healthy older adults possessing the ε4 allele and hence may serve as an early cognitive marker for DAT even in the absence of deficits in more global measures of cognition.
Method
Participants
Thirty young adults were recruited from Washington University Psychology Department participant pool and received course credit as compensation for participation. A total of 105 healthy older adults and 48 very mild DAT individuals with a Clinical Dementia Rating (CDR) of 0.5 were recruited from Washington University Alzheimer’s Disease Research Center (ADRC) and compensated $40 for their participation in this and other tasks in a large psychometric battery. The study was approved by the Institutional Review Board at Washington University in St. Louis School of Medicine and all participants provided their informed consents at the beginning of the experiment. As shown in Table 1, the healthy older adults and very mild DAT individuals were matched on age and years of education.
All ADRC participants were screened for depression, untreated hypertension, reversible dementias, and other disorders which could potentially produce cognitive impairment. The inclusion and exclusion criteria for DAT are consistent with the criteria of National Institute of Neurological and Communications Disorders and Stroke—Alzheimer’s disease and Related Disorders Association (McKhann et al., 1984). The severity of dementia was assessed according to the Washington University CDR scale (Morris, 1993; Morris et al., 1988), with CDR 0, 0.5, 1, 2, and 3 representing no dementia, very mild dementia, mild dementia, moderate dementia, and severe dementia, respectively. The CDR is based on a 90-minute clinical interview that directly assesses the participant and also relies on information from a close collateral source concerning the participant. This interview assesses potential changes in participants’ cognitive abilities in the areas of memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care relative to previous behavior. Details regarding the assessment and recruitment procedures have been previously described (Berg et al., 1998; Morris et al., 2001). Both the reliability of the CDR (Burke et al., 1988) and the validity of the diagnosis based upon autopsy by this research team have been excellent (93% diagnostic accuracy) and well documented (e.g., Berg et al., 1998).
It is important to note that we are particularly interested in the mildest form of DAT in the present study, and so we only report data from the individuals with a CDR of 0.5. Indeed, recent studies (see Storandt et al., 2002, 2006) found that these individuals performed as well as, if not slightly better than, individuals diagnosed with mild cognitive impairment (e.g., Petersen et al., 2001) based on standardized psychometric test performance.
In addition to participating in the memory exclusion experiment, all ADRC participants completed a two-hour battery of psychometric tests as part of a longitudinal study of cognitive performance in healthy aging and Alzheimer’s disease. Because young adults were not recruited through the ADRC, they did not receive the psychometric battery. These psychometric testing sessions always occurred within a one-year window of the testing session for the memory exclusion task. On average, the psychometric testing session was conducted 81 (SD = 150) and 61 (SD = 99) days ahead of the memory exclusion task for healthy older adults and very mild DAT individuals, respectively. In order to take into account the participants’ varied lag between the time they did the psychometric tests and the time they did the memory exclusion experiment, in all of our regression analyses, we entered the mean signed interval between psychometric testing session and memory exclusion testing session (hereafter called session lag) in the first step of each regression model.
Memory was assessed with Logical Memory, Forward and Backward Digit Span, and Associate Memory subtests from Wechsler Memory Scale (WMS; Wechsler, 1987) and the Selective Reminding Free Recall Test (Grober et al., 1988), general intelligence with Information and Similarities subtests of Wechsler Adult Intelligence Scale (WAIS; Wechsler, 1955; 1997), visual perceptual-motor abilities with Crossing Off (Botwinick & Storandt, 1973) and Trail Making A and B (Armitage, 1945), and semantic/lexical retrieval with Word Fluency Test S-P (Thurstone & Thurstone, 1949) and the Animal Naming Test (Goodglass & Kaplan, 1983). These psychometric tests are scored such that higher scores indicate better performance except the Trail Making A and B tasks, where higher scores indicate poorer performance. In addition to these standard psychometric measures, we also included three span measures (reading, computational and rotation) for assessing working memory capacity (Engle et al., 1999). The findings of the psychometric tests and span measures are summarized in Table 1. As expected, very mild DAT individuals performed more poorly than healthy older adults on most tests.
Apparatus and Materials
The experiment was programmed using E-prime (Schneider, Eschman & Zuccolotto, 2002) and run on a Dell desktop computer with a standard 15-inch monitor. All stimuli and instructions were presented in black print in 18 point Courier font on a white background.
Two sets of 48 words were selected from the E-Lexicon Project database (Balota et al., 2007) for the two testing sessions. They were matched on word length, number of syllables, log HAL word frequency, orthographic neighborhood size, mean lexical decision reaction time, and mean naming reaction time. Their lexical characteristics are presented in Table 2.
Table 2.
Lexical Characteristics of Stimuli
| First Session | ||||||
|---|---|---|---|---|---|---|
| Required Response in the Memory Exclusion Task | New | Old | ||||
| Presentation Frequency in the Distractor List | 0X | 1X | 3X | 0X | 1X | 3X |
| Word Length | 5.00 | 5.33 | 5.00 | 4.92 | 5.00 | 5.00 |
| Log HAL Word Frequency | 8.66 | 8.42 | 8.32 | 8.49 | 8.27 | 8.44 |
| Orthographic Neighborhood Size | 3.92 | 5.67 | 7.50 | 4.42 | 4.83 | 4.17 |
| Number of Syllable | 1.50 | 1.50 | 1.50 | 1.42 | 1.50 | 1.33 |
| Normed Lexical Decision Reaction Time | 609.61 | 609.45 | 617.21 | 602.95 | 601.66 | 625.71 |
| Normed Naming Reaction Time | 624.94 | 621.52 | 612.86 | 620.00 | 593.57 | 585.59 |
| Second Session | ||||||
|---|---|---|---|---|---|---|
| Required Response in the Memory Exclusion Task | New | Old | ||||
| Presentation Frequency in the Distractor List | 0X | 1X | 3X | 0X | 1X | 3X |
| Word Length | 4.92 | 5.17 | 5.17 | 5.08 | 5.00 | 5.17 |
| Log HAL Word Frequency | 8.50 | 8.53 | 8.66 | 8.28 | 8.41 | 8.43 |
| Orthographic Neighborhood Size | 7.00 | 2.17 | 6.50 | 5.92 | 5.67 | 7.00 |
| Number of Syllable | 1.67 | 1.67 | 1.50 | 1.58 | 1.50 | 1.67 |
| Normed Lexical Decision Reaction Time | 613.91 | 619.48 | 618.61 | 631.92 | 606.04 | 611.41 |
| Normed Naming Reaction Time | 615.53 | 611.93 | 605.64 | 614.94 | 588.51 | 622.47 |
Design and Procedure
Participants took part in the study as part of a four-hour battery of tasks examining memory and attention performance across the lifespan. The battery was divided into 2 two-hour segments that were given to participants during a two-week interval. Participants completed one session of the current experiment in the first segment of the battery and the other session in the second segment. Each session took about 15 minutes to complete. The structures of the two sessions are displayed in Figure 1. As noted, participants were given two sets of words: One 26-item (24 experimental + 2 primacy buffers) word set was rated for pleasantness or self relevance and the other 50-item (48 experimental + 2 primacy buffers) word set was read aloud (see below and Appendix A for the details about the list composition.) In the first session the reading task was presented first and followed immediately by the rating task; in the second session this order was reversed. A different matched set of words was used in the second session. Because this counterbalancing variable did not produce any reliable interaction with the targeted variables, the data reported below are collapsed across the two sessions. Words from both tasks were then presented as test items in the memory exclusion task, in which participants were instructed to respond “yes” only to the words they previously rated and “no” to all other words. Thus, the rated list was the target list whereas the read list was the distractor list. Whereas each target was presented once and no distractor was presented on the 26-item target list, we varied the presentation frequency of targets and distractors on the 50-item distractor list. One-fourth of the distractors were presented once, one-fourth, thrice, and one-fourth of targets were also presented once, one-fourth, thrice. Appendix A presents all stimuli and their conditions.
In the reading task, each item was individually presented and stayed on the computer screen until participants read the word aloud. Participants were asked to respond as quickly and as accurately as possible. After a blank screen for 1000 ms, the next trial was presented. This task was composed of 50 trials (see Appendix A): two primacy buffer words, six words read once and also rated, six words read thrice and also rated, six words read once and not seen in the rating task, and six words read thrice and also not seen in the rating task.
During the rating task, each item was individually presented until participants gave the item a self relevance rating (first session) or pleasantness rating (second session) by pressing a key on the number pad. Both rating scales ranged from 1 to 7, with 1 being personally irrelevant or very unpleasant and 7 being highly relevant or very pleasant to the participants. Responses were self paced with a 1000-ms blank screen after the rating. This task was composed of 24 trials (see Appendix A) plus two primacy buffer words. While none of the items were repeated in the rating task, six of the 24 items were also read once, six were also read thrice, and 12 were not presented in the reading task. After the reading and rating tasks, participants completed an 18-trial self-paced two-digit addition and subtraction arithmetic task to minimize recency effects. The final phase of both experiments was the memory exclusion task. Participants were instructed to respond “yes” only to words seen in the rating task and “no” to all other words. Verbal and written instructions stressed that only the words in the rating task should be given a “yes” response even if other words seemed familiar. The exact wordings of the instruction were quoted as follow. (The contents in italic were presented in the first session and those within the parentheses were presented in the second session.)
“We would now like to test your memory for ONLY those words that you previously gave Relevance (Pleasantness) Ratings for. Please do not be misled by the words which you only named in the first (second) part of the experiment. Press the “L” key for an item that you DID NOT previously give a relevance (pleasantness) rating and the “A” key for items that you DID give a relevance (pleasantness) rating.”
The participants were instructed to respond as quickly and as accurately as possible. A 1000-ms blank screen intervened after the keypress and before the next item was presented. This task consisted of 50 trials (see Appendix A). The experimenters made sure that the participants understood the instruction of this memory exclusion task before they began the task.
Results
First, we present mixed-factor ANOVAs on the hit rates, false alarm rates, d’s (memory accuracy) and Cs (response criterion) as a function of participant group (young, healthy old and very mild DAT) to examine whether memory exclusion performance differs as a function of healthy aging and very mild DAT and whether these effects are modulated by the target/distractor presentation frequency. Second, we present regression analyses for determining whether memory exclusion d’s add to the discrimination between healthy aging and early-stage DAT after various cognitive abilities (as reflected by span and psychometric measures) are taken into account. Third, the hit rates, false alarm rates, d’s and Cs are also analyzed as a function of ε4 status for our healthy older adults in order to investigate whether the presence of the ε4 allele predicts memory exclusion performance. We also present regression analyses for determining whether memory exclusion d’s add to the discriminative power of ε4 status for healthy older adults after various cognitive abilities are taken into account.
