Abstract
New walking patterns can be learned over short time scales (i.e., adapted in minutes) using a split-belt treadmill that controls the speed of each leg independently. This leads to storage of a modified motor pattern that is expressed as an aftereffect in regular walking conditions and must be de-adapted to return to normal. Here we asked whether the nervous system adapts a general walking pattern that is used across many speeds or a specific pattern affecting only the two speeds experienced during split-belt training. In experiment 1, we tested three groups of healthy adult subjects walking at different split-belt speed combinations and then assessed aftereffects at a range of speeds. We found that aftereffects were largest at the slower speed that was used in split-belt training in all three groups, and it decayed gradually for all other speeds. Thus adaptation appeared to be more strongly linked to the slow walking speed. This result suggests a separation in the functional networks used for fast and slow walking. We tested this in experiment 2 by adapting walking to split belts and then determining how much fast regular walking washed out the slow aftereffect and vice versa. We found that 23–38% of the aftereffect remained regardless of which speed was washed out first. This demonstrates that there is only partial overlap in the functional networks coordinating different walking speeds. Taken together, our results suggest that there are some neural networks for controlling locomotion that are recruited specifically for fast versus slow walking in humans, similar to recent findings in other vertebrates.
INTRODUCTION
Humans alter their locomotor patterns for different situations—the pattern used for pavement is different from those used for ice, snow, or sand. Adaptation, or short time-scale learning, can occur in minutes to adjust the walking pattern to meet the demands of new, predictable situations. Is this type of adaptation specific to the situation that drove it or general to a broader range of walking conditions? This question is important because it addresses whether the neural circuitry used for walking is shared across different conditions. On a practical level, this information is critical for optimizing rehabilitation because the goal is to train a general walking pattern used in many different situations. Thus we sought to determine the range of activities across which adapted walking patterns generalize (i.e., the “dynamic range” of adaptation).
We and others have previously studied walking adaptation using a split-belt treadmill, which has two belts that can control the walking speed of each leg independently (Jensen et al. 1998; Prokop et al. 1995; Reisman et al. 2005). Practicing split-belt walking results in changes in interlimb coordination. This modified walking pattern is stored and results in an aftereffect when normal walking conditions are restored (i.e., “tied belts”) (Choi and Bastian 2007; Morton and Bastian 2006; Reisman et al. 2005, 2007). Recent experiments have shown that split-belt adaptation is quite specific: there is limited generalization to other locomotor tasks (e.g., backward walking) (Choi and Bastian 2007) and contexts (e.g., over-ground walking) (Reisman et al. 2009). Other walking adaptation tasks have shown varied amounts of generalization. When adapting the walking pattern to step on a moving platform, aftereffects are specific to the context and task; there is no generalization of the aftereffect between forward and backward walking (Reynolds and Bronstein 2004). On the other hand, Lam and Dietz (2004) showed that subjects who learned a different locomotor task—an obstacle avoidance strategy during treadmill walking—could generalize the acquired performance to other forms of treadmill walking, like downhill walking or walking with additional weight attached to the leg. The degree of similarity between locomotor tasks that is required for complete generalization of a learned or adapted pattern remains unknown.
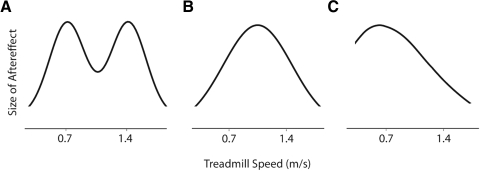
To address this issue, we investigated the generalization of split-belt adaptation across different speeds of walking. We chose to examine different speeds because these can be altered in an incremental fashion. Previously, Kitazawa et al. (1997) showed that prism adaptation during a reaching task was speed-specific: the aftereffects when the prisms were removed were largest when reaching was performed at the same speed as what was adapted. In other words, the closer the speed of reaching resembled that during adaptation, the greater the generalization. We hypothesized that walking adaptation would also be speed-specific with the largest aftereffects occurring at the speeds that were trained. One possibility is that a bimodal function would describe generalization with the largest aftereffects occurring at the speeds of the slow and fast belts during adaptation (Fig. 1 A). Alternatively, the generalization function could resemble a Gaussian distribution, peaking at an intermediate speed between the two trained belt speeds (Fig. 1B). Last, the generalization function could favor one of the trained speeds—e.g., it could be skewed to the right, with a peak at the slow belt speed (Fig. 1C) or to the left with a peak at the fast speed (not shown). This would suggest that adaptation is associated with the training speed of one leg more than the other.
Fig. 1.
Three predicted shapes of speed generalization functions. A: bimodal with peaks at each of the belt speeds during split-belt adaptation (i.e., 0.7 and 1.4 m/s). B: unimodal with the peak centered between the split-belt speeds (i.e., 1.05 m/s). C: skewed to the right with the peak centered on the slow belt speed (i.e., 0.7 m/s).
METHODS
Subjects
Sixty-four healthy adult volunteers [30.3 ± 8.6 (SD) yr] participated in this study with one subject participating in two experiments. All participants gave informed written consent prior to participating and the experimental protocols were approved by the Johns Hopkins Institutional Review Board.
Experimental setup and design
Subjects walked on a custom-built split-belt treadmill (Woodway, Waukesha, WI) with two separate belts driven by independent motors—these belts could be driven at same speed (“tied belt”) or at different speeds (“split belt”). Speed commands for each belt were sent to the treadmill via a computer interface written in MatLab (Mathworks, Natick, MA). Subjects were positioned in the middle of the treadmill with one foot on each belt. They held onto a front rail that was adjusted to elbow height and wore a safety harness around their chest that was suspended from the ceiling. The safety harness did not support body weight during walking. At the beginning of each trial, the belts were stationary and, with the exception of the baseline tied-belt trials at the beginning of the experiment and the first split-belt trial, subjects were not told what speeds the belts would be going. Once the belts started moving, subjects were instructed to look at an experimenter standing in front of the treadmill.
