Abstract
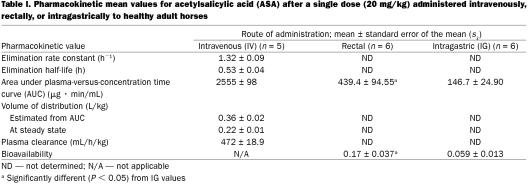
Six healthy adult horses (5 mares and 1 stallion) were given a single dose of acetylsalicylic acid (ASA), 20 mg/kg of body weight, by intravenous (IV), rectal, and intragastric (IG) routes. Serial blood samples were collected via jugular venipuncture over a 36-h period, and plasma ASA and salicylic acid (SA) concentrations were determined by high-performance liquid chromatography. After IV administration, the mean elimination rate constant of ASA (± the standard error of the mean) was 1.32 ± 0.09 h−l, the mean elimination half-life was 0.53 ± 0.04 h, the area under the plasma concentration-versus-time curve (AUC) was 2555 ± 98 μg · min/mL, the plasma clearance was 472 ± 18.9 mL/h/kg, and the volume of distribution at steady state was 0.22 ± 0.01 L/kg. After rectal administration, the plasma concentration of ASA peaked at 5.05 ± 0.80 μg/mL at 0.33 h, then decreased to undetectable levels by 4 h; the plasma concentration of SA peaked at 17.39 ± 5.46 μg/mL at 2 h, then decreased to 1.92 ± 0.25 μg/mL by 36 h. After rectal administration, the AUC for ASA was 439.4 ± 94.55 μg · min/mL and the bioavailability was 0.17 ± 0.037. After IG administration, the plasma concentration of ASA peaked at 1.26 ± 0.10 μg/mL at 0.67 h, then declined to 0.37 ± 0.37 μg/mL by 36 h; the plasma concentration of SA peaked at 23.90 ± 4.94 μg/mL at 4 h and decreased to 0.85 ± 0.31 μg/mL by 36 h. After IG administration, the AUC for ASA was 146.70 ± 24.90 μg · min/mL and the bioavailability was 0.059 ± 0.013. Administration of a single rectal dose of ASA of 20 mg/kg to horses results in higher peak plasma ASA concentrations and greater bioavailability than the same dose given IG. Plasma ASA concentrations after rectal administration should be sufficient to inhibit platelet thromboxane production, and doses lower than those suggested for IG administration may be adequate.
Introduction
Acetylsalicylic acid (ASA) is a nonsteroidal anti-inflammatory drug (NSAID) that has antipyretic, analgesic, and potent antiplatelet properties. Its pharmacokinetics have been evaluated after intravenous (IV), oral, and rectal administration in humans (1,2,3), after oral administration in cattle (4), and after oral and IV administration in horses (5,6). Acetylsalicylic acid is poorly absorbed from the gastrointestinal tract of horses after oral administration and disappears rapidly from the plasma (5). The half-life (t1/2) of ASA after IV administration to horses is 0.11 h (6). Acetylsalicylic acid is rapidly converted to salicylic acid (SA) via deacetylation by endogenous esterases and by hepatic mechanisms. Doses of 35 mg/kg administered orally every 2 h are necessary to maintain effective anti-inflammatory plasma concentrations (5). Because of its poor absorption and short half-life, ASA is not used routinely for treatment of inflammatory conditions of horses.
Acetylsalicylic acid is used for the treatment and prevention of arterial thrombotic diseases in humans and has likewise been recommended for the treatment of equine diseases that have arterial thrombosis as part of their pathogenesis; such diseases include laminitis, endotoxemia, disseminated intravascular coagulation, and thromboembolic colic (7,8,9). Arterial thrombus formation is initiated by damage to vascular endothelium and propagated by interaction of platelets with the damaged vessel wall. Stimulated platelets produce thromboxane, which potentiates platelet aggregation and causes local vasoconstriction. Acetylsalicylic acid irreversibly inhibits platelet cyclooxygenase by covalent acetylation of the enzyme (6,10). Because platelets are non-nucleated cells and are incapable of synthesizing additional cyclooxygenase, platelet thromboxane production will be inhibited for the life of any circulating platelets exposed to ASA (8,10). Administration of a single oral dose of ASA (20 mg/kg) to horses decreases the plasma thromboxane concentration by 68% to 93% for 3 d (7).
