Abstract
While connections between inhibitory interneurons are common circuit elements, it has been difficult to define their signal processing roles because of the inability to activate these circuits using natural stimuli. We overcame this limitation by studying connections between inhibitory amacrine cells in the retina. These interneurons form spatially extensive inhibitory networks that shape signaling between bipolar cell relay neurons to ganglion cell output neurons. We investigated how amacrine cell networks modulate these retinal signals by selectively activating the networks with spatially defined light stimuli. The roles of amacrine cell networks were assessed by recording their inhibitory synaptic outputs in bipolar cells that suppress bipolar cell output to ganglion cells. When the amacrine cell network was activated by large light stimuli, the inhibitory connections between amacrine cells unexpectedly depressed bipolar cell inhibition. Bipolar cell inhibition elicited by smaller light stimuli or electrically activated feedback inhibition was not suppressed because these stimuli did not activate the connections between amacrine cells. Thus the activation of amacrine cell circuits with large light stimuli can shape the spatial sensitivity of the retina by limiting the spatial extent of bipolar cell inhibition. Because inner retinal inhibition contributes to ganglion cell surround inhibition, in part, by controlling input from bipolar cells, these connections may refine the spatial properties of the retinal output. This functional role of interneuron connections may be repeated throughout the CNS.
INTRODUCTION
Earlier work in several neural circuits suggests that inhibitory connections between interneurons modulate inhibitory signaling. Inhibitory networks likely tune the spatial extent and timing of inhibition, especially in circuitry that processes sensory signals. Anatomical studies demonstrate connections between interneurons in the insect antennal lobe (Distler et al. 1998), superior colliculus (Schmidt et al. 2001), and visual cortex (Kisvarday et al. 1993). Physiological studies suggest that interneuron connections may modulate spatial visual processing in the thalamus (Sanchez-Vives et al. 1997; Zhu and Lo 1999) and visual cortex (Shevelev et al. 2006). However, the functional roles of these serial connections have been difficult to determine because these inhibitory networks were not anatomically defined and could not be directly physiologically activated. These shortcomings are overcome by using the retina, where inhibitory amacrine cell networks can be naturally activated with light and the basic anatomical circuits, in which they function, are well defined.
We investigated how serial connections between amacrine cell (AC) interneurons shape inhibition to bipolar cells (BCs). BCs are critical relay neurons that connect the input and output stages of the retina. BC output is gated by AC inhibition (Eggers and Lukasiewicz 2006b; Freed et al. 2003; O'Brien et al. 2003; Shields and Lukasiewicz 2003; Volgyi et al. 2002). Although the basic connection between ACs and BCs is well known, this inhibitory gating is complex and not well understood because of the diversity of BC and AC types. BCs receive inhibitory input from GABAergic and glycinergic ACs (Dong and Werblin 1998; Euler and Masland 2000; Lukasiewicz and Werblin 1994; Pan and Lipton 1995), mediated by glycine, GABAA, and GABAC receptors (GABARs)— the latter is a unique type of ionotropic GABAR highly expressed by BCs in the retina (Eggers and Lukasiewicz 2006a; Eggers et al. 2007; Euler and Wässle 1998; Koulen et al. 1998; McCall et al. 2002). Our earlier work demonstrates that AC inhibition varies in different classes of BCs, attributable, in part, to distinct complements of GABA and glycine receptors (Eggers et al. 2007). Additionally, connections between inhibitory ACs, that have been anatomically demonstrated (Dowling and Boycott 1966; Dowling and Werblin 1969; Greferath et al. 1993; Klump et al. 2009; Vaughn et al. 1981; Wong-Riley 1974; Zhang et al. 2004) can shape the magnitude (Eggers and Lukasiewicz 2006a; Eggers et al. 2007) and timing (Roska et al. 1998; Zhang et al. 1997) of inhibition in the retina. However, given the contribution of AC inhibition to receptive field surrounds, surprisingly little is known about how these inhibitory networks affect the spatial processing of visual information in distinct retinal signaling pathways. Here we functionally define these inhibitory networks by recording AC-mediated inhibition in different classes of BCs. Using spatially defined light stimuli, we are able to selectively activate components of the inhibitory networks.
When we activated BC inhibition with light stimuli of varying sizes, we found that connections between ACs limit the spatial extent of BC inhibition. The extent of this shaping varied between different BC pathways. Because BCs contribute to the receptive field surround of ganglion cells (GCs), the spatial tuning of BC inhibition should contribute to spatial processing in the retina.
METHODS
Preparation of mouse retinal slices
Animal protocols were approved by the Washington University School of Medicine Animal Studies Committee. The experimental techniques were similar to those described previously (Eggers and Lukasiewicz 2006a). Mice (C57BL/6J strain; Jackson Laboratories; Bar Harbor, ME) 28–90 days of age were killed using carbon dioxide and their eyes enucleated, and the cornea, lens, and vitreous were removed. The eyecup was incubated for 20 min in dissection and storage solution (see following text) with 0.5 mg/ml hyaluronidase (Sigma, St. Louis, MO). The hyaluronidase solution was replaced with cold, oxygenated storage solution, the retina was dissected out of the eyecup, and 250 μM slices were prepared from the isolated retina and maintained in oxygenated storage solution at room temperature.