As noted above, we wanted to test the utility of the memory exclusion measures in discriminating the healthy older adults and very mild DAT individuals above and beyond (a) the session lag between psychometric testing and memory exclusion experiment, as well as, (b) the other cognitive measures. In all of the following regression analyses for healthy older adults vs. very mild DAT individuals and for APOe4+ vs. APOe4− individuals, the session lag was entered uniquely as a predictor in the first step of regression models before entering the cognitive measure in the second step and the memory exclusion measure in the third step.
Participant Group Difference in Memory Exclusion Performance
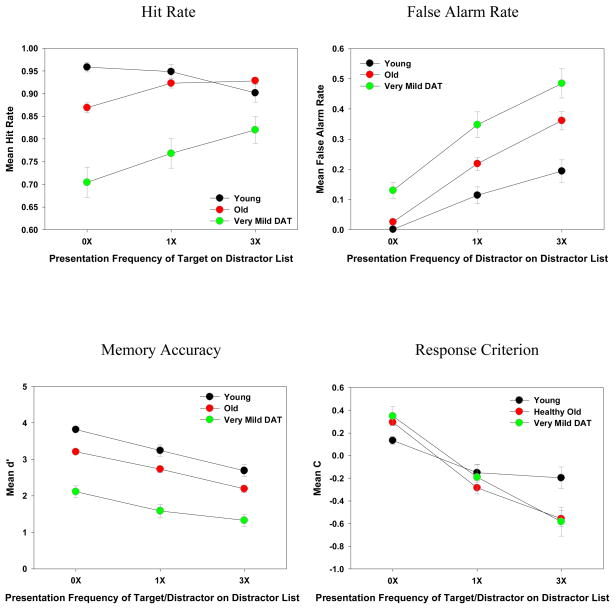
The mean hit rate, false alarm rate, d′ and C as a function of target/distractor presentation frequency on the distractor list and participant group are depicted in Figure 2. The main effects are discussed only when the interaction was not significant. Unless otherwise specified, all effects are significant at p < .05. The effect sizes in ηp2 are reported for all effects.
Figure 2.
Mean proportion of “yes” responses to the targets (hit rate) and to the distractors (false alarm rate), mean d′ (memory accuracy) and mean C (response criterion) as a function of target/distractor presentation frequency on the distractor list and participant group (young, old and very mild DAT). Error bars represent standard error of the mean.
Hit Rates
The main effects of Frequency and Group were both significant [F (2, 360) = 8.99, MSE = .01, ηp2 = .05, and F (2, 180) = 27.15, MSE = .05, ηp2 = .23, respectively]. The significant Frequency × Group interaction [F (4, 360) = 9.36, MSE = .01, ηp2 = .09] indicates that as the targets were presented more frequently on the distractor list, both healthy older adults and very mild DAT individuals showed an increase in hit rates (all Fs > 13.07, ηp2s > .15). In contrast, interestingly, young adults showed a reliable decrease in hit rates [F (2, 58) = 4.60, MSE = .01, ηp2 = .14] (see Figure 2). For healthy older adults and very mild DAT individuals, the hit-rate increments from 0X to 1X to 3X conditions were all significant (all Fs > 4.68, ηp2s > .09) except the 1X vs. 3X hit-rate difference for healthy older adults [F (1, 104) = .33, MSE = .01, ηp2 = .00]. For young adults, although the hit-rate decrement from 0X to 1X condition was not significant [F (1, 29) = .43, MSE = .003, ηp2 = .01], the decrements from 0X to 3X and from 1X to 3X were both reliable (both Fs > 4.27, ηp2s > .13).
False Alarm Rates
The main effects of Frequency and Group were both significant [F (2, 360) = 116.25, MSE = .11, ηp2 = .39, and F (2, 180) = 13.27, MSE = .11, ηp2 = .13, respectively]. The significant Frequency × Group interaction [F (4, 360) = 2.74, MSE = .03, ηp2 = .03] indicates that as the distractors were presented more frequently, the false-alarm increment for healthy older adults (0X-1X: 19% and 1X-3X: 14%) was statistically equivalent to that for very mild DAT individuals (0X-1X: 21% and 1X-3X: 14%, all Fs < 1, ηp2s < .01, see Figure 2). However, in comparison to these two groups, young adults produced a smaller change between the 0X and 1X conditions (11%, both Fs > 3.89, ps < .051, ηp2s > .02). In addition, although the 1X-3X increment for young adults was numerically smaller (8%), compared to the other two groups, this difference did not reach significance (both Fs < 2.67, ηp2s < .02).
d′
The main effects of Frequency and Group were both significant [F (2, 360) = 72.60, MSE = .46, ηp2 = .29, and F (2, 180) = 43.59, MSE = 1.86, ηp2 = .33, respectively], but the Frequency × Group interaction was not [F (4, 360) = .97, MSE = .46, ηp2 = .01]. As illustrated in Figure 2, memory accuracy decreased as a function of target/distractor presentation frequency (all Fs > 32.29, ηp2s > .12) and participant groups (all Fs > 13.95, ηp2s > .10). It should be noted that there was a larger difference in overall d′ [1.03, F (1, 151) = 52.57, MSE = .67, ηp2 = .26] between healthy older adults and very mild DAT individuals, relative to the overall difference between young and older adults [0.54, F (1, 133) = 13.95, MSE = .49, ηp2 = .10].
Criterion
The main effect of Frequency was significant [F (2, 360) = 114.26, MSE = .16, ηp2 = .39] but the main effect of Group was not [F (2, 180) = .62, MSE = .72, ηp2 = .01]. The Frequency × Group interaction was significant [F (4, 360) = 6.34, MSE = .16, ηp2 = .07]. As depicted in Figure 2, all three groups became more liberal in their response criteria when the targets/distractors were presented more frequently (all Fs > 7.60, ηp2s > .21). However, follow-up analyses showed that for both healthy older adults and very mild DAT individuals, response criterion differences were all significant among the three levels of presentation frequency (i.e., 0X-1X, 1X-3X, and 0X-3X, all Fs > 24.08, ηp2s > .19). In contrast, young adults’ response criteria were only significant for 0X-1X [F (1, 29) = 11.55, MSE = .10, ηp2 = .29] and 0X-3X differences [F (1, 29) = 9.84, MSE = .25, ηp2 = .16], but not for 1X-3X difference [F (1, 29) = .30, MSE = .11, ηp2 = .01].
CDR Status Discrimination by Memory Exclusion Performance
To examine the discriminative power of participants’ memory exclusion performance on healthy aging vs. the earliest detectable signs of dementia (i.e., CDR 0—healthy older adults vs. CDR 0.5—very mild DAT), we performed a series of stepwise logistic regression analyses, in which the session lag was entered in the first step. Each of the 15 individual working memory span or psychometric measures and the composite d′ measure was entered in the second and third steps of the regression equation, respectively (see Table 3). This composite d′ measure was the d′ averaged across the 1X and 3X target/distractor presentation frequency conditions. These analyses allowed us to test the discriminative power of memory exclusion d′ on CDR status after the discriminative power of each working memory span or psychometric measure was taken into account. Following the procedures in prior studies (e.g., Duchek et al., 2006; Rubin et al., 1998), the working memory span and psychometric measures entered in these analyses were all first standardized using the mean performance of healthy older adults (CDR 0 group). As shown in Table 3, the composite d′ measure was still significant and increased the correct classification rates for CDR status by 6.7% on average even after each of the general cognitive abilities had been taken into account. Apart from considering each of the 15 individual psychometric and working memory span measures, we quantified (a) a general cognitive measure by averaging the z-scores of all of these 15 measures, (b) a general memory measure by averaging the z-scores of the logical memory, associate memory and WAIS Information, and (c) a general attention measure by averaging the z-scores of the forward digit span and word fluency. As shown in Table 3, the composite d′ measure was still significant and increased the correct classification rates for CDR status by 6.2% on average after these composite scores had been taken into account.3
Table 3.
Logistic Regression Analyses of d′ on predicting CDR status (0 vs. 0.5)
| For X, after partialing out the session lag | For composite d′, after partialing out X | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable X | CCR | χ2(2) | Odd Ratio | Nagelkerke’s R2 | CCR | χ2(3) | Odd Ratio | Nagelkerke’s R2 |
| Composite d′ | 76.2 | 36.05* | .35* | .30 | ||||
| General Cognitive Measure | 76.8 | 37.80* | .08* | .31 | 80.8 | 49.54* | .48* | .39 |
| General Memory Measure | 77.2 | 36.08* | .26* | .31 | 80.0 | 46.54* | .48* | .39 |
| General Attention Measure | 69.0 | 9.79* | .52* | .09 | 80.7 | 38.61* | .35* | .33 |
| WMS Logical Memory | 74.6 | 22.20* | .45* | .20 | 83.1 | 41.56* | .41* | .36 |
| WMS Digit Forward | 68.3 | 5.11^ | .80 | .05 | 76.8 | 36.08* | .34* | .32 |
| WMS Digit Backward | 69.0 | 6.93* | .71^ | .07 | 78.9 | 37.62* | .35* | .33 |
| WMS Associate Memory | 78.6 | 42.35* | .31* | .36 | 80.7 | 52.82* | .48* | .43 |
| Selective Reminding Free Recall | 82.6 | 55.85* | .33* | .45 | 84.0 | 61.91* | .56* | .49 |
| WAIS-R Information | 71.0 | 12.55* | .58* | .12 | 76.6 | 36.62* | .37* | .31 |
| WAIS-R Similarities | 69.2 | 10.31* | .65* | .10 | 79.5 | 37.64* | .37* | .32 |
| Crossing Off | 70.8 | 9.91* | .35* | .09 | 77.1 | 39.15* | .35* | .34 |
| Trail Making A | 70.6 | 7.42* | 1.23 | .07 | 76.9 | 37.46* | .36* | .32 |
| Trail Making B | 73.8 | 19.47* | 1.81* | .18 | 78.7 | 44.38* | .37* | .38 |
| Reading Span | 71.3 | 24.42* | .43* | .21 | 78.0 | 48.14* | .38* | .39 |
| Rotation Span | 72.1 | 13.99* | .57* | .13 | 77.9 | 36.09* | .41* | .32 |
| Computational Span | 71.1 | 13.78* | .52* | .13 | 79.9 | 38.65* | .38* | .32 |
| Animal Naming | 71.8 | 18.86* | .46* | .18 | 78.2 | 40.26* | .39* | .35 |
| Word Fluency S-P | 69.0 | 11.16* | .57* | .10 | 78.6 | 41.97* | .57* | .35 |
p < .05,
p < .10. Composite d′ was computed by averaging the d′ across 1X and 3X conditions. CCR = correct classification rates for CDR 0 and 0.5 status. The χ2 column indicates the χ2 statistics for the full regression model and the odd ratio column indicates the odd ratio for the d′ as a predictor variable in the third step of the logistic regression analyses. Nagelkerke’s R2 indicates the effect size.