Arterial thrombosis may occur concurrently with gastrointestinal diseases, such as postoperative ileus and proximal enteritis, that prevent the use of orally administered drugs. An alternative route of administration is needed in these instances. Administration of rectal suppository forms of ASA is effective in humans when oral administration is impractical (3). Drugs such as metronidazole and indomethacin have been shown to be absorbed after rectal administration in horses (11,12). We hypothesized that the bioavailability of ASA after rectal administration to horses would be comparable or superior to that seen after oral administration. The purposes of the studies reported here were to determine the pharmacokinetics of ASA after a single IV dose of 20 mg/kg and to compare the plasma concentrations of ASA and SA and the bioavailability of ASA after a single intragastric (IG) and a single rectal dose of 20 mg/kg as a test of this hypothesis.
Materials and methods
Animals
We used 6 healthy adult horses (5 mares and 1 stallion), of various breeds, with weights ranging from 463 to 586 kg, for all experiments. The horses were determined to be clinically normal before each experiment by physical examination and a complete blood count. Serum biochemical testing was not performed. The horses were housed in box stalls for 24 h before and during the first 12 h of each experiment. They were fasted for 24 h before and during the first 12 h of experiment 2 (rectal administration), to mimic the clinical situation that occurs in horses with gastrointestinal disease that prevents oral intake, but were allowed free choice of coastal bermudagrass hay at all times during experiments 1 (IV administration) and 3 (IG administration). Each horse participated in all 3 experiments. There was at least a 10-d interval between experiments. The use of the horses in this study was approved by the Institutional Animal Care and Use Committee of the University of Florida.
Drug administration
In each experiment, the horses received a single ASA dose of 20 mg/kg of body weight by IV, rectal, or IG administration.
For the IV experiment, an indwelling catheter (Abbocath; Abbott Hospitals, North Chicago, Illinois, USA) was placed into a jugular vein after aseptic preparation of the catheter site. The calculated dose of reference standard ASA (Sigma Chemical Company, St. Louis, Missouri, USA) was dissolved in 120 mL of warm ethanol (absolute ethyl alcohol; Florida Distillers Company, Lake Alfred, Florida, USA) immediately before injection. The ethanol/ASA solution was injected through the jugular catheter over a 4-min period.
In the rectal-administration experiment, contents were manually removed from the distal 65 cm of the rectum immediately before the ASA was administered. Boluses of ASA, 15.6 g (The Butler Company, Columbus, Ohio, USA), were crushed and suspended in 40 mL of tap water. A 30 french Foley catheter was inserted 40 cm into the rectum, and the ASA suspension was injected through the catheter with a 60-mL catheter-tip syringe. The syringe was rinsed with an additional 30 mL of water, which was then injected through the catheter. The horses were monitored for defecation throughout the first 12 h of sampling, and the time to 1st defecation was recorded.
In the IG-administration experiment, ASA boluses were crushed and suspended in approximately 120 mL of tap water and administered by nasogastric tube. The tube was flushed with an additional 120 mL of water.
Collection and handling of samples
Blood was collected from the jugular vein into chilled 14-mL evacuated glass tubes containing 14.0 mg of potassium oxalate and 17.5 mg of sodium fluoride (Vacutainer; Becton Dickinson, Rutherford, New Jersey, USA). Samples were taken immediately before and at 0.08, 0.17, 0.25, 0.33, 0.50, 0.67, 1, 1.5, 2, 3, 4, 6, 8, 12, 24, and 36 h after ASA administration in all the experiments. Additional samples were collected at 0.03 and 0.12 h after IV administration. Time 0 was recorded as the time when half the dose had been administered. Samples were collected from either jugular vein after rectal or IG administration. After IV administration, all samples were collected from the jugular vein opposite to that used for injection. The blood samples were immediately placed on ice slush. All samples were centrifuged within 30 min of collection, at 3000 × g for 20 min at 4°C, and the plasma was harvested and frozen in polypropylene tubes (Sarstedt, Princeton, New Jersey, USA) at −70°C. Plasma ASA and SA concentrations were determined within 72 h of sample collection by means of high-performance liquid chromatography (HPLC).
Determination of plasma ASA and SA concentrations
Plasma ASA and SA concentrations were determined by HPLC according to a modification of the method of Mason and Gillflan (13). Samples were kept chilled at all times during the assay procedure to prevent hydrolysis of ASA to SA. Acetylsalicylic acid and SA were extracted from 0.5 mL of plasma by the addition of 0.2 mL of 50% v/v phosphoric acid containing 0.25 g/mL of ammonium sulfate and 8.0 mL of 50% v/v ethyl acetate in butyl chloride. Samples were agitated on a horizontal shaker for 2 min, then centrifuged at 4°C for 10 min at 3000 × g. The organic supernatant was removed and transferred to 13 × 100-mm glass tubes. The tubes were placed on ice slush in an evaporation rack (Evap-O-Rac; Cole-Parmer, Niles, Illinois, USA), and the organic layer was evaporated to near dryness under air stream. The remaining organic layers were individually evaporated to dryness to prevent sublimation of the ASA. Immediately thereafter, the samples were resuspended in 2 mL of the mobile phase for HPLC or stored dry at −70°C and analyzed by HPLC within 24 h of extraction.
For HPLC, we used a dual-piston reciprocating pump (LKB 2150 HPLC Pump; LKB Instruments, Gaithersburg, Maryland, USA), a variable wavelength detector (Spectro Flow 757; Kratos Analytical Instruments, Ramsey, New Jersey, USA) operated at 237 nm, and a stainless steel column (25 cm × 4.6 mm) packed with reverse-phase chromatographic medium (5-μm particles) (Microsorb C18; Rainin Instrument Company, Woburn, Massachusetts, USA). The mobile phase consisted of a mixture of HPLC water (71% v/v), acetonitrile (28% v/v), and acetic acid (1% v/v). The mobile-phase flow rate was 1 mL/min. Under these conditions, retention times were about 6.7 min for ASA and 8.6 min for SA.
Standards were prepared by adding ASA or SA stock solutions (1 mg ASA or SA/mL distilled water) to normal equine plasma. Standards were extracted and analyzed exactly as test samples. A standard curve was constructed by linear regression analysis, and ASA and SA concentrations in test samples were determined from the standard curve. The lowest limits of detection for the assay were 0.5 μg/mL for ASA and 0.125 μg/mL for SA.
Pharmacokinetic analysis
The following equations were fit to the ASA concentration-versus-time data for each experiment for each horse. For calculations of pharmacokinetic parameters, measured plasma concentrations that were below detectable limits were considered to be zero. A weighted, nonlinear fit of the mathematical model to the data was done with the use of a computer algorithm that minimized the sum of the squared deviations (14). Thus,
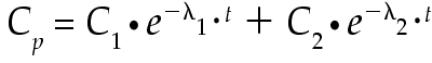 |
where Cp is the plasma ASA concentration, e is the base of the Napierian logarithms, C1 and C2 are the 0 time intercepts of the 2 exponential components, λ1 is the distribution rate constant, λ2 is the overall elimination rate constant (Kel), and t is the time in hours after dose administration. The pharmacokinetic parameters were calculated on the basis of noncompartmental kinetics (15). The area under the plasma concentration-versus-time curve (AUC) for IV administration was calculated as follows.
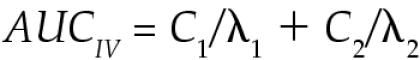 |
Plasma clearance was calculated as the dose divided by the AUC. The area under the moment curve (AUMC) for IV administration was calculated as follows.
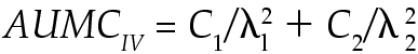 |
The volume of distribution at steady state [Vd(ss)] was calculated as the dose multiplied by the AUMC divided by the square of the AUC, as follows.
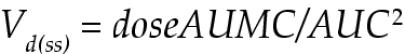 |
The plasma ASA concentration-versus-time data after rectal or IG administration of ASA were estimated as follows.
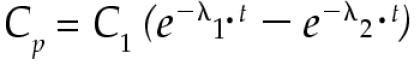 |
The AUC for rectal or IG administration was calculated as follows.
The bioavailability (F) of ASA after rectal or IG administration was calculated as follows.
A paired Student's t-test was used to determine significant differences between rectal and IG pharmacokinetic values and peak plasma concentrations of ASA. Probabilities of less than 5% (P < 0.05) were considered significant.
Results
One horse had an increase in respiratory rate and signs of mild abdominal pain after IV injection of the ethanol/ASA solution. These signs were transient and resolved without treatment within 45 min.
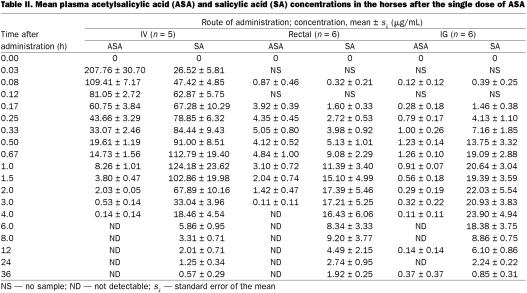
The pharmacokinetic values for ASA and the plasma concentrations of ASA and SA after IV administration of ASA were calculated from data obtained from 5 horses; the IV data from the 6th horse were discarded owing to perivascular injection of a portion of the ASA solution. After IV administration, the mean plasma concentration (± standard error of the mean) of ASA peaked at 207.76 ± 30.70 μg/mL at 0.03 h and decreased to undetectable levels by 6 h (Figure 1). The plasma clearance was 472 ± 18.9 mL/h/kg and the tl/2 0.53 ± 0.04 h (Table I). The mean plasma SA concentration was 26.52 ± 5.81 μg/mL at 0.03 h, reached a maximum of 124.18 ± 23.62 μg/mL at 1 h, and then decreased to 0.57 ± 0.29 μg/mL by 36 h (Table II).
Figure 1. Mean plasma concentration of acetylsalicylic acid (ASA) in 5 horses given a single intravenous dose (20 mg/kg) of ASA (experiment 1). The curve represents the fit of the mathematical model to the mean concentration at each time.
Table I.
Table II.
After rectal administration of ASA, the plasma concentration of ASA was 0.87 ± 0.46 μg/mL and that of SA 0.32 ± 0.21 μg/mL at 0.08 h. The ASA concentration peaked at 5.05 ± 0.80 μg/mL at 0.33 h and decreased to undetectable levels by 4 h. The SA concentration peaked at 17.39 ± 5.46 μg/mL at 2 h and decreased to 1.92 ± 0.25 μg/mL by 36 h. The AUC for ASA was 439.4 ± 94.55 μg · min/mL, and the bioavailability was 0.17 ± 0.037. The greatest individual peak concentration (665.16 μg/mL) and bioavailability (0.24) for ASA were in the male horse. In the female horses, the peak concentrations ranged from 181.24 to 586.95 μg/mL and the bioavailability from 0.07 to 0.23.
The time to defecation after rectal administration of ASA had no apparent effect on the peak plasma ASA concentration. One horse passed feces containing crushed ASA approximately 3 min after rectal administration; the peak plasma ASA concentration in this horse was 3.79 μg/mL. In 2 horses with a time to defecation of 47 min in 1 horse and 3 h in the other, the peak plasma ASA concentrations were 8.74 and 4.68 μg/mL, respectively. In the remaining 3 horses, the time to defecation was greater than 12 h, and the peak plasma ASA concentrations were 3.06, 5.76, and 7.40 μg/mL, respectively.
After IG administration, ASA and SA were first detectable in plasma at 0.08 h, at mean concentrations of 0.12 ± 0.12 and 0.39 ± 0.25 μg/mL, respectively. The mean ASA concentration reached a maximum of 1.26 ± 0.10 at 0.67 h and declined to 0.37 ± 0.37 μg/mL by 36 h. The mean SA concentration reached a maximum of 23.90 ± 4.94 μg/mL at 4 h and decreased to 0.85 ± 0.31 μg/mL by 36 h. The AUC of ASA was 146.7 ± 24.90 μg · min/mL and the bioavailability 0.059 ± 0.013 after IG administration.
Discussion
This study demonstrated that the bioavailability of ASA was approximately 3 times greater (0.17 ± 0.037 versus 0.059 ± 0.013; P = 0.02) after rectal administration to fasted horses than after IG administration to nonfasted horses, and the peak plasma ASA concentration was approximately 4 times greater (5.05 ± 0.80 μg/mL versus 1.26 ± 0.10 μg/mL; P = 0.007). The ASA was eliminated rapidly after IV administration. The tl/2 value of 0.53 ± 0.04 h was higher than the tl/2 of 0.11 ± 0.02 h reported by Lees and colleagues (6) after IV administration of 19 mg/kg of lysine acetylsalicylate to horses. The difference may be due to the different formulations of ASA used in the 2 studies.
In humans, absorption of drugs, including ASA, is usually poorer after rectal administration than after oral administration (3,16). In horses, peak serum concentrations of metronidazole are lower when the drug is given rectally than when an equivalent dose is given by the IG or oral route (12). There may be several reasons for the enhanced absorption and higher bioavailability of ASA after rectal administration in this study. Gastrointestinal absorption of ASA is determined by the rate of dissolution, the luminal pH, and the rate of gastric emptying (17). The solubility of ASA is limited in the normal acid environment of the equine stomach and proximal small intestine (18,19). In the less acidic environment of the rectum (pH 6.0 to 6.5) (18), ASA solubility is increased, which should result in greater absorption (20). In human subjects with oral administration, plasma ASA concentrations peaked sooner, at levels approximately 48% higher, when sodium bicarbonate was administered with ASA than when ASA was administered alone (21).
Drugs that are administered in the distal rectum in humans may be subject to reduced first-pass hepatic metabolism (22). The rectum of the horse is approximately 20 to 30 cm long. Venous drainage of the orad half occurs via the caudal mesenteric vessels, which empty into the portal circulation. Venous outflow from the the distal portion of the rectum, however, drains into the caudal vena cava via the internal iliac or pudendal veins, thus bypassing the portal circulation (23). It is unlikely that a reduction in first-pass hepatic metabolism after rectal administration contributed significantly to the higher plasma concentrations of ASA in this study, because the ASA suspension was deposited approximately 10 cm orad to the rectum (in the terminal small colon). A portion of drug, however, could have spread to and been absorbed from the caudal rectum (22), avoiding first-pass hepatic metabolism.
The low absorption of ASA after IG administration may be due to the feeding of hay to the horses before and during the IG-administration experiments. The presence of food in the stomach can lead to slower gastric emptying and increased drug binding by organic matter (24,25,26). In humans, ASA absorption is delayed and plasma ASA concentrations are lower when the drug is administered after a meal (24). Absorption of orally administered phenylbutazone was reduced by approximately 50% in horses fed hay before and after the drug's administration (25). The absorption of rectally administered drugs can also be lowered by binding of the drug to organic matter in the gastrointestinal tract (16). The influence of such drug binding was reduced in this study because feces were removed from the rectum before drug administration; this may have contributed to the increased bioavailability of ASA administered rectally compared with that after oral administration. Similarly, fecal material would be decreased in the rectum of horses with gastrointestinal diseases in which food intake is reduced or absent. Although we realize that the study design (the horses being nonfasted for IG administration) created a condition that would lower the absorption of intragastrically administered ASA, the IG administration of ASA to nonfasted horses would likely mimic the clinical situation in which horses are not routinely fasted before oral administration of NSAIDs.
The peak plasma SA concentrations after IG administration were higher than those after rectal administration of ASA. The presence of food in the stomach of the nonfasted horses would delay gastric emptying and may have resulted in increased gastric retention of ASA. ASA is rapidly hydrolyzed to SA within the gastrointestinal tract (26). Consequently, larger intestinal concentrations of SA would have been available for absorption, resulting in higher plasma concentration.
Oral administration of NSAIDs, including ASA, can result in gastric irritation and ulceration (27,28). Damage to the gastric mucosa may result more from the physical interaction of the drug with the mucosa than from the inhibition of local prostaglandin synthesis. In rats, 100 mg of ASA/kg in a single dose caused significant gastric mucosal irritation when administered orally but no gastric damage when administered rectally (28). Thus, rectal administration of ASA may prevent the gastric mucosal damage that can occur after oral administration. We did not examine the rectal mucosa of our horses after rectal administration of ASA. Long-term rectal administration of ASA, however, has been shown to cause anorectal ulceration and stricture in humans (29,30).
Orally administered ASA significantly inhibits plasma thromboxane production and prolongs bleeding time in horses (6,7,8,9,31). Oral administration of ASA is not always feasible in horses with gastrointestinal disease. Currently in the United States, there is no commercially available preparation of ASA that can be given intravenously. Although an IV formulation of SA is available, SA does not inhibit platelets (10). To our knowledge, plasma or tissue concentrations of ASA necessary to inhibit thromboxane production in horses have not been determined. Baxter (7) showed that a single IG dose of ASA of 5 to 20 mg/kg administered to fasted horses significantly inhibited thromboxane production for 3 to 5 d, in a dose-dependent manner. Although we compared the bioavailability of ASA after rectal administration to fasted horses with that after IG administration to nonfasted horses, Kopp and associates (9) demonstrated that 17 mg/kg of ASA given orally once daily to nonfasted horses significantly reduced serum thromboxane B2 concentrations, to barely detectable levels, during the administration period.
We have shown that a single rectal dose of ASA of 20 mg/kg in fasted horses results in higher peak plasma ASA concentrations and greater bioavailability than the same dose given IG to nonfasted horses. Our findings, when combined with those of Kopp and associates (9), suggest that rectal administration of ASA should result in adequate inhibition of serum thromboxane production when oral administration is contraindicated. Therefore, under these experimental conditions, our hypothesis that the bioavailability of ASA after rectal administration would be comparable or superior to that after oral administration is supported. Additionally, lower doses than those suggested for IG administration may be sufficient.
Footnotes
Address all correspondence and reprint requests to Dr. M.P. Brown; telephone: (352) 392-4700, ext. 4000; fax: (352) 392-8289; e-mail: brownmu@mail.vetmed.ufl.edu
College of Veterinary Medicine, Journal Series Number 615.
Received May 27, 2002. Accepted June 25, 2003.
References
- 1.Cham BE, Ross-Lee L, Bochner F, Imhoff DM. Measurement and pharmacokinetics of acetylsalicylic acid by a novel high performance liquid chromatographic assay. Ther Drug Monit 1980;2:365–371. [PubMed]
- 2.Muir N, Nichols JD, Clifford JM, Stillings MR, Hoare RC. The influence of dosage form on aspirin kinetics: implications for cardiovascular use. Curr Med Res Opin 1997;13:547–553. [DOI] [PubMed]
- 3.Kanto J, Klossner J, Mantyla R, Yrjana T. Bioavailability of rectal aspirin in neurosurgical patients. Acta Anaesth Scand 1981;75:25–26. [DOI] [PubMed]
- 4.Gingerich DA, Baggot JD, Yeary RA. Pharmacokinetics and dosage of aspirin in cattle. J Am Vet Med Assoc 1975;167:945–948. [PubMed]
- 5.Davis LE. Clinical pharmacology of salicylates. J Am Vet Med Assoc 1980;176:65–66. [PubMed]
- 6.Lees P, Ewins CP, Taylor JBO, Sedgwick AD. Serum thromboxane in the horse and its inhibition by aspirin, phenylbutazone and flunixin. Br Vet J 1987;143:462–476. [DOI] [PubMed]
- 7.Baxter GM. Effect of aspirin on ex vivo generation of thromboxane in healthy horses. Am J Vet Res 1987;48:13–16. [PubMed]
- 8.Cambridge H, Lees P, Hooke RE, Russell CS. Antithrombotic actions of aspirin in the horse. Equine Vet J 1991;23:123–127. [DOI] [PubMed]
- 9.Kopp KJ, Moore JN, Byars TD, Brooks P. Template bleeding time and thromboxane generation in the horse: effects of three non- steroidal anti-inflammatory drugs. Equine Vet J 1985;17:322–324. [DOI] [PubMed]
- 10.Deykin D. Antithrombotic therapy. Postgrad Med 1979;65: 135–146. [DOI] [PubMed]
- 11.Delbeke FT, Debackere M, Vynckier L. Disposition of human drug preparations in the horse. l. Rectally administered indomethacin. J Vet Pharmacol Ther 1991;14:145–149. [DOI] [PubMed]
- 12.Garber JL, Brown MP, Gronwall RR, Merritt K. Pharmacokinetics of metronidazole after rectal administration in horses. Am J Vet Res 1993;54:2060–2063. [PubMed]
- 13.Mason WD, Gillflan R. Revised method for determination of aspirin and salicylic acid in human plasma by high pressure liquid chromatography. Anal Lett 1983;16:903–912.
- 14.Caeci MS, Cacheris WP. Fitting curves to data. Byte 1984;9:340–362.
- 15.Gibaldi M, Perrier D. Pharmacokinetics. New York: Marcel Dekker, 1982:409–417.
- 16.Gibaldi M. Biopharmaceutics and clinical pharmacokinetics. Philadelphia: Lea and Febiger, 1991:113–118.
- 17.Dromgoole SH, Furst DE. Salicylates. In: Evans WE, Schentag JJ, Jusko WJ, eds. Applied pharmacokinetics. Principles of therapeutic drug monitoring. Vancouver: Applied Therapeutics, Inc, 1994:32–1,32–34.
- 18.Argenzio RA, Southworth M, Stevens CE. Sites of organic acid production and absorption in the equine gastrointestinal tract. Am J Physiol 1974;226:1043–1050. [DOI] [PubMed]
- 19.Murray MJ, Schusser GF. Measurement of 24-h gastric pH using an indwelling pH electrode in horses unfed, fed, and treated with ranitidine. Equine Vet J 1993;25:417–424. [DOI] [PubMed]
- 20.Feldman M, Cryer B. Aspirin absorption rates and platelet inhibition times with 325-mg buffered aspirin tablets (chewed or swallowed intact) and with buffered aspirin solution. Am J Cardiol 1999;84:404–409. [DOI] [PubMed]
- 21.Mason WD, Winer N. Kinetics of aspirin, salicylic acid, and salicyluric acid following oral administration of aspirin as a tablet and two buffered solutions. J Pharm Sci 1981;70: 262–265. [DOI] [PubMed]
- 22.van Hoogdalem EJ, de Boer AG, Breimer DD. Pharmacokinetics of rectal drug administration, part I. Clin Pharmacokinet 1991;21:11–26. [DOI] [PubMed]
- 23.Schummer A, Nickel R, Sack WO. The viscera of the domestic mammals. New York: Springer-Verlag, 1979.
- 24.Mason WD, Winer N. Influence of food on aspirin absorption from tablets and buffered solutions. J Pharm Sci 1983;72: 819–821. [DOI] [PubMed]
- 25.Sullivan M, Snow DH. Factors affecting absorption of non- steroidal anti-inflammatory agents in the horse. Vet Rec 1982;110:554–558. [DOI] [PubMed]
- 26.Toothaker RD, Welling PG. The effect of food on drug bioavailability. Ann Rev Pharmacol Toxicol 1980;20:173–199. [DOI] [PubMed]
- 27.Lees P, Higgins AJ. Clinical pharmacology and therapeutic uses of non-steroidal anti-inflammatory drugs in the horse. Equine Vet J 1985;17:83–96. [DOI] [PubMed]
- 28.Ligumsky M, Sestieri M, Karmeli F, Zimmerman E, Okon E, Rachmilewitz D. Rectal administration of nonsteroidal antiinflammatory drugs. Gastroenterology 1990;98:1245–1249. [PubMed]
- 29.van Gossum A, Zalcman M, Adler M, Penny M, Houben J, Cremer M. Anorectal stenosis in patients with prolonged use of suppositories containing paracetamol and acetylsalicylic acid. Dig Dis Sci 1993;38:1970–1977. [DOI] [PubMed]
- 30.D'Haens G, Debackere M, Vynckier L. Proctitis and rectal stenosis induced by nonsteroidal anti-inflammatory suppositories. J Clin Gastroenterol 1993;17:207–212. [DOI] [PubMed]
- 31.Trujillo O, Rios A, Maldonado R, Rudolph W. Effect of oral administration of acetylsalicyclic acid on haemostasis in the horse. Equine Vet J 1981;13:205–206. [DOI] [PubMed]