Abstract
Purpose:
A previous study showed decreased uropathogen adherence using a novel anti-fouling coating consisting of mussel adhesive protein mimics conjugated to poly(ethylene glycol). We assessed the ability of methoxy polyethylene glycol-dihydroxyphenylalanine (Nerites Corp. Ltd., Madison, Wisconsin) coated ureteral stents to resist bacterial adherence, infection development and encrustation in a rabbit model of uropathogenic Escherichia coli cystitis.
Materials and Methods:
Sof-Flex® stent curls that were uncoated and coated with 3 coatings, including Surphys™ 002, 008 and 009, respectively, and uncoated Percuflex Plus® stents were inserted transurethrally into the bladder of 50 male New Zealand White rabbits (Charles River Laboratories, Montreal, Quebec, Canada), followed by instillation of uropathogenic E. coli strain GR12 (107 cfu). Urine was examined for bacteria on days 0, 1, 3 and 7, and for cytokine levels on day 7. On day 7 the animals were sacrificed. Stent curls and bladders were harvested for analysis. In a parallel experiment stents were challenged in vitro for 7 days with GR12 in human urine.
Results:
Surphys 009 coated devices showed decreased urine and stent bacterial counts compared to those in controls. Eight of 10 rabbits in the Surphys 009 group had sterile urine by day 3 vs 1 in each control group (p = 0.013), while stent adherent organisms were decreased by more than 75%. While no statistical differences were found in encrustation and bladder inflammation across the groups, immune scoring was lowest in the uncoated Sof-Flex control and Surphys 009 groups (p = 0.030).
Conclusions:
Surphys 009 strongly resisted bacterial attachment, resulting in improved infection clearance over that of uncoated devices. However, this did not translate to decreased encrustation, which appeared to be independent of infection in this model.
Keywords: ureter, stents, rabbits, Escherichia coli, dihydroxyphenylalanine
Bacterial attachment and biofilm formation are serious problems associated with urinary stents and catheters since they lead to chronic infections that cannot be resolved without device removal.1,2 Despite numerous strategies to prevent these events, including anti-fouling and antimicrobial coatings, dietary and urinary modification, and oral antibiotics, nothing has yet proved efficacious.3-6 The urinary tract has multiple strategies for dealing with invading microorganisms but the presence of a foreign device provides a novel nonhost surface to which they can attach and form a biofilm. This is supported by studies highlighting the ability of normally nonuropathogenic microorganisms to readily cause device associated UTIs.7-9
Recently we performed an in vitro study using surfaces coated with PEG conjugated to a synthetic mimic of MAPs.10 MAP mimics consisted of tripeptides of DOPA, the key adhesive component in MAPs. These peptides served as anchors and were coupled to the terminus of a 5,000 Da mPEG-DOPA3. Coated surfaces inhibited attachment by greater than 94% for 7 uropathogenic bacterial strains. This led to the current study of the ability of DOPA anchored coatings to resist bacterial attachment, infection and encrustation in a rabbit model of cystitis. Escherichia coli infections comprise more than half of all urinary tract device associated infections, making it the most prevalent pathogen in such episodes.4,11
MATERIALS AND METHODS
The study was approved by the University of Western Ontario animal use subcommittee. We used 6Fr SF stents, 5Fr Kumpe angled catheters, Bentson guidewires and 6Fr PP stents. Stent curls were coated at Nerites Corp. (table 1). Curls were treated for 24 hours, rinsed in distilled water and packaged aseptically. Male New Zealand White Rabbits were used.
Table 1.
Coating solutions and conditions used to treat SF stent curls
| S002 | S008 | S009 | |
|---|---|---|---|
| Buffer: | |||
| K2SO4 (M) | 0.6 | 0.3 | 0.6 |
| 3-N-(morpholino) propane sulfonic acid | 0.1 | 0.05 | 0.1 |
| pH | 9.0 | 6.0 | 6.0 |
| Temperature (C) | 50 | 25 | 45 |
Transurethral Stent Curl and UPEC Instillation
A total of 50 rabbits were randomized to 1 of 5 stent curl groups, including UnSF control 1, uncoated PP control 2, and S002, S008 and S009 coated SF groups (fig. 1). Rabbits were maintained on 12-hour light/dark cycling with free access to food and water. Each animal was anesthetized using acepromazine (0.5 mg/kg) and inhalation isoflurane, and surgically prepared. A 5Fr angled catheter was guided into the bladder transurethrally via ultrasound and a flexible tip Bentson guidewire was tunneled through. The catheter was withdrawn and the angled portion was removed. A stent curl was placed over the guidewire and inserted using the cut catheter as a pusher. The guidewire was removed, the bladder was drained, 1 × 107 cfu E. coli GR12 in 1.5 ml saline were injected and 0.5 ml sterile saline were injected to clear bacteria from the catheter. The animal was recovered and given the anti-inflammatory meloxicam (0.2 mg/kg).