Whole cell recordings
Whole cell patch recordings were made from BCs from retinal slices as described previously (Eggers and Lukasiewicz 2006a). Light-evoked inhibitory postsynaptic currents (L-IPSCs) were recorded from retinal BCs voltage clamped to 0 mV, the reversal potential for currents mediated by nonselective cation channels. Feedback-IPSCs (fIPSCs) were recorded by briefly (500 ms) depolarizing a voltage-clamped rod BC from −60 to 0 mV. Because of limitations in the amount of time fIPSCs could be recorded, only one antagonist condition could be recorded in each cell. Liquid junction potentials of 20 mV were corrected at the beginning of each recording. Electrodes were pulled from borosilicate glass (1B150F-4; World Precision Instruments, Sarasota, FL) on a P97 Flaming/Brown puller (Sutter Instruments, Novato, CA) and had resistances of <5 MΩ. Patchit software (White Perch Software, Somerville, MA) was used to generate voltage command outputs and acquire data. The data were digitized and stored with a personal computer using a Labmaster DMA data-acquisition board (Scientific Solutions, Solon, OH). For L-IPSC recordings, mice were dark-adapted overnight, and all dissection and recording procedures were performed under infrared illumination to preserve the light sensitivity of the preparations. L-IPSC recordings were made in extracellular solution heated to 32°C, using thin stage and inline heaters (Cell Microcontrols, Norfolk, VA). fIPSC recordings were made using light-adapted slices at room temperature. Responses were filtered at 1 kHz with the four-pole Bessel filter on the Axopatch 200B (Axon Instruments, Foster City, CA) and sampled at 2 kHz.
Solutions and drugs
The control solution used for dissection, storage contained (in mM) 137 NaCl, 2.5 KCl, 1 MgCl2, 2.5 CaCl2, 28 glucose, and 10 HEPES, was adjusted to pH 7.4 with NaOH and bubbled with O2. The extracellular recording solution used to examine light-evoked currents contained (in mM) 125 NaCl, 2.5 KCl, 1 MgCl2, 1.25 NaH2PO4, 2 CaCl2, 20 glucose, and 26 NaHCO3 and was bubbled with 95% O2-5% CO2. The intracellular solution contained (in mM) 120 Cs gluconate, 1 MgCl2, 10 HEPES, 10 TEA-Cl, 10 phosphocreatine-Na2, 4 Mg-ATP, 0.5 Na-GTP, and 0.1 or 10 EGTA and was adjusted to pH 7.2 with CsOH. Antagonists were applied to the slice chamber using a gravity-driven superfusion system. To isolate aspects of the inhibitory receptor inputs, bicuculline methobromide (50 μM) to block GABAARs, (1,2,5,6-tetrahydropyridine-4yl) methyphosphinic acid (TPMPA, 50 μM) to block GABACRs, and strychnine (500 nM) were used. Unless otherwise indicated, all chemicals were obtained from Sigma (St. Louis, MO).
Morphological identification of retinal cell classes
BCs were labeled with sulforhodamine B (0.005%) dissolved in the intracellular solution. They were classified as either rod, on cone, or off cone BCs, based on their dendritic and axonal morphologies, and the stratification of their somas in the inner nuclear layer and their axon terminals within the on and off sublaminae of the inner plexiform layer (Ghosh et al. 2004). Photomicrographs were recorded using a Photometric Coolsnap ES camera (Roper Scientific, Tucson, AZ) and Metamorph software (Molecular Devices, Downingtown, PA).
Light stimuli
Full-field light stimuli were evoked using a light-emitting diode (LED, Agilent HLMP-3950, λpeak = 565 nm, Palo Alto, CA) positioned near the microscope stage. Stimulus intensity (1.85 × 103 photon·μm2−1·s−1) and duration were controlled by current applied to the LED. Spatially defined light stimuli were generated using VisionWorks software (VRG, Durham, NH) to produce bars of increasing width for mapping spatial fields. These light stimuli were sent to a Plus XGA, DLP Projector (Projection Direct, Poulsbo, WA). The output of the projector was focused onto a fiber optic cable (Schott Fiber Optics, Southbridge, MA) that was appropriately placed to send focused light into the camera port of the Eclipse E600FN microscope (Nikon, Japan). The DLP-generated patterns are then projected onto the surface of the slice using a 4× objective, with an intensity of 0.1 μW/cm2 for cone BCs and 0.005 μW/cm2 for rod BCs. For the area response function (ARF), the charge transfer of L-IPSCs in response to 10 different sizes of light stimuli were used: 25, 75, 125, 175, 225, 275, 325, 425, 625, and 825 μm.
Data analysis and statistics
Clampfit (Axon Instruments, Foster City, CA) software was used to create average response records and to measure the peak, charge transfer (Q, pA*ms) and decay time (D37, defined in the following text) of fIPSCs and the Q of L-IPSCs. To determine changes in current, we measure the charge transfer (Q), which is the integral of current in a cell. This is important because GABAARs and GABACRs mediate currents with very different time courses, and measurements of the peak current may not represent all changes in response magnitude (Eggers and Lukasiewicz 2006b). Because the decay time could not be easily fit with either a single- or double-exponential curve, we determined the decay time by computing the time at which the fIPSC declined to 37% of its peak amplitude (D37). Student's t-test (2-tailed, unequal variance) were used to compare response characteristics before and after drug application. ANOVA tests, with a Scheffe post hoc comparison, were used to determine differences between populations of cells. Differences were considered significant when P ≤ 0.05 and, unless otherwise stated, represent results from a t-test. All average data are reported as means ± SE.
A suppression index (SI) that represents the suppression of L-IPSCs by large light stimuli was calculated from the charge transfer of the response to different stimulus sizes (ARF). The SI is the maximum response divided by the response to the largest light stimulus (825 μm); an index of 1 indicates no suppression. We also determined what light stimulus size gave the maximum L-IPSC for each recorded BC. To determine the response of BC L-IPSCs across a range of stimulus sizes, the Qs of L-IPSCs for each BC were normalized to the maximum Q. The ARFs for each cell were then aligned at their maximum response, and the stimulus sizes were calculated as a percentage of the stimulus size that gave the maximal response to enabling comparisons across BC classes.
To compare recordings across cells in different pharmacological conditions (Fig. 8), average ARFs were fit with various curves using Sigmaplot. Using these curve fitting values, new curves were calculated in Excel. The equation for the modified Gaussian is as follows: F(x) = y0 + a*exp(−0.5*abs[(x − x0)/b^c]), where y0 is the y intercept, x0 is the x value at maximum y, a is the maximum y value, b determines the steepness of the function and c the width of the peak.
Fig. 8.
