Abstract
Expression in Xenopus oocytes and electrophysiology was used to test for transport activity of the five sucrose transporter (SUT) homologs from rice. Expression of OsSUT1 and OsSUT5 resulted in sucrose-dependent currents that were analyzed by two-electrode voltage clamping. We examined the transport kinetics, substrate specificity and pH dependence of sucrose transport and K0.5 for sucrose. OsSUT1 showed similar features to those of other type II SUTs from monocots examined previously, with a K0.5 value of 7.50 mM at pH 5.6. In contrast, OsSUT5 had a higher substrate affinity (K0.5 = 2.32 mM at pH 5.6), less substrate specificity and less pH dependence compared with all type II SUTs tested to date. Regulation of the rice SUTs, as well as ZmSUT1 from maize and HvSUT1 from barley, by reduced (GSH) and oxidized (GSSG) forms of glutathione was tested. GSSG and GSH were found to have no significant effect on the activity of sucrose transporters when expressed in Xenopus oocytes. In conclusion, differences in transport activity between OsSUT1 and OsSUT5 indicate that type II SUTs have a range of transport activities that are tuned to their function in the plant.
Keywords: Electrophysiology, Oryza sativa, OsSUT1, OsSUT5, Rice, Sucrose transporter
Introduction
Sucrose is the main carbohydrate transported in the phloem of most plants including rice (Hayashi and Chino 1990). The long-distance transport of sucrose in the phloem is important to supply non-photosynthetic or developing tissues (sink tissues) such as roots, developing leaves, flowers and seeds with fixed carbon. Sucrose transporters (SUTs) are important for both phloem loading in source tissue and sucrose uptake into some sink cells. SUTs utilize the transmembrane proton gradient to drive sucrose uptake into the cytoplasm (Giaquinta 1977). The first SUT cDNA was cloned by functional expression in yeast and found to encode a membrane protein with 12 transmembrane spans and sucrose: H+ symporter activity (Riesmeier et al. 1992). More recently, the availability of complete genome sequences revealed that plants encode small gene families of related SUTs.
The SUT family can be divided into three main clades: type I, II and III (Aoki et al. 2003). Type I SUTs are exclusive to eudicots while type II and III SUTs are found in all plants. This is interesting because in eudicot species type I SUTs are required for loading sucrose into the phloem: examples are StSUT1 in potato (Riesmeier et al. 1994) and AtSUC2 in Arabidopsis (Gottwald et al. 2000). In addition, other type I SUTs function in sucrose uptake into sink cells, for example AtSUC1 is required for normal pollen function and is also expressed in trichomes and in roots (Sivitz et al. 2008). Several type I SUTs are well characterized and their transport activities can be compared. For example, all type I SUTs analyzed to date have similar substrate specificity (Chandran et al. 2003, Sivitz et al. 2007). However, extreme differences in substrate affinity are found for type I SUTs: AtSUC9 has much higher substrate affinity than other type I SUTs (Sivitz et al. 2007).
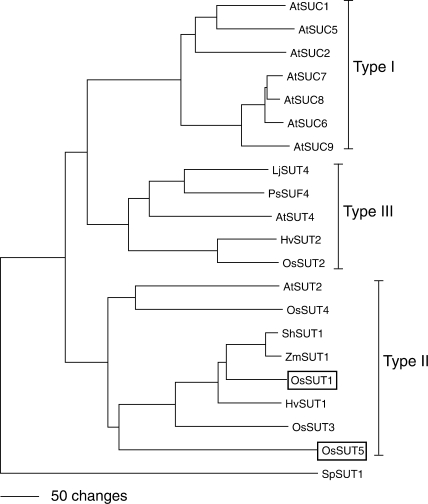
Monocot species such as rice do not encode type I SUTs, and it has been hypothesized that they use type II SUTs for phloem loading. It has now been shown that a mutation in the maize ZmSUT1 (type II) gene results in defective phloem loading and carbohydrate accumulation in leaves (Slewinski et al. 2009). Several monocot type II SUTs have been analyzed using electrophysiology, and all are orthologous to OsSUT1. Type II SUTs from barley (HvSUT1) and sugarcane (ShSUT1) are much more selective for sucrose and have lower substrate affinity compared with type I SUTs (Sivitz et al. 2005, Reinders et al. 2006). The rice genome encodes five SUTs, four are type II and one (OsSUT2) is type III (Aoki et al. 2003). OsSUT1 and OsSUT3 were shown to function as SUTs by expression in yeast (Hirose et al. 1997, Aoki et al. 2003) but none of the rice SUTs has been characterized in detail. Based on the phylogenetic tree (Fig. 1), we would predict that OsSUT1 has similar transport properties to its closest homologs: HvSUT1, ShSUT1 and ZmSUT1 (Carpaneto et al. 2005, Sivitz et al. 2005, Reinders et al. 2006). Recent studies suggested a potential role of OsSUT1 in phloem loading of sucrose (Hirose et al. 1997, Matsukura et al. 2000, Scofield et al. 2007). However, Ishimaru et al. (2001) reported that antisense inhibition of OsSUT1 does not result in a decrease in phloem loading, indicating that other SUTs have a redundant function in rice or that a symplasmic pathway (through plasmodesmata) for phloem loading exists in rice. Little information is available concerning the function of the other four rice SUTs. Gene expression of rice SUT family members has been studied through reverse transcription–PCR (RT–PCR) analysis. OsSUT1 mRNA was found in internode-1 (Scofield et al. 2007), germinating seeds, sink and source leaves and leaf sheaths, but little in roots (Aoki et al. 2003). OsSUT2 is expressed at similar levels in all tissues tested (Aoki et al. 2003). OsSUT3 transcripts accumulated the most in sink leaves (Aoki et al. 2003), preferentially in exposed internode-1, and was absent from the enclosed region (Scofield et al. 2007). OsSUT4 showed preferential expression in sink leaves (Aoki et al. 2003), and expression was also found in the stem (Scofield et al. 2007). OsSUT5 is expressed most in sink leaves and is the only rice SUT not expressed significantly in germinating seeds (Aoki et al. 2003) or internode-1 (Scofield et al. 2007).