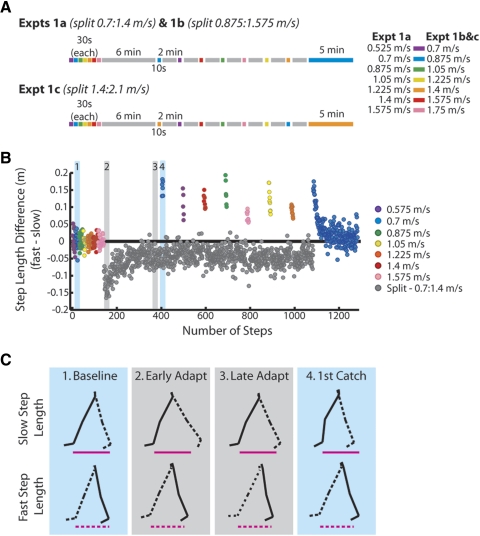
Generally, each experiment began with a series of baseline (tied belt) trials at different speeds. Subjects were then adapted to the split belts. The leg that was made to move faster was randomly assigned. Once they were completely adapted, between 1 and 8 short (10 s) tied-belt catch trials were interspersed within the split-belt period. Following this was a postadaptation (tied belt) period, during which aftereffects were washed out (see Fig. 2A).
Fig. 2.
A: experimental protocol: tied-belt trials are in different colors (see legend for exact speeds) and split-belt trials are in gray. All experiments started with baseline tied-belt trials, followed by a 6-min adaptation period and alternating tied-belt catch trials (10 s) and split-belt re-adaptation periods (2 min). The order of catch trials was randomized with the exception of the 1st, which was always at the speed of the slow belt. Split-belt speeds for experiment 1a were 0.7:1.4 m/s, 1b were 0.875:1.575 m/s, and 1c were 1.4:2.1 m/s. The experiment ended with 5 min of tied-belt walking at the slow speed (i.e., the “wash-out”), and the size of the aftereffect at the beginning of this trial was compared with the 1st catch to ensure the stability of the catch trials throughout the experiment. B: single subject data from experiment 1a. Each dot represents 1 step; colored and gray dots correspond to tied- and split-belt trials, respectively. A step length difference of 0 indicates symmetry. Note that the catch trials vary in size according to the speed, and the asymmetry at the beginning of the final wash-out period was similar to the size to the 1st catch trial. Shaded regions marked with numbers 1–4 are highlighted in C. C: slow and fast step lengths during baseline (1), adaptation (2 and 3), and the 1st catch trial (4). The leg on the slow belt (“slow leg”) is shown in solid lines; the fast leg is shown in dashed lines. For reference, slow and fast step lengths at baseline are denoted by magenta lines below stick figures in all 4 conditions. Step length asymmetry in early adaptation was mainly due to a longer step on the slow side. After 6 min of adaptation, step lengths were equalized. The aftereffect in the 1st catch showed the opposite asymmetry as in early adaptation: the slow step was shortened and the fast step was lengthened.
EXPERIMENT 1.
In this experiment, we constructed generalization curves for three different sets of adaptation speeds. In experiment 1a (n = 20), subjects were adapted at split-belt speeds of 0.7 and 1.4 m/s and tied-belt aftereffects were tested at seven different speeds in 0.175-m/s increments between 0.525 and 1.575 m/s. For reference, preferred walking speed for adults is around 1.2 m/s (Dingwell and Marin 2006), and, for treadmill locomotion, the walk-to-run transition occurs around 2.1 m/s (Segers et al. 2006). We selected the training and aftereffect speeds to cover a range of comfortable walking speeds without coming close to the walk-to-run transition. In experiment 1b (n = 15), subjects were adapted at split-belt speeds of 0.875 and 1.575 m/s, and tied aftereffects were tested at seven speeds between 0.7 and 1.75 m/s. In experiment 1c (n = 11), subjects were adapted at split-belt speeds of 1.4 and 2.1 m/s, and tied aftereffects were tested at the same speeds as in experiment 1b (see Fig. 2A for schematic). Although the walk-to-run transition occurs around 2.1 m/s when belts are tied, during split-belt walking at 1.4:2.1 m/s, all subjects adopted a walking gait. For all subjects, each experiment began with a baseline period, where the relevant speeds of walking were tested for 30 s each. This was followed by a 6-min split-belt adaptation period and then a series of seven to eight 10-s catch trials at different tied-belt speeds, each alternating with 2-min split-belt re-adaptation trials. The order of the catch trials was random with the exception of the first, which was always at the speed of the slow belt (i.e., experiment 1a: 0.7 m/s; experiment 1b: 0.875 m/s; experiment 1c: 1.4 m/s). This was done to allow comparisons between the size of the first catch trial and the last wash-out trial (also at the slow belt speed) to ensure that the size of this catch trial was stable. Subjects were not included in the analysis if the catch trials at the beginning and end of the experiment were significantly different in size or if there was a high negative correlation between aftereffect size and catch trial number (i.e., if the catch trials systematically decreased in size over the course of the experiment). This was done to ensure that the initial catch trial (at the speed of the slow belt) was not exaggerated due to being the first catch in the experiment. Using these criteria, we included 11/20 subjects in experiment 1a, 10/15 subjects in experiment 1b, and 10/11 subjects in experiment 1c. Because we excluded nearly half of the subjects in experiment 1a, we wished to determine if including these subjects would change our main results. We show this comparison in Supplementary Fig. S11—notice here that the two generalization functions are nearly identical. Thus, while we excluded these subjects in an effort to be conservative in interpreting our results, the inclusion of these subjects did not change our findings.
We hypothesized that a number of subjects were excluded in experiment 1a because the first aftereffect was exaggerated due to the surprise and instability experienced by subjects the first time they experienced the aftereffect. To counteract this “surprise” effect, we inserted an additional catch trial at the slow-belt speed after 4 min of adaptation. The purpose of this was to simply expose subjects to the feeling of switching between tied and split belts, and this extra initial catch trial was not included in the data analysis. The extra catch was inserted into the experiment for 6/20 subjects in experiment 1a and all subjects in experiments 1 b and c.
