Abstract
Three dogs reared on a dairy farm with a high incidence for Brucella abortus were serologically positive for B. abortus and no other Brucella spp. The identity of the organism was confirmed to be B. abortus by AMOS (abortus melitensis ovis suis)-polymerase chain reaction with specific primers for B. canis. One hundred percent homology of the canine isolate and the bovine pathogen isolated from the farm was demonstrated. The only possible source of infection was infected cattle on the same farm. It is suggested that dogs be routinely included in brucellosis surveillance and eradication programs.
Canine brucellosis is an infectious disease caused by Brucella canis, a small Gram negative aerobic coccobacillus (1). Brucella canis was first identified in 1966 as a causative agent of canine abortion (2). Despite B. canis being the major organism, infection with other Brucella species (B. abortus and B. suis) has been extensively investigated (3). Naturally acquired B. abortus infection in dogs associated with infected cattle has been reported and horizontal, dog-to-dog, cattle-to-dog, dog-to-cattle, and dog-to-human transfer of the disease has been demonstrated (4).
In the Korean peninsula, dairy cattle brucellosis has been shown to be caused by B. abortus biotype 1 (5). This communication demonstrates, for the first time, the transmission of brucellosis from cattle to farm dogs in South Korea.
During the course of this study, 1-year-old mixed breed, female dogs were housed at the Department of Public Health, Chonbuk National University, Chonju City, Korea, in the Chonbuk Province. The dogs were reared in very close proximity with 131 dairy cattle 1 y prior to the study, such that they had access to aborted fetuses and placentae. In that farm, the 1st outbreak of dairy brucellosis peaked from March 6, 2002 to April 14, 2002, as demonstrated by the tube agglutination test (TAT) and Rose Bengal test (RBT). A total of 84 heads of dairy cattle were slaughtered under the government brucellosis surveillance program during 2001. However, canine brucellosis was not part of the surveillance program. Sampling of the dogs started 5 wk after slaughter of positive cattle. The dogs were investigated bacteriologically; serologically; and by B. abortus, melitensis, ovis, suis AMOS (abortus melitensis ovis suis)-polymerase chain reaction (PCR), and also tested for B. canis. One dog raised in a brucellosis-free farm served as a negative control animal.
One milliliter of blood was collected from each dog and cultured according to the methods and criteria described by Alton et al (6). Serum samples from the dogs were screened by the RBT and plate agglutination test (PAT) using B. abortus strain 1119-3 whole cell antigen (6,7). Genomic DNA for AMOS PCR was extracted from each cultured blood sample using a genomic DNA extraction kit (Accuprep; Bioneer Company, Chonbuk, Chonju, South Korea). The procedure used by Ewalt and Bricker (8) was followed for the rest of the protocol. Following 4 d of incubation, smooth, pinpoint, glistening, bluish, translucent colonies were observed on the cultured plates derived from all the dogs.
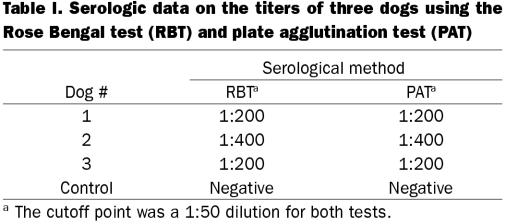
Sera from all 3 1-year-old dogs were found positive by RBT and PAT, as shown in Table I. One dog had a reciprocal antibody titer of ≤ 1:400, both in RBT and PAT, and 2 other dogs had a reciprocal antibody titer of ≤ 1:200. Serum from the control dog was found negative by both RBT and PAT.
Table I.
The predicted 498 base pairs (bp) DNA band was demonstrated from DNA extracted from all 3 dogs (Figure 1, lanes 4–6). The control dog was consistently negative (lane 3). There was no evidence of B. canis.