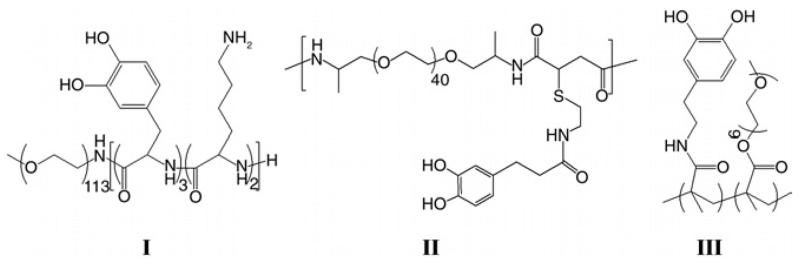
Figure 1.
Polymer used to coat SF ureteral stents was S002 (I), S008 (II) and S009 (III).
Sample Collection and Analysis
Urine was collected on days 1 and 3 via suprapubic aspiration. On day 7 the animals were sacrificed using intramuscular ketamine (5 mg/kg) and xylazine (35 mg/kg), followed by intracardiac pentobarbital (150 mg/kg). They were opened via a midline incision. The bladders were opened, urine was collected and stent curls were removed. Bladders were fixed in 10% formalin for pathological evaluation. E. coli were quantified via plating on brain heart infusion agar. Cytokines were determined on day 7 using Luminex® bead based technology.12
To quantify stent adherent bacteria the sections were sonicated for 10 minutes in saline and dilution plated. To determine encrustation the segments were dried and the material was removed and weighed. For microscopic analysis representative segments were gold coated and analyzed using an S-4500 field emission scanning electron microscope (Hitachi, Tokyo, Japan). EDX was done using the attached Quartz XOne EDX system (Quartz Imaging Corp., Vancouver, British Columbia, Canada).
As described by Cadieux et al,13 bladders were assessed for inflammation by a blinded pathologist by scoring from 0 to 4 based on the level of polymorphonuclear cells per high power field and the degree of necrosis. For all samples the score was 0—fewer than 15 polymorphonuclear cells, 1—15 to 50, 2—50 to 300, 3—greater than 300 and 4—necrosis present.
In Vitro Challenge
One cm stent segments were each incubated in 2 ml human pooled urine10 (pH 6.5) containing 106 cfu/ml E. coli GR12 for 12 hours at 37C and 70 rpm. Every 12 hours for the next 7 days the devices were removed, rinsed and placed in 2 ml fresh sterile human pooled urine. On day 7 the devices were rinsed in sterile phosphate buffered saline, sonicated and dilution plated, as described.
Statistical Analysis
Results were analyzed using Prism® 4. We analyzed bacterial counts, encrustation, protein, cytokines and immune scores using ANOVA and Dunnett's multiple comparison test. For comparisons of the number of infected animals and samples approaching bacterial thresholds we used the chi-square and Fisher exact tests with significance considered at p <0.05.
RESULTS
Tables 2 and 3 list data on culture, pathological findings, encrustation, urinary protein and cytokine expression in all 50 animals.
Table 2.
Urinary and stent adherent UPEC GR12 count and encrustation
| Urine (cfu/ml) |
|||||
|---|---|---|---|---|---|
| Stent Group (animal No.) | Day 1 | Day 3 | Day 7 | Stent (cfu/cm2) | Encrustation (mg/cm2) |
| UnSF: | |||||
| 1 | 2.4 × 105 | 9.0 × 107 | 1.2 × 108 | 1.2 × 108 | 4.2 |
| 2 | 1.9 × 105 | 1.4 × 103 | 1.7 × 106 | 8.2 × 106 | 7.7 |
| 3 | 7.3 × 106 | 2.4 × 108 | 1.0 × 108 | 2.8 × 107 | 6.4 |
| 4 | 1.2 × 105 | 2.9 × 105 | 2.4 × 105 | 3.0 × 106 | 3.1 |
| 5 | 4.4 × 103 | 3.3 × 104 | 0 | 2.5 × 104 | 2.2 |
| 6 | Not available | 1.8 × 107 | 6.9 × 107 | 15.1 | |
| 7 | Not available | 1.3 × 106 | 3.2 × 103 | 1.9 | |