APOe4 Difference in Memory Exclusion Performance
The ε4 status was available for 96 of our 105 healthy older participants. The individuals who possess at least 1 ε4 allele are categorized as APOe4+, ε4 carriers (N = 69) and those who do not are categorized as APOe4−, ε4 noncarriers (N = 27). Table 4 displays the demographic characteristics and working memory span and psychometric measure performance as a function of ε4 status for healthy older adults. As shown these individuals are well matched on age and years of education. As in the initial analyses, we first submitted the mean hit rate, false alarm rate, d′ and C to a 2 (APOe4: ε4+ or ε4−) × 2 (Frequency: 0X, 1X or 3X) mixed-factor ANOVA. Only the effects associated with APOe4 are discussed.
Table 4.
Demographic characteristics and performance in psychometric measures as a function of two ε4 status for healthy older adults
| APOe4− (N = 69) | APOe4+ (N = 27) | p | |||||
|---|---|---|---|---|---|---|---|
| Mean | N | SD | Mean | N | SD | ||
| Age | 75.19 | 69 | 8.18 | 73.30 | 27 | 9.10 | 0.33 |
| Years of Education | 15.01 | 68 | 2.83 | 15.37 | 27 | 3.12 | 0.59 |
| Mini-Mental State Exam | 29.12 | 66 | 1.13 | 29.19 | 27 | 1.04 | 0.80 |
| WMS Logical Memory | 11.86 | 65 | 3.60 | 11.06 | 26 | 4.08 | 0.36 |
| WMS Digit Forward | 6.60 | 65 | 1.27 | 6.73 | 26 | 1.61 | 0.68 |
| WMS Digit Backward | 5.03 | 65 | 1.44 | 4.62 | 26 | 1.39 | 0.21 |
| WMS Associate Memory | 14.70 | 66 | 3.59 | 15.19 | 27 | 3.59 | 0.56 |
| Selective Reminding Free Recall | 31.56 | 66 | 5.43 | 28.74 | 27 | 6.81 | 0.04 |
| WAIS-R Information | 22.14 | 65 | 3.78 | 21.67 | 26 | 3.99 | 0.46 |
| WAIS-R Similarities | 25.55 | 66 | 3.84 | 25.44 | 27 | 4.92 | 0.92 |
| Crossing Off | 168.82 | 66 | 42.85 | 177.85 | 27 | 39.69 | 0.35 |
| Trail Making A | 35.91 | 66 | 14.01 | 29.73 | 27 | 10.54 | 0.59 |
| Trail Making B | 90.20 | 65 | 36.60 | 83.73 | 26 | 38.65 | 0.05 |
| Reading Span | 7.29 | 69 | 1.68 | 7.12 | 26 | 1.42 | 0.64 |
| Rotation Span | 8.26 | 66 | 3.47 | 9.04 | 24 | 3.04 | 0.33 |
| Computational Span | 8.93 | 69 | 3.59 | 7.56 | 27 | 3.29 | 0.09 |
| Animal Naming | 19.91 | 65 | 5.68 | 19.38 | 26 | 7.03 | 0.71 |
| Word Fluency S-P | 33.98 | 66 | 10.96 | 32.22 | 27 | 11.29 | 0.49 |
Note. The p column indicates the p-value associated with the independent t statistics for the comparison between APOe4+ and APOe4− groups.
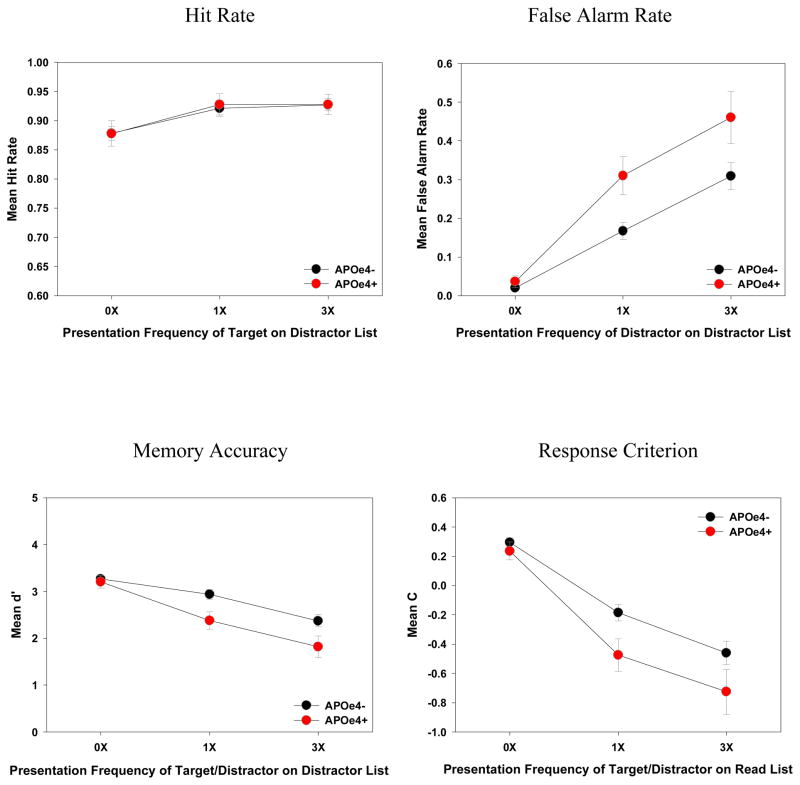
Hit Rates
Neither the main effect nor the interaction associated with APOe4 was significant (both Fs < 1, ηp2s < .01, see Figure 3).
Figure 3.
Mean proportion of “yes” responses to the targets (hit rate) and to the distractors (false alarm rate), mean d′ (memory accuracy) and mean C (response criterion) as a function of target/distractor presentation frequency on the distractor list and healthy older adult group (APOe4+ and APOe4-). Error bars represent standard error of the mean.
False Alarm Rates
The main effects of APOe4 and Frequency were significant [F (1, 94) = 7.14, MSE = .09, ηp2 = .07 and F (2, 188) = 93.23, MSE = .03, ηp2 = .50, respectively]. The APOe4 × Frequency interaction was also significant [F (2, 188) = 4.17, MSE = .03, ηp2 = .04]. The APOe4 effect was significant only when the distractor presentation frequency was 1X [14%, F (1, 94) = 9.26, MSE = .04, ηp2 = .09] and 3X [15%, F (1, 94) = 4.75, MSE = .09, ηp2 = .05], but not when it was 0X [2%, F (1, 94) = 1.27, MSE = .01, ηp2 = .01], thereby suggesting an enhanced level of distraction as a consequence of repetition for the APOe4 group.
d′
The main effects of APOe4 and Frequency were significant [F (1, 94) = 5.94, MSE = 1.45, ηp2 = .06 and F (2, 188) = 56.25, MSE = .45, ηp2 = .37, respectively]. The APOe4 × Frequency interaction was also significant [F (2, 188) = 3.55, MSE = .45, ηp2 = .04]. The APOe4 effect was significant only when the target/distractor presentation frequency was 1X [.56, F (1, 94) = 7.71, MSE = .79, ηp2 = .08] and 3X [.55, F (1, 94) = 4.73, MSE = 1.25, ηp2 = .05], but not when it was 0X [.06, F (1, 94) = .20, MSE = .36, ηp2 = .00], again suggesting a drop in memory exclusion accuracy as a consequence of presentation frequency for the APOe4 group.
Criterion
The main effects of APOe4 and Frequency were both significant [F (1, 94) = 4.62, MSE = .52, ηp2 = .05, and F (2, 188) = 96.71, MSE = .16, ηp2 = .51, respectively], but the APOe4 × Frequency interaction was not [F (2, 188) = 2.00, MSE = .16, ηp2 = .02]. As shown in Figure 3, ε4 carriers (-.32) generally used a more liberal response criterion than ε4 noncarriers (-.12) in their recognition decision.
APOe4 Discrimination by Memory Exclusion Performance
As shown in Table 4, ε4 carriers showed poorer performance in selective reminding free recall and computational span and better performance in Trail Making B than ε4 noncarriers. It should be noted that the marginalAPOe4 effect on the computational span replicated the findings of Rosen et al. (2002) although the effect they reported was also marginally significant. It is important to note that two of the three tests that discriminate between the ε4 carriers and ε4 noncarriers tap more executive control measures (i.e., Computational Span and Trail Making B) than declarative memory measures (i.e., Selective Reminding Free Recall). To verify whether the APOe4 effect in memory exclusion performance still occurs after taking into account the difference in working memory span and psychometric measures between ε4+ and ε4− healthy older adults, we performed a series of stepwise logistic regression analyses, with ε4 status as the criterion measure. This procedure was identical to the CDR 0 vs. 0.5 analyses discussed above, but now the criterion variable was the ε4 status. As shown in Table 5, even after the session lag and each of the cognitive abilities have been taken into account the composite d′ measure was still significant and on average increased the correct classification rates for ε4 status by 4.0%. As was done for the CDR 0 vs. 0.5 logistic regression analyses, we also quantified the general cognitive, general memory and general attention measures by using the same method. As indicated in Table 5, the composite d′ measure was still significant and increased the correct classification rates for ε4 status by 5.0% on average after the session lag and these composite scores had been taken into account.4
Table 5.
Logistic Regression Analyses of d′ on predicting ε4 status (4+ vs. 4−)
| For X, after partialing out session lag | For composite d′, after partialing out X | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable X | CCR | χ2(2) | Odd Ratio | Nagelkerke’s R2 | CCR | χ2(3) | Odd Ratio | Nagelkerke’s R2 |
| Composite d′ | 74.0 | 8.49* | .52* | .12 | ||||
| General Cognitive Measure | 70.8 | 2.90 | .51 | .04 | 75.0 | 9.34* | .53* | .13 |
| General Memory Measure | 69.9 | 2.59 | .89 | .04 | 75.3 | 9.48* | .51* | .14 |
| General Attention Measure | 69.9 | 2.48 | .95 | .04 | 75.3 | 9.47* | .51* | .14 |
| WMS Logical Memory | 70.3 | 3.49 | .74 | .05 | 78.0 | 10.86* | .50* | .16 |
| WMS Digit Forward | 70.3 | 2.10 | 1.09 | .03 | 72.5 | 10.90* | .46* | .16 |
| WMS Digit Backward | 70.3 | 3.48 | .75 | .05 | 73.6 | 11.73* | .47* | .17 |
| WMS Associate Memory | 69.9 | 3.26 | 1.24 | .05 | 74.2 | 10.52* | .50* | .15 |
| Selective Reminding Free Recall | 74.2 | 6.80* | .62* | .10 | 76.3 | 13.06* | .52* | .19 |
| WAIS-R Information | 69.9 | 2.86 | .85 | .04 | 77.4 | 9.79* | .51* | .14 |
| WAIS-R Similarities | 69.9 | 2.52 | .95 | .04 | 75.3 | 9.60* | .51* | .14 |
| Crossing Off | 68.8 | 3.14 | 1.44 | .05 | 72.0 | 10.31* | .50* | .15 |
| Trail Making A | 70.3 | 6.03* | .57^ | .09 | 76.9 | 14.06* | .47* | .21 |
| Trail Making B | 70.3 | 2.65 | .82 | .04 | 76.9 | 10.58* | .48* | .16 |
| Reading Span | 73.7 | 1.79 | .88 | .03 | 73.7 | 8.69* | .51* | .13 |
| Rotation Span | 72.2 | 2.63 | 1.24 | .04 | 73.3 | 8.31* | .54* | .13 |
| Computational Span | 70.8 | 3.95 | .67 | .06 | 72.9 | 10.87* | .51* | .15 |
| Animal Naming | 70.3 | 2.17 | .90 | .03 | 75.8 | 9.97* | .49* | .15 |
| Word Fluency S-P | 69.9 | 2.86 | .85 | .04 | 72.0 | 10.11* | .50* | .15 |
p < .05,
p < .10. Composite d′ was computed by averaging the d′ across 1X and 3X conditions. CCR = correct classification rates for ε4 status. The χ2 column indicates the χ2 statistics for the full regression model and the odd ratio column indicates the odd ratio for the d′ as a predictor variable in the third step of the logistic regression analyses. Nagelkerke’s R2 indicates the effect size.