Suppression by serial connections increases with increasing light stimulus size. A: the average response of rod BCs in control solution where serial synapses are active (as in Fig. 6A1), shows a peak at intermediate light sizes (n = 19). The average response was fit with a modified Gaussian curve to enable comparisons across groups of cells. Responses for all curves were normalized to the maximum control response. B: the average response of rod BC in bicuculline, where serial synapses have been blocked, shows an increase with increasing light size (n = 8). Here the response has been fit with a sigmoidal function for comparison. C: the average GABAAR-mediated contribution to the total light response estimated by subtracting the response in bicucuclline+TPMPA from the response in TPMPA (n = 6). D: using the fitted curves in A–C, we can calculate how the total inhibition to rod BCs when serial connections are blocked is compared with the response when serial connections are active. The average light size at maximum was used to make the x axis. In A–D, the response is normalized to the maximum response in control. In A–C, the stimulus is normalized to light size that gives the maximum response in control.
RESULTS
Serial connections suppress inhibition to rod BCs
We first analyzed the effects of serial connections on rod BCs by using a full-field stimulus that covers the entire retinal slice and activates the extensive AC network. Bath application of the GABAAR antagonist bicuculline increases the charge transfer of inhibition (Q, see methods) onto rod BCs (Fig. 1C, 189 ± 74%, n = 11, P < 0.05). Both GABAARs and GABACRs contribute to GABAergic L-IPSCs in rod BCs (Fig. 1A), so one might expect that blocking GABAARs would decrease inhibition. However, the increase in inhibition elicited by bicuculline was attributed to the enhanced GABAergic signaling between ACs and rod BC terminals that was mediated by the GABAC receptors (Eggers and Lukasiewicz 2006a). These findings suggest that GABAAR-mediated connections between ACs normally suppress lateral rod BC L-IPSCs, in agreement with our previous results (Eggers and Lukasiewicz 2006a; Eggers et al. 2007).
Fig. 1.
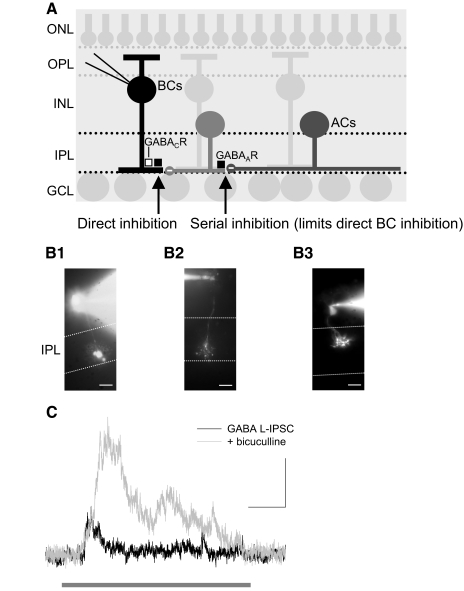
Retinal inhibitory circuits. A: bipolar cells (BCs) in the inner nuclear layer (INL) receive excitatory input from photoreceptors located in the outer nuclear layer (ONL) through synapses in outer plexiform layer (OPL). BCs then release glutamate onto downstream ganglion cells (GCs) and amacrine cells (ACs). BCs also receive direct inhibition from ACs to GABAA (■) and GABAC (□) receptors (R). Inhibitory synapses are marked by a minus sign. This inhibition is regulated by serial connections between inhibitory ACs in the retina, mediated by GABAARs (■). Both direct and serial inhibition modulate glutamate release from BCs onto GCs in the GC layer (GCL), the output neurons of the retina. B: BC types were identified as rod (B1), on cone (B2), or off cone (B3) BCs by labeling with sulforhodamine (scale bar, 10 μm). ···, borders of the IPL. C: GABAergic light-evoked inhibitory postsynaptic currents (L-IPSCs) recorded from a rod BC (in the presence of strychnine). Because GABAAR-mediated serial connections suppress BC inhibition, blocking the connections with a GABAAR antagonist (bicuculline) leads to an increase in L-IPSCs (189 ± 74%, n = 11, P < 0.05). The full-field light-emitting diode (LED) light stimulus (1000 ms) is marked (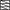 ). Scale bars are 200 ms and 5 pA.
). Scale bars are 200 ms and 5 pA.
Serial connections differentially regulate local and lateral L-IPSCs in all BC classes
Full-field light stimulates a spatially large AC network, leading to the activation of serial connections between ACs (Fig. 1). Narrow-field light stimuli will activate a very small segment of the AC network and may not significantly activate connections between ACs. We tested whether serial connections are selectively regulated by light stimulus size by comparing rod BC L-IPSCs activated by narrow-field (25 μm) and by wide-field (825 μm) light stimuli when serial connections were either intact or disrupted by bicuculline (Fig. 2).
Fig. 2.
Serial connections limit wide-field but not narrow-field BC L-IPSCs. Wide-field (825 μm, A) and narrow-field (25 μm, B) light stimuli (A1 and B1 and thick dark gray bar in traces) were applied to rod, on cone, and off cone BCs. Bicuculline (50 μM) was added to block GABAARs. In all BC types, blocking GABAARs increased the charge transfer (Q) of wide-field L-IPSCs (A and C), suggesting that serial inhibitory connections between ACs limit wide-field light activated L-IPSCs (rod, P < 0.05; on, P < 0.05; off, P < 0.05). In contrast, blocking GABAARs decreased the Q of narrow-field L-IPSCs (B and D), suggesting narrow-field light activated only direct connections (rod, n = 10, P < 0.001; on, n = 6, P < 0.01; off, n = 5, P < 0.05). Inset in B1 is the trace in B1 at an increased scale. The scale bars are 200 ms and 10 pA in A, 1 and 3, B, 1 and 3; 5 pA in A2 and B2. The dotted line represents control in C and D.