| 8 | Not available | 1.3 × 104 | 0 | 7.9 × 104 | 12.4 |
| 9 | 0 | 0 | 0 | 0 | 45.0 |
| 10 | 1.0 × 104 | 2.2 × 105 | 7.7 × 105 | 1.8 × 107 | 38.6 |
| Mean | 1.1 × 106 | 4.1 × 107 | 2.4 × 107 | 2.4 × 107 | 13.7 |
| S002: | |||||
| 11 | 5.3 × 106 | 3.1 × 106 | 8.0 × 107 | 8.1 × 107 | 31.5 |
| 12 | 7.0 × 103 | 0 | 7.0 × 103 | 4.4 × 103 | 22.0 |
| 13 | Not available | 0 | 0 | 6.8 × 102 | 4.9 |
| 14 | 1.8 × 108 | 1.7 × 107 | 1.3 × 107 | 6.4 × 107 | 51.7 |
| 15 | 1.3 × 103 | 1.2 × 107 | 1.7 × 107 | 1.9 × 108 | 54.3 |
| 16 | 0 | 0 | 0 | 3.8 × 102 | 26.6 |
| 17 | 4.3 × 105 | 1/2 × 107 | 4.7 × 107 | 4/4 × 107 | 21.9 |
| 18 | 0 | 0 | 1.3 × 103 | 0 | 45.3 |
| 19 | 3.3 × 103 | 5.7 × 105 | 1.0 × 107 | 6.0 × 106 | 29.1 |
| 20 | 1.3 × 103 | 1.3 × 107 | 1.4 × 108 | 5.1 × 106 | 8.0 |
| Mean | 1.8 × 107 | 5.7 × 106 | 3.1 × 107 | 3.9 × 107 | 29.5 |
| S008: | |||||
| 21 | 8.5 × 104 | Not available | 0 | 5.7 × 102 | 11.0 |
| 22 | 2.0 × 103 | 0 | 0 | 7.8 × 103 | 44.4 |
| 23 | 0 | 1.4 × 105 | 1.2 × 108 | 7.0 × 107 | 1.5 |
| 24 | 0 | 9.0 × 107 | 1.7 × 108 | 6.2 × 107 | 0.1 |
| 25 | Not available | 1.0 × 106 | 1.1 × 107 | 9.5 × 105 | 23.4 |
| 26 | 0 | 0 | 0 | 2.6 × 102 | 20.0 |
| 27 | 4.3 × 103 | 2.6 × 104 | 1.6 × 105 | 5.5 × 105 | 44.8 |
| 28 | 0 | 21. × 105 | 2.9 × 106 | 5.4 × 106 | 63.1 |
| 29 | Not available | 5.3 × 107 | 1.4 × 108 | 3.0 × 107 | 4.5 |
| 30 | 0 | 0 | 1.7 × 103 | 2.6 × 103 | 17.1 |
| Mean | 1.1 × 104 | 1.6 × 107 | 4.4 × 107 | 1.8 × 107 | 23.0 |
| S009: | |||||
| 31 | 1.8 × 103 | 0 | 0 | 6.7 × 106 | 5.2 |
| 32 | 0 | 0 | 0 | 7.2 × 104 | 41.4 |
| 33 | 1.3 × 105 | 2.1 × 108 | 1.6 × 108 | 4.8 × 107 | 1.2 |
| 34 | 0 | 0 | 0 | 9.5 × 104 | 6.0 |
| 35 | Not available | 0 | 0 | 7.6 × 104 | 30.6 |
| 36 | 0 | 0 | 0 | 1.5 × 102 | 41.5 |
| 37 | 1.8 × 107 | 1.0 × 108 | 3.0 × 107 | 5.4 × 106 | 17.8 |
| 38 | 1.4 × 106 | 0 | 0 | 1.1 × 103 | 25.2 |
| 39 | 0 | 0 | 0 | 0 | 99.4 |
| 40 | 0 | 0 | 0 | 8.4 × 102 | 60.5 |
| Mean | 2.1 × 106 | 3.1 × 107 | 1.9 × 107 | 6.1 × 106 | 32.9 |
| PP: | |||||
| 41 | Not available | 2.1 × 104 | 2.7 × 104 | 11.0 | |
| 42 | 1.2 × 105 | 6.0 × 103 | 1.5 × 103 | 2.1 × 103 | 68.8 |
| 43 | 1.4 × 106 | 6.3 × 107 | 2.1 × 107 | 3.4 × 107 | 9.9 |
| 44 | 0 | 0 | 0 | 7.7 × 101 | 19.9 |
| 45 | 7.7 × 106 | 1.7 × 107 | 2.1 × 107 | 6.7 × 106 | 31.4 |
| 46 | 8.7 × 105 | 21. × 106 | 2.9 × 108 | 9.0 × 106 | 19.0 |
| 47 | 7.0 × 105 | 8.0 × 105 | 1.6 × 108 | 5.1 × 106 | 38.7 |
| 48 | 0 | 1.2 × 107 | 6.0 × 107 | 1.0 × 107 | 52.8 |
| 49 | 1.1 × 106 | 1.8 × 106 | 1.4 × 107 | 2.1 × 107 | 71.1 |
| 50 | 8.5 × 105 | 8.3 × 105 | 21. × 107 | 3.4 × 106 | 52.8 |
| Mean | 1.4 × 106 | 1.1 × 107 | 5.8 × 107 | 8.9 × 106 | 37.5 |
Table 3.
Infected animals on days 1, 3 and 7
| No. Pos/Total No. | No. Greater Than 105 cfu/ml | |
|---|---|---|
| UnSF (day): | ||
| 1 | 6/7 | 4 |
| 3 | 7/8 | 4 |
| 7 | 7/10 | 7 |
| S002 (day): | ||
| 1 | 7/9 | 3 |
| 3 | 6/10 | 6 |
| 7 | 8/10 | 6 |
| S008 (day): | ||
| 1 | 3/8 | 0* |
| 3 | 6/9 | 5 |
| 7 | 7/10 | 6 |
| S009 (day): | ||
| 1 | 4/9 | 3 |
| 3* | 2/10 | 2 |
| 7* | 2/10 | 2 |
| PP (day): | ||
| 1 | 7/9 | 7 |
| 3 | 8/9 | 7 |
| 7 | 9/10 | 7 |
p <0.05.