Discussion
In the present study, we used a memory exclusion paradigm to assess the memory abilities of healthy young adults, healthy older adults and very mild DAT individuals. We were also interested in whether performance in this task could discriminate the healthy older adults who possess the ε4 alleles from those who do not. Unlike previous studies, we manipulated both target and distractor presentation frequencies on the distractor list and counterbalanced the presentation order of the target list and distractor list in the study phase. (The list order did not produce any reliable interaction with the targeted variables, and hence the interference effects observed across groups in the present study occur equally in a proactive and retroactive manner.) We shall now turn to a brief summary of the results, while noting a few unexpected but intriguing patterns. After this summary, we will discuss the theoretical implications of these results. Finally, we will discuss the implication of the current findings for the effect of APOe4 in memory exclusion abilities of nondemented, healthy older adults.
Summary of the Results
Hit Rates
Both healthy older adults and very mild DAT individuals showed higher hit rates when the targets were presented in both the target list and the distractor list than when they were only presented in the target list. Interestingly, as shown in Figure 2, young adults actually showed lower hit rates when the targets were presented more frequently on the distractor list than when they were not or were presented only once. The drop in hit rates occurred even when the overall hit rate of young adults was near ceiling level (about 90%). In contrast, the boost in hit rates for healthy older adults and very mild DAT individuals suggests that they benefited from the familiarity produced by repetition on the distractor list. Of course, it is unlikely that young adults’ decline in hit rates over the target presentation frequency on the distractor list indicates that they had poorer memory than the other two groups. In contrast, given the memory exclusion instruction that participants were told to give “yes” responses only to words presented in the target list, young adults appeared to be monitoring the source of each test item and suppressing the highly activated items that had possibly been from the wrong source (i.e., the thrice presented items on the distractor list). In other words, it is possible that the increased salience of the targets that were repeated thrice on the distractor list may have led the young adults to perform an additional check of the source to insure that their high level of familiarity was not due to the item presentation on the distractor list. This might lead them to exclude these targets more often on the memory exclusion task and have lower hit rates than when the targets were presented only once or not presented at all in the distractor list. In contrast, healthy older adults and very mild DAT individuals may be less likely to monitor the source of these targets, such that they appear to just go with the increase in the distractor familiarity in responding “yes” to these items. Hence, the multiple presentations of the targets, despite being on the distractor list, boosted their familiarity and in turn, the hit rates of healthy older adults and very mild DAT individuals.
False Alarm Rates
The false alarm data also suggested differential reliance on item familiarity in the memory exclusion task among the three participant groups. Although all three groups showed an increase in false alarm rates as a function of distractor presentation frequency, the increase was greatest for very mild DAT individuals, followed by healthy older adults and in turn, young adults (see Figure 2). This generally replicated the ironic effect of distractor repetition reported by Jacoby (1999) with young and older adults. Although very mild DAT individuals showed overall higher false alarm rates (32%) than healthy older adults (20%), the increase in false alarms as a function of distractor repetition (0X vs. 3X) was identical in both groups (35% and 34%, respectively). In other words, very mild DAT individuals did not show a disproportional increase in false alarm when the distractors were more frequent. In terms of dual-process theory of recognition memory (e.g., Mandler, 1980), our findings were consistent with previous studies (e.g., Balota et al., 2002; Jacoby, 1999). Of course, if one collapses across distractor frequency, the overall increase in false alarm rates across three groups suggests that very mild DAT individuals have a greater deficit in recollection-based retrieval than healthy older adults, who in turn have greater deficit in recollection-based retrieval than young adults.
Because of hit-rate differences between the healthy older adults and very mild DAT individuals, it is possible that the present results could be accommodated simply by changes in memory strength for the items across groups. Hence, we performed a stepwise linear regression analysis in which we predicted false alarm rate after hit rate was partialed out. This analysis showed that participant group (healthy old vs. very mild DAT) strongly predicted overall false alarm rate after we partialed out the variance due to overall hit rates [Change in F (1, 150) = 21.88, R2 change = .13]. Similar results were also found when only the hit and false alarm rates of the 1X and 3X conditions were considered [Change in F (1, 150) = 19.20, R2 change = .11]. Thus, very mild DAT individuals showed an overall higher false alarm rate than healthy older adults even when their hit rates had been controlled.
Turning to the APOe4 effect for healthy older adults, we found that relative to ε4 noncarriers, ε4 carriers were more likely to falsely recognize the distractors as targets. Similar to the DAT-related difference in false alarms, the APOe4 effect on false alarms was virtually identical whether the distractors were presented once or thrice. Because no APOe4 difference was found in hit rates, we did not perform the stepwise linear regression analysis for predicting false alarm rates after partialing out the variance due to hit rates.
Memory Exclusion Performance
We quantified the memory exclusion performance by computing d′, which took into account both hit and false alarm rates. As expected, we found that the overall memory exclusion performance dropped as targets and distractors were presented more frequently on the distractor list. Averaged across the presentation frequency, we found that young adults showed the best memory exclusion performance, followed by healthy older adults, and finally very mild DAT individuals. This group difference was not modulated by presentation frequency of targets and distractors on the distractor list. The fact that healthy older adults and very mild DAT individuals differed by more than 1 SD (d′ difference of 2.71 vs. 1.68) in exclusion performance supports the idea that very mild DAT individuals are likely to have a more severe breakdown in their attentional control system than healthy older adults. More importantly, after taking standard psychometric measures and working memory spans into account, the composite d′ (i.e., averaged across 1X and 3X) still significantly predicted the CDR status for healthy older adults (0) and very mild DAT individuals (0.5). Hence, the CDR-group difference in memory exclusion abilities could not be solely attributed to differences in general cognitive and declarative memory abilities in these two groups (see Table 3) and the memory exclusion performance could be utilized in discriminating very mild DAT individuals from healthy older adults.
Regarding the APOe4 effect, we also found that relative to ε4 noncarriers, ε4 carriers showed worse memory exclusion performance. However, unlike the age- and DAT-related difference, this APOe4 effect occurred only when the targets and distractors were presented at least once on the distractor list. The magnitude of the APOe4 effect remained the same even when the targets and distractors were presented more frequently on the distractor list. After taking standard psychometric measures and working memory spans into consideration, composite d′ (i.e., averaged across 1X and 3X) still significantly predicted ε4 status for healthy older adults. Indeed, as shown in Table 5, the correct classification rate of composite d′ was higher than all of the other psychometric or span measures. Thus, even within the healthy older adults, ε4 carriers showed worse memory exclusion performance than ε4 noncarriers, after taking into account their differences in general cognitive or episodic memory abilities.
It should be noted that some of the psychometric measures we included in our battery also tap attentional control abilities (e.g., Trailmaking B). After partialling out these measures, memory exclusion abilities still discriminated the healthy older adults vs. very mild DAT individuals and the APOe4+ vs. APOe4− healthy older adults. We would argue that Trailmaking B does not stress the attentional control system as much as the exclusion paradigm because there is a direct conflict between a controlled recollection process and a more automatic familiarity-based process in our memory exclusion task. Hence, our measure is likely to be more sensitive than the traditional psychometric measure in tapping the attentional control system.
Finally, it is also worth noting a limitation in our study. As mentioned in the Method section, there were varied time lags between the session of psychometric testing and the session of the memory exclusion task for healthy older adults and very mild DAT individuals. Although we took this into account by partialling out the mean signed interval between psychometric testing session and memory exclusion in our regression analyses, it is possible that the changes in performance over time may not be linear. Of course, to completely avoid the ambiguity for the interpretation of the data, future research should conduct both psychometric testing and memory exclusion task within the same testing session.
Implications for Memory Deficit Hypothesis and Attentional Control Hypothesis
As noted by Hodges (2000), the onset of Alzheimer’s disease is characterized by an initial loss of episodic memory, followed by other cognitive functions, such as attentional control and working memory. This memory deficit hypothesis has been supported by studies using DRM false memory paradigm. Using an episodic recognition test, Budson et al. (2002, 2003) found that mild DAT individuals showed a greater reduction in both hit and false alarm rates than age-matched healthy older adults. Balota et al. (1999) and Waldie and Kwong See (2003) replicated this pattern, although in these two studies the recognition memory performance was confounded with the level of prior free recall. These results support the memory deficit hypothesis: because mildly demented individuals failed to encode list memory in the DRM false memory paradigm, the presentation of highly associated items (e.g., blanket, pillow, bed) did not boost the familiarity of related lures (e.g., sleep). As a result, mild DAT individuals were less susceptible to falsely recognize the related lures than healthy older adults.
Although the memory deficit hypothesis may be able to explain some aspects of the DAT related change in the DRM paradigm, there are also some aspects that are a bit more difficult to accommodate. For example, after matching the level of veridical recall between healthy older adults and very mild DAT individuals, Balota et al. (1999) found that the level of false recall was higher for very mild DAT individuals than for healthy older adults, contrary to the opposite pattern reported in recognition memory studies. (It should be noted that the very mild DAT individuals in Balota et al. have mean MMSE scores on the order of 27, whereas the mild DAT individuals in Budson et al., 2002, 2003, have mean MMSE scores of approximately 22–23.) Assuming that participants’ memory abilities can be equated when the levels of veridical recall are matched between two groups, memory deficit per se would appear to be insufficient in accounting for the false-recall difference between healthy older adults and very mild DAT individuals. Instead, these results suggest a role for an attentional control deficit in the DAT individuals (Balota & Faust, 2001; Perry & Hodges, 1999). Relative to healthy older adults, very mild DAT individuals are less likely to control the extraneous activation of highly familiar related lures, such that they are more likely to output them directly, and in turn produce more false-recall responses.
In the current study, we further tested the attentional control hypothesis by using item repetition, rather than semantic associations among study items, to increase distractor familiarity. Rather than having participants study a list of words that are all associated with the distractors (i.e., nonstudied related lures), we increased tension between recollection and familiarity by directly repeating the targets and distractors on the distractor list, a list that the participants are instructed to exclude in the memory exclusion paradigm. Several observations in our experiment support the role of changes in attentional control mechanisms across our groups. First, the increase in hit rates for healthy older adults and very mild DAT individuals as targets were repeated on the distractor list indicates that these two groups were not deficient in sensitivity to repetition on the study list. This rules out the possibility that these individuals did not encode and store the information well during the study phase. Second, participant group (healthy old vs. very mild DAT) significantly predicted false alarm rates after partialing out the variance in hit rates. Similar to the interpretation of Balota et al. (1999), memory deficit per se could not explain this group difference in memory exclusion errors. Rather, it is more likely that healthy older adults and especially very mild DAT individuals failed to adequately tune their attentional control system to the relevant information during retrieval. In fact, if the DAT-related decline in memory exclusion was due entirely to memory deficit, one could expect both hit and false alarm rates to be lower for very mild DAT individuals than for healthy older adults, thereby rendering the d′ not to be a useful discriminator for DAT discrimination. Third, after we partialled out the variance due to various declarative memory psychometric measures, we found that memory exclusion performance still discriminated the very mild DAT individuals from healthy older adults. Given that (a) healthy older adults and very mild DAT individuals have problems in attention tasks, (b) it has been found that memory is dependent on an intact attentional control system and (c) there is evidence in our experiment that these individuals did encode the study items successfully, we believe that the pure memory account of cognitive deficits in DAT needs to be revisited. Although it is not easy to tease apart the contribution of declarative memory and attention, we clearly have demonstrated that there is also a role for changes in attentional control systems that are particularly salient in Jacoby’s memory exclusion paradigm.
Because we also found a d′ difference between healthy older adults and very mild DAT individuals in the 0X condition (see Figure 2), one could argue that item recognition memory itself may be sufficient to discriminate the two participant groups. However, given the context of memory exclusion task (e.g., task instruction), the d′ in our 0X condition does not necessarily reflect one’s accuracy of item recognition memory. This point is supported by two additional findings. First, the 0X d′ significantly discriminated the two participant groups [χ2 (3) = 53.48; odd ratio = .23; Nagelkerke’s R2 = .44] even after the session lag as well as the variance of WMS logical memory psychometric measure, which is typically regarded as a measure of item memory, were partialled out. The correct classification rate in this analysis increased from 74.6% to 82.4%. Second, we also assessed participants’ item recognition memory performance in one of the experiments of our large cognitive battery. In that experiment, participants studied a list of 24 words and did an immediate item recognition memory test in which they were asked to discriminate the studied words from the nonstudied ones. We obtained the typical findings: healthy older adults showed the same level of hit rates [.77 vs. .74, F (1, 135) = .38, MSE = .04, ηp2 = .00], lower false alarm rates [.12 vs. .21, F (1, 135) = 7.00, MSE = .03, ηp2 = .05] and lower d′ [2.47 vs. 1.97, F (1, 135) = 4.22, MSE = 1.67, ηp2 = .03] than very mild DAT individuals.5 More importantly, after partialing out the variance of session lag as well as item recognition d′, we found that the 0X d′ yielded in our memory exclusion experiment could still significantly discriminate the two participant groups [χ2 (3) = 47.34; odd ratio = .20; Nagelkerke’s R2 = .42]. The correct classification rate in this analysis increased from 69.6% to 83.0%. Similar pattern was found for composite d′ that averaged across 1X and 3X conditions [χ2 (3) = 34.45; odd ratio = .36; Nagelkerke’s R2 = .32], with the correct classification rate being increased from 69.6% to 77.0%. Hence, we argue that even the 0X d′ in our memory exclusion task involves cognitive control abilities in addition to pure memory abilities. This provides converging evidence for our argument that memory deficit hypothesis per se may not account for the present findings.
It is worth noting the implication of our findings on the differential deficits of item vs. source memory in DAT. Instead of a breakdown in the attentional control system, one could also attribute the reduced memory exclusion abilities of our very mild DAT individuals to their preserved item memory but impaired source memory. We believe that the dichotomy of intact item vs. impaired source memory is entirely compatible with our claim. Indeed, attentional mechanisms are important for laying down source information at encoding and in turn, using it at retrieval (see Schacter et al., 1994; Troyer et al., 1999). Although the exclusion paradigm is ideally suited to tapping these operations during retrieval, we believe that encoding differences could also play a role. That is, relative to the healthy older adults, the deficit in memory exclusion may occur at both encoding—very mild DAT individuals may fail to encode the source memory when they study the list items in the first place and/or at retrieval—very mild DAT individuals may fail to use their source memory to discriminate the items from different lists. In this light one might ask “where” memory ends and “attentional control” begins in a given task. However, we believe that for most declarative memory tasks, such a dichotomy is overstated. Indeed, McCabe et al. (2008) have recently demonstrated that virtually all of the age-related change in standard episodic memory tasks can be accounted for age-related changes in executive operations tapped by standard working memory measures. All of these findings strongly suggest that in addition to the memory deficit of DAT, one also needs to consider how a breakdown of their attentional control system contributes to their episodic memory performance.
Further Evidence for the Effect of DAT in Memory Exclusion Performance from Previous Studies
The detrimental effect of DAT on memory exclusion performance was consistent with the findings reported in previous studies using the process dissociation procedure. Here, we focus on data in the past “exclusion” conditions that are analogous to the 1X condition in the present study. Using a stem completion task, Koivisto et al. (1998, see also Knight, 1998) showed that relative to healthy older adults, mild DAT individuals were more likely to generate studied words to the test stems even though they were instructed to exclude them and produce different responses. Smith and Knight (2002, Experiment 2) showed that relative to healthy older adults, mild DAT individuals were less likely to resist from generating the identical exemplars for the same category in a category exemplar generation task. Kessels et al. (2005) generalized the DAT-related increase in memory exclusion errors to a spatial memory task. Using a series of precautions to avoid the methodological limitations that occurred in the previous studies (e.g., ceiling/floor effect and comprehension of instruction), Adam et al. (2005) showed that in a continuous stem completion task with repeating stem cues, DAT individuals were more likely to produce the same response to repeated stem cues than healthy older adults even though they were explicitly instructed to produce a different response to the repeated cues. All of these results converged on the conclusion that DAT individuals failed to exclude memory by controlling the irrelevant yet highly familiar information, thereby providing support for the role of attentional control during retrieval. Since the mean MMSE scores provided in most of these studies were lower than 23, it is likely that the DAT participants were at the mild level. By contrast, because our very mild DAT individuals yielded mean MMSE scores of 27 (see Table 1), the current study shows that even individuals in the very early stages of DAT already demonstrate a subtle deficit in memory exclusion performance, highlighting the fact that attentional control could deteriorate in concordance with memory functioning.
Implication for the effect of APOe4 on executive function and attentional control
The presence of the ε4 allele of the APOe4 genotype has been reliably reported to be a risk factor for the development of DAT (e.g., Blacker, 1997). Previous studies have reported the detrimental effect of APOe4 in memory and cognitive functioning of non-demented individuals, such as visual selective attention (e.g., Parasuraman et al., 2000), episodic memory (e.g., Bondi et al., 1995, 1999; Levy et. al., 2004; Lind et al., 2006; Wilson et al., 2002) and prospective memory (e.g., Driscoll et al., 2005, but see Duchek et al., 2006). However, it should be noted that the absence of an APOe4 effect on cognitive tasks has also been reported (e.g., Cohen et al., 2001; Collie et al., 2001; Jorm et al., 2007; Smith et al., 1998). In 2004, Small et al.’s meta-analysis indicated that relatively little evidence has been reported investigating the effect of ε4 status on executive control functions, but what has been reported has produced a relatively large effect. Since then, several APOe4 studies have provided further evidence for the modulation of APOe4 alleles on cognitive performance pertinent to attentional control. For example, Rosen et al. (2005, see also Nilsson et al., 2006) showed that in a category fluency task ε4 carriers generated fewer exemplars and category clusters, and took longer time to access the clusters than ε4 noncarriers. They attributed this to ε4 carriers’ reduced attentional capacity interfering with their covertly shifting attention among subcategories in the category fluency task. Packard et al. (2007) and Albert et al. (2007) found that ε4 carriers showed stronger Stroop interference in response latency than ε4 noncarriers. To perform the Stroop task, participants need to suppress the irrelevant information (e.g., color name) and access the relevant one (e.g., ink color) (see also Mutter et al., 2005; Spieler et al., 1996). We would argue that surpassing the familiarity in a memory exclusion task may tap similar mechanisms, as supported by Sommers and Huff’s (2003) showing a direct relationship between Stroop interference effects and the ability to control familiarity in memory performance. Using a modified Stroop task with components of response inhibition and cognitive switching, Wetter et al. (2005) found that ε4 carriers made more intrusion errors than ε4 noncarriers.
Based on our nondemented healthy older adult sample (see Table 4), we also found that ε4 carriers performed worse than ε4 noncarriers in the selective reminding free recall task and computational span task, replicating the findings of some previous studies (e.g., Helkala et al., 1995 and Klages & Fisk, 2002 for selective reminding and Rosen et al., 2002 for computational span) but not of the others (e.g., Rosen et al., 2002 for selective reminding). It is not clear why this discrepancy occurred given that the version we used in our study (i.e., Grober et al., 1988) was methodologically similar to the one used in Rosen et al. (i.e., Buschke’s, 1973, version).
As mentioned in the Introduction, memory exclusion performance can be regarded as an indicator of attentional control. To decide whether a test item appeared on the target list, one needs to focus attention on recollecting the relevant information on the target list and inhibiting the irrelevant information on the distractor list. We found that relative to ε4 noncarriers, ε4 carriers were less likely to reject highly familiar distractors and make more exclusion errors. (Whether the distractors were presented only once or thrice on the distractor list, however, did not modulate the APOe4 effect on false alarm.) Since attentional control is an executive function (e.g., see Daniels et al., 2007), our current findings provide additional evidence for the negative effect of APOe4 allele on the executive functioning of nondemented healthy older adults.
Finally, according to Small et al.’s (2004) meta-analysis, the magnitude of cognitive deficits associated with ε4+ is inversely related to age. For example, Smith et al. (1998) reported that ε4 carriers under 80 years of age showed poorer delayed recall performance than ε4 noncarriers, but the pattern was opposite for those ε4 carriers and noncarriers over 80 years of age. Duchek et al. (2006) showed a positive correlation between age and prospective memory performance for ε4 carriers but a negative correlation between age and prospective memory performance for the ε4 noncarriers. This is consistent with the claim that the presence of the ε4 allele as a risk factor of Alzheimer’s disease actually declines with increasing age and is related to the earlier onset of DAT (Blacker, 1997; Farrer et al., 1997; but see Riley et al., 2000, for an opposite view). Using 80 as an age cutoff to divide each of ε4-carrier and ε4-noncarrier healthy older adults into two groups, we did find that the APOe4 effect on the false alarm rates was significant and numerically greater for healthy older adults younger than 80 [.29 vs. .15, F (1, 66) = 8.49, MSE = .03, ηp2 = .10] than for those at or older than 80 [.22 vs. .20, F (1, 26) = .10, MSE = .03, ηp2 = .02]. Although the age-related modulation of the APOe4 effect is intriguing, these data must be interpreted with caution given the small sample of adults over 80 who are ε4+ (N = 7) and await further replication with larger samples of participants.
Summary and Conclusion
The present study examined the ability to control familiarity-based information in a memory exclusion paradigm in healthy young, older adults, and individuals with early-stage DAT, and compared the potential predictive power of performance in this task to standard psychometric performance in discriminating between healthy aging and the earliest detectable form of DAT, and between APOe4 genotype in healthy older adults. We found that memory exclusion performance (as reflected by d′) decreased across participant groups (young > healthy old control > very mild DAT) and as indicated by regression analyses, d′ significantly increased the discriminative power for healthy older adults vs. very mild DAT individuals above and beyond standard psychometric measures for general cognitive abilities. For healthy older adults, memory exclusion d′ was also lower for ε4 carriers than for ε4 noncarriers even after partialing out baseline psychometric performance. Therefore, it appears that engaging attentional control systems by pitting recollection-based information against familiarity-based information in the exclusion paradigm may be a particularly useful procedure for the early detection of DAT, and individuals who are likely to convert to DAT.
Acknowledgments
This work was supported by National Institute on Aging Grant PO1AG003991. We thank John Morris and the Clinical Core at the Washington University Alzheimer’s Disease Research Center for their careful recruitment and description of the healthy older adults and individuals with dementia of the Alzheimer’s type, Alison Goate for the APOe4 genotyping, Martha Storandt for providing the psychometric performance, Nathan Rose for helping develop the computer program, and Amy Heidbreder, Betsy Hemphill, Jeff Templeton, and Brian Weber for testing the participants. Portions of these findings were presented at the Cognitive Aging Conference, Atlanta, Georgia, April 2008.
Appendix A
Experimental Stimulus List
| First Session | |||||
|---|---|---|---|---|---|
| Item | Distractor List | Target List | Item | Distractor List | Target List |
| bowl | 0X | 1X | carpet | 0X | 0X |
| cotton | 0X | 1X | clay | 0X | 0X |
| drum | 0X | 1X | cloud | 0X | 0X |
| hive | 0X | 1X | eagle | 0X | 0X |
| kidney | 0X | 1X | knee | 0X | 0X |
| lamb | 0X | 1X | lemon | 0X | 0X |
| pond | 0X | 1X | sail | 0X | 0X |
| razor | 0X | 1X | shotgun | 0X | 0X |
| stairs | 0X | 1X | slug | 0X | 0X |
| tiger | 0X | 1X | swamp | 0X | 0X |
| tunnel | 0X | 1X | token | 0X | 0X |
| wheat | 0X | 1X | turtle | 0X | 0X |
| hook | 1X | 1X | bucket | 1X | 0X |
| onion | 1X | 1X | duck | 1X | 0X |
| pillow | 1X | 1X | feather | 1X | 0X |
| taxi | 1X | 1X | oven | 1X | 0X |
| throne | 1X | 1X | snake | 1X | 0X |
| towel | 1X | 1X | throat | 1X | 0X |
| glove | 3X | 1X | ankle | 3X | 0X |
| leaf | 3X | 1X | bench | 3X | 0X |
| roof | 3X | 1X | castle | 3X | 0X |
| shelf | 3X | 1X | hose | 3X | 0X |
| toilet | 3X | 1X | moss | 3X | 0X |
| walnut | 3X | 1X | subway | 3X | 0X |
| Second Session | |||||
|---|---|---|---|---|---|
| Item | Distractor List | Target List | Item | Distractor List | Target List |
| beef | 0X | 1X | alley | 0X | 0X |
| bicycle | 0X | 1X | belt | 0X | 0X |
| bull | 0X | 1X | cherry | 0X | 0X |
| exam | 0X | 1X | creek | 0X | 0X |
| gravel | 0X | 1X | lime | 0X | 0X |
| lace | 0X | 1X | olive | 0X | 0X |
| needle | 0X | 1X | piano | 0X | 0X |
| salad | 0X | 1X | pony | 0X | 0X |
| shirt | 0X | 1X | seal | 0X | 0X |
| stove | 0X | 1X | sheep | 0X | 0X |
| tribe | 0X | 1X | trumpet | 0X | 0X |
| wallet | 0X | 1X | wagon | 0X | 0X |
| bubble | 1X | 1X | arrow | 1X | 0X |
| cave | 1X | 1X | chicken | 1X | 0X |
| cliff | 1X | 1X | frog | 1X | 0X |
| coal | 1X | 1X | ozone | 1X | 0X |
| elbow | 1X | 1X | puppet | 1X | 0X |
| kitten | 1X | 1X | yarn | 1X | 0X |
| anchor | 3X | 1X | bread | 3X | 0X |
| bacon | 3X | 1X | cone | 3X | 0X |
| cake | 3X | 1X | jewelry | 3X | 0X |
| closet | 3X | 1X | lion | 3X | 0X |
| nail | 3X | 1X | pencil | 3X | 0X |
| worker | 3X | 1X | rifle | 3X | 0X |
Footnotes
Based on the implicit memory literature (e.g., Gabrieli et al., 1999), DAT individuals showed smaller repetition priming effect than healthy older adults in production implicit memory tests (e.g., word-stem completion) but not in identification implicit memory tests (e.g., perceptual identification). Accordingly, one might argue that it could be differences in the task demands (recall-production vs. recognition-identification) that produced the discrepancy between Balota et al. (1999) and Budson et al. (2000, 2002, 2003). However, as demonstrated in the present study, we still found a difference in memory exclusion performance between healthy older adults and very mild DAT individuals in an episodic recognition task. Hence, the differential DAT severity was more likely the cause for the discrepancy.
We also conducted the same set of analyses on the mean arithmetic difference between hit rates and false alarm rates in each condition for each group and obtained the statistically identical findings as those reported here for d’s.
Similar analyses were also performed for composite false alarm rates (i.e., false alarms averaged across 1X and 3X conditions). Unlike the d′ predictor which was significant after partialing out each of the individual or composite predictors (see Table 3), the false-alarm predictor was no longer significant after partialing out the general cognitive measure, general memory measure, WMS associate memory and selective reminding free recall. After taking each of psychometric measures into account, the mean change in classification rate (1.2%) was lower than that for d′ (6.7%). The mean Nagelkerke’s R2 change (.05) was also lower than that for d′ (.19). Similarly, after taking each of composite measures into account, the mean change in classification rate (2.7%) was lower than that for d′ (6.2%). The mean Nagelkerke’s R2 change (.03) was also lower than that for d′ (.13). This shows that the difference-score measures, such as d′, are not necessarily less powerful than single point measures, such as false alarm rates, in discriminating the healthy older adults from the very mild DAT individuals.
Similar analyses were also performed for composite false alarm rates (i.e., false alarms averaged across 1X and 3X conditions). Similar to d′ predictors, all of the false-alarm predictors were also significant after partialing out each of the individual or composite predictors (see Table 5). However, after taking each of individual psychometric measures into account, the mean change in classification rate (3.1%) was slightly lower than that for d′ (4.0%). The mean Nagelkerke’s R2 change (.12) was about the same as that for d′ (.11). After taking each of composite measures into account, the mean change in classification rate (2.1%) was lower than that for d′ (5.0%). The mean Nagelkerke’s R2 change (.10) was virtually identical to that for d′ (.10). Again, this pattern shows that the difference-score measures, such as d′, may not be necessarily less powerful than single point measures, such as false alarm rates, in discriminating the APOe4+ vs. APOe4− individuals. The higher sensitivity in d′ may be due to the fact that participants did not only need to exclude the distractors but also need to correctly accept the targets because it had also been repeatedly presented in the distractor list.
The young adults and healthy older adults showed equivalent hit rates [.78 vs. .77, F (1, 124) = .05, MSE = .04, ηp2 = .00], false alarm rates [.124 vs. .118, F (1, 124) = .04, MSE = .02, ηp2 = .00] and d′ [2.50 vs. 2.47, F (1, 124) = .02, MSE = 1.72, ηp2 = .00]. The absence of age-related difference in the item recognition memory test was not without precedence (e.g., Craik & McDowd, 1987).
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/neu.