Bicuculline significantly increased rod BC L-IPSCs elicited by wide-field light stimuli, suggesting that these responses were normally suppressed by serial connections (Fig. 2A2), similar to our results with full-field stimuli (Fig. 1C). Even though bicuculline blocked GABAARs on the rod BC, the L-IPSCs increased because of augmented GABACR-mediated signaling from disinhibited ACs. In contrast, L-IPSCs elicited by narrow-field light stimuli were not enhanced by the addition of bicuculline. Instead bicuculline decreased the L-IPSCs (Fig. 2B2) by blocking GABAARs on BC terminals. Although the rod BC L-IPSCs in response to narrow-field stimulation were relatively small, the small response amplitudes are attributable to the activation of only a small part of the inhibitory receptive field, and they consistently responded to the application of bicuculline, confirming that they were L-IPSCs. These findings indicate that local rod BC inhibition was not suppressed by GABAAR-mediated serial connections.
There are > 20 types of amacrine cells and ≥10 types of BCs that have been anatomically identified in mammalian retinas (Ghosh et al. 2004; Masland 2001). Different BCs form the parallel signaling pathways in the visual system (Wassle 2004). Variations in AC types and interneuron connections could distinctly regulate parallel signaling pathways in the retina. We focused on the three major classes that include all these BC types. On cone and off cone BCs receive inputs from cone photoreceptors and depolarize in response to increments and decrements of light, respectively (Fig. 1B, 2 and 3). Rod BCs receive inputs from rod photoreceptors and depolarize in response to light increments (Fig. 1B1). We have shown previously that these three major BC pathways receive distinct contributions of inhibitory inputs and may be contacted by separate presynaptic ACs (Eggers et al. 2007). Here we determined whether serial connections between ACs were different in these three BC pathways.
We compared the influence of serial connections on inhibition evoked by narrow and wide-field light stimuli in on and off cone BCs to that in rod BCs. In all BC classes, bicuculline significantly increased L-IPSCs elicited by wide-field light stimuli (Fig. 2, A and C), while bicuculline decreased L-IPSCs elicited by narrow-field light stimuli (Fig. 2, B and D). There was no significant difference among BC classes in their responses to bicuculline for either wide-field (ANOVA P = 0.388) or narrow-field stimulation (ANOVA P = 0.369). Our findings suggest that suppression of inhibition by serial AC connections was common a mechanism across these three BC pathways. This serial inhibition likely regulates the spatial sensitivity of inhibition in the retina. Given the diversity of ACs and the paucity of data on anatomical connectivity, it is possible that separate types of amacrine cells mediate this role in different parallel BC pathways.
Feedback inhibition is not affected by serial connections in rod BCs
Local feedback inhibition (Fig. 3) results from the activation of a single rod BC terminal, which then receives feedback inhibitory input from multiple A17 ACs (Grunert and Hughes 1993; Kim et al. 1998; Nelson and Kolb 1985; Sandell et al. 1989). Because serial connections differentially control narrow- and wide-field light-induced inhibition (Fig. 2), then serial connections may also differentially affect local feedback inhibition and lateral inhibition. However, a previous report on goldfish retina (Vigh et al. 2005) suggests that these local fIPSCs are controlled by serial AC connections; this conflicts with our findings for narrow- versus wide-field-evoked L-IPSCs (Fig. 2). To determine whether local inhibition, like lateral inhibition, is also regulated by serial connections, we elicited local fIPSCs by depolarizing the rod BCs when serial connections were either intact or pharmacologically disrupted.
Fig. 3.
Local feedback inhibition to rod BCs is not suppressed by GABAAR-mediated serial connections. Feedback-IPSCs (fIPSCs) to rod BCs were measured by depolarizing a rod BC briefly (500 ms) from −60 to 0 mV. fIPSCs were partially blocked in different rod BCs by both (1,2,5,6-tetrahydropyridine-4yl) methyphosphinic acid (TPMPA; A and C, n = 5; Q, P < 0.001) and bicuculline (B and C, n = 4; Q, P < 0.001, different cells than in A), suggesting they are mediated by both GABAARs and GABACRs. Blocking GABACRs significantly decreased the decay of the currents (C, P < 0.001), while blocking GABAARs significantly decreased the peak of the currents (C, P < 0.0001). However, blocking GABAARs caused no increase in the currents, suggesting these local fIPSCs are not suppressed by serial connections. Traces were normalized to the baseline at the end of the trace, after current had decayed. Scale bars are 100 ms and 10 pA (A), 20 pA (B).
When we depolarized rod BCs, we evoked GABAergic fIPSCs that were mediated by both GABAARs and GABACRs. Both the GABACR antagonist TPMPA and the GABAAR antagonist bicuculline partially blocked the fIPSC (Fig. 3, A and B). These findings are consistent with previous anatomical and physiological evidence (Dong and Werblin 1998; Fletcher and Wässle 1999; Hartveit 1999; but see Chavez et al. (2006). Unlike lateral inhibition elicited by full- and wide-field light stimuli, the local feedback inhibition was not suppressed by serial connections. Bicuculline, which disrupts serial connections (Fig. 1C), did not increase the fIPSC. Instead bicuculline decreased the fIPSC by a similar amount as TPMPA (Fig. 3C) and showed no evidence of disinhibition, similar to our results using narrow-field light stimuli. Blocking the GABACR-mediated component decreased the decay of the fIPSC, and blocking the GABAAR-mediated component decreased the peak of the fIPSC, consistent with the distinct temporal properties of GABAAR and GABACR-mediated IPSCs (Eggers and Lukasiewicz 2006b). Together with our L-IPSC findings with narrow-field stimuli (Fig. 2), these results suggest that serial connections differentially regulate local and lateral inhibition in rod BCs.