Urine and Stent Culture
At stent curl placement most animals had a low urine volume. Overall urine was collected on day 0 from 18 animals and results were culture negative. Urine was successfully acquired from 42, 46 and 50 of 50 animals on days 1, 3 and 7, respectively. Overall at least 70% of all UnSF and PP control urine samples were positive with more than half containing clinically relevant numbers (greater than 105 cfu/ml). Only 1 animal per control group had sterile urine throughout the study. Seven and 9 of 10 animals had infected urine on day 7 in the UnSF and PP groups, respectively. In contrast, 52.9% of all coated samples were positive (p = 0.0002). Overall 7 animals had sterile urine throughout the study.
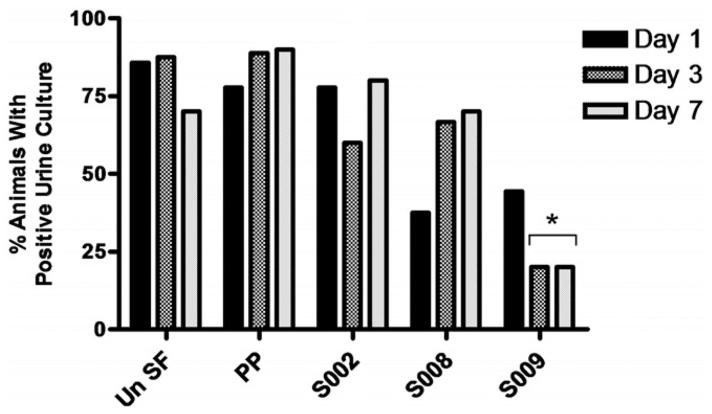
On day 1 S008 was the only group with any significant culture difference from controls with clinically relevant bacterial levels in 0 of 8 rabbits compared to 4 of 7 UnSF and 7 of 9 PP controls (p = 0.026 and 0.002, respectively). However, as the study progressed, this changed and S009 became the only group with significant decreases. On days 3 and 7 only 2 of 10 animals in the S009 group had infected urine (same animals on each day) compared to 14 of 19 controls on day 3 and 15 of 20 on day 7 (p = 0.008 and 0.003, respectively). These differences were significant not only for all control animals combined, but also for the UnSF and PP groups separately (fig. 2). A significant decrease was also observed in terms of clinically relevant cultures with 11 of 17 and 14 of 20 controls positive on days 3 and 7 compared to 2 of 10 in the S009 group on each day (p = 0.03 and 0.013, respectively). Thus, in 8 of 10 animals in the S009 group the infection had cleared from urine by day 3 compared to only 1 animal in each control group.
Figure 2.
Percent of positive urine cultures per stent group on days 1, 3 and 7. Un SF, UnSf. Asterisk indicates p <0.05.
For stent curl analysis organisms were cultured from 47 of 50 devices overall with 1 sterile device in 1 animal in each of the S002, S009 and UnSF groups. Following a similar urinary trend, S009 devices showed the fewest attached bacteria with 75.2% and 32.0% decreases compared to the UnSF and PP groups (6.05 × 106 vs 2.44 × 107 and 8.89 × 106 cfu/cm2, respectively). However, these decreases did not achieve statistical significance when all groups were compared (p = 0.26). Since 105 cfu/ml is commonly used as a clinical urinary marker for significant UTI, we compared devices across groups in similar fashion using a 105 cfu/cm2 threshold value. Only 3 of 10 S009 group devices showed that degree of bacterial attachment but at least 6 of 10 devices did so in all other groups.
Encrustation
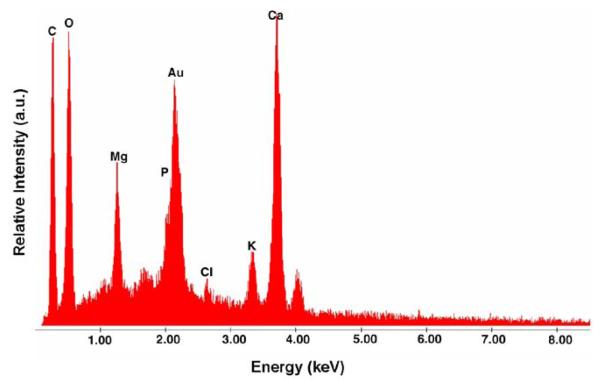
Even in the absence of infection rabbit urine contains many precipitates, chiefly phosphates and carbonates,14,15 which we confirmed using EDX (figs. 3 and 4, A). Encrustation levels varied widely across animals even in the same stent group (range 0.13 to 99.35 mg) (fig. 4, B and C). Overall the average amount of stent curl encrustation was greater in all groups compared to that in UnSF controls with PP control devices the most heavily encrusted at almost 3-fold higher. However, differences across all groups were not statistically significant (p = 0.15). To further determine whether urinary bacteria levels influenced encrustation development independent of stent group the encrustation results of all 50 animals were separated into 4 groups based on the day 7 urinary cfu/ml as well as a fifth group consisting of devices removed from animals with sterile urine at each study time point. The 4 groups based on the day 7 urinary count consisted of those containing 0 (but not sterile throughout the study), less than 105, 105 to 108 and greater than 108 cfu/ml. Mean encrustation was highest for devices removed from animals with sterile urine cultures throughout the study and lowest for devices removed from those with the highest urinary bacterial counts, that is greater than 108 cfu/ml (39.39 vs 9.29 mg/cm2), representing a 4.24-fold difference (p = 0.028). The remaining groups showed no significant differences.
Figure 3.
Rabbit urine typical elemental composition
Figure 4.
Rabbit urine precipitative nature (A), and mildly (B) and heavily (C) encrusted stents
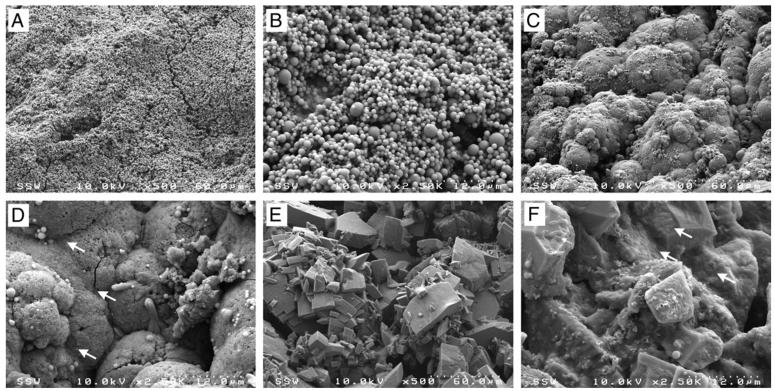
Encrustation structure and quantity varied depending on the degree of infection (fig. 5). Encrustation removed from a sterile urinary environment showed material arranged largely as microspheres, while that removed from infected animals differed substantially and appeared to contain extensive extracellular matrix material throughout the encrustation (fig. 5). Furthermore, bacteria were observed on the surface of infected devices but not on devices with sterile urine on study day 7 (fig. 5, D and F). These findings were consistent across all samples analyzed.
Figure 5.
Scanning electron microscopy reveals various encrustation architectures and organizations depending on infection presence and degree in stents on day 7 in animal 8 with sterile urinary environment (A and B), and in animals 47 (C and D) and 24 (E and F) with infected environment. Arrows indicate bacteria.
Protein and Cytokine Levels, and Bladder Pathology
Protein levels were equal and highest in the UnSF and S002 groups at 15.5 and 15.3 mg/ml, respectively, significantly higher than in the remaining S008, S009 and PP groups, which were about 33% decreased (10.1, 10.4 and 10.4 mg/ml, p = 0.006). For cytokine analysis we initially screened a number of anti-human proinflammatory cytokine antibodies for their ability to cross-react with rabbit homologues. TGF-α and VEGF consistently cross-reacted and were subsequently quantified in all day 7 samples. Control groups had the highest levels of each cytokine, specifically PP (105.6 pg/ml TGF-α and 132.6 pg/ml VEGF) followed by UnSF (59.9 and 118.3 pg/ml, respectively). In comparison to UnSF the S002, S008 and S009 groups showed 13.4%, 28.7% and 19.4% decreases in TGF-α, and 75.8%, 51.2% and 68.0% decreases in VEGF, respectively. These differences did not achieve significance but they showed a consistent trend among Surphys coated devices. When animals were grouped based on encrustation, no differences were found for either cytokine. However, the 2 cytokines showed significant differences when grouped by day 7 E. coli levels or by those attached to stents. TGF-α and VEGF levels were 2.1 and 6.0-fold higher in animals with clinically relevant bacterial counts in day 7 urine (p = 0.002 and 0.003), and 2.0 and 5.2-fold higher in samples from animals with greater than 105 cfu/cm2 stent adherent organisms (p = 0.003 and 0.005, respectively).
Bladders were examined histologically to assess inflammation. Figure 6 shows a representative image of each scoring category. Control group UnSF and group S009 had the least amount of inflammation with an identical mean of 0.65, significantly lower than in the S002, S008 and PP groups (1.9, 1.2 and 1.1, p = 0.018). Values were also grouped based on encrustation and E. coli levels, and a pattern developed that was consistent with cytokine levels. While encrustation alone had no significant effect on the immune score, vs those below the thresholds scores were more than double in animals with greater than 105 cfu/ml E. coli in day 7 urine (1.45 vs 0.66, p = 0.002) or with greater than 105 cfu/cm2 adherent to the stent surface (1.49 vs 0.61, p = 0.0005).
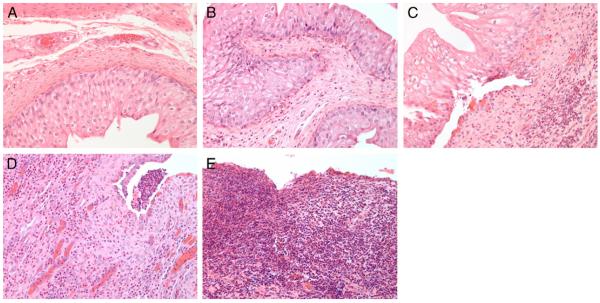
Figure 6.
Representative images show relative degree of inflammation and immune cell infiltration used to categorize bladders by immune score 0 (A), 1 (B), 2 (C), 3 (D) and 4 (E). Reduced from ×200.
In Vitro Challenge
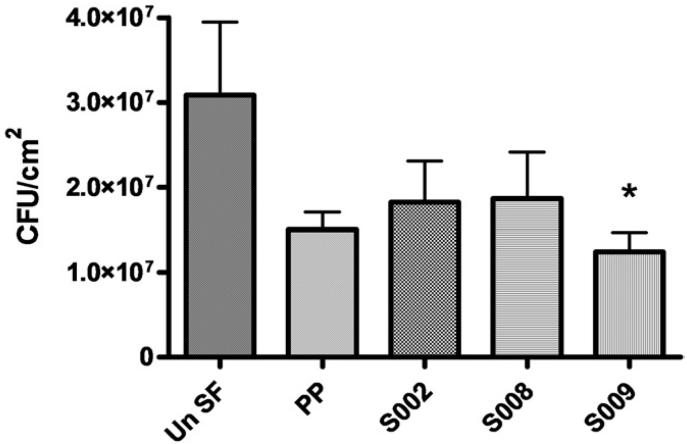
To augment the in vivo data we performed a 7-day in vitro challenge in the same stent segment groups using UPEC GR12 in human urine. Group S009 again showed the greatest resistance to GR12 attachment and it was the only group that showed a statistically significant 59.9% decrease compared to the UnSF group (1.24 × 107 vs 3.09 × 107 cfu/cm2, Dunnett's test p <0.05, fig. 7). Although the remaining groups also showed varying levels of decreased GR12 adherence compared to UnSF, none were significant.
Figure 7.
In vitro attachment of UPEC GR12 to stent segments during 7-day incubation in pooled human urine inoculated with 106 cfu/ml on day 0. Un SF, UnSf. Asterisk indicates p <0.05.
DISCUSSION
While most uncomplicated UTIs can be successfully eradicated using current antimicrobial therapies, those involving urinary stents and catheters are generally cleared only using antimicrobials with device removal.1,2,4 Furthermore, these infections have the potential to develop into chronic conditions, promote antibiotic resistance and spread rapidly in health care centers and nursing homes.16,17 Improvements resulting in significantly fewer infections associated with their use would be a clinical triumph.
A previous in vitro study by our group showed that surfaces coated with mPEG-DOPA3, a novel compound based on PEG and an adhesive peptide inspired by marine mussels, strongly resisted the attachment of numerous uropathogens, including UPEC.10 These findings prompted the current study, in which we investigated the efficacy of this class of coatings to decrease UPEC infection in a 7-day rabbit model of UTI.
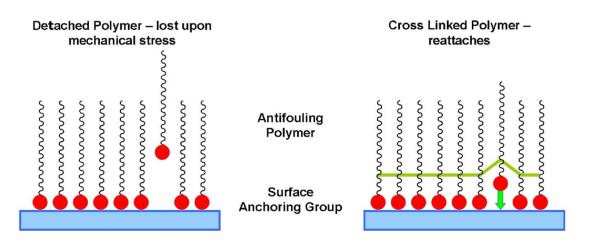
Polymers for coating the stent curls were selected from a library of DOPA anchored antifouling polymers. A goal was to determine the effect of linear (S002) vs cross-linked (S008 and S009) polymer coatings in regard to coating durability and function (fig. 1). Molecular DOPA forms reversible bonds to some biomaterial surfaces and, therefore, separates from a surface when a sufficiently strong force on the order of several hundred pN breaks those bonds.18 After a coating such as S002, which is composed of singly attached polymeric chains of linear PEG, detaches from the surface the exposed uncoated area is an ideal locus for protein or cell adsorption. New cross-linked polymers (S008 and S009) offer greater durability as coatings because, unlike a single polymeric DOPA molecule, they have many more attachment points spread over a larger area (fig. 8). Completely removing a complex cross-linked polymer coating requires much more force than detaching a single chain molecule such as DOPA. As a result, although mechanical stress might detach a single catechol, the polymer film as a whole remains in place, giving the single loose catechol the opportunity to reattach.
Figure 8.
Enhanced antifouling coating stability may be achieved by cross-linked polymer films
The different results in this study among the 3 coating groups may largely be explained by these structural differences. Coating S002 has only a single attachment point per polymer and, thus, it was more susceptible to mechanical perturbation and detachment. This could account for that group having no beneficial results throughout the study, actually finishing with more positive urine cultures, infected animals and higher stent adherent bacteria than uncoated controls. Coating S008 consists of long PEG chains with individual DOPA units attached at various intervals throughout, presenting PEG as a series of loops and trains. Thus, while any detached DOPA molecules would remain connected to the rest of the coating, they could become exposed to the surrounding environment and provide strong attachment points for bacteria and biomolecules. This may explain why S008 appeared to work well initially with clinically relevant urine counts in no animals on day 1, only to be similar to the control groups with respect to all bacterial counts by day 7. In contrast, coating S009 showed significant improvements in several categories over all other groups. This coating was clearly the most resistant to UPEC attachment and the only one that showed significant decreases in infection compared to controls. Notably in 8 animals in that group all E. coli had cleared from urine by day 3 vs only 1 in each control group, 2 in the S002 group and 3 in the S008 group. Also, similar trends of lower urinary and stent cfu were observed for S009. Finally, while only 1 animal in each of the other groups had sterile urine throughout the study, the S009 group had 5. The S009 coating comprises a series of linear PEG strands cross-linked together in a sheet structure by an acrylic backbone and held firmly attached to the surface by interspersed DOPA units. This structure may explain the success of the coating since detached DOPA units are held close to the surface to promote reattachment and away from the surrounding urinary milieu (fig. 8).
We examined 2 commercially available stents, SF and PP, in addition to stents coated with DOPA containing polymers. Since these 2 devices are the most commonly used stents in North America, their use in our study is most reflective of current clinical urological practice and enabled us to determine whether one was better than the other to prevent infection and/or encrustation in this model. Our findings show that neither device had any statistically significant benefit over the other in any category studied.
In the rabbit model carbonates and phosphates constantly precipitate out of solution even in the absence of infection. Since no significant differences were observed across groups, no device and/or coating could be deemed to be better at resisting its development in this model. However, a clear inference could be made in terms of comparing animals with a sterile urinary environment throughout the study to those that were heavily infected on day 7 (highest vs lowest encrustation degree). These data strongly suggest that severely infected animals (greater than 108 cfu/ml) did not produce typical encrustation promoting urine, which was perhaps attributable to the higher degree of cytokine expression and subsequent leukocyte infiltration in those animals. Finally, not all organisms buried in the encrustation can be accurately quantified since methods to disrupt and dissolve encrustation are not entirely successful and can kill many organisms that are present. Ultimately most of the organisms enumerated likely represent those that were attached directly to the device or on the outer surface of the encrustation. This may explain why S009 had adherent organisms on almost all stents but no urinary organisms in 8 of 10 by day 3. Those bacteria were adherent to the stent but trapped under the encrustation. Despite this complication S009 still showed a 75% decrease in overall adherent bacteria and only 3 of 10 curls in that group had greater than 105/ml compared to at least double that in all other groups, including the PP group (7 of 10).
Cytokine expression and bladder inflammation were theoretically driven by the stent curl and its surface, encrustation and urinary and stent UPEC levels. The first 2 factors did not have a role across stent groups since the curl was common to all animals and encrustation covered all exposed curl surfaces shortly after placement. Furthermore, animals grouped based on encrustation alone and compared for cytokine expression and immune scoring showed no significant differences. Thus, urinary and stent levels of E. coli GR12 were the only major factors that alone significantly affected the inflammatory determinants measured. In each case the expected trend of more bacteria and more inflammation was followed. Overall group S009 had the least amount of overall inflammation when considering all 3 parameters measured, consistent with its lowest urinary infection rate and fewest stent adherent organisms.
The development of a device associated UPEC UTI in this model can be hypothesized to occur in a certain way. Immediately after device and bacterial instillation the animal mounts an immune response and the instilled UPEC attach to any surface present, namely the stent curl, urinary crystals and bladder epithelium. While most crystals and infected uroepithelial cells are cleared, thereby eliminating much of the infection, the device remains. Thus, stent adherent organisms escape clearance. Depending on infection severity stent encrustation continuously forms and adherent organisms replicate and promote the infection. Thus, if too few organisms adhere, most are cleared from the urinary tract and there will not be enough to maintain active infection in the bladder. Also, since encrustation is continually deposited on the device, organisms on the device may become covered and, although they are still alive and detectable upon stent removal, they are unable to drive the infection. This explains how animals such as those in the S009 group with sterile urine cultures on days 3 and 7 still had positive stent cultures when the encrustation was removed.
CONCLUSIONS
Stents coated with S009 were the only ones able to resist UPEC attachment to the point where most rabbits could clear the UTI. Thus, S009 shows great potential as a future anti-fouling coating for use in humans to prevent device related infection. Also, the success of S009 compared to that of the other polymers tested suggests that coating a surface with a multifunctional cross-linked coating aids in coating durability and longevity.
ACKNOWLEDGMENTS
Brad Kobe, Surface Science Western, performed stent curl scanning electron microscopy analysis. SF stents, Kumpe catheters and Bentson guidewires were provided by Cook Urological, Spencer, Indiana. Urine collection and culture, and bladder analysis materials were obtained from VWR Canlab, Mississauga, Ontario, Canada. Cytokine analysis materials were obtained from Invitrogen™. Coating polymers were obtained from Nerites Corp. Ltd.
Supported by the American Urological Association Foundation Research Scholar program (PAC), Nerites Corp., Ltd., Lawson Health Research Institute, National Institutes of Health Grants P41RR02301 (Bio-medical Research Technology Program, National Center for Research Resources) and P41GM66326 (National Institute of General Medical Sciences), University of Wisconsin, National Institutes of Health P41GM66326, P41RR02301, RR02781 and RR08438, National Science Foundation DMB-8415048, OIA-9977486 and BIR- 214394, and United States Department of Agriculture.
Abbreviations and Acronyms
- DOPA
3,4-dihydroxyphenylalanine
- EDX
energy dispersive x-ray analysis
- MAP
marine mussel adhesive protein
- mPEG
methoxy PEG
- PEG
poly(ethylene glycol)
- PP
Percuflex Plus
- S002
Surphys 002
- S008
Surphys 008
- S009
Surphys 009
- SF
Sof-Flex
- TGF-α
transforming growth factor α
- UnSF
uncoated Sof-Flex
- UPEC
uropathogenic Escherichia coli
- UTI
urinary tract infection
- VEGF
vascular endothelial growth factor
REFERENCES
- 1.Beiko DT, Knudsen BE, Watterson JD, et al. Uri-nary tract biomaterials. J Urol. 2004;171:2438. doi: 10.1097/01.ju.0000125001.56045.6c. [DOI] [PubMed] [Google Scholar]
- 2.Chew BH, Denstedt JD. Technology insight: Novel ureteral stent materials and designs. Nat Clin Pract Urol. 2004;1:44. doi: 10.1038/ncpuro0014. [DOI] [PubMed] [Google Scholar]
- 3.Denstedt JD, Reid G, Sofer M. Advances in ureteral stent technology. World J Urol. 2000;18:237. doi: 10.1007/pl00007074. [DOI] [PubMed] [Google Scholar]
- 4.Jacobsen SM, Stickler DJ, Mobley HL, et al. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin Microbiol Rev. 2008;21:26. doi: 10.1128/CMR.00019-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schierholz JM, Beuth J, Rump A, et al. Novel strategies to prevent catheter-associated infections in oncology patients. J Chemother. 2001;13:239. doi: 10.1179/joc.2001.13.Supplement-2.239. [DOI] [PubMed] [Google Scholar]
- 6.Schierholz JM, Yucel N, Rump AF, et al. Antiinfective and encrustation-inhibiting materials— myth and facts. Int J Antimicrob Agents. 2002;19:511. doi: 10.1016/s0924-8579(02)00096-1. [DOI] [PubMed] [Google Scholar]
- 7.Pierce GE. Pseudomonas aeruginosa, Candida albicans, and device-related nosocomial infections: implications, trends, and potential approaches for control. J Ind Microbiol Biotechnol. 2005;32:309. doi: 10.1007/s10295-005-0225-2. [DOI] [PubMed] [Google Scholar]
- 8.Schierholz JM, Beuth J. Implant infections: a haven for opportunistic bacteria. J Hosp Infect. 2001;49:87. doi: 10.1053/jhin.2001.1052. [DOI] [PubMed] [Google Scholar]
- 9.Yetkin G, Otlu B, Cicek A, et al. Clinical, microbiologic, and epidemiologic characteristics of Pseudomonas aeruginosa infections in a university hospital, Malatya, Turkey. Am J Infect Control. 2006;34:188. doi: 10.1016/j.ajic.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Ko R, Cadieux PA, Dalsin JL, et al. First prize: novel uropathogen-resistant coatings inspired by marine mussels. J Endourol. 2008;22:1153. doi: 10.1089/end.2008.0049. [DOI] [PubMed] [Google Scholar]
- 11.Kehinde EO, Rotimi VO, Al-Hunayan A, et al. Bacteriology of urinary tract infection associated with indwelling J ureteral stents. J Endourol. 2004;18:891. doi: 10.1089/end.2004.18.891. [DOI] [PubMed] [Google Scholar]
- 12.Vignali DA. Multiplexed particle-based flow cytometric assays. J Immunol Methods. 2000;243:243. doi: 10.1016/s0022-1759(00)00238-6. [DOI] [PubMed] [Google Scholar]
- 13.Cadieux PA, Chew BH, Knudsen BE, et al. Triclosan loaded ureteral stents decrease Proteus mirabilis 296 infection in a rabbit urinary tract infection model. J Urol. 2006;175:2331. doi: 10.1016/S0022-5347(06)00252-7. [DOI] [PubMed] [Google Scholar]
- 14.Kiwull-Schone H, Kalhoff H, Manz F, et al. Food mineral composition and acid-base balance in rabbits. Eur J Nutr. 2005;44:499. doi: 10.1007/s00394-005-0553-z. [DOI] [PubMed] [Google Scholar]
- 15.Watterson JD, Cadieux PA, Beiko DT, et al. Oxalate-degrading enzymes from Oxalobacter formi-genes: a novel device coating to reduce urinary tract biomaterial-related encrustation. J Endourol. 2003;17:269. doi: 10.1089/089277903322145431. [DOI] [PubMed] [Google Scholar]
- 16.Lobel B, Patard JJ, Guille F. Nosocomial infections in urology. Ann Urol (Paris) 2003;37:339. [PubMed] [Google Scholar]
- 17.Smith PW, Seip CW, Schaefer SC, et al. Microbiologic survey of long-term care facilities. Am J Infect Control. 2000;28:8. doi: 10.1016/s0196-6553(00)90005-1. [DOI] [PubMed] [Google Scholar]
- 18.Lee H, Scherer NF, Messersmith PB. Single-molecule mechanics of mussel adhesion. Proc Natl Acad Sci U S A. 2006;103:12999. doi: 10.1073/pnas.0605552103. [DOI] [PMC free article] [PubMed] [Google Scholar]