EXPERIMENT 2.
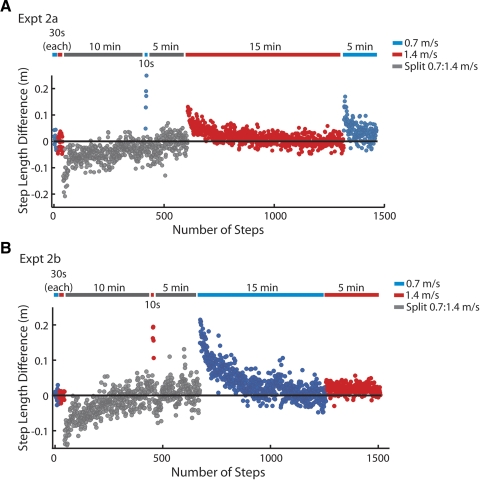
In this experiment, we tested whether subjects could store separate aftereffects for slow and fast walking (n = 19). All subjects did baseline walking at slow (0.7 m/s) and fast (1.4 m/s) speeds for 30 s each on tied belts and were adapted to split belts (0.7:1.4m/s) for 10 min. Subsequently, one group of subjects (n = 10 – experiment 2a) had a 10-s catch trial at 0.7 m/s to measure the size of the aftereffect. This group was then re-adapted to the split belts for 5 min and then carried out two postadaptation trials: 15 min of tied belt fast walking (1.4 m/s) to completely wash-out the fast aftereffect and 5 min of slow walking (0.7 m/s). We compared the size of the remaining aftereffect during slow walking to the size of the first slow catch trial to determine the proportion of the aftereffect that was not washed out by fast walking. A second group of subjects (n = 9 – experiment 2b) did the converse experiment: following baseline and 10 min of adaptation, this group did a 10-s catch trial at the fast speed (1.4 m/s), was re-adapted to the split belts for 5 min, did 15 min of tied-belt slow walking, and then did 5 min of tied-belt fast walking to determine if any part of the fast aftereffect was not washed out by slow walking. Also see Fig. 6, A and B, top, for schematic.
Fig. 6.
Paradigm used to test the independence of aftereffects at different walking speeds. A, top: experimental paradigm; bottom: corresponding single subject data. Number of steps is shown on the x axis. Subjects were adapted at a 2:1 speed ratio (0.7:1.4 m/s) for 10 min. We then assessed walking with the treadmill belts tied at the slow belt speed (0.7 m/s) and re-adapted them for another 5 min. The fast aftereffect was washed-out during 15 min of tied fast walking (1.4 m/s), and then the slow aftereffect was reassessed. This was done to determine how much fast walking washed out the slow aftereffect. The positive step length difference present at the end of the slow wash-out period in the stride-by-stride plot indicates that fast walking did not wash-out the slow aftereffect completely. B: the reverse experiment from A—paradigm is shown at top, single subject data at bottom (different subject from A). Here we wished to determine how much slow walking washed out the fast aftereffect. There was a small aftereffect remaining at the tied fast speed following 15 min of slow wash-out—note that the red dots at the beginning of the final fast walking period are above the reference line marking baseline walking symmetry.
DATA COLLECTION.
Kinematic data were collected at 100 Hz using Optotrak (Northern Digital, Waterloo, ON). Infrared-emitting markers were placed bilaterally over the fifth metatarsal head (toe), lateral malleolus (ankle), lateral femoral epicondyle (knee), greater trochanter (hip), iliac crest (pelvis), and acromion process (shoulder). The coordinate system was aligned such that the x axis was parallel to the treadmill belts, the y axis was parallel to the vertical line, and the z axis was parallel to the horizontal line perpendicular to the x-y plane. Pressure sensors taped beneath the heel and ball of each foot were used to record times of foot contact and lift off. Voltages reflecting treadmill belt speeds were recorded directly from treadmill motor output. Marker position and analog data (foot pressure sensors and treadmill speed) were synchronized and sampled simultaneously using Optotrak software at 100 and 1,000 Hz, respectively.
DATA ANALYSIS.
By convention, we refer to the leg that is adapted on the slow belt as the “slow leg” and the leg on the fast belt as the “fast leg,” even during tied-belt walking. In this experiment, we decided to focus on a spatial measure of interlimb coordination—step length—which was calculated as the anterior-posterior distance between the ankle markers of each leg at heel strike (Fig. 2C). Slow step length refers to the step length measured at heel strike of the slow leg; fast step length refers to the step length measured at heel strike of the fast leg. The difference between fast step length and slow step length was used as an indication of gait symmetry. The difference in step lengths at baseline was subtracted from all data to remove any baseline offset in symmetry. Therefore a step length difference of zero corresponds with the subject's baseline symmetry (this is referred to as “symmetric” walking). If the difference was positive or negative, the walking pattern deviated from baseline symmetry and was referred to as “asymmetric” (i.e., resembling a limp, as in early split-belt adaptation and catch trials).
We focused on step length (a spatial measure), as opposed to a temporal measure like double support duration (Reisman et al. 2005) because we found that the temporal parameters tended to be less stable over the course of experiment 1. In other words, the aftereffect in the last wash-out period was significantly smaller than that in the first catch trial. While it is not clear why temporal measures of walking symmetry were less stable, it is becoming clear that temporal and spatial parameters can be adapted and stored separately (Choi et al. 2009). Due to this limitation, we were unable to assess the dynamic range of generalization for temporal parameters in the current study.
Statistical analysis
EXPERIMENT 1.