Serial GABAergic and glycinergic AC connections differentially regulate BC pathways
Our findings suggest that ACs receive GABAA receptor-mediated inputs from other GABAergic ACs. Most of the previous studies of the inner plexiform layer suggest that GABACRs are found solely on BCs. However, there is a report of GABACRs on rabbit GCs (Rotollo and Dacheux 2003), raising the possibility that they are also found on some ACs. We tested whether a component of AC L-IPSCs was mediated by GABACRs when we activated the AC network with full-field illumination. If GABACRs mediate inhibition between ACs, then a component of the AC L-IPSCs should be apparent after blocking both GABAA and glycine receptors. Inhibition to ACs was completely blocked by bicuculline and strychnine, indicating that GABAC receptors do not mediate AC L-IPSCs (Fig. 4, A and D, n = 8). Furthermore, AC L-IPSCs were not enhanced after serial connections were disrupted by bicuculline, as we observed in BCs. Instead bicuculline decreased AC L-IPSCs (63 ± 10% of control, n = 16), confirming that GABACRs do not contribute to AC L-IPSCs. These results are in agreement with previous receptor localization (Enz et al. 1996; Koulen et al. 1998) and electrophysiological (Hsueh et al. 2008; Menger and Wässle 2000; Zhou and Dacheux 2004; Zhou and Fain 1995) data.
Fig. 4.
ACs receive inputs onto GABAA and glycine receptors but not GABAC receptors. A: when both glycine and GABAA receptors were blocked, all of the inhibitory current in ACs was blocked. B: in this AC example, little of the current was blocked by strychnine, suggesting it receives primarily GABAAR-mediated. C: in this AC example, little of the current was blocked by bicuculline, suggesting that the current was primarily mediated by glycine receptors. In all figures, the scale bars are 10 pA and 200 ms. D: average proportion of the total current carried by glycine receptors, GABAARs and GABACRs.
Glycine is the other major inhibitory transmitter that is used by roughly half of the ACs. In rabbit, ACs receive inputs from glycinergic and GABAergic ACs (Hsueh et al. 2008). We tested whether this was also the case in mouse by recording AC L-IPSCs after blocking either GABAA or glycine receptors. We found one class of ACs received primarily GABAAR-mediated input. In this AC class, bicuculline blocked most of the current and strychnine had little, if any, effect (Fig. 4B, 14/34 cells had <20% glycine receptor current). Another class of ACs received primarily glycine-receptor-mediated input; strychnine blocked the most of the current, and bicuculline had little, if any effect (Fig. 4C, 5/34 cells had <20% GABAAR current). A third class of AC received a mixture of glycine and GABAAR-mediated inputs (Fig. 4D, 15/34 cells). These results suggest that both glycine receptors and GABAARs mediate serial connections, but their relative contributions vary with AC class.
BCs also receive inputs from narrow-field glycinergic ACs, in addition to inputs from wide-field GABAergic ACs (Eggers et al. 2007). The glycinergic input varied with BC class; off cone BCs received a large glycinergic input, on cone BCs received no glycinergic input, and rod BCs received a minor input. If glycinergic ACs receive GABAergic input, then blocking GABAAR-mediated serial connections could increase glycinergic input to BCs. GABAergic ACs have wide field processes and signal across the IPL within a sublamina while glycinergic ACs have narrow field processes and signal vertically between IPL sublaminae. Thus similar to the situation with GABAAR-mediated serial connections, GABAergic regulation of glycinergic ACs is a lateral, serial inhibition of a local inhibitory connection, as in Fig. 1. Using wide-field light stimuli, we determined whether GABAergic AC connections modulate glycinergic ACs by comparing BC L-IPSCs before and after serial connections were disrupted with bicuculline. We isolated the glycinergic input to BCs by blocking GABACRs with TPMPA.
In rod BCs, bicuculline did not increase the glycinergic L-IPSCs. These findings suggest that GABAAR-mediated serial connections affect only GABA, but not glycine, release onto rod BCs (Fig. 5, A and D) in agreement with our previous results (Eggers and Lukasiewicz 2006a). Because bicuculline also blocks GABAA receptors on rod BCs, the small decrease in the L-IPSC could have obscured a glycine-mediated enhancement. Given the minor GABAAR-mediated inhibition received by rod BC, this offsetting effect is unlikely. We found no evidence for serial inhibition of a glycinergic input to on cone BCs (Fig. 5, B and D), consistent with the observations that on cone BCs do not receive glycinergic input (Eggers et al. 2007; Ivanova et al. 2006). Conversely, in off cone BCs, blocking serial connections with bicuculline increased glycinergic L-IPSCs (Fig. 5, C and D). Thus the increase in L-IPSCs in off cone BCs (Fig. 2), observed when serial connections were blocked was attributable to increased input from both GABAergic and glycinergic ACs. These findings suggest that in addition to asymmetries in excitatory and glycinergic input between on and off cone BCs, there is an additional asymmetry in regulation by serial connections. In off cone BCs, GABAergic and glycinergic inputs are both influenced by wide-field GABAergic ACs, but in on cone bipolar cells, only GABAergic inputs are influenced.
Fig. 5.
GABAAR-mediated serial connections limit only GABACR-mediated input in rod and on cone BCs but also limit glycineR-mediated input in off cone BCs. Light (825 μm) was applied to all BC types. In rod and on cone BCs (A and B), when GABACRs were 1st blocked using TPMPA, adding bicuculline caused no increase in the Q of L-IPSCs (C and D), showing that the GABAAR-mediated serial connections only limit GABACR-mediated inputs (rod, n = 6, P < 0.05; on, n = 4, P < 0.001). In contrast, in off cone BCs, the addition of bicuculline still caused an increase in L-IPSC Q (D) due to the increase in glycine-receptor-mediated inputs (off, n = 4, P < 0.05). The change with bicuculline in the presence of TPMPA was significantly greater in off cone BCs than rod and on cone BCs (ANOVA P < 0.001, Scheffe on vs. off P < 0.001, rod vs. off P < 0.01). Scale bars are 200 ms and 5 pA (A and B; 10 pA (D).