References
- Adam S, Van der Linden M, Collette F, Lemauvais L, Salmon E. Further exploration of controlled and automatic memory processes in early Alzheimer’s disease. Neuropsychology. 2005;19:420–427. doi: 10.1037/0894-4105.19.4.420. [DOI] [PubMed] [Google Scholar]
- Albert M, Moss MB, Blacker D, Tanzi R, McArdle JJ. Longitudinal change in cognitive performance among individuals with mild cognitive impairment. Neuropsychology. 2007;21:158–169. doi: 10.1037/0894-4105.21.2.158. [DOI] [PubMed] [Google Scholar]
- Armitage SG. An analysis of certain psychological tests used for the evaluation of brain injury. Psychological Monographs. 1945;60(1 Whole No 177):1–48. [Google Scholar]
- Atkinson RC, Juola JF. Search and decision processes in recognition memory. In: Krantz DH, Atkinson RC, Duncan LR, Suppes P, editors. Contemporary developments in mathematical psychology: Learning, memory, and thinking. Oxford, England: Oxford University Press; 1974. pp. 243–293. [Google Scholar]
- Bäckman L, Small BJ, Fratiglioni L. Stability of the preclinical episodic memory deficit in Alzheimer’s disease. Brain. 2001;124:96–102. doi: 10.1093/brain/124.1.96. [DOI] [PubMed] [Google Scholar]
- Balota DA, Burgess GC, Cortese MJ, Adams DR. Word-frequency mirror effect in young, old, & early stage Alzheimer’s disease: Evidence for two processes in episodic recognition performance. Journal of Memory & Language. 2002;46:199–226. [Google Scholar]
- Balota DA, Cortese MJ, Duchek JM, Adams D, Roediger HL, McDermott KB, Yerys BE. Veridical and false memory in healthy older adults and in Dementia of the Alzheimer’s Type. Cognitive Neuropsychology. 1999;16:361–384. [Google Scholar]
- Balota DA, Dolan PO, Duchek JM. Memory changes in healthy young and older adults. In: Tulving E, Craik FIM, editors. The Oxford Handbook of Memory. New York, NY: Oxford University Press; 2000. pp. 395–410. [Google Scholar]
- Balota DA, Faust M. Attention in dementia of the Alzheimer’s type. In: Boller F, Cappa S, editors. Handbook of Neuropsychology. 2. NY: Elsevier Science; 2001. pp. 51–80. [Google Scholar]
- Balota DA, Yap MJ, Cortese MJ, Hutchison KA, Kessler B, Loftis B, Neely JH, Nelson DL, Simpson GB, Treiman R. The English Lexicon Project. Behavior Research Methods. 2007;39:445–459. doi: 10.3758/bf03193014. [DOI] [PubMed] [Google Scholar]
- Berg L, McKeel DW, Jr, Miller JP, Storandt M, Rubin EH, Morris JC, Baty J, Coats M, Norton J, Goate AM, Price JL, Gearing M, Mirra SS, Saunders AM. Clinicopathologic studies in cognitively healthy aging and Alzheimer’s disease: Relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Archives of Neurology. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- Blacker D. The genetics of Alzheimer’s disease: Progress, possibilities, and pitfalls. Harvard Review of Psychiatry. 1997;5:234–237. doi: 10.3109/10673229709000306. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Salmon DP, Galasko D, Thomas RG, Thal LJ. Neuropsychological function and apolipoprotein E genotype in the preclinical detection of Alzheimer’s disease. Psychology and Aging. 1999;14:295–303. doi: 10.1037//0882-7974.14.2.295. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Salmon DP, Monsch AU, Galasko D, Butters N, Klauber MR, Thal LJ, Saitoh T. Episodic memory changes are associated with the APOE-ε4 allele in nondemented older adults. Neurology. 1995;45:2203–2206. doi: 10.1212/wnl.45.12.2203. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Serody AB, Chan AS, Eberson-Shumate SC, Delis DC, Hansen LA, Salmon DP. Cognitive and neuropathologic correlates of Stroop Color-Word Test performance in Alzheimer’s disease. Neuropsychology. 2002;16:335–343. doi: 10.1037//0894-4105.16.3.335. [DOI] [PubMed] [Google Scholar]
- Botwinick J, Storandt M. Age differences in reaction time as a function of experience, stimulus intensity, and preparatory interval. Journal of Genetic Psychology. 1973;123:209–217. doi: 10.1080/00221325.1973.10532679. [DOI] [PubMed] [Google Scholar]
- Brown LB, Storandt M. Sensitivity of category cued recall to very mild dementia of the Alzheimer’s type. Archives of Clinical Neuropsychology. 2000;15:529–534. [PubMed] [Google Scholar]
- Budson AE, Daffner KR, Desikan R, Schacter DL. When false recognition is unopposed by true recognition: Gist-based memory distortion in Alzheimer’s disease. Neuropsychology. 2000;14:277–287. doi: 10.1037//0894-4105.14.2.277. [DOI] [PubMed] [Google Scholar]
- Budson AE, Sitarski J, Daffner KR, Schacter DL. False recognition of pictures versus words in Alzheimer’s disease: The distinctiveness heuristic. Neuropsychology. 2002;16:163–173. doi: 10.1037//0894-4105.16.2.163. [DOI] [PubMed] [Google Scholar]
- Budson AE, Sullivan AL, Daffner KR, Schacter DL. Semantic vs. phonological false recognition in aging and Alzheimer’s disease. Brain and Cognition. 2003;51:251–261. doi: 10.1016/s0278-2626(03)00030-7. [DOI] [PubMed] [Google Scholar]
- Burke WJ, Miller JP, Rubin EH, Morris JC, Coben LA, Duchek JM, Wittels IG, Berg L. Reliability of the Washington University Clinical Dementia Rating. Archives of Neurology. 1988;45:31–32. doi: 10.1001/archneur.1988.00520250037015. [DOI] [PubMed] [Google Scholar]
- Buschke H. Selective reminding for analysis of memory and learning. Journal of Verbal Learning and Verbal Behavior. 1973;12:543–550. [Google Scholar]
- Caselli RJ, Reiman EM, Osborne D, Hentz JG, Baxter LC, Hernandez JL, Alexander GG. Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE ε4 allele. Neurology. 2004;62:1990–1995. doi: 10.1212/01.wnl.0000129533.26544.bf. [DOI] [PubMed] [Google Scholar]
- Castel AD, Balota DA, Hutchison KA, Logan JM, Yap MJ. Spatial attention and response control in healthy younger and older adults and individuals with Alzheimer’s disease: Evidence for disproportionate selection breakdowns in the Simon task. Neuropsychology. 2007;21:170–182. doi: 10.1037/0894-4105.21.2.170. [DOI] [PubMed] [Google Scholar]
- Cohen AL, West R, Craik FIM. Modulation of the prospective and retrospective components of memory for intentions in younger and older adults. Aging, Neuropsychology, and Cognition. 2001;8:1–13. [Google Scholar]
- Collie A, Maruff P, Shafiq-Antonacci R, Smith M, Hallup M, Schofield PR, Masters CL, Currie J. Memory decline in healthy older people: Implications for identifying mild cognitive impairment. Neurology. 2001;56:1533–1538. doi: 10.1212/wnl.56.11.1533. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Craik FIM, McDowd JM. Age differences in recall and recognition. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1987;13:474–479. [Google Scholar]
- Craik FIM, Lockhart RS. Levels of processing: A framework for memory research. Journal of Verbal Learning & Verbal Behavior. 1972;11:671–684. [Google Scholar]
- Daniels K, Toth J, Jacoby L. The aging of executive functions. In: Bialystok E, Craik FIM, editors. Lifespan cognition: Mechanisms of change. New York, NY: Oxford University Press; 2006. pp. 96–111. [Google Scholar]
- Driscoll I, McDaniel MA, Guynn MJ. Apolipoprotein E and prospective memory in normally aging adults. Neuropsychology. 2005;19:28–34. doi: 10.1037/0894-4105.19.1.28. [DOI] [PubMed] [Google Scholar]
- Duchek JM. Encoding and retrieval differences between young and old: The impact of attentional capacity usage. Developmental Psychology. 1984;20:1173–1180. [Google Scholar]
- Duchek JM, Balota DA, Cortese M. Prospective memory and apolipoprotein E in healthy aging and early stage Alzheimer’s disease. Neuropsychology. 2006;20:633–644. doi: 10.1037/0894-4105.20.6.633. [DOI] [PubMed] [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, Conway ARA. Working memory, short-term memory, and general fluid intelligence: A latent-variable approach. Journal of Experimental Psychology: General. 1999;128:309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer’s disease: A meta-analysis. JAMA: Journal of the American Medical Association. 1997;278:1349–1356. [PubMed] [Google Scholar]
- Gabrieli JDE, Vaidya CJ, Stone M, Francis WS, Thompson-Schill SL, Fleischman DA, Tinklenberg JR, Yesavage JA, Wilson RS. Convergent behavioral and neuropsychological evidence for a distinction between identification and production forms of repetition priming. Journal of Experimental Psychology: General. 1999;128:479–498. doi: 10.1037//0096-3445.128.4.479. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. Boston Naming Test. PA: Lea & Febiger; 1983. [Google Scholar]
- Grober E, Buschke H, Crystal HA, Bang S, Dresner R. Screening for dementia by memory testing. Neurology. 1988;38:900–903. doi: 10.1212/wnl.38.6.900. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: A review and a new view. In: Bower GH, editor. The psychology of learning and motivation: Advances in research and theory. San Diego, CA: Academic Press; 1988. pp. 193–225. [Google Scholar]
- Hay JF, Jacoby LL. Separating habit and recollection in young and older adults: Effects of elaborative processing and distinctiveness. Psychology and Aging. 1999;14:122–134. doi: 10.1037//0882-7974.14.1.122. [DOI] [PubMed] [Google Scholar]
- Helkala EL, Koivisto K, Hänninen T, Vanhanen M, Kervinen K, Kuusisto J, Mykkänen L, Kesäniemi YA, Laakso M, Riekkinen P., Sr The association of apolipoprotein E polymorphism with memory: A population-based study. Neuroscience Letters. 1995;191:141–144. doi: 10.1016/0304-3940(95)11575-h. [DOI] [PubMed] [Google Scholar]
- Hodges JR. Memory in the dementias. In: Tulving E, Craik FIM, editors. The Oxford handbook of memory. New York, NY: Oxford University Press; 2000. pp. 441–459. [Google Scholar]
- Jacoby LL. A process dissociation framework: Separating automatic from intentional uses of memory. Journal of Memory and Language. 1991;30:513–541. [Google Scholar]
- Jacoby LL. Ironic effects of repetition: Measuring age-related differences in memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1999;25:3–22. doi: 10.1037//0278-7393.25.1.3. [DOI] [PubMed] [Google Scholar]
- Jennings JM, Jacoby LL. An opposition procedure for detecting age-related deficits in recollection: Telling effects of repetition. Psychology and Aging. 1997;12:352–361. doi: 10.1037//0882-7974.12.2.352. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Hashtroudi S, Lindsay DS. Source monitoring. Psychological Bulletin. 1993;114:3–28. doi: 10.1037/0033-2909.114.1.3. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Mather KA, Butterworth P, Anstey KJ, Christensen H, Easteal S. APOE genotype and cognitive functioning in a large age-stratified population sample. Neuropsychology. 2007;21:1–8. doi: 10.1037/0894-4105.21.1.1. [DOI] [PubMed] [Google Scholar]
- Kessels RPC, Feijen J, Postma A. Implicit and explicit memory for spatial information in Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders. 2005;20:184–191. doi: 10.1159/000087233. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Anderson BA, Barch DM, Jacoby LL. The effects of encoding strategy and cognitive training on age-related changes in prefrontal activity. Poster presented at the 37th Annual Meeting of the Society for Neuroscience; San Diego, CA. 2007. [Google Scholar]
- Klages J, Fisk JD. The relation between APOE status and neuropsychological memory test performance: an analysis of the Canadian Study of Health and Aging. Brain & Cognition. 2002;49:201–204. [PubMed] [Google Scholar]
- Knight RG. Controlled and automatic memory processes in Alzheimer’s disease. Cortex. 1998;34:427–435. doi: 10.1016/s0010-9452(08)70765-2. [DOI] [PubMed] [Google Scholar]
- Koivisto M, Portin R, Seinelä A, Rinne J. Automatic influences of memory in Alzheimer’s disease. Cortex. 1998;34:209–219. doi: 10.1016/s0010-9452(08)70748-2. [DOI] [PubMed] [Google Scholar]
- Levy JA, Bergeson J, Putnam K, Rosen V, Cohen R, Lalonde F, Mirza N, Linker G, Sunderland T. Context-specific memory and apolipoprotein E (ApoE) ε4: Cognitive evidence from the NIMH prospective study of risk for Alzheimer’s disease. Journal of the International Neuropsychological Society. 2004;10:362–370. doi: 10.1017/S1355617704103044. [DOI] [PubMed] [Google Scholar]
- Light LL, Prull MW, La Voie DJ, Healy MR. Dual-process theories of memory in old age. In: Perfect TJ, Maylor EA, editors. Models of cognitive aging. Oxford: Oxford University Press; 2000. pp. 238–300. [Google Scholar]
- Lind J, lngvar M, Persson J, Sleegers K, Van Broeckhoven C, Adolfsson R, Nilsson LG, Nyberg L. Parietal cortex activation predicts memory decline in apolipoprotein E-ε4 carriers. Neuroreport. 2006;17:1683–1686. doi: 10.1097/01.wnr.0000239954.60695.c6. [DOI] [PubMed] [Google Scholar]
- Lord FM. Elementary models for measuring change. In: Harris CW, editor. Problems in measuring change. Madison: University of Wisconsin Press; 1963. [Google Scholar]
- Mandler G. Recognizing: The judgment of previous occurrence. Psychological Review. 1980;87:252–271. [Google Scholar]
- McCabe DP, Roediger HL, McDaniel MA, Balota DA, Hambrick DZ. The relationship between working memory capacity, executive functioning, and general fluid intelligence: Converging evidence for an executive attention construct. 2008 doi: 10.1037/a0017619. Submitted to Cognitive Psychology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA work group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morris JC, McKeel DW, Jr, Fulling K, Torack RM, Berg L. Validation of clinical diagnostic criteria for Alzheimer’s disease. Annals of Neurology. 1988;24:17–22. doi: 10.1002/ana.410240105. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early-stage Alzheimer’s disease. Archives of Neurology. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- Mutter SA, Naylor JC, Patterson ER. The effects of age and task context on Stroop task performance. Memory & Cognition. 2005;33:514–530. doi: 10.3758/bf03193068. [DOI] [PubMed] [Google Scholar]
- Nilsson LG, Adolfsson R, Bäckman L, Cruts M, Nyberg L, Small BJ, Van Broeckoven C. The influence of APOE status on episodic and semantic memory: data from a population-based study. Neuropsychology. 2006;20:645–657. doi: 10.1037/0894-4105.20.6.645. [DOI] [PubMed] [Google Scholar]
- Norman KA, Schacter DL. False recognition in younger and older adults: Exploring the characteristics of illusory memories. Memory & Cognition. 1997;25:838–848. doi: 10.3758/bf03211328. [DOI] [PubMed] [Google Scholar]
- Packard CJ, Westendorp RGJ, Stott DJ, Caslake MJ, Murray HM, Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Jolles J, Perry IJ, Sweeney BJ, Twomey C. Association between apolipoprotein ε4 and cognitive decline in elderly adults. Journal of the American Geriatrics Society. 2007;55:1777–1785. doi: 10.1111/j.1532-5415.2007.01415.x. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Greenwood PM, Alexander GE. Alzheimer’s disease constricts the dynamic range of spatial attention in visual search. Neuropsychologia. 2000;38:1126–1135. doi: 10.1016/s0028-3932(00)00024-5. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Greenwood PM, Sunderland T. The apolipoprotein E gene, attention, and brain function. Neuropsychology. 2002;16:254–274. doi: 10.1037//0894-4105.16.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasuraman R, Haxby JV. Attention and brain function in Alzheimer’s disease: A review. Neuropsychology. 1993;7:242–272. [Google Scholar]
- Perry RJ, Hodges JR. Attention and executive deficits in Alzheimer’s disease: A critical review. Brain. 1999;122:383–404. doi: 10.1093/brain/122.3.383. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: Early detection of dementia: Mild cognitive impairment (an evidence-based review) Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- Prull MW, Dawes LLC, Martin AM, III, Rosenberg HF, Light LL. Recollection and familiarity in recognition memory: Adult age differences and neuropsychological test correlates. Psychology and Aging. 2006;21:107–118. doi: 10.1037/0882-7974.21.1.107. [DOI] [PubMed] [Google Scholar]
- Riley KP, Snowdon DA, Saunders AM, Roses AD, Mortimer JA. Cognitive function and apolipoprotein E in very old adults: Findings from the Nun Study. Journal of Gerontology. 2000;55:S69–S75. doi: 10.1093/geronb/55.2.s69. [DOI] [PubMed] [Google Scholar]
- Roediger HL, McDermott KB. Creating false memories: Remembering words not presented in lists. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1995;21:803–814. [Google Scholar]
- Rogosa DR, Willett JB. Demonstrating the reliability of the difference score in the measurement of change. Journal of Educational Measurement. 1983;20:335–343. [Google Scholar]
- Rosen VM, Bergeson JL, Putnam K, Harwell A, Sunderland T. Working memory and apolipoprotein E: What’s the connection? Neuropsychologia. 2002;40:2226–2233. doi: 10.1016/s0028-3932(02)00132-x. [DOI] [PubMed] [Google Scholar]
- Rosen VM, Sunderland T, Levy J, Harwell A, McGee L, Hammond C, Bhupali D, Putnam K, Bergeson J, Lefkowitz C. Apolipoprotein E and category fluency: Evidence for reduced semantic access in healthy normal controls at risk for developing Alzheimer’s disease. Neuropsychologia. 2005;43:647–658. doi: 10.1016/j.neuropsychologia.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Rubin EH, Storandt M, Miller P, Kinscherf DA, Grant EA, Morris JC, Berg L. A prospective study of cognitive function and onset of dementia in cognitively healthy elders. Archives of Neurology. 1998;55:395–401. doi: 10.1001/archneur.55.3.395. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Osowiecki D, Kaszniak AW, Kihlstrom JF, Valdiserri M. Source memory: Extending the boundaries of age-related deficits. Psychology and Aging. 1994;9:81–89. doi: 10.1037//0882-7974.9.1.81. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime User’s Guide. Pittsburgh, PA: Psychology Software Tools Inc; 2002. [Google Scholar]
- Small BJ, Rosnick CB, Fratiglioni L, Bäckman L. Apolipoprotein E and cognitive performance: A meta-analysis. Psychology and Aging. 2004;19:592–600. doi: 10.1037/0882-7974.19.4.592. [DOI] [PubMed] [Google Scholar]
- Smith GE, Bohac DL, Waring SC, Kokmen E, Tangalos EG, Ivnik RJ, Petersen RC. Apolipoprotein E genotype influences cognitive “phenotype” in patients with Alzheimer’s disease but not in healthy control subjects. Neurology. 1998;50:355–362. doi: 10.1212/wnl.50.2.355. [DOI] [PubMed] [Google Scholar]
- Smith JA, Knight RG. Memory processing in Alzheimer’s disease. Neuropsychologia. 2002;40:666–682. doi: 10.1016/s0028-3932(01)00137-3. [DOI] [PubMed] [Google Scholar]
- Sommers MS, Huff LM. The effects of age and dementia of the Alzheimer’s type on phonological false memories. Psychology and Aging. 2003;18:791–806. doi: 10.1037/0882-7974.18.4.791. [DOI] [PubMed] [Google Scholar]
- Spieler DH, Balota DA, Faust ME. Stroop performance in healthy younger and older adults and in individuals with dementia of the Alzheimer’s type. Journal of Experimental Psychology: Human Perception and Performance. 1996;22:461–479. doi: 10.1037//0096-1523.22.2.461. [DOI] [PubMed] [Google Scholar]
- Storandt M, Grant EA, Miller JP, Morris JC. Rates of progression in mild cognitive impairment and early Alzheimer’s disease. Neurology. 2002;59:1034–1041. doi: 10.1212/wnl.59.7.1034. [DOI] [PubMed] [Google Scholar]
- Storandt M, Grant EA, Miller JP, Morris JC. Longitudinal course and neuropathologic outcomes in original vs. revised MCI and in pre-MCI. Neurology. 2006;67:467–473. doi: 10.1212/01.wnl.0000228231.26111.6e. [DOI] [PubMed] [Google Scholar]
- Thomas AK, Sommers MS. Attention to item-specific processing eliminates age effects in false memories. Journal of Memory and Language. 2005;52:71–86. [Google Scholar]
- Thurstone LE, Thurstone TG. Examiner manual for the SRA Primary Mental Abilities Test. Chicago: Science Research Associates; 1949. [Google Scholar]
- Troyer AK, Winocur G, Craik FIM, Moscovitch M. Source memory and divided attention: Reciprocal costs to primary and secondary tasks. Neuropsychology. 1999;13:467–474. [PubMed] [Google Scholar]
- Tun PA, Wingfield A, Rosen MJ, Blanchard L. Response latencies for false memories: Gist-based processes in normal aging. Psychology and Aging. 1998;13:230–241. doi: 10.1037//0882-7974.13.2.230. [DOI] [PubMed] [Google Scholar]
- Waldie BD, Kwong See ST. Remembering words never presented: False memory effects in Dementia of the Alzheimer’s type. Aging, Neuropsychology, and Cognition. 2003;10:281–297. [Google Scholar]
- Watson JM, Balota DA, Sergent-Marshall SD. Semantic, phonological, and hybrid veridical and false memories in healthy older adults and in individuals with dementia of the Alzheimer type. Neuropsychology. 2001;15:254–268. [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale. Oxford, England: Psychological Corporation; 1955. [Google Scholar]
- Wechsler D. Manual: Wechsler Memory Scale-Revised. San Antonio, Texas: Psychological Corporation; 1987. [Google Scholar]
- Wechsler D. Administration and scoring manual. 3. San Antonio, TX: Psychological Corporation; 1997. Wechsler Adult Intelligence Scale. [Google Scholar]
- Wetter SR, Delis DC, Houston WS, Jacobson MW, Lansing A, Cobell K, Salmon DP, Bondi MW. Deficits in inhibition and flexibility are associated with the APOE ε4 allele in nondemented older adults. Journal of Clinical and Experimental Neuropsychology. 2005;27:943–952. doi: 10.1080/13803390490919001. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Barnes LL, Beckett LA, Aggarwal NT, Cochran EJ, Berry-Kravis E, Bach J, Fox JH, Evans DA, Bennett DA. The apolipoprotein E ε4 allele and decline in different cognitive systems during a 6-year period. Archives of Neurology. 2002;59:1154–1160. doi: 10.1001/archneur.59.7.1154. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Recognition memory ROCs for item and associative information: The contribution of recollection and familiarity. Memory & Cognition. 1997;25:747–763. doi: 10.3758/bf03211318. [DOI] [PubMed] [Google Scholar]