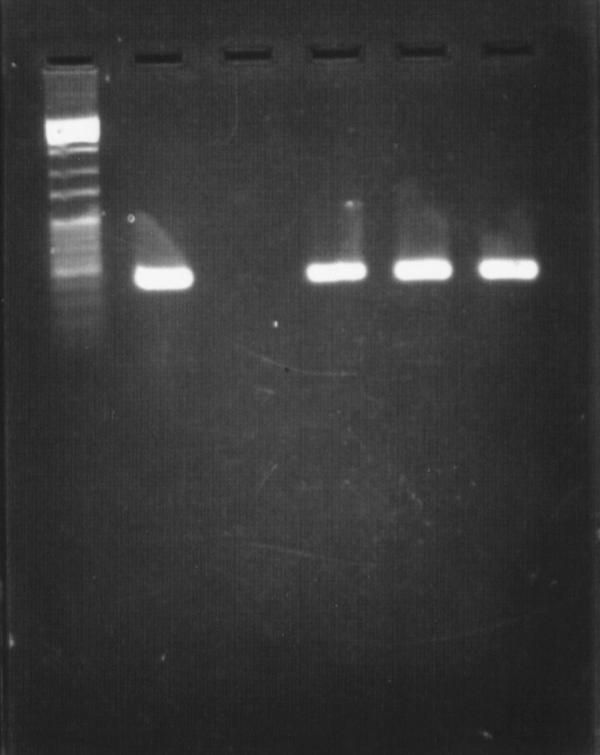
Figure 1. Amplification of DNA in the AMOS (abortus melitensis ovis suis)-polymerase chain reaction (PCR) assay. Lane 1, 1000 base pairs (bp) DNA marker; lane 2, DNA extracted from standard B. abortus colony; lane 3, DNA extracted from blood; lane 4, DNA extracted from one of the cultured blood samples (dog #2). A 498 bp amplification was consistently shown as predicte d, lanes 5 and 6.
Canine brucellosis is a contagious bacterial disease that is characterized by abortions in females and epididymitis in males. The disease is insidious and many dogs are asymptomatic (9,10). In this study the only clinical sign noted in the dogs was mild fever of 38.5°C. Infected dogs shed the organisms into the environment via urine, vaginal secretions, ejaculates, aborted fetuses, or feces (11,12). The disease is important from the public health standpoint since human infections have occurred as a result of laboratory accidents or close contact when feeding or handling Brucella-infected dogs (13).
Canine brucellosis is currently diagnosed by serology and blood culture (2). The main serological tests used for the diagnosis of Brucella infection are RBT as a screening test and complement fixation test (CFT) as a confirmatory test. The RBT is more sensitive than the CFT when testing culture-positive animals (14). In many countries, the PAT, which may give false-negative results, is the routine test and is sometimes the only serological test used (15). The PAT was originally developed to provide a rapid test that would complement the results of the TAT (11). Our report was based on data derived from bacteriological culture, RBT, and PAT assays. Further confirmation was by positive AMOS PCR using rigorously tested B. abortus-specific primers. A predicted 498 bp amplicon was consistently demonstrated, in agreement with previously published data (16).
Brucella abortus infection in dogs has been reported under experimental and field conditions (17). Seroconversion, based on immediate results, can occur as early as 4 to 14 d after exposure but is not necessarily coincident with positive culture. Seronegative culture-positive dogs have also been described (2), similar to the situation in cattle (18). Seropositivity may persist for up to 3 y (11), but the maximum duration has not been demonstrated. This is important, because infected dogs can shed organisms into the environment via urine, vaginal secretions, aborted fetuses, or feces.
Brucella abortus positive vaginal discharges have been reported to persist for up to 42 d after abortion or parturition, but the duration of shedding and the number of organisms in the discharge are not known. If the situation in dogs is similar to that in cattle, 108 to 1013 organisms/g may be present in parturient canine material (19). The infective dose for dogs is approximately 106 to 1010 organisms/g (17). It is reasonable to speculate that aborted material and infected vaginal discharges of cattle could be a factor in the spread of Brucella from cattle to dog and vice-versa. The zoonotic aspects of B. abortus infection from dogs must, therefore, be considered when investigating reactor cattle herds and human brucellosis. Elimination of reactor cattle only may not necessarily eradicate the disease.
Brucellosis continues to be a major problem in Korea despite the existence of a ‘test and slaughter’ strategy program for eradication. There is close contact between dogs and people, and dogs are obviously at high risk in brucellosis-infected farms. Accordingly, it may be prudent to keep dogs away from farms known to be infected with B. abortus or to include them in all eradication programs. In addition, we have demonstrated that dogs may be a valuable indicator (sentinel) of brucellosis in cattle, at least under South Korean conditions.
Footnotes
Acknowledgments
This research project was supported by Chonbuk National University, Post-Doc Program (2002).