Fig. 1.
Phylogenetic tree of sucrose transporters (SUTs). The protein sequences of selected SUTs were aligned using ClustalX followed by Neighbor–Joining analysis in PAUP 4.0 to create the tree. The tree was rooted using the SpSUT1 sucrose transporter homolog from Schizosaccharomyces pombe (Reinders and Ward 2001). Type I, II, III classification follows the nomenclature in Aoki et al. (2003). The accession numbers for the peptide sequences are Arabidopsis AtSUC1 (At1g71880), AtSUC2 (At1g22710), AtSUT2/AtSUC3 (At2g02860), AtSUT4 (At1g09960), AtSUC5 (At1g71890), AtSUC6 (At5g43610), AtSUC7 (At1g66570), AtSUC8 (At2g14670), AtSUC9 (At5g06170); barley HvSUT1 (CAJ20123), HvSUT2 (CAB75881); fission yeast SpSUT1 (O14091); lotus LjSUT4 (CAD61275); maize ZmSUT1 (BBA83501); pea PsSUF4 (ABB30162); rice OsSUT1 (Os03g07480), OsSUT2 (Os12g44380), OsSUT3 (Os10g26740), OsSUT4 (Os02g58080), OsSUT5 (Os02g36700); and sugarcane ShSUT1 (AAV41028).
It would be useful to know the range of sucrose transport activities (transport mechanism, substrate affinity and specificity, and regulatory properties) present in type II SUTs in rice, especially considering the possibility that they function redundantly in phloem loading. In this report we tested the activity of the five rice SUT homologs by expression in Xenpous oocytes and two-electrode voltage clamping (TEVC). OsSUT1 and OsSUT5 were functional in this system and therefore were selected for further analysis. Unfortunately, expression of OsSUT2, OsSUT3 and OsSUT4 did not produce substrate- dependent inward currents. OsSUT5 has an almost identical size to OsSUT1, with 65% sequence identity (Aoki et al. 2003) and a distinct expression pattern (Aoki et al. 2003; Scofield et al. 2007). We report a comparison of substrate specificity, transport kinetics, regulation by extracellular pH and results from experiments to test the effects of reduced (GSH) and oxidized (GSSG) glutathione on sucrose transport activity by OsSUT1 and OsSUT5.
Results
OsSUT1 and OsSUT5 are proton-coupled symporters
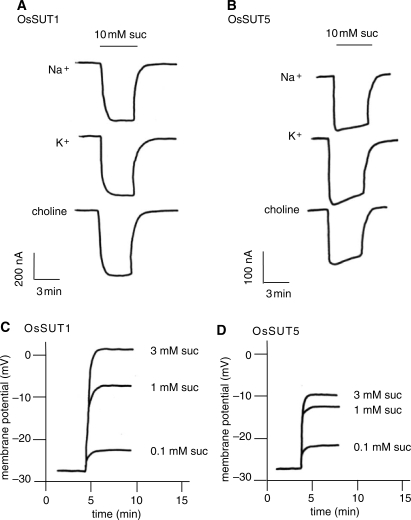
In order to study their transport activity, each of the five rice SUTs was expressed in Xenopus oocytes and analyzed by TEVC. External application of sucrose induced inward currents in oocytes expressing OsSUT1 and OsSUT5, consistent with cation-coupled sucrose transport (Fig. 2A, B). No currents were induced in oocytes expressing OsSUT2, OsSUT3 and OsSUT4. The standard recording solution (Na Ringer) contained 115 mM Na+ and 2 mM K+. To determine if the inward currents were carried by H+, the experiment was repeated with K Ringer solution (containing 115 mM K+ and no Na+) or choline Ringer solution (without K+ or Na+). For all of the solutions tested, the currents induced by sucrose in OsSUT1 (Fig. 2A) or OsSUT5 (Fig. 2B) were similar, indicating that K+ or Na+ are not required for sucrose-induced inward currents and that H+ acts as the coupling ion.
Fig. 2.
Sucrose-induced currents and membrane depolarization in oocytes expressing OsSUT1 and OsSUT5. (A, B) In Na+, K+ and choline Ringer solutions at pH 5.6, SUT-expressing oocytes were voltage clamped at −40 mV and currents were recorded. Each trace represents a current induced by 10 mM sucrose in a modified Ringer solution. (C, D) Membrane potential was recorded using an oocyte impaled with a single electrode, referenced against the bath electrodes. Membrane depolarization was observed when the oocyte was perfused with Na Ringer solution at pH 5.6 containing sucrose at the indicated concentrations.
When membrane potential was measured rather than clamped, proton-coupled sucrose symport was expected to cause a membrane depolarization, and this was observed in both OsSUT1- (Fig. 2C) and OsSUT5- (Fig. 2D) expressing oocytes. The resting potential was similar (around −27 mV) for oocytes expressing either transporter. Increasing the concentration of sucrose resulted in increasing depolarization from the resting potential for both transporters (Fig. 2C, D). Because sucrose is not charged, sucrose-induced depolarization is consistent with a H+-coupled transport mechanism for both OsSUT1 and OsSUT5.