We first determined that the sizes of aftereffects measured during catch trials were stable over the course of the experiment using one-tailed t-test to compare step length difference from the first three steps of the first catch trial (at 0.7 m/s) and the last wash-out period (also at 0.7 m/s) within each subject. Subjects with a P > 0.05, indicating that the aftereffect was not significantly different at the beginning and end of the experiment, were included in the analysis. As an additional criterion, Pearson correlation coefficients were calculated between aftereffect size and catch trial order for each subject. Subjects with high correlations (r2 >0.7) in the negative direction were not included in the analysis because this indicated that the aftereffect diminished systematically with time. We also checked group data to confirm that there was no systematic decrease in aftereffect size over the course of the experiment via a Pearson correlation coefficient (experiment 1a: r2 = 0.03; 1b: r2 = 0.01; 1c: r2 = 0.02). One-way repeated-measures ANOVAs with seven levels (aftereffect speeds) were performed to test significance within each generalization function in Fig. 3. Planned comparisons compared the size of aftereffects at each speed to that at the slow belt speed. One-way repeated-measures ANOVAs (7 levels) were also used to compare stride duration and the coefficient of variation of stride duration during baseline walking at different speeds in Fig. 4. Post hoc analysis of significant main effects was conducted using Fisher's LSD. Paired t-tests were used to compare characteristics of fast and slow walking (i.e., in Fig. 5).
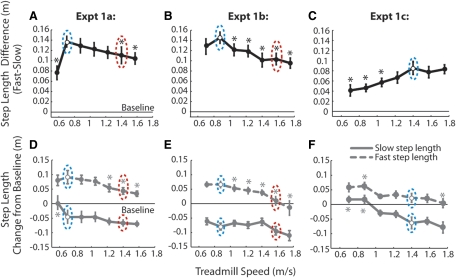
Fig. 3.
Speed generalization functions for step length difference (A–C) and step length changes from baseline (D–F). Eleven subjects were included in the analysis for experiment 1a (A and D), 10 for experiment 1b (B and E), and 10 for experiment 1c (C and F). Data shown are means ± SE. Blue dashed circles indicate the speed of the slow belt during adaptation; red circles indicate the speed of the fast belt during adaptation. Reference lines are drawn at zero to denote step symmetry during baseline trials (preadaptation). *, aftereffects that are significantly different from that at the slow belt speed (P < 0.05).
Fig. 4.
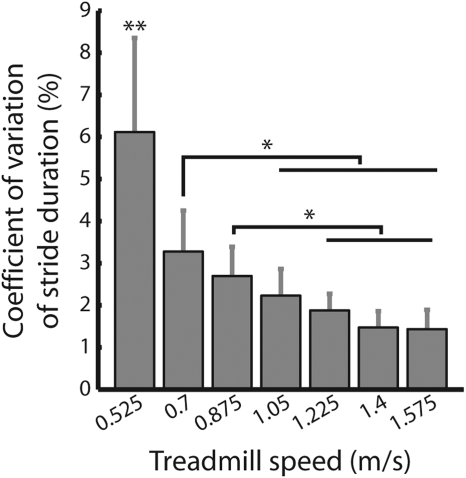
Coefficient of variation of stride duration. This was calculated for each subject as (SDstride_duration/meanstride_duration)*100 at each speed. Group averages are shown (means ± SE). All data were taken from baseline trials of experiment 1a (n = 11). **, SDs at this speed were significantly different from all other speeds (P < 0.01); *, specific differences between other speeds (P < 0.05).
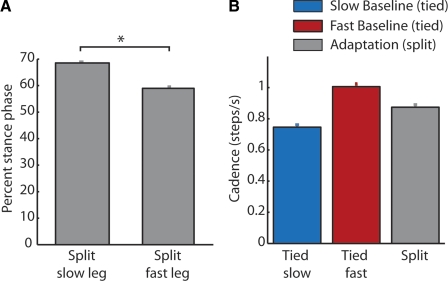
Fig. 5.
Possible explanations for why the speed generalization functions peak at the slow belt speed. All data were taken from experiment 2 (n = 19). A: the slow leg spends significantly more time in stance than the fast leg (P < 0.01). The percentage of the step cycle occupied by stance phase (i.e., percent stance time—mean ± SE) is shown for the slow and the fast leg during adaptation at 0.7:1.4 m/s. B: the cadence during adaptation cannot explain why the largest aftereffects are seen at slow speeds. Mean cadence in step/s (±SE) is plotted for slow (0.7 m/s) and fast (1.4 m/s) baseline and adaptation (0.7:1.4 m/s) periods. Cadence during adaptation is mid-way between the fast and slow cadence. A paired t-test comparing the differences between tied fast and adaptation cadence and tied slow and adaptation cadence was not significant (P = 0.38).
EXPERIMENT 2.
For each of the two experiments (2 a and b), one-way repeated-measures ANOVAs with seven levels were performed on averaged data from baseline slow, baseline fast, first three strides of catch, first three strides of first wash-out, last three strides of first wash-out, first three strides of second wash-out, and last three strides of second wash-out. Fisher's post hoc analysis was used to evaluate differences between aftereffects during catch trials and wash-out periods with respect to the appropriate baseline trial (slow or fast). In addition, a t-test was used to compare the proportion of the aftereffect remaining in the first three strides of the second wash-out period between experiments 2 a and b.
For all experiments, t-test and Pearson correlation analyses were performed using MatLab. Repeated-measures ANOVAs, planned comparisons, and post hoc tests were calculated using Statistica. The α-level was set at 0.05.
RESULTS
Split-belt walking resulted in changes in interlimb coordination as expected (Reisman et al. 2005). When first exposed to the split belts in early adaptation, subjects showed a pronounced limp, quantified as a step length asymmetry relative to baseline (Fig. 2, B and C). Step length symmetry improved by late adaptation, and subjects showed significant aftereffects with the reverse asymmetry when normal tied-belt walking conditions were restored. In the first set of experiments (experiment 1), we tested aftereffects at several different tied-belt speeds, which were interspersed throughout adaptation (Fig. 2A). During this period, subjects were not aware that they were switching between tied and split belts. Rather they reported that each tied-belt catch trial felt like it was a split-belt trial with the opposite belt configuration (i.e., the fast leg felt like it was now on the slow belt and vice versa). Moreover, each catch trial at a different speed felt like there was a different ratio between belt speeds. Therefore it was difficult to anticipate the belt configurations in upcoming trials.
To ensure that subjects did not anticipate upcoming trials, we only included those who showed a stable catch from the beginning to the end of the experiment. Figure 2B shows data from a subject who was included in the analysis for experiment 1a—note that while aftereffects varied in size with treadmill speed, the aftereffect in the end wash-out period (at 0.7 m/s) was the same size as the aftereffect in the first catch trial (also at 0.7 m/s).
Within each subject, average aftereffect size was determined from the mean of the first three strides in each catch trial, discounting the initial starting stride. These values were averaged across all subjects to give the group generalization functions shown in Fig. 3. Asterisks mark aftereffects that are significantly different from that at the slow-belt speed (P < 0.05). Our hypothesis was that the largest aftereffects would occur at the speeds that were trained; that is, when belts were tied at either the speed of the slow belt or the fast belt during adaptation (Fig. 1A). However, Fig. 3A shows that the generalization function was skewed to the right as in Fig. 1C. While aftereffects were present at all speeds tested, the largest aftereffects were observed around the speed of the slow belt (maximum aftereffect at 0.7 m/s—circled in blue). These aftereffects at the slow speed were significantly larger than those at the fast belt speed (1.4 m/s—circled in red; P = 0.02).
In experiment 1a, we noticed that that the aftereffect decreased in size quite suddenly on the left side of the curve: the aftereffect at 0.575 m/s was significantly smaller than that at 0.7 m/s (P < 0.01—see Fig. 3A). This slow speed also made people to walk differently than at the other speeds tested. The stride duration was markedly more variable (Fig. 4), and we suspected that it was not as natural of a walking pattern compared with the other speeds. We were concerned that the significant decrease in aftereffect at this speed in experiment 1a (Fig. 3A) might be an artifact of the variable stride durations. Experiment 1b was designed to ask whether the decrease in aftereffect size was still present if the slowest speed was a more natural walking speed (i.e., 0.7 m/s). In that experiment, we adapted subjects to slightly faster split-belt speeds: 0.875:1.575 m/s and found that the aftereffect showed a strong trend toward decreasing on the left side of the curve (P = 0.07; Fig. 3B). Thus the shape of the generalization function was largely similar in experiments 1, a and b, with the maximum aftereffect at the slow belt speed (circled in blue in Fig. 3, A and B).
Following experiment 1, a and b, it remained unclear to what extent the size of the aftereffect depended on the adaptation speeds. That is, was the maximum of the generalization function centered around the slow-belt speed specifically or did the largest aftereffects simply occur at slower walking speeds? To address this question, we adapted a third group of subjects to split-belt speeds of 1.4:2.1 m/s (experiment 1c—Fig. 3C) and assessed aftereffects at the same tied-belt speeds as in experiment 1b. In this case, we found that the generalization function reached a maximum at the slow belt speed (1.4 m/s—circled in blue) and aftereffects diminished in size at slower walking speeds. Therefore maximum aftereffects occurred at speeds near that of the slow belt even when the slow belt speed was quite fast (i.e., 1.4 m/s).
The step length differences shown in Fig. 3, A–C, could reflect changes in the slow step length, the fast step length, or both. We wished to determine how each contributed to the overall difference. Figure 3, D–F, shows the changes in individual step lengths compared with baseline. Note that the contributions of individual step lengths to the overall step length difference changed depending on the speed at which the aftereffect was tested—the change in slow step length relative to baseline tended to decrease at slower speeds whereas the change in fast step length decreased at faster speeds. Therefore overall step length differences (i.e., aftereffects) at the slow-belt speed (blue circles) were determined by changes in both the slow and the fast step lengths, aftereffects at faster speeds were dominated by changes in the slow step length, and aftereffects at slower speeds were dominated by changes in the fast step length.
Why were the largest aftereffects observed at speeds near that of the slow belt during adaptation and not, for instance, near the fast-belt speed or in between the two belt speeds? We can see from the individual step length differences (Fig. 3, D–F) that aftereffects in the fast step length diminish as treadmill speed increases and those in the slow step length diminish as treadmill speed decreases. However, both step lengths show moderate to large aftereffects at the speed of the slow belt, and thus the largest step-length asymmetries occur at this speed. So, why do the larger aftereffects on each side converge near the slow-belt speed? It could be that the adapted gait pattern was most closely associated with the slow belt speed. For instance, during adaptation the slow leg spends more time in stance (Prokop et al. 1995; Reisman et al. 2005). We verified this finding in our experiment and found that stance time was significantly different, composing 68% of the step cycle on the slow side, but only 57% on the fast side (Fig. 5A); therefore it is possible that the sensory cues from the slow belt might be more salient during adaptation. Another possibility could be that the step cadence (step/s) during adaptation was closer to the cadence during tied slow walking than tied fast walking. However, we ruled out this possibility by showing that the cadence was, in fact, between that during tied slow and fast walking (Fig. 5B). A paired t-test comparing the differences between tied fast and adaptation cadence and tied slow and adaptation cadence was not significant (P = 0.38).
Because aftereffect size for the fast and slow legs varied with speed, we wondered whether there is partial separation of the functional networks for fast and slow walking. If so, we would predict that a portion of the slow aftereffect would remain following complete wash-out of the fast aftereffect and vice versa. This was tested in experiment 2. Figure 6 shows single subject data that supports this hypothesis. In Fig. 6A, 15 min of fast tied walking caused the aftereffect to be washed-out completely (see red dots). Nonetheless, when the speed of the tied belts was changed to the slow belt speed (blue dots), a portion of the slow aftereffect remained. That is, the slow aftereffect was only partially washed-out with fast walking. In the converse experiment, the subject in Fig. 6B held onto a very small portion of the fast aftereffect as shown by the red dots above baseline following 15 min of slow walking (blue dots). Therefore in this single subject data, it is clear that more of the aftereffect remained in Fig. 6A. This is particularly notable considering the fact that more steps were taken during the faster first wash-out period in experiment 2a compared with experiment 2b (compare length of first wash-out period between Fig. 6, A and B).
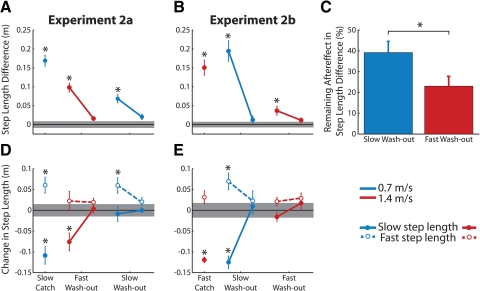
Figure 7 shows group data from experiment 2 and confirms the trends observed in the single subject data (asterisks mark significant differences from baseline symmetry). In experiment 2, a and b, aftereffects were abolished at the end of the first wash-out (Fig. 7, A and B). However, when we reassessed step length differences at different speeds (i.e., in the 2nd washout period), significant aftereffects remained in both cases (experiment 2a: P < 0.01; experiment 2b: P = 0.03). In experiment 2a (Fig. 7A), the remaining slow aftereffect was 38.2 ± 5.6% (mean ± SE) of the catch trial and in experiment 2b (Fig. 7B), the remaining fast aftereffect was 23.1 ± 4.7% of the catch (see also Fig. 7C).
Fig. 7.
Aftereffects remain following wash-out at a different speed. All data plotted are from tied-belt trials—adaptation data are not shown. A: step length differences (fast step length minus slow step length) from experiment 2a: data shown are averages (±SE) of the slow catch trial (blue), the beginning and end of the fast wash-out period (red), and the beginning and end of the slow wash-out period (blue). Baseline symmetry is marked by the reference line at 0. The gray shaded region denotes the SE during fast and slow baseline walking. Asterisks, significant differences from baseline. B: step length differences from experiment 2b: data shown are averages of the fast catch trial (red), the beginning and end of the slow wash-out period (blue), and the beginning and end of the fast wash-out period (red). C: mean percentage of aftereffect (±SE) remaining at the beginning of the 2nd wash-out period in experiment 2a (blue bar) and 2b (red bar). Percentages were calculated with respect to the size of the catch trial (which was 100%). D: baseline-subtracted step lengths are shown for experiment 2a. A value of 0 on the y axis would occur if step lengths were the same size as in baseline trials. Slow step lengths are marked by solid lines/closed circles; fast step lengths are marked by dashed lines/open circles. Asterisks correspond to the data point below and indicate significant differences from baseline step length. E: individual fast and slow step lengths are shown, as in C, for experiment 2b.
Why does part of the aftereffect remain? Recall from Fig. 3, D–F that aftereffects at the slow belt speed were due to changes in both fast and slow step lengths, whereas aftereffects at faster speeds were primarily due to changes in the slow step length. In experiment 2a (Fig. 7D), it is the portion of the aftereffect that was unique to slow walking—the change in fast step length—that was not washed out by fast walking. The shared component of the aftereffects (i.e., the change in slow step length) was washed-out.
Based on this result, we predicted that in the reverse experiment (experiment 2b—Fig. 7E), the fast aftereffect would be more fully abolished by the slow wash-out period. We found that while the fast aftereffect was not completely washed-out (23.1% of the original catch trial remained – see Fig. 7, B and C), this was significantly less than the size of the aftereffect that remained in experiment 2a (38.2%—see Fig. 7, A and C; P = 0.04). Moreover neither the fast nor the slow step lengths were significantly different from baseline at the beginning of the final wash-out in experiment 2b (P > 0.05; Fig. 7D). Because aftereffects for the slow and fast step lengths were either equally sized or larger during slow walking, we were able to wash-out the smaller fast aftereffect more completely with slow walking.
Discussion
Our results indicate that the plasticity associated with locomotor adaptation is speed-specific in humans. Not only does the adapted walking pattern generalize incompletely across different speeds of walking, but washing out aftereffects at one speed (e.g., 1.4 m/s) does not abolish the aftereffects at a different speed (e.g., 0.7 m/s). These findings suggest that the functional circuits controlling different speeds of walking in humans may be more distinct than previously thought.
We found that aftereffects were largest when they were tested at the speed of the slow belt and diminished as we moved away from this speed in either direction (i.e., faster or slower). In experiment 1a, aftereffects decreased significantly in size between the slow belt speed (0.7 m/s) and the next slowest speed tested (0.525 m/s—see Fig. 3A). We also determined that stride durations while walking at 0.525 m/s were significantly more variable than at any of the other speeds tested (Fig. 4). We were concerned that the reason for this significant decrease in aftereffect size on the left side of the generalization function was due to the very slow speed of walking, which allowed more time for subjects to make on-line feedback corrections to their movements (which was reflected in the increased variability). However, when we shifted the adaptation speeds in experiment 2b (Fig. 3B), we saw that the general shape of the generalization function was conserved—specifically, there was still a diminishing trend on the left side of the curve even though the slowest speed (0.7 m/s) was now a more natural walking speed. Therefore these smaller aftereffects at the slowest speeds are likely not solely due to having more opportunity to make feedback corrections.
Perhaps a more intriguing question is why the aftereffects diminished on the right side of the generalization function, as speeds increased. Specifically, why were aftereffects at the slow speed greater than those at the fast speed, which was also experienced during split-belt adaptation? One possibility is that the aftereffects at the slow speed were the first ones tested, whereas the order of the remaining catch trials was randomized. However, this is unlikely because we only included subjects in the analysis if they had a stable aftereffect across the experiment. In other words, we re-tested the aftereffect at the slow belt speed at the end of the experiment and only included subjects who did not show significant differences between the first and last catch trials (both at the speed of the slow belt).
Another possible explanation is related to the sensory cues associated with slow walking. It is well known that sensory cues providing information about the environmental context can be powerful in determining how well an adapted motor pattern generalizes to other untrained movements (Kitazawa et al. 1997; Krakauer et al. 2006; Morton and Bastian 2004; Reisman et al. 2009). If the slow walking context is more salient during adaptation, this could explain the increased generalization at this speed. One way that the slow walking context could be more salient is if step cadence during adaptation was close to that at the tied slow speed. However, this was not the case because cadence during adaptation was between that during slow and fast walking (Fig. 5B). Another way that the slow context could be more salient during adaptation is if the nervous system attends more to the rate at which the slow leg is extended through stance. Indeed, in adaptation, the slow leg is clearly in stance longer than the fast leg (Fig. 5A) (also see Reisman et al. 2005). It is possible that the nervous system resolves the discrepancy in belt speeds on the two sides by attributing more importance to the treadmill belt it encounters longer (i.e., the slow side). If so, this would explain the greater generalization of split-belt adaptation to slow walking.
It is also possible that fast walking aftereffects are diminished because of a ceiling effect. As step length increases with faster walking, eventually a limit would be reached which could also limit aftereffect size. However, it is unlikely that we reached that ceiling with the speeds tested in the present study. Grillner and colleagues (1979) showed that foot excursion continues to increase linearly up to a walking speed of 2.2 m/s, which is well above the speeds that we tested. Furthermore, if the shape of the generalization functions was determined by mechanical constraints during faster walking, we would predict that aftereffects would always be larger at slow speeds regardless of the adaptation speeds. However, this was not the case because we found that following adaptation at 1.4:2.1 m/s (Fig. 3C), subjects showed the largest aftereffects at the speed of the slow belt (1.4 m/s), which was a relatively fast walking speed. Importantly, the aftereffect at 1.4 m/s was significantly larger than that at slower speeds of walking. It should also be noted that the smaller aftereffect size when subjects were adapted at 1.4:2.1 m/s (compare Fig. 3, C to A and B) is likely due to the smaller ratio between the belt speeds—a 2:3 belt speed ratio was used here, compared with a ∼1:2 ratio in experiments 1 a and b. Thus in summary, we suggest that the large slow aftereffects are because of salient context cues from the slow belt and differences in the relative amounts of adaptation on each side. We do not believe that this is due to a ceiling effect limiting aftereffect size.
One surprising result from this study is that while significant aftereffects occurred at all speeds tested, sometimes a speed difference of <0.2 m/s away from the slow-belt speed was sufficient for a significant decrease in the size of the aftereffect (see Fig. 3, A and B). Moreover, in experiment 2, we discovered that washing-out aftereffects completely at one speed (e.g., 1.4 m/s) did not abolish aftereffects at a different speed (e.g., 0.7 m/s). This suggests that there is a partial separation in the functional networks controlling fast and slow walking. Furthermore, our result showing a more complete wash-out of the aftereffect when the initial wash-out period was at the slow speed (experiment 2b) suggests that fast walking encompasses more components of slow walking than vice versa. Note that the slow and fast steps are both in error during the slow aftereffect, whereas only the slow step was in error in the fast aftereffect (compare beginning of 1st wash-out periods in Fig. 7, D and E). Therefore, an element of the slow aftereffect was not expressed during fast walking, which may indicate a separation in the neural control of each.
Where in the nervous system could a separation in the control of fast and slow walking occur? One possibility is the cerebellum, which is known to be critical for locomotor adaptation both in humans (Morton and Bastian 2006) and cats (Yanagihara and Kondo 1996). The cerebellum receives afferent signals from the dorsal spinocerebellar pathways, which provide information about the actual movement of the legs (Bosco et al. 2005, 2006; Poppele et al. 2003). It also receives input from the ventral spinocerebellar tract, which reflects the activity of spinal networks that generate stepping (i.e., intended movement) (Arshavsky et al. 1978). Because of these inputs, the cerebellum has been implicated in generating comparisons between predicted and actual movement outcomes and recalibrating predictive feedforward control, which is necessary for split-belt adaptation (Bastian 2006). Our results suggest that the functional networks performing these predictive calibrations may be partially distinct for different speeds of walking.
If there is a separation in the control of fast and slow walking, it also could occur at the level of spinal central pattern generators (CPGs). Currently, one proposed model of the neural control of locomotion stipulates a two-level CPG consisting of a discrete top-level rhythm generator setting the frequency (i.e., cadence) of movement and a continuum of bottom-level pattern formation units recruiting the appropriate muscle synergies for each task (reviewed in McCrea and Rybak 2008). If our results are due to separation at the spinal level, then a modification of this model would be warranted. Rather than viewing the rhythm generator as a discrete network of pace-setting interneurons, the top level may be a continuum of interneurons that can be recruited separately to coordinate different speeds of locomotion.
Recent investigations of spinal interneuronal activity during zebrafish swimming also support this model. McLean and colleagues (2008) showed that different classes of interneurons are recruited at different locomotor speeds: ones that are recruited at slow swimming frequencies are silenced at fast frequencies and vice versa. Importantly, this group showed that the swimming behavior did not exhibit large discrete changes, like changes in muscle activity timing, while transitions were made between different interneuronal sets. Rather significant changes in the neural control were accompanied by gradual, subtle changes in behavior. Based on these findings, they suggested that a continuum of pattern generating networks is recruited differently for different tasks in a smooth and gradual manner. It is not clear to what extent these findings in zebrafish can be extended to humans; however, principles of motor control are highly conserved across species (Dickinson 2006; Pearson 1993; Selverston 1999), thus it is likely that patterns of interneuronal recruitment may also be conserved. Indeed, McLean et al. (2008)'s proposal of a continuum of pattern generating networks coordinating different speeds of walking is consistent with the incomplete generalization across the speeds in the present study. Moreover, if functional networks for fast and slow walking are only partially overlapping, this would explain the remaining aftereffect following the wash-out of the adaptation at a different speed.
Not only do the present results add to the understanding of adaptive reorganization of neuronal circuits, but our characterization of the dynamic range of locomotor adaptation is also an important step toward optimizing rehabilitation treatments. We have previously shown that the split-belt treadmill can be used to correct walking asymmetries in adults and children with hemiparesis as a result of stroke (Reisman et al. 2007) or hemispherectomy (Choi et al. 2009). However, until the present study, it was unknown which treadmill speeds should be trained to achieve the largest aftereffects. Here we show that the largest aftereffects occur at the speed that was trained on the slow belt. Thus we recommend that, for the purposes of rehabilitation, split-belt training speeds should be selected such that the slow belt speed is near the preferred over-ground walking speed.
GRANTS
This work was supported by National Institutes of Health grants F32 NS-063642 and R01 HD-048741.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank A. Gurbani, R. Pallegadda, and P. Trautman for assistance with data collection.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Arshavsky YI, Gelfand IM, Orlovsky GN, Pavlova GA. Messages conveyed by spinocerebellar pathways during scratching in the cat. II. Activity of neurons of the ventral spinocerebellar tract. Brain Res 151: 493–506, 1978 [DOI] [PubMed] [Google Scholar]
- Bastian AJ. Learning to predict the future: the cerebellum adapts feedforward movement control. Curr Opin Neurobiol 16: 645–649, 2006 [DOI] [PubMed] [Google Scholar]
- Bosco G, Eian J, Poppele RE. Kinematic and non-kinematic signals transmitted to the cat cerebellum during passive treadmill stepping. Exp Brain Res 167: 394–403, 2005 [DOI] [PubMed] [Google Scholar]
- Bosco G, Eian J, Poppele RE. Phase-specific sensory representations in spinocerebellar activity during stepping: evidence for a hybrid kinematic/kinetic framework. Exp Brain Res 175: 83–96, 2006 [DOI] [PubMed] [Google Scholar]
- Choi JT, Bastian AJ. Adaptation reveals independent control networks for human walking. Nat Neurosci 10: 1055–1062, 2007 [DOI] [PubMed] [Google Scholar]
- Choi JT, Vining EP, Reisman DS, Bastian AJ. Walking flexibility after hemispherectomy: split-belt treadmill adaptation and feedback control. Brain 132: 722–733, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson PS. Neuromodulation of central pattern generators in invertebrates and vertebrates. Curr Opin Neurobiol 16: 604–614, 2006 [DOI] [PubMed] [Google Scholar]
- Dingwell JB, Marin LC. Kinematic variability and local dynamic stability of upper body motions when walking at different speeds. J Biomech 39: 444–452, 2006 [DOI] [PubMed] [Google Scholar]
- Grillner S, Halbertsma J, Nilsson J, Thorstensson A. The adaptation to speed in human locomotion. Brain Res 165: 177–182, 1979 [DOI] [PubMed] [Google Scholar]
- Jensen L, Prokop T, Dietz V. Adaptational effects during human split-belt walking: influence of afferent input. Exp Brain Res 118: 126–130, 1998 [DOI] [PubMed] [Google Scholar]
- Kitazawa S, Kimura T, Uka T. Prism adaptation of reaching movements: specificity for the velocity of reaching. J Neurosci 17: 1481–1492, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Mazzoni P, Ghazizadeh A, Ravindran R, Shadmehr R. Generalization of motor learning depends on the history of prior action. PLoS Biol 4: e316, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam T, Dietz V. Transfer of motor performance in an obstacle avoidance task to different walking conditions. J Neurophysiol 92: 2010–2016, 2004 [DOI] [PubMed] [Google Scholar]
- McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev 57: 134–146, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean DL, Masino MA, Koh IY, Lindquist WB, Fetcho JR. Continuous shifts in the active set of spinal interneurons during changes in locomotor speed. Nat Neurosci 11: 1419–1429, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Prism adaptation during walking generalizes to reaching and requires the cerebellum. J Neurophysiol 92: 2497–2509, 2004 [DOI] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci 26: 9107–9116, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KG. Common principles of motor control in vertebrates and invertebrates. Annu Rev Neurosci 16: 265–297, 1993 [DOI] [PubMed] [Google Scholar]
- Poppele RE, Rankin A, Eian J. Dorsal spinocerebellar tract neurons respond to contralateral limb stepping. Exp Brain Res 149: 361–370, 2003 [DOI] [PubMed] [Google Scholar]
- Prokop T, Berger W, Zijlstra W, Dietz V. Adaptational and learning processes during human split-belt locomotion: interaction between central mechanisms and afferent input. Exp Brain Res 106: 449–456, 1995 [DOI] [PubMed] [Google Scholar]
- Reisman DS, Block HJ, Bastian AJ. Interlimb coordination during locomotion: what can be adapted and stored? J Neurophysiol 94: 2403–2415, 2005 [DOI] [PubMed] [Google Scholar]
- Reisman DS, Wityk R, Silver K, Bastian AJ. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain 130: 1861–1872, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman DS, Wityk R, Silver K, Bastian AJ. Split-belt treadmill adaptation transfers to overground walking in persons poststroke. Neurorehabil Neural Repair 23: 735–744, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds RF, Bronstein AM. The moving platform aftereffect: limited generalization of a locomotor adaptation. J Neurophysiol 91: 92–100, 2004 [DOI] [PubMed] [Google Scholar]
- Segers V, Aerts P, Lenoir M, De CD. Spatiotemporal characteristics of the walk-to-run and run-to-walk transition when gradually changing speed. Gait Posture 24: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- Selverston A. General principles of rhythmic motor pattern generation derived from invertebrate CPGs. Prog Brain Res 123: 247–257, 1999 [DOI] [PubMed] [Google Scholar]
- Yanagihara D, Kondo I. Nitric oxide plays a key role in adaptive control of locomotion in cat. Proc Natl Acad Sci USA 93: 13292–13297, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.