Address all correspondence and reprint requests to Dr. Ibulaimu Kakoma; telephone: (217) 333-1859; fax: (217) 333-0346; e-mail: kakomai@uiuc.edu
Received January 29, 2003. Accepted April 28, 2003.
References
- 1.Kerwin SC, Lewis DD, Hribernik TN, et al. Diskospondylitis associated with Brucella canis infection in dogs: 14 cases (1980–1991). J Am Vet Med Assoc 1992;201:1253–1257. [PubMed]
- 2.Rifkin GD, Supena RB, Axelson JA. Brucella canis bacteremia: a case with negative B. canis agglutinins. Am J Med Sci 1978;276:113–115. [PubMed]
- 3.Barr SC, Elite BE, Roy AF, et al. Brucella suis biotype 1 infection in a dog. J Am Vet Med Assoc 1986;189:689–687. [PubMed]
- 4.Forbes LB. Brucella abortus infection in 14 farm dogs. J Am Vet Med Assoc 1990;196:911–916. [PubMed]
- 5.Davis DS, Heck FC, Williams JD, et al. Interspecific transmission of Brucella abortus from experimentally infected coyotes (Canis latrans) to parturient cattle. J Wildl Dis 1988;24:533–537. [DOI] [PubMed]
- 6.Chung JS, Cho YJ, Park CK. Isolation and biotyping of Brucella abortus from dairy cattle in Kyungbuk area, Korea. Kor J Vet Res 1988;28:339–343.
- 7.Alton GG, Jones LM, Pietz DF. Laboratory techniques in brucellosis. 2nd ed, World Health Organization, Geneva, Switzerland, 1975. [PubMed]
- 8.Baek BK, Lim KH, Hur J, Choi MJ. Immunological responses in pigs immunized with inactivated Brucella suis. Kor J Vet Publ Hlth 2000;24:133–142.
- 9.Ewalt DR, Bricker BJ. Validation of the abbreviated Brucella AMOS PCR as a rapid screening method for differentiation of Brucella abortus field strain isolates and the vaccine strains, 19 and RB51. J Clin Microbiol 2000;38:3085–3086. [DOI] [PMC free article] [PubMed]
- 10.Smeak DD, Olmstead ML, Hohn RB. Brucella canis osteomyelitis in two dogs with total hip replacements J Am Vet Med Assoc 1987;191:986–989. [PubMed]
- 11.Zoha SJ, Carmichael LE. Serological responses of dogs to cell wall and internal antigens of Brucella canis. Vet Microbiol 1982;7:35–50. [DOI] [PubMed]
- 12.George WB. Handbook of zoonoses, second edition, Section A: Bacterial, Rickettsial, Chlamydial and Mycotic. Boca Raton, Florida: CRC Press, 1994.
- 13.Serikawa T, Muraguchi T, Nakao N. A survey of dogs from Gifu and Shiga area for Brucella canis. J Vet Sci 1977;39:635–642. [DOI] [PubMed]
- 14.Schoenemann J, Lutticken R, Scheibner E. Brucella canis infection in man. Dtsch Med Wochenschr 1986;3:20–22. [DOI] [PubMed]
- 15.Blasco JM, Garin-Bastuji B, Marin CM, et al. Efficacy of different Rose Bengal and complement fixation antigens for the diagnosis of Brucella melitensis infection in sheep and goats. Vet Rec 1994;134:415–420. [DOI] [PubMed]
- 16.Lucero NE, Bolpe JE. Buffered plate antigen test as a screening test for diagnosis of human brucellosis. J Clin Microbiol 1998;36:1425–1427. [DOI] [PMC free article] [PubMed]
- 17.Bricker BJ, Halling SM. Differentiation of Brucella abortus bv. 1, 2, and 4, Brucella melitensis, Brucella ovis, and Brucella suis bv. 1 by PCR. J Clin Microbiol 1994;32:2660–2666. [DOI] [PMC free article] [PubMed]
- 18.Pidgeon GL, Scanlan CM, Miller WR, et al. Experimental infection of dogs with Brucella abortus. Cornell Vet 1987;77:339–347. [PubMed]
- 19.Stemshorn BW, Forbes LB, Eagesome MD. A comparison of standard serological tests for the diagnosis of bovine brucellosis in Canada. Can J Comp Med 1985;49:391–394. [PMC free article] [PubMed]