Glycinergic serial circuits also may inhibit GABAergic ACs, as we observed glycinergic inputs to some ACs. If these circuits exist, then their blockade with strychnine should enhance GABAergic inputs to BCs. However, in our experiments strychnine never enhanced inputs to BCs. Instead, strychnine either decreased inhibition (off cone BCs: −58 ± 6%, n = 8; rod BCs: −48 ± 6%, n = 12) or had no effect (on cone BCs: −12 ± 9%, n = 8), suggesting that glycinergic serial circuits do not influence BC inhibition. It is possible that an increase in off cone and rod BC GABAergic inhibition might be obscured by the blockade of glycinergic input to BCs. This is unlikely because, we never observed the expected L-IPSC increase in on cone BCs that lack glycineRs.
Serial connections spatially tune light-evoked inhibition to BCs
If serial connections determine how BC L-IPSCs respond to different light stimulus sizes, then the spatial tuning of inhibition should be different when serial connections are active compared with when they are inactive. In the absence of serial connections, increasing the size of light stimuli should enhance BC L-IPSCs by activating a larger network of ACs. However, if serial connections are functional, then they will limit the spatial extent of the AC network, and L-IPSCs will not increase with light stimulus size.
To determine how serial connections affect the spatial extent of BC inhibition, we recorded L-IPSCs in response to different sizes of light bars (25–825 μm, see methods) when serial connections are either active (control) or blocked (bicuculline). We have normalized the data to the light stimulus giving the maximum response for each cell to more clearly compare the relationships between increasing bar size and the amount of BC inhibition across cell classes. A similar procedure was previously used to compare the receptive field surrounds of GCs (Cook and McReynolds 1998; Sagdullaev and McCall 2005). As different types of BCs may receive inputs from different presynaptic ACs, normalization allows us to compare the behavior of BCs across cell classes (rod, on cone, and off cone) and within cell classes (the multiple types of on and off cone BCs). For all three major classes of BCs, the average area response functions (ARF, see methods) of the L-IPSCs were spatially tuned when serial connections were active. The responses were suppressed at larger bar sizes (Fig. 6A), and the maximum response occurred at intermediate bar sizes. These findings suggest that serial connections limit the extent of inhibition. When serial connections were blocked with bicuculline, spatial tuning was eliminated and L-IPSCs in all BC classes increased with increasing bar size (Fig. 6B). Bicuculline abolished the suppression (Fig. 6B) observed with the largest bar sizes in control conditions (Fig. 6A).
Fig. 6.
The spatial responses of BC L-IPSCs are suppressed at large light stimulus sizes in control conditions but not when serial connections are blocked by bicuculline. Light stimuli of 10 different sizes (25 μm–825 μm) were applied to BCs in control and bicuculline, and the Q of each L-IPSC was measured. A: in all BC types in control, intermediate-sized light stimuli in (examples at 325 μm shown here) gave the maximum L-IPSC (A) instead of the largest light size (825 μm). The average ARFs of all BCs recorded were normalized to the maximum light response for each BC and to the light size where the maximum response was elicited (A4). ARFs showed a peak at an intermediate-sized light stimuli for all BC types in control (rod, n = 19; on, n = 14; off, n = 9). B: when GABAAR-mediated serial connections are blocked, BC L-IPSCs show no suppression. In the same cells as in A, 1–3, bicuculline was added to block serial connections. In contrast to the recordings in control conditions, in all BC types, the largest L-IPSCs were observed at the largest light stimulus size (825 μm) instead of the intermediate-sized light stimuli (325 μm shown here). The average ARFs of all BCs recorded in bicuculline were calculated (B4), and showed an increasing light response with increasing light stimulus size (rod, n = 11; on, n = 5; off, n = 5). This suggests that the spatial tuning seen in control conditions is due to limiting BC L-IPSCs by serial connections. Scale bars are 200 ms and 5 pA (A, 1 and 2, and B, 1 and 2); 25 pA (A3 and B3).
To determine if all BC classes were similarly affected by serial inhibition, we quantified the differences observed in the presence or absence of serial connections by calculating the suppression index (SI, 1 indicates no suppression, see methods). In all BC types, the SI was significantly >1 in control conditions, indicating that large light stimuli suppressed BC inhibition (Fig. 7A). We also determined the actual light stimulus size that gave the maximum response for each BC class to ascertain whether serial inhibition differentially affected the extent of inhibition. The light stimulus size that gave the maximal response in control was significantly smaller than the largest light stimulus used (Fig. 7B). However, when the serial connections were blocked by bicuculline, the SI was always 1, indicating that there was no suppression (Fig. 7A) and that the maximum response was evoked by the largest bar size (Fig. 7B).
Fig. 7.
Serial connections significantly suppress BC L-IPSCs and create a maximum response at intermediate light stimuli sizes. A suppression index (SI, A) and the light stimulus size that elicited the maximum L-IPSC (B) were calculated from the ARFs of each recorded BC in control and bicuculline. A: in control conditions, the SI was significantly >1 for all BC types (rod, n = 19; P < 0.0001; on, n = 14, P < 0.01; off, n = 9, P < 0.01), showing significant suppression of large-sized light stimuli, while in bicuculline, the SI = 1, showing no suppression (rod, n = 11, P = 0.8; on, n = 5, P = 1; off, n = 5, P = 0.3). B: similarly, in control, the size of light stimulus eliciting the maximal L-IPSC was significantly less than the maximum light stimulus size (825 μm) in all BC types (rod, P < 0.0001; on, P < 0.0001; off, P < 0.01). In bicuculline, the light stimulus size that elicited the maximal L-IPSC was not different from 825 μm, the largest size of light stimulus used (rod, P = 0.2; on, P = 1; off, P = 0.2). Thus when serial connections were blocked by bicuculline, the suppression of L-IPSCs by large light stimuli was not observed.
Although all BCs showed the same general trend, there were some differences between the BC classes. We found that the light stimulus size that evoked the maximum control response in off cone BCs was greater than that observed in rod BCs (ANOVA P < 0.05, off vs. rod P < 0.05, on vs. off NS). This difference suggests that the inhibitory AC networks that modulate off cone and rod BC outputs are different.
BC inhibition is created by a balance between direct inhibition and serial inhibition
Because serial connections activated by wide-field illumination are mediated by GABAARs, we estimated the spatial extent of serial inhibition between ACs by comparing the responses of rod BC L-IPSCs in either the absence (Fig. 8A) or the presence of bicuculline (B, bicuculline data were normalized to control data for each cell), where the difference shows inhibition suppressed by serial connections. This comparison underestimates the role of serial inhibition because bicuculline blocks both serial connections between ACs and direct inhibition to rod BCs. To more accurately assess the extent of serial connections, we estimated the amount of GABAAR-mediated direct inhibition to a rod BC. The direct GABAAR component was estimated by subtracting the responses recorded in TPMPA and bicuculline from those recorded in TPMPA (Fig. 8C). There may be some caveats with this calculation because TPMPA can also increase the glutamatergic output of rod BCs, which could in turn increase the GABAergic output of ACs onto BC and other ACs, enhancing both BC inhibition and suppression of BC inhibition simultaneously. However this is unlikely because our previous results from rod BCs in the GABACR null mouse showed no significant increase in GABAA R-mediated L-IPSCs, suggesting this effect is not large (Eggers and Lukasiewicz 2006a). Our previous work demonstrates that the GABACR null mouse is the genetic equivalent of blocking GABACRs with TPMPA as we have showed that no compensatory changes occur in the retina due to the elimination of GABACRs (Eggers and Lukasiewicz 2006a; McCall et al. 2002; Sagdullaev et al. 2006). This method should then give us the closest estimate possible of the direct role of GABAARs on the BCs.
The estimate of the direct GABAAR-mediated inhibition (Fig. 8C) allowed us to more accurately calculate rod BC inhibition in the absence of serial connections. The total inhibition received by a rod BC is the response measured when serial connections were blocked (bicuculline, Fig. 8B), plus the direct GABAAR-mediated response (Fig. 8C) originally missed when serial connections were blocked. With this measure, we can more effectively estimate how serial connections affect the spatial tuning of rod BC L-IPSCs.
Comparison of the total inhibition curve (Fig. 8D) with the curve obtained when serial connections are active shows the differences between the two curves with increasing bar size. The increased divergence between the two curves represents the increased activation of inhibitory connections between ACs. These findings show that the peak of the control light response is determined by the balance of the total inhibition curve and the suppression of inhibition caused by GABAAR-mediated serial connections.
DISCUSSION
Here we show that interneuron circuits shape spatial processing in the retina. These serial circuits limit the spatial extent of BC inhibition by suppressing inhibition in response to large light stimuli. As inhibitory inputs regulate excitation between BCs and GCs, this suggests that large light stimuli will decrease inhibition of BCs, increasing BC excitatory output and subsequently increasing GC spiking. Because the spatial properties of inhibition in the inner retina are important in determining the spatial response characteristics of GCs (Cook and McReynolds 1998; Flores-Herr et al. 2001; Ichinose and Lukasiewicz 2005; O'Brien et al. 2003; Volgyi et al. 2002), these interneuron connections may shape the spatial properties of the retinal output that is sent to higher visual centers. Similar instances of inhibitory interneuron actions have been suggested elsewhere (Sanchez-Vives et al. 1997; Shevelev et al. 2006; Zhu and Lo 1999), suggesting that the interneuronal control of inhibitory circuits may be common across the CNS.
Interneuron circuits regulate the timing and spatial extent of BC inhibition
In addition to the serial inhibitory effects on spatial processing reported here, previous studies in salamander retina show that inhibitory interneuron connections can regulate the timing of retinal signaling. Presynaptic inhibition at BC terminals by GABAergic ACs modulates the timing of excitation in GCs (Dong and Werblin 1998). Serial connections between GABAergic and glycinergic ACs set the kinetics of this presynaptic inhibition in BCs to alter the time course of excitatory signals in GCs (Roska et al. 1998; Zhang et al. 1997). Together with our results, these studies suggest that these anatomically defined interneuron circuits (Dowling and Boycott 1966; Dowling and Werblin 1969; Greferath et al. 1993; Klump et al. 2009; Vaughn et al. 1981; Wong-Riley 1974; Zhang et al. 2004) can modulate both the temporal and spatial aspects of inner retinal inhibition.
Are serial inhibitory circuits common to all BC pathways?
There are many types of BCs and ACs in the mammalian retina. Emerging evidence suggests that there are also functional differences in parallel signaling pathways that may be attributed, in part, to distinct BC and AC circuit elements. In spite of this diversity, there are common features that are present in all rod and on cone BCs and in all off cone BCs. For example, synaptic inputs are mediated in all on BCs by mGlu6Rs and in all off cone BC by ionotropic AMPA/Kainate receptors, respectively.
Do similar common rules exist for inhibitory signaling within the inner plexiform layer? Our findings suggest that this is the case across the major BC pathways. We observed that GABAergic connections between ACs shape the spatial tuning of inhibition in all of the major classes of BC (Fig. 6). Consistent with our model for serial inhibitory circuits, we observed a similar spatially dependent suppression of L-IPSCs in some ACs (data not shown), presumably attributable to GABAergic connections between ACs.
Anatomical basis for spatial tuning
It is difficult to assign the spatial tuning of inhibition that we observed in BCs to specific classes of ACs because the BC L-IPSCs can be attributed to many types of ACs. In the case of on and off cone BCs, the ACs that comprise these circuits are poorly characterized. However, in the case of rod BCs, we know that a significant portion of their GABAergic input comes from the A17 AC. We found that the optimal stimulus that gave the maximum inhibition to rod BCs in our slice preparation was 354 ± 25 μm (Fig. 7). Assuming that the optimal stimulus, centered on the rod BC, activates A17 ACs on either side of the BC, then the stimulus dimensions are well described by the spatial extent of the A17 AC processes, which are ∼150–200 μm (Menger and Wässle 2000). If these A17 ACs are connected to each other to form serial inhibitory networks, then the inhibitory input to rod BCs will decrease as the A17 ACs significantly inhibit each other.
Because our studies focused on inhibitory inputs to BC terminals, the retinal slice was the optimal preparation to record BC L-IPSCs. However, the slice preparation is not without limitations as lateral connections can be cut during slicing. We were aware of the limitations of the slice preparation and used relatively thick retinal slices (250 μm) to maintain spatial connections as much as possible. Furthermore, previous studies have effectively looked at spatial processing using the slice preparation (Cohen 1998; Ichinose and Lukasiewicz 2005; Roska et al. 1998; Zhang and Wu 2009), and several have found qualitatively similar results when directly comparing responses from slice and eyecup preparations (Cook et al. 1998; Zhang et al. 1997). While we present clear evidence for serial interactions, we cannot rule out that the dimensions of these interactions were underestimated by the slicing procedure.
Effects of activating the far GC surround
We have shown that serial circuits shape the spatial profile of BC inhibition. Earlier works demonstrated that BC inhibition contributes to the GC surround (Cook and McReynolds 1998; Flores-Herr et al. 2001; Ichinose and Lukasiewicz 2005; O'Brien et al. 2003). For on GCs, illumination of the receptive field center increases GC spiking, whereas illumination of the receptive field inhibitory surround decreases GC spiking (Barlow and Levick 1976; Enroth-Cugell and Lennie 1975). The inhibitory receptive field is generally thought to increase with increasing distance from the receptive field center up to some maximum value that causes decreases in GC spiking. However, our results suggest that large stimuli will activate serial connections, resulting in a decrease in the inhibitory surround dimensions. This will lead to an increase in GC spiking, instead of the usual suppression of spiking, and may also explain earlier findings.
Previous studies have suggested that the organization of the GC surround is complex. GC responses have been shown to increase when sustained light stimuli extend beyond the receptive field surround, presumably due to the reduction of inhibitory surround input (Li et al. 1992; O'Brien et al. 2003). The reversals of the surround effect in GCs, from suppression to enhancement (Li et al. 1992; O'Brien et al. 2003), began when stimuli exceeded 2–4 visual degrees. This corresponds to a 400- to 800-μm-diam stimulus in the cat retina (Eckhorn et al. 2006), which is in good agreement with our stimulus dimensions that suppress inhibitory inputs to BCs (Fig. 7). O'Brien et al. (2003) attributed the decrease in GC surround responses to serial connections between GABAergic and glycinergic ACs, which might predict glycinergic inputs to on BCs seen in cat that are not present in the mouse. Alternatively, because O'Brien et al. (2003) were recording ganglion cell voltage responses, they could not directly distinguish between presynaptic and direct inhibitory inputs and could have been measuring effects of direct inhibitory inputs to GCs. Despite potential differences due to species and amacrine cell types, this is analogous to the mechanism we propose for the GABAergic inhibitory connections to BCs.
Other studies also show that large or distant surround stimuli can produce increases in GC responses. In cat GCs, rapidly changing stimuli in the far periphery increase GC spiking (Barlow et al. 1977; Geffen et al. 2007; McIlwain 1964; Passaglia et al. 2001), presumably by decreasing surround inhibition. This response modulation was sensitive to barbiturates, suggesting the involvement of GABAARs (McIlwain 1964) in agreement with our results. Although these far surround studies used rapidly changing stimuli, while we used constant stimuli, they still suggest inhibitory interactions of far peripheral stimuli with GC surround. However, because both the studies using constant and changing stimuli did not directly isolate BC inhibition, they were unable to determine how presynaptic modulation of GC inputs affected their spatial sensitivity. By measuring BC inhibition directly, we determined that BC L-IPSCs show spatial tuning that will influence GC responses.
Differences between BC pathways
Rod, on and off cone BC pathways form parallel signaling pathways determined by rod and cone inputs and the distinct postsynaptic filtering of photoreceptor inputs. The kinetics of inputs to BC pathways are specifically shaped by subtypes of glutamate receptors (Ashmore and Copenhagen 1980; DeVries 2000; Schnapf and Copenhagen 1982). In a similar manner, the outputs of these BC pathways are modulated by differing contributions of glycine receptor (Eggers et al. 2007; Ivanova et al. 2006), GABAAR and GABACR (Eggers et al. 2007; Lukasiewicz and Shields 1998; Sagdullaev et al. 2006)-mediated inhibition. Here we show an additional difference between on and off BC pathways, variations in the regulation of BC inhibition by serial synapses. off BCs receive both glycinergic and GABAergic input that is regulated by GABAAR-mediated AC connections. However, in on BCs, only GABAergic input is regulated by serial inhibition. These differences between pathways may explain, in part, differences seen between the center-surround organization of on and off GCs (Chichilnisky and Kalmar 2002). on GCs had a larger excitatory receptive field than off GCs. The excitatory receptive field is a balance between the center and surround responses in GCs. If off BCs inhibition is less spatially tuned by serial inhibition, as in our data suggest, then they will provide more inhibition to off GCs and contribute to a smaller receptive field center.
Roles of interneuron connections in the nervous system
We show that interneuron circuits shape spatial processing in the retina. Similar functional roles may exist in other sensory circuits. Serial connections have been observed in other visual areas of the brain, such as SC (Schmidt et al. 2001), LGN (Sanchez-Vives et al. 1997; Zhu and Lo 1999), and visual cortex (Kisvarday et al. 1993; Shevelev et al. 2006). It is possible that interneuron connections also limit the spatial extent of inhibition in these areas to enhance spatial tuning. Similar connections may also serve a similar role in the insect antennal lobe (Distler et al. 1998), where the inhibitory circuits may enhance differences between olfactory stimuli. These interneuron connections could be a common theme used by different systems to enhance the tuning of sensory signals.
GRANTS
This work was supported by National Eye Institute Grants EY-018131 to E. D. Eggers, EY-08922 to P. D. Lukasiewicz, and EY-02687 to the Washington University Dept. of Ophthalmol and grants from the Research to Prevent Blindness, McDonