Abstract
To test the hypothesis that the pulmonary vascular pressures of Thoroughbred and Standardbred horses behave similarly during exertion. Measurements were made on 5 Thoroughbred and 5 Standardbred horses on a treadmill at rest and during 3-minute exercise intervals at speeds predicted to produce 75%, 90%, and 100% maximal heart rate. Left forelimb acceleration, heart rate, esophageal pressure, and pulmonary artery pressure were measured continuously. Pulmonary capillary and wedge pressures were measured during intermittent occlusion of the pulmonary artery. Breathing rate and gait frequency were the fundamental frequencies of the esophageal pressure and limb acceleration signals respectively. The ratio of speed:gait frequency gave stride length. The effects of exertion and breed were evaluated using two-way analysis of variance. Exertion produced significant increases in pulmonary artery (P = 0.001), capillary (P= 0.002), and wedge (P= 0.005) pressures. No significant effect of breed was detected on pulmonary artery pressure, but at exertion pulmonary capillary and wedge pressures were 15% (P= 0.03) and 23% (P= 0.04) greater in Thoroughbreds, respectively. Treadmill speed was ~12% greater (P= 0.04), stride length was ~25% greater (P= 0.0003), gait frequency was ~10% less (P= 0.006), breathing rate was ~10% less (P= 0.001), and heart rate was ~6% less (P= 0.06) for Thoroughbreds. There was no effect of breed on inspiratory or expiratory esophageal pressure although mean esophageal pressure was ~2 mmHg greater (P= 0.03) in exercising Standardbreds. In conclusion, pulmonary capillary and wedge pressures are greater in Thoroughbreds than in Standardbreds at similar fractions of maximal heart rate. This is compatible with the higher incidence of exercise-induced pulmonary hemorrhage observed in Thoroughbreds.
Introduction
Pulmonary arterial pressure more than doubles in horses during strenuous exertion (1,2,3,4,5). This pressure increase is much greater than that observed in most other mammals that have been studied (6,7,8,9) and might be a crucial factor in the etiology of conditions that could limit equine athletic performance, such as equine exercise-induced pulmonary hemorrhage (EIPH) and limitation of oxygen diffusion (10,11,12,13,14,15,16).
The incidence of EIPH reported in Thoroughbreds is higher than that reported in Standardbreds (17,18,19,20). This could be due to greater pulmonary vascular pressures in exercising Thoroughbreds (16). Values reported for the mean pulmonary arterial pressure in horses at maximal exertion vary from 70 mmHg (4) to 115 mmHg (21). This wide range could be attributed to inherent differences between the populations studied (such as breed), but it might also be caused by differences between the fitness levels of the subjects; the gait at which the subjects worked; the level of exertion at which measurements were made; or other systematic differences, such as the method of pressure measurement. These methodological inconsistencies in the existing literature prevent substantiation of the thesis that Thoroughbreds have greater pulmonary vascular pressures than Standardbreds.
The purpose of this study was to investigate whether Standardbred and Thoroughbred horses have similar pulmonary vascular pressures when exercised under similar circumstances. We hypothesized that the pulmonary vascular pressures of Thoroughbred horses would be greater than that of Standardbred horses exercising at similar fractions of maximal heart rate (HRmax).
Materials and methods
Horses
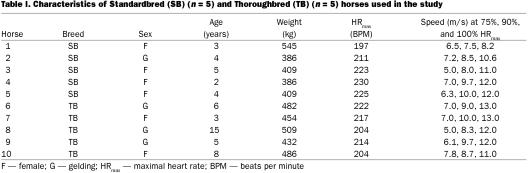
Data from 5 Standardbreds were compared with data from 5 Thoroughbreds (Table I). Horses were determined to be in good condition and athletically fit based on a physical examination and hemogram. Prior to the study, normal laryngeal function was confirmed by video endoscopy at rest. Horses were trained 5 to 6 d per week on a treadmill for 2 mo prior to the study. Heart rate was measured continuously using a device (Hippocard PEH 200; Kentucky Equine Research, Versaille, Kentucky, USA) attached to electrodes secured under the girth strap. Maximal heart rate was determined for each horse by incrementally increasing treadmill speed until a plateau in heart rate was reached. Within the 14 d prior to each experiment, each horse was exercised for 3 or more episodes (~3 min duration) at different speeds predicted to produce submaximal heart rate, and heart rate was recorded. Heart rate was then regressed on treadmill speed, and speeds predicted to produce 75%, 90%, and 100% HRmax were determined. These fractions of HRmax are equivalent to ~60%, 83%, and 100% of maximal oxygen uptake, respectively (22). Horses were shod with stainless steel/polyurethane shoes (Slypner; Slypner Athletic Horseshoes, Claremont, New Hampshire, USA). All procedures complied with applicable U.S. federal and state governmental regulations and were approved by Cornell University's Institutional Animal Care and Use Committee.
Table I.
Food was withheld for 3 h prior to each experiment. Observations were made initially with the horse standing quietly and then during exertion on the treadmill. Each horse performed an exercise protocol consisting of a warm-up for 4 min trotting at 4 m/s followed by sequential 3-minute exercise intervals at speeds predicted to produce 75%, 90%, and 100% HRmax. The treadmill was horizontal throughout and horses walked for ~2 min between each interval of exertion.
Instrumentation for experiments
An accelerometer (Model 226C piezoelectric accelerometer; Endevco, San Juan Capistrano, California, USA) was strapped to the metacarpal region of the left forelimb. The heart rate was measured in the same way as it was when HRmax was determined. A 7-Fr solid-state pressure transducer-tipped catheter (SPR-538; Millar Instruments, Houston, Texas, USA) was passed into the esophagus via a flexile nasogastric tube. Its tip was placed 135 to 145 cm from the nares in a location previously found to produce similar tracheal and esophageal pressure waveforms during inspiratory effort against obstructed nares. Catheter sites were prepared aseptically and infiltrated with local anesthetic. A 10-Fr catheter introducer (USCI Angiocath Systems, Tewksbury, Massachusetts, USA) was placed in the left jugular vein and a 2nd 7-Fr transducer-tipped catheter (SPR-546; Millar Instruments), modified by the addition of a balloon (volume ~2.5 mL air) just proximal to the transducer, was placed via the jugular introducer into the pulmonary artery. The diameter of the balloon was ~15 mm when inflated in atmospheric pressure, this dimension was reduced < 20% by exposure to pressure of 150 mmHg greater than atmospheric pressure (corresponding to supra maximal pressures in the pulmonary artery). Location of the transducer was verified by the characteristic pressure waveforms in the right ventricle and pulmonary artery. The tip was positioned so balloon inflation produced arterial occlusion. Wedging of the catheter was confirmed after each occlusion by observation of the characteristic arterial and balloon pressure waveforms on a thermal ray recorder (Gould Electronics, Cleveland, Ohio, USA). Incomplete wedging produced high-frequency noise in the arterial pressure and/or oscillation in the balloon pressure; this was corrected by adding a small volume (0.1 to 0.3 mL) of air to the balloon.
The transducer-tipped catheters were calibrated at 0, 75, and 150 mmHg before and after each experiment using a mercury sphygmomanometer (Lumiscope Company, East Brunswick, New Jersey, USA). Calibration of the transducer-tipped catheters in the 150 to −150 mmHg range was confirmed after each experiment using a U-shaped column of mercury. The hydrostatic pressure difference between the level of the catheter tip in the vascular bed and the level of the root of the main pulmonary artery was measured at the end of each experiment by continuously recording the pressure while withdrawing the tip into the right ventricle from its experimental location in the pulmonary vasculature (4). This pressure difference was used to standardize all pressures to the level of the root of the pulmonary trunk. Both pressure transducer-tipped catheters were phase-matched up to 30 Hz.
Data collection and analysis
Accelerometer and pressure signals were collected at 250 Hz, stored on computer disc, and analyzed using software (LabView, version 5.0; National Instruments, Austin, Texas, USA). All pressure data were low-pass filtered at 12 Hz before analysis. To derive the transmural pulmonary arterial pressure (Pta) signal, the esophageal pressure signal was subtracted from the raw arterial pressure signal. The Pta tracing, thus derived, was used subsequently to calculate all of the vascular pressures reported here. Pulmonary capillary pressure was calculated by the arterial occlusion technique (4,23,24,25,26). The instant of occlusion was defined as that point after balloon inflation when the pulmonary arterial pressure tracing deviated from the expected waveform without occlusion (26). Three occlusions from the last 1.5 min at each level of exertion were analyzed and the results averaged. Mean Pta was the arithmetic mean of the transmural pressure signal over 2 to 6 s just prior to occlusion. Mean transmural wedge pressure (Ptw) was the arithmetic mean of the transmural pressure signal over 2 to 6 s after the pressure signal approximated a plateau. A mono-exponential curve was fitted to all data points of the transmural pressure signal within 3 to 6 s after occlusion, and the pressure on this curve at the instant of occlusion was defined as transmural pulmonary capillary pressure (Ptc) (16). Peak inspiratory, peak expiratory, and mean esophageal pressures were averaged over ~16 s near the end of exertion at each level.
By applying fast Fourier transformations to data from the last minute of exertion at each level, fundamental frequencies were determined for the esophageal pressure (breathing rate) and limb acceleration (gait frequency). The ratio of treadmill speed to gait frequency gave stride length.
A paired t-test was used to determine, a priori, whether the heart rates measured during data collection at each level of exertion were different from the target heart rates. A student t-test was used to identify if there was a breed effect on either the actual or the target HRmax observed. Mean pulmonary vascular pressures, expressed both as absolute values and as percentages of resting values, were analyzed using a two-way analysis of variance (ANOVA) blocked on breed and exercise intensity, followed by Tukey's multiple comparison procedure (for exercise intensity). The two-way interaction terms (breed and exercise intensity) were used as the error terms. Missing data were replaced by their least-squares estimates. The heart rate, and the fundamental frequencies of the esophageal pressure and limb acceleration were also subjected to a two-way ANOVA blocked on breed and exercise intensity, followed by Tukey's multiple comparison procedure for exercise. A significant difference between means was indicated when P < 0.05 (2-sided).
Results
Target HRmax was 212 ± 3.6 beats/min for the Thoroughbred horses and 217 ± 5.9 beats/min for the Standardbred horses; no difference was detected between these values and the heart rates achieved during the experiments at this level of exertion. Likewise, there were no significant differences between the target heart rates and the actual heart rates of Standardbreds and Thoroughbreds at 75% and 90% HRmax. All of the horses galloped at 90% and 100% HRmax, but some trotted or cantered at 75% HRmax. One horse (#1) was excluded from analysis involving breathing rate because its gait frequency and breathing rate were observed to be in the ratio of 2:1. The accelerometer also failed on this horse so that gait frequency and its derivatives are missing. Horse 1 had the slowest HRmax and slowest speed to achieve HRmax; nevertheless, pressure data from this horse were not outliers and were not excluded from analysis.
Effects of breed
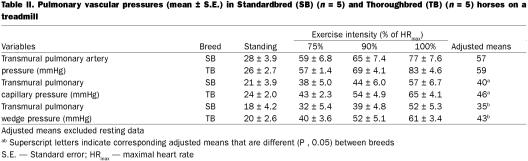
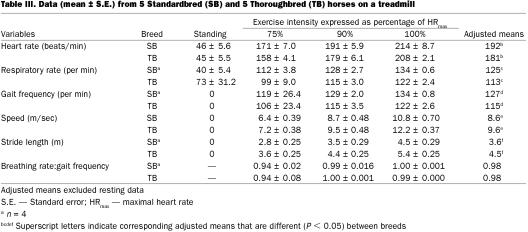
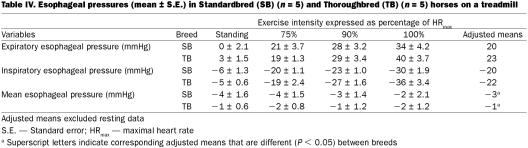
After controlling for exercise intensity in the analysis of variance, we detected no significant effect of breed on Pta, but mean Ptc and Ptw were 15% (P = 0.03) and 23% (P = 0.04) greater in Thoroughbreds, respectively (Table II). Heart rate was ~6% less (P = 0.06), breathing rate was ~10% less (P = 0.001), gait frequency was ~10% less (P = 0.006), treadmill speed was ~12% greater (P = 0.04), and stride length was ~25% greater (P = 0.003) for Thoroughbreds (Table III. No significant difference between the 2 breeds was detected, in the ratio of breathing frequency to gait frequency. Restricting the data to galloping horses by excluding the data collected at 75% HRmax did not change which parameters were significantly different between breeds. After controlling for exercise intensity using ANOVA, there was no effect of breed on inspiratory or expiratory esophageal pressure (Table IV). However, the mean esophageal pressure of Standardbreds was 2 mmHg lower than that of Thoroughbreds (P = 0.03).
Table II.
Table III.
Table IV.
Effects of exercise
After accounting for the effects of breed in the ANOVA, exertion produced significant increases in Pta (P = 0.001), Ptc (P = 0.002), and Ptw (P = 0.005) with respect to standing values.
After accounting for the effects of breed in the ANOVA, gait frequency at 90% (adjusted least-squares mean = 120 strides per min) and 100% HRmax (126 strides per min) was significantly greater (P = 0.01) than at 75% HRmax (114 strides per min). Likewise, respiratory frequency at 90% (114 breaths per min) and 100% HRmax (120 breaths per min) was significantly greater (P = 0.03) than that at 75% HRmax (102 breaths per min). The ratio of breathing frequency to gait frequency was similar at 75% (0.94), 90% (0.96), and 100% HRmax (1.00).
After accounting for the effect of breed using ANOVA, the esophageal pressure was greater during expiration at 75% (20 mmHg), 90% (29 mmHg), and 100% HRmax (37 mmHg) compared with the resting value (1 mmHg) (P = 0.003). Esophageal pressure was lower during inspiration at 75% (−19 mmHg), 90% (−25 mmHg), and 100% HRmax (−33 mmHg) compared with the resting value (−5 mmHg) (P = 0.005). There was no effect of exercise on mean esophageal pressure.
Discussion
Pulmonary vascular pressures increased with exertion in both breeds. Although mean Pta was not different between breeds, both mean Ptc and Ptw were ~20% greater in Thoroughbreds than in Standardbreds at exertion. This suggests that transmural capillary pressure in Thoroughbreds is more likely to exceed the pressure at where stress failure can occur, which is compatible with the higher incidence of EIPH that has been reported in Thoroughbreds. Although the qualitative changes in pulmonary vascular pressures were similar in Thoroughbreds and Standardbreds, the quantitative differences were enough to suggest that extrapolation of pulmonary vascular pressures between breeds is unwarranted. Because the horses all galloped at the greater levels of exertion, the difference in mean Ptc and Ptw cannot be attributed to differences in the type of gait.
Inspection of Table II suggests that the pressure gradient across the whole pulmonary vascular bed (Pta − Ptw) at maximal exertion is similar regardless of breed. These data also suggest that the distribution of pre-capillary (mean Pta − Ptc) and post-capillary (mean Ptc − Ptw) pressure gradients and, hence, the corresponding distribution of resistance to flow, are also independent of breed. The greater increase in pulmonary vascular pressures in Thoroughbreds is, thus, probably largely attributable to greater left atrial pressure in this breed at exertion. Confirmation of this would require direct measurement of left atrial pressure in both breeds at similar levels of exertion. The etiology of the greater pulmonary vascular pressures observed in Thoroughbreds is unclear.
With the exception of 1 horse, breathing rate:gait frequency was ~1.00 at all levels of exertion, hence breathing and stride were largely entrained in this ratio even in those horses that trotted or cantered at 75% HRmax (27). Although exertion caused a decrease in inspiratory pleural (esophageal) pressure and an increase in expiratory pleural pressure, there was no effect on mean pleural pressure. Although inspiratory and expiratory pleural pressures were not different between breeds, Standardbreds had mean esophageal pressures 2 mmHg greater than Thoroughbreds; however, even if this difference were confirmed in other horses, we think that it is unlikely to be biologically important. The vascular pressures reported here are transmural pressures and are the instantaneous difference between pulmonary intravascular and esophageal pressures; the difference in mean esophageal pressure between Standardbreds and Thoroughbreds is too small to be an important component of the vascular transmural pressure differences between the breeds. The general similarity of esophageal pressures in both breeds at each fraction of HRmax is compatible with similar work of breathing and supports the extrapolation of airway mechanical observations from one breed to another in normal horses. One Standardbred (#1, Table I) was observed to have gait frequency and breathing rate in a ratio of 2:1; this individual had the slowest HRmax and the slowest speed when achieving HRmax. This is compatible with the reduced energetic efficiency when gait and breathing are not entrained 1:1.
The smaller Standardbreds had faster heart rates, faster gaits, faster breathing rates, slower speeds, and shorter stride lengths at similar fractions of maximal heart rate. Hence, at similar levels of exertion at the gallop, Thoroughbreds travel faster than Standardbreds by using a much longer stride length that operates at a slower frequency.
We conclude that Ptc and Ptw are greater in Thoroughbreds than in Standardbreds at similar levels of exertion and that this is compatible with the higher incidence of EIPH in Thoroughbreds. This conclusion is predicated on the assumption that the fractions of HRmax used in these experiments produce similar levels of exertion in these breeds. Confirmation of these results using speeds that clamp other independent variables, such as oxygen consumption, may be warranted. During racing, speeds often exceed those that produce HRmax. Exhaustion toward the end of a race may be associated with a shorter gait length, increased gait frequency, and uncoupling of breathing and gait. For these reasons, extrapolation of these results to racing animals should be undertaken with caution.
Footnotes
Acknowledgment
This work was funded by the Japan Racing Association.
Address all correspondence and reprint requests to Dr. N.G. Ducharme; telephone: (607) 253-3170; fax: (607) 253-3271; e-mail: ngd1@cornell.edu
Received May 23, 2002. Accepted February 19, 2003.
References
- 1.Erickson BK, Erickson HH, Coffman JR. Pulmonary artery, aortic, and oesophageal pressure changes during high intensity exercise in the horse: a possible relation to exercise-induced pulmonary haemorrhage. Equine Vet J 1990;9:47–52. [DOI] [PubMed]
- 2.Erickson BK, Erickson HH, Coffman JR. Pulmonary artery and aortic pressure changes during high intensity treadmill exercise in the horse: effect of furosemide and phentolamine. Equine Vet J 1992;24:215–219. [DOI] [PubMed]
- 3.Manohar M, Hutchens E, Coney E. Pulmonary haemodynamics in the exercising horse and their relationship to exercise-induced pulmonary haemorrhage. Br Vet J 1993;149:419–428. [DOI] [PubMed]
- 4.Sinha AK, Gleed RD, Hakim TS, Dobson A, Shannon KJ. Pulmonary capillary pressure during exercise in horses. J Appl Physiol 1996;80:1792–1798. [DOI] [PubMed]
- 5.Jackson JA, Ducharme NG, Hackett RP, et al. Effects of airway obstruction on transmural pulmonary artery pressure in exercising horses. Am J Vet Res 1997;58:897–903. [PubMed]
- 6.Slonin NB, Ravin O, Balchum OJ, Dressler SH. The effects of exercise in the supine position on the pulmonary arterial pressure of five normal subjects. J Clin Invest 1954;33: 1022–1030. [DOI] [PMC free article] [PubMed]
- 7.Elkins RC, Milnor WR. Pulmonary vascular response to exercise in the dog. Circ Res 1971;29:591–599. [DOI] [PubMed]
- 8.Damato AN, Galante JG, Smith WM. Hemodynamic response to treadmill exercise in normal subjects. J Appl Physiol 1966;21:959–966. [DOI] [PubMed]
- 9.Newman JH, Cochran CP, Roselli RJ, Parker RE, King LS. Pressure and flow changes in the pulmonary circulation in exercising sheep: evidence for elevated microvascular pressure. Am Rev Respir Dis 1993;147:921–926. [DOI] [PubMed]
- 10.Bayly WM, Hodgson DR, Schulz DA, Dempsey JA, Gollnick PD. Exercise-induced hypercapnia in the horse. J Appl Physiol 1989;67:1958–66. [DOI] [PubMed]
- 11.Hodgson DR, Rose RJ, Kelso TB, McCutcheon LJ, Bayly WM, Gollnick PD. Respiratory and metabolic responses in the horse during moderate and heavy exercise. Pflugers Arch 1990;417:73–78. [DOI] [PubMed]
- 12.West JB, Mathieu-Costello O, Jones JH, et al. Stress failure of pulmonary capillaries in racehorses with exercise-induced pulmonary hemorrhage. J Appl Physiol 1993;75:1097–1109. [DOI] [PubMed]
- 13.Erickson HH, Lowe BS. Mechanisms of exercise-induced pulmonary hemorrhage in the equine athlete. Biomed Sci Instrum 1994;30:33–38. [PubMed]
- 14.Birks EK, Mathieu-Costello O, Fu Z, Tyler WS, West JB. Very high pressures are required to cause stress failure of pulmonary capillaries in thoroughbred racehorses. J Appl Physiol 1997;82:1584–92. [DOI] [PubMed]
- 15.Ducharme NG, Hackett RP, Gleed RD, et al. Pulmonary capillary pressure in horses undergoing alteration of pleural pressure by imposition of various upper airway resistive loads. Equine Vet J 1999;30:27–33. [DOI] [PubMed]
- 16.Gleed RD, Ducharme NG, Hackett RP, et al. Effects of furosemide on pulmonary capillary pressure in horses exercising on a treadmill. Equine Vet J 1999;30:102–106. [DOI] [PubMed]
- 17.Pascoe JR, Ferraro GL, Cannon JH, Arthur RM, Wheat JD. Exercise-induced pulmonary hemorrhage in racing thoroughbreds: a preliminary study. Am J Vet Res 1981;42:703–707. [PubMed]
- 18.Raphel CF, Soma LR. Exercise-induced pulmonary hemorrhage in Thoroughbreds after racing and breezing. Am J Vet Res 1982;43:1123–1127. [PubMed]
- 19.MacNamara B, Bauer S, Iafe J. Endoscopic evaluation of exercise-induced pulmonary hemorrhage and chronic obstructive pulmonary disease in association with poor performance in racing Standardbreds. J Am Vet Med Assoc 1990;196:443–445. [PubMed]
- 20.Lapointe JM, Vrins A, McCarvill E. A survey of exercise-induced pulmonary haemorrhage in Quebec standardbred racehorses. Equine Vet J 1994;26:482–485. [DOI] [PubMed]
- 21.Jones JH, Smith BL, Birks EK, Pascoe JR, Hughes TR. Left atrial and pulmonary artery pressures in exercising horses. FASEB J 1992;6:A2020.
- 22.Evans DL, Rose RJ. Maximum oxygen uptake in racehorses:changes with training state and prediction from submaximal cardiorespiratory measurements. In: Gillespie JR, Robinson NE, eds. Equine Exercise Physiology. Davis: ICEEP Publications, 1987;2:52–67.
- 23.Holloway H, Perry M, Downey J, Parker J, Taylor A. Estimation of effective pulmonary capillary pressure in intact lungs. J Appl Physiol 1983;54:846–851. [DOI] [PubMed]
- 24.Cope DK, Allison RC, Parmentier JL, Miller JN, Taylor AE. Measurement of effective pulmonary capillary pressure using the pressure profile after pulmonary artery occlusion. Crit Care Med 1986;14:16–22. [DOI] [PubMed]
- 25.Hakim TS, Maarek JM, Chang HK. Estimation of pulmonary capillary pressure in intact dog lungs using the arterial occlusion technique. Am Rev Respir Dis 1989;140:217–224. [DOI] [PubMed]
- 26.Gilbert E, Hakim TS. Derivation of pulmonary capillary pressure from arterial occlusion in intact conditions. Crit Care Med 1994;22:986–993. [DOI] [PubMed]
- 27.Lafortuna CL, Reinach E, Saibene F. The effects of locomotor-respiratory coupling on the pattern of breathing in horses. J Physiol 1996;492:587–596. [DOI] [PMC free article] [PubMed]