Differences in substrate affinity for OsSUT1 and OsSUT5
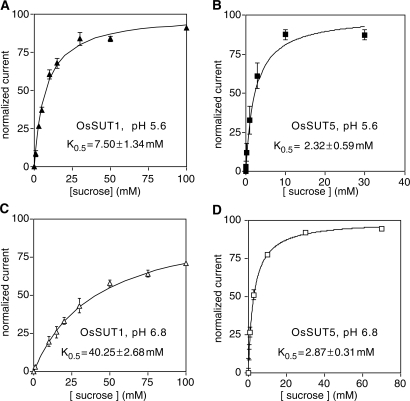
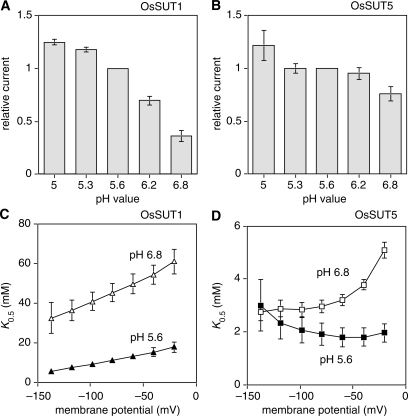
To examine the substrate affinity of OsSUT1 and OsSUT5, kinetic analysis was performed using TEVC (Fig. 3). At a membrane potential of −117 mV and extracellular pH of 5.6 and 6.8, the sucrose-induced currents mediated by OsSUT1 and OsSUT5 were measured. By fitting the Michaelis–Menten equation to these data, the K0.5 values for the two rice sucrose transporters were determined. At both pH 5.6 and 6.8, OsSUT5 had a lower K0.5 value than that of OsSUT1, indicating a higher affinity of OsSUT5 for sucrose (Fig. 3). OsSUT5 was also less affected by the external pH level. The measured K0.5 values of OsSUT5 were similar at pH 5.6 (Fig. 3B; K0.5 = 2.32 mM) and pH 6.8 (Fig. 3D; K0.5 = 2.87 mM). In contrast, the K0.5 value of OsSUT1 was more pH dependent, with values of 7.50 mM at pH 5.6 (Fig. 3A) and 40.25 mM at pH 6.8 (Fig. 3C).
Fig. 3.
Kinetic analysis of sucrose transport by OsSUT1 and OsSUT5. Sucrose-dependent currents were recorded under voltage-clamped conditions at a membrane potential of −117 mV. Currents were normalized to Vmax and plotted against the substrate concentration. Substrates were applied in Na Ringer solution at pH 5.6 or pH 6.8. Results were fitted to the Michaelis–Menten equation, indicated by the line. Values correspond to the mean ± SE of data from 4–9 oocytes. (A) OsSUT1, pH 5.6. (B) OsSUT5, pH 5.6. (C) OsSUT1, pH 6.8. (D) OsSUT5, pH 6.8.
OsSUT1 and OsSUT5 have slight substrate specificity differences
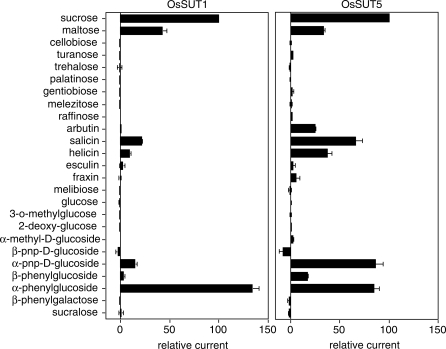
A series of glucosides were tested as potential substrates for OsSUT1 and OsSUT5. OsSUT1 displayed almost the same substrate specificity pattern (Fig. 4) as HvSUT1 (Sivitz et al. 2005, Sivitz et al. 2007) and ShSUT1 (Reinders et al. 2006, Sivitz et al. 2007), consistent with their close relationship in the phylogenetic tree (Fig. 1). They all transport sucrose, maltose, salicin, helicin, α-phenylglucoside and α-paranitrophenyl-d-glucoside. OsSUT5 differed from OsSUT1 in its ability to transport two substrates. Arbutin was transported by oocytes expressing OsSUT5 but not OsSUT1. Also, α-paranitrophenyl-d-glucoside was transported at a much higher rate by OsSUT5 compared with OsSUT1 (Fig. 4), HvSUT1 (Sivitz et al. 2005) or ShSUT1 (Reinders et al. 2006).
Fig. 4.
Substrate specificity of OsSUT1 (A) and OsSUT5 (B). Substrate-dependent currents were at a membrane potential of −117 mV. Substrates were applied at a concentration of 10 mM in Na Ringer solution, pH 5.6 except for fraxin (1 mM) and esculin (5 mM) that were added at their limits of solubility. Currents were normalized to currents for 10 mM sucrose to eliminate the influence of expression level differences between oocytes. Values correspond to the mean ± SE of data from 3–5 oocytes.
OsSUT1 and OsSUT5 have distinct pH and voltage dependence
SUTs usually display larger inward currents at lower extracellular pH, and this was observed for both OsSUT1 (Fig. 5A) and OsSUT5 (Fig. 5B). The K0.5 values of SUTs are usually pH dependent, and at higher pH the K0.5 values are always voltage dependent. The effect of extracellular pH on the K0.5 of OsSUT1 for sucrose was typical (Fig. 5A). At pH 5.6 the K0.5 for sucrose was much lower and less voltage dependent compared with pH 6.8 (Fig. 5C). Results for OsSUT5 were different from those of any SUT analyzed previously. At more polarized membrane potentials, typical of plant cells, the K0.5 of OsSUT5 for sucrose was similar at both pH 5.6 and 6.8 (Fig. 5D, also Fig. 3B, D). At the lower pH value the K0.5 of OsSUT5 for sucrose was less voltage dependent compared with pH 6.8 (Fig. 5D).
Fig. 5.
pH dependence and voltage dependence of transport activity of OsSUT1 and OsSUT5. (A, B) Sucrose-dependent currents were measured at a membrane potential of −137 mV. The sucrose concentration in Na Ringer solution was 50 mM for OsSUT1 (A) and 25 mM for OsSUT5 (B). (C, D) K0.5 values of sucrose-dependent currents were measured at pH 5.6 and pH 6.8, plotted against the membrane potential. Values for OsSUT1 (C) and OsSUT5 (D) are the mean ± SE from 4–9 oocytes.
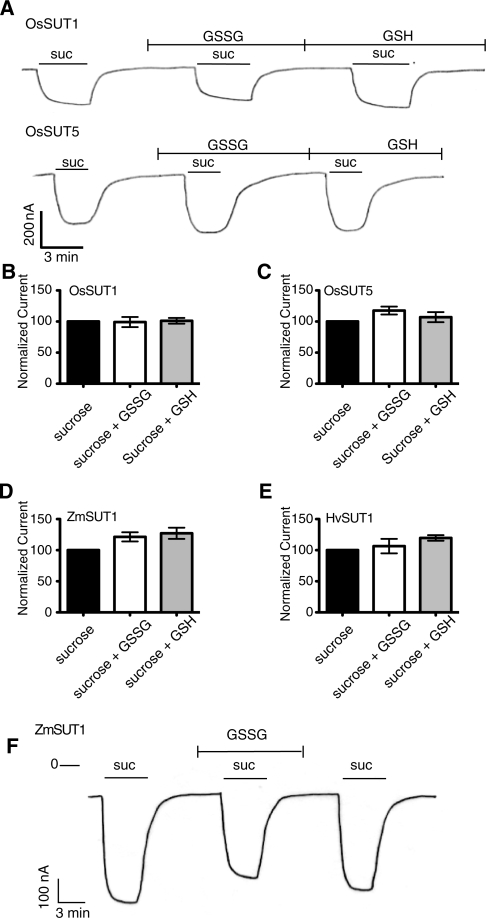
Effect of GSSG and GSH on the transport activity of OsSUT1, OsSUT5, HvSUT1 or ZmSUT1
Glutathione is one of the most abundant antioxidants in plants, and the redox state of glutathione is thought to reflect the metabolic state of the plant (Wormuth et al. 2007). Recently (Krügel et al. 2008) reported that 1 mM GSSG increased ZmSUT1 activity in oocytes by 1.8-fold. However, in our experiments, GSSG or GSH at 1 mM had no significant effect on OsSUT1 and only a small effect on OsSUT5 (Fig. 6A–C). Therefore. we tried to confirm that GSSG increases the activity of ZmSUT1 in oocytes. Despite the similar conditions used here, GSSG or GSH had a smaller effect on ZmSUT1 activity (Fig. 6D) compared with the previous report (Krügel et al. 2008). In our experiments GSSG increased sucrose transport of ZmSUT1 by 1.2-fold and GSH had a similar effect. For HvSUT1 (Fig. 6E) currents induced by sucrose in the presence of GSSG were similar to those of controls. GSH had a small stimulatory effect. To confirm that the lack of effect of GSSG was not due to the slightly different conditions used in Fig. 6A–E compared with the original report (Krügel et al. 2008), the following experiment was conducted. Oocytes expressing ZmSUT1 were recorded in solution A (100 mM NaCl, 2 mM KCl, 2 mM MgCl2, 0.2 mM CaCl2 and 10 mM MES-Tris pH 5.5). The addition of GSSG to solution A lowered the pH to 4.3 and the pH was readjusted to 5.5 with NaOH. Sucrose (20 mM) in either solution A or solution A with GSSG induced inward currents of similar magnitude (Fig. 6F) using conditions identical to those of Krügel et al. (2008). In conclusion, a small effect of glutathione could not be excluded but we did not observe the large increase in sucrose transport due to GSSG reported by Krügel et al. (2008).
Fig. 6.
Effect of oxidized (GGSG) and reduced (GSH) glutathione on sucrose transport activity of four SUTs expressed in Xenopus oocytes. All experiments were performed at pH 5.6 in modified Na Ringer solution with a holding potential of −40 mV, except in F. (A) Currents were recorded from Xenopus oocytes expressing OsSUT1 (top) or OsSUT5 (bottom). GSH and GSSG were applied at 1 mM concentration in Na Ringer solution readjusted to pH 5.6 with NaOH. Sucrose (10 mM) was applied extracellularly where indicated. For B–E data are presented as normalized mean sucrose-dependent current ± SE. (B) Average sucrose-dependent currents for OsSUT1 recorded as in A. In the absence of glutathione the average current was −83 nA (n = 4 oocytes). (C) Average sucrose-dependent current for OsSUT5-expressing oocytes in the absence of glutathione was −188 nA (n = 5 oocytes). (D) Average sucrose-dependent current for ZmSUT1-expressing oocytes in the absence of glutathione was −224 nA (n = 10 oocytes). (E) Average sucrose-dependent current for HvSUT1-expressing oocytes was 294 nA (n = 6 oocytes). (F) Sucrose-dependent currents were recorded from oocytes expressing ZmSUT1 as described by Krügel et al. (2008). Oocytes in solution A (100 mM NaCl, 2 mM KCl, 2 mM MgCl2, 0.2 mM CaCl2 and 10 mM MES-Tris pH 5.5) at a holding potential of −70 mV. GSSG (1 mM) in solution A was applied where indicated. Sucrose (20 mM) in either solution A or solution A with GSSG was applied where indicated.
Discussion
Relatively little is known about the transport activities of monocot SUTs compared with those in eudicots. Monocots encode multiple type II SUTs (while eudicots only have one), and discovery of differences in transport activity may provide insight into the structure–function relationship for this transporter family. Transport activity of the five SUT homologs from rice was tested by expression in Xenopus oocytes and TEVC. Only OsSUT1 and OsSUT5 showed sucrose-dependent inward currents in oocytes, and these were further analyzed. The five cDNAs were also expressed in yeast (not shown). In addition to OsSUT1 and OsSUT5, we expected OsSUT3 to be functional in yeast since it has been previously reported to allow the yeast strain SuSy7 to grow on sucrose (Aoki et al. 2003). In our experiments, when OsSUT3 was expressed in yeast we did not observe significant [14C]sucrose uptake. Perhaps the OsSUT3 cDNA sequence that was optimized for expression in oocytes was not expressed well in yeast. Only OsSUT1, OsSUT5 and OsSUT2 conferred significant [14C]sucrose uptake in yeast. OsSUT2 is a type III SUT; members of this group are generally localized to the vacuole membrane in plants (Endler et al. 2006, Reinders et al. 2008). When expressed in yeast, it is common for type III SUTs to mislocalize to the plasma membrane and show [14C]sucrose uptake; for example AtSUT4 was characterized in this way (Weise et al. 2000). The only type III SUT that was found to localize to the plasma membrane in Xenopus oocytes enabling analysis by electrophysiology was LjSUT4 (Reinders et al. 2008). Therefore, it is not surprising that OsSUT2 was found to be functional in yeast but not in Xenopus oocytes. It appears that each plant (both monocot and eudicot) only contains one type III SUT gene. However, there is evidence for differences in function or localization. StSUT4 was found to be localized to the plasma membrane in potato (Chincinska et al. 2008). In addition, PsSUF4 from pea is a type III sucrose transporter, closely related to LjSUT4, and has been described as having sucrose transporter activity that is uncoupled from H+ (Zhou et al. 2007). In this study we focused on a detailed characterization of OsSUT1 and OsSUT5.
Substrate specificity of OsSUT1 and OsSUT5
The substrate specificity of all type I SUTs studied to date is very similar. Natural sugars including sucrose, maltose, turanose, arbutin, salicin, helicin, esculin and fraxin, and synthetic sugars such as α-methyl-glucoside, α- and β-glucosides are transported by both AtSUC2 (Chandran et al. 2003) and AtSUC9 (Sivitz et al. 2007). In contrast, our study shows different substrate specificities within type II SUTs. OsSUT1 showed almost identical substrate specificity compared with its orthologs HvSUT1 (Sivitz et al. 2005) and ShSUT1 (Reinders et al. 2006). However, OsSUT5 appeared less selective than previously reported type II SUTs, transporting arbutin and α-paranitrophenyl-d-glucoside to a larger extent (Fig. 4). It is not known if the native substrate of SUTs is limited to sucrose, or if other glucosides found to be transported in a heterologous system are also transported in plants. However, analysis of substrate specificity may be useful in studying the structure–function relationship in SUTs. Correlation of substrate specificity data with sequence differences would be useful to generate hypotheses concerning amino acid positions involved with substrate binding. The function of specific amino acids in substrate binding or translocation could then be tested by determining the effects of mutagenesis at these positions on substrate specificity.
Substrate affinity of SUTs
Large differences in substrate affinity have been found between type I SUTs. AtSUC2 has a K0.5 of 1.44 mM for sucrose at pH 5.0 (Chandran et al. 2003) and it is responsible for phloem loading (Gottwald et al. 2000). In contrast, AtSUC9, another type I SUT, has a 20-fold higher apparent affinity, with a K0.5 of 0.066 mM at the same pH, and is expressed mainly in sink tissues (Sivitz et al. 2007). Among type II SUTs tested to date, the K0.5 values for sucrose are similar at pH 5.6, with 2.3 mM for OsSUT5 (Fig. 3B), 3.7 mM for ZmSUT1 (Carpaneto et al. 2005), 7.5 mM for OsSUT1 (Fig. 3A), 8.3 mM for ShSUT1 (Reinders et al. 2006) and 10.6 mM for HvSUT1 (Sivitz et al. 2005). The substrate affinity of SUTs may be fine-tuned for the location of the transporters in plants. In the apoplast surrounding most plant cells, sucrose is maintained at a low concentration. For example, the sucrose concentration in the apoplast of Vicia faba guard cells was in the range of 0.5 mM (Outlaw and De Vlieghere-He 2001). The sucrose concentration in the apoplast from the mesophyll of several species including Plantago major was ≤1 mM (Nadwodnik and Lohaus 2008). However, a relatively high sucrose concentration is expected in the cell wall spaces near veins where phloem loading occurs (Giaquinta et al. 1983). If this is the case, plants might use specific SUTs at specific locations, for example high affinity SUTs in areas with low extracellular sucrose concentrations and lower affinity SUTs at high sucrose concentration sites. This situation might have developed independently in eudicots and monocots. For rice SUTs studied here, OsSUT1 (low affinity) is expressed in the phloem and is suggested to function in phloem loading (Matsukura et al. 2000), while OsSUT5 (higher affinity) is expressed preferentially in sink tissues (Aoki et al. 2003). Information on the remaining three SUTs from rice (OsSUT2, OsSUT3 and OsSUT4) is required to understand the full range of SUT activities in rice.
Difference in pH dependence of OsSUT1 and OsSUT5
Sucrose transport through SUTs is driven by the transmembrane electrochemical H+ gradient. Lower extracellular pH increases sucrose transport for all SUTs with the exception of AtSUC9 (Sivitz et al. 2007). Low extracellular pH also regulates most SUTs by modifying substrate affinity, and all SUTs tested previously have lower K0.5 values at lower extracellular pH (Boorer et al. 1996, Sivitz et al. 2005, Reinders et al. 2006). This regulation (pH dependence of the K0.5) is thought to be an additional mechanism, besides the generation of a proton motive force that stimulates the symport of H+ and sucrose uptake by SUTs (Chandran et al. 2003). From a physiological perspective, the activation of H+ pumps would both increase the electrochemical proton gradient to drive sucrose transport and acidify the extracellular space which would decrease the K0.5 for sucrose transport.
OsSUT1 (Fig. 5A, C) displayed a very similar pH dependence to ShSUT1 (Reinders et al. 2006), again reflecting their close phylogenetic relationship (Fig. 1). Interestingly, sucrose- induced current though OsSUT5 (Fig. 5B) was much less pH dependent (at −137 mV). Fig. 5D shows that at negative membrane potentials typical of plant cells, OsSUT5 remains a high affinity transporter even at more alkaline pH values. The lack of this form of regulation in OsSUT5 is interesting because it indicates that OsSUT5 has a high affinity for sucrose even when proton pumping is not very active. Differences in the primary structure between OsSUT1 and OsSUT5 are expected to underlie this difference in regulation by pH. It could be caused by differences in amino acid sequence at a single or multiple positions. Obtaining additional functional information from OsSUT5 orthologs would be useful to try to correlate differences in sequence with differences in activity or regulation.
SUT regulation
Aside from regulation by extracellular pH, there is little evidence that SUT activity is regulated at the protein level. We know that SUTs are capable of forming homo- and heterodimers (or multimers) (Reinders et al. 2002a, Reinders et al. 2002b, Schulze et al. 2003) but no change in transport activity had been ascribed to multimerization. GSSG increases sucrose transport activity in yeast, and oxidative redox reagents including GSSG cause recruitment of SlSUT1 (LeSUT1) to the plasma membrane in yeast (Krügel et al. 2008). GSSG was also shown to increase the activity of ZmSUT1 when expressed in oocytes. In our experiments GSSG had a much smaller effect (Fig. 6). The addition of GSSG or GSH acidified Na Ringer solution, therefore we readjusted the pH of solutions containing glutathione to pH 5.6 using NaOH. Using GSSG solutions with lower pH would result in larger sucrose-induced current. This may explain the apparent greater effect of GSSG on ZmSUT1 activity in the previous report (Krügel et al. 2008). In our experiments GSSG or GSH had only minimal effects on the four type II SUTs tested. In addition, we saw no evidence of an opposite effect of GSSG and GSH as might be expected if SUTs were regulated through cysteine reduction/oxidation.
In conclusion, we present the first detailed analysis of the transport activity of two SUTs from rice, OsSUT1 and OsSUT5. Both are electrogenic, H+-coupled sucrose transporters. We show that OsSUT1 has a low affinity for sucrose and OsSUT5 has a higher affinity for sucrose. Both OsSUT1 and OsSUT5 are highly selective for sucrose over other glucosides. We noted that unlike other type II SUTs, OsSUT5 transports arbutin. OsSUT5 was very distinctive because its apparent substrate affinity (K0.5) was insensitive to pH at physiological membrane potentials. This is different from every other SUT characterized to date. The unique properties of OsSUT5 may reflect its function in sink tissue.
Materials and Methods
Cloning of OsSUT1 and OsSUT5
The complete open reading frame of OsSUT1 was amplified by PCR using primers 5′-atggctcgcggcagcggggccggag and 5′-tcagtgaccgccgcccatgctgac with OsSUT1 in pBluescript SK(−) (Aoki et al. 2003) as template and TA-cloned into pCR8/GW/TOPO (Invitrogen, Carlsbad, CA, USA). The resulting clone was recombined in vitro with the Gateway-compatible oocyte vector p002/GW to yield p002/GW-OsSUT1. Xenopus expression-optimized clones were synthesized corresponding to the amino acid sequences of OsSUT2, OsSUT3, OsSUT4 and OsSUT5, and cloned into Gateway vector pENTR221 by Blue Heron Biotechnology, Inc. The amino acid sequence of OSSUT5 was based on the consensus of available sequence information (genomic DNA-derived cDNA sequence NP_001047217, cDNA sequence BAC67165 and TIGR consensus sequence TA57893_4530). There are two amino acid changes (M26T; D226N) compared with the sequence published by Aoki et al. (2003). The pENTR221-OsSUT5 clone was recombined with p002/GW to produce p002/GW-OsSUT5. The mMessage mMachine kit (Applied Biosystems/Ambion, Austin, TX, USA) was used to prepare cRNA for injection of Xenopus oocytes.
Heterologous expression
Stage V and VI Xenopus laevis oocytes were separated using a 2 h incubation in Barth’s medium [88 mM NaCl, 1 mM KCl, 0.33 mM Ca(NO3)2, 0.41 mM CaCl2, 0.82 mM MgSO4, 2.4 mM NaHCO3, 10 mM HEPES, pH 7.6, 100 μg ml−1 penicillin and 100 μg ml−1 streptomycin] containing 10 mg ml−1 collagenase A (Roche Applied Science, Indianapolis, IN, USA). The oocytes were then washed five times in 1 mg ml−1 bovine serum albumin in Barth’s medium. The oocytes were injected with 50 nl (1 ng nl−1) of OsSUT1 or OsSUT5 cRNA and incubated at 15°C in Barth’s medium supplemented with 10 μg ml−1 gentamycin. Electrophysiological experiments were performed 3–4 d after the RNA injection.
Electrophysiological methods
Oocytes were bathed in modified Na Ringer solution (115 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5 mM MES-Tris, with the pH indicated in the figure legends) with continuous perfusion at 1 ml ml−1. Recording pipets, filled with 1 M KCl, with resistances between 1 and 3 MΩ were used. Currents were measured using the TEVC technique with a Dagan TEV 200A amplifier (Dagan Corp., Minneapolis, MN, USA). Currents were filtered on-line at 200 Hz and digitized at 2,000 Hz using pClamp 5.5.1 (Axon Instruments, Inc., Union City, CA, USA). The holding potential was −40 mV except where indicated, and voltage pulses from −157 to 58 mV were applied for 203 ms. Steady-state currents are presented as the mean current between 150 and 200 ms following the onset of voltage pulses. Substrate-dependent currents were obtained by subtracting an average of background currents before and after substrate application. Substrates were added at the concentrations indicated in the figure legends.
For the experiment presented in Fig. 6F, oocytes were recorded in solution A (100 mM NaCl, 2 mM KCl, 2 mM MgCl2, 0.2 mM CaCl2 and 10 mM MES-Tris pH 5.5) (Krügel et al. 2008). After addition of GSSG to 1 mM, the pH was readjusted to pH 5.5 with NaOH. In this experiment the holding potential was −70 mV.
Membrane potential recording was done using one channel of the Dagan TEV 200A in recording mode. Oocytes were bathed in Na Ringer solution at pH 5.6 and sucrose-induced depolarization was recorded using a chart recorder.
Funding
This work was supported by the Department of Energy [DE-FG02-07ER15886 to J.M.W.].
Acknowledgements
We thank Dr. Robert Furbank (CSIRO) for supplying the OsSUT1 cDNA.
Glossary
Abbreviaitions
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- RT–PCR
reverse transcription–PCR
- SUT
sucrose transporter
- TEVC
two-electrode voltage clamping.
References
- Aoki N, Hirose T, Scofield GN, Whitfeld PR, Furbank RT. The sucrose transporter gene family in rice. Plant Cell Physiol. 2003;44:223–232. doi: 10.1093/pcp/pcg030. [DOI] [PubMed] [Google Scholar]
- Boorer KJ, Loo DD, Frommer WB, Wright EM. Transport mechanism of the cloned potato H+/sucrose cotransporter StSUT1. J. Biol. Chem. 1996;271:25139–25144. doi: 10.1074/jbc.271.41.25139. [DOI] [PubMed] [Google Scholar]
- Carpaneto A, Geiger D, Bamberg E, Sauer N, Fromm J, Hedrich R. Phloem-localized, proton-coupled sucrose carrier ZmSUT1 mediates sucrose efflux under the control of the sucrose gradient and the proton motive force. J. Biol. Chem. 2005;280:21437–21443. doi: 10.1074/jbc.M501785200. [DOI] [PubMed] [Google Scholar]
- Chandran D, Reinders A, Ward JM. Substrate specificity of the Arabidopsis thaliana sucrose transporter AtSUC2. J. Biol. Chem. 2003;278:44320–44325. doi: 10.1074/jbc.M308490200. [DOI] [PubMed] [Google Scholar]
- Chincinska IA, Liesche J, Krugel U, Michalska J, Geigenberger P, Grimm B, et al. Sucrose transporter StSUT4 from potato affects flowering, tuberization, and shade avoidance response. Plant Physiol. 2008;146:515–528. doi: 10.1104/pp.107.112334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endler A, Meyer S, Schelbert S, Schneider T, Weschke W, Peters SW, et al. Identification of a vacuolar sucrose transporter in barley and Arabidopsis mesophyll cells by a tonoplast proteomic approach. Plant Physiol. 2006;141:196–207. doi: 10.1104/pp.106.079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. Possible role of pH gradient and membrane ATPase in the loading of sucrose into sieve tubes. Nature. 1977;267:369–370. [Google Scholar]
- Giaquinta RT, Lin W, Sadler NL, Franceschi VR. Pathway of phloem unloading of sucrose in corn roots. Plant Physiol. 1983;72:362–367. doi: 10.1104/pp.72.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwald JR, Krysan PJ, Young JC, Evert RF, Sussman MR. Genetic evidence for the in planta role of phloem-specific plasma membrane sucrose transporters. Proc. Natl Acad. Sci. USA. 2000;97:13979–13984. doi: 10.1073/pnas.250473797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Chino M. Chemical composition of phloem sap from the uppermost internode of the rice plant. Plant Cell Physiol. 1990;31:247–251. [Google Scholar]
- Hirose T, Imaizumi N, Scofield GN, Furbank RT, Ohsugi R. cDNA cloning and tissue specific expression of a gene for sucrose transporter from rice (Oryza sativa L.) Plant Cell Physiol. 1997;38:1389–1396. doi: 10.1093/oxfordjournals.pcp.a029134. [DOI] [PubMed] [Google Scholar]
- Ishimaru K, Hirose T, Aoki N, Takahashi S, Ono K, Yamamoto S, et al. Antisense expression of a rice sucrose transporter OsSUT1 in rice (Oryza sativa L.) Plant Cell Physiol. 2001;42:1181–1185. doi: 10.1093/pcp/pce148. [DOI] [PubMed] [Google Scholar]
- Krügel U, Veenhoff LM, Langbein J, Wiederhold E, Liesche J, Friedrich T, et al. Transport and sorting of the Solanum tuberosum sucrose transporter SUT1 is affected by posttranslational modification. Plant Cell. 2008;20:2497–2513. doi: 10.1105/tpc.108.058271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukura C, Saitoh T, Hirose T, Ohsugi R, Perata P, Yamaguchi J. Sugar uptake and transport in rice embryo. Expression of companion cell-specific sucrose transporter (OsSUT1) induced by sugar and light. Plant Physiol. 2000;124:85–93. doi: 10.1104/pp.124.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadwodnik J, Lohaus G. Subcellular concentrations of sugar alcohols and sugars in relation to phloem translocation in Plantago major, Plantago maritima, Prunus persica, and Apium graveolens. Planta. 2008;227:1079–1089. doi: 10.1007/s00425-007-0682-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw WH, Jr, De Vlieghere-He X. Transpiration rate. An important factor controlling the sucrose content of the guard cell apoplast of broad bean. Plant Physiol. 2001;126:1716–1724. doi: 10.1104/pp.126.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinders A, Schulze W, Kuhn C, Barker L, Schulz A, Ward JM, et al. Protein–protein interactions between sucrose transporters of different affinities colocalized in the same enucleate sieve element. Plant Cell. 2002a;14:1567–1577. doi: 10.1105/tpc.002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinders A, Schulze W, Thaminy S, Stagljar I, Frommer WB, Ward JM. Intra- and intermolecular interactions in sucrose transporters at the plasma membrane detected by the split-ubiquitin system and functional assays. Structure. 2002b;10:763–772. doi: 10.1016/s0969-2126(02)00773-6. [DOI] [PubMed] [Google Scholar]
- Reinders A, Sivitz AB, Hsi A, Grof CP, Perroux JM, Ward JM. Sugarcane ShSUT1: analysis of sucrose transport activity and inhibition by sucralose. Plant Cell Environ. 2006;29:1871–1880. doi: 10.1111/j.1365-3040.2006.01563.x. [DOI] [PubMed] [Google Scholar]
- Reinders A, Sivitz AB, Starker CG, Gantt JS, Ward JM. Functional analysis of LjSUT4, a vacuolar sucrose transporter from Lotus japonicus. Plant Mol. Biol. 2008;68:289–299. doi: 10.1007/s11103-008-9370-0. [DOI] [PubMed] [Google Scholar]
- Reinders A, Ward JM. Functional characterization of the alpha-glucoside transporter Sut1p from Schizosaccharomyces pombe, the first fungal homologue of plant sucrose transporters. Mol. Microbiol. 2001;39:445–454. doi: 10.1046/j.1365-2958.2001.02237.x. [DOI] [PubMed] [Google Scholar]
- Riesmeier JW, Willmitzer L, Frommer WB. Isolation and characterization of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO J. 1992;11:4705–4713. doi: 10.1002/j.1460-2075.1992.tb05575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesmeier JW, Willmitzer L, Frommer WB. Evidence for an essential role of the sucrose transporter in phloem loading and assimilate partitioning. EMBO J. 1994;13:1–7. doi: 10.1002/j.1460-2075.1994.tb06229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze WX, Reinders A, Ward J, Lalonde S, Frommer WB. Interactions between co-expressed Arabidopsis sucrose transporters in the split-ubiquitin system. BMC Biochem. 2003;4:3. doi: 10.1186/1471-2091-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield GN, Hirose T, Aoki N, Furbank RT. Involvement of the sucrose transporter, OsSUT1, in the long-distance pathway for assimilate transport in rice. J. Exp. Bot. 2007;58:3155–3169. doi: 10.1093/jxb/erm153. [DOI] [PubMed] [Google Scholar]
- Sivitz AB, Reinders A, Johnson ME, Krentz AD, Grof CP, Perroux JM, et al. Arabidopsis sucrose transporter AtSUC9. High-affinity transport activity, intragenic control of expression, and early flowering mutant phenotype. Plant Physiol. 2007;143:188–198. doi: 10.1104/pp.106.089003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivitz AB, Reinders A, Ward JM. Analysis of the transport activity of barley sucrose transporter HvSUT1. Plant Cell Physiol. 2005;46:1666–1673. doi: 10.1093/pcp/pci182. [DOI] [PubMed] [Google Scholar]
- Sivitz AB, Reinders A, Ward JM. Arabidopsis sucrose transporter AtSUC1 is important for pollen germination and sucrose-induced anthocyanin accumulation. Plant Physiol. 2008;147:92–100. doi: 10.1104/pp.108.118992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slewinski TL, Meeley R, Braun DM. Sucrose transporter1 functions in phloem loading in maize leaves. J. Exp. Bot. 2009;60:881–892. doi: 10.1093/jxb/ern335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise A, Barker L, Kuhn C, Lalonde S, Buschmann H, Frommer WB, et al. A new subfamily of sucrose transporters, SUT4, with low affinity/high capacity localized in enucleate sieve elements of plants. Plant Cell. 2000;12:1345–1355. doi: 10.1105/tpc.12.8.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormuth D, Heiber I, Shaikali J, Kandlbinder A, Baier M, Dietz KJ. Redox regulation and antioxidative defence in Arabidopsis leaves viewed from a systems biology perspective. J. Biotechnol. 2007;129:229–248. doi: 10.1016/j.jbiotec.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Qu H, Dibley KE, Offler CE, Patrick JW. A suite of sucrose transporters expressed in coats of developing legume seeds includes novel pH-independent facilitators. Plant J. 2007;49:750–764. doi: 10.1111/j.1365-313X.2006.03000.x. [DOI] [PubMed] [Google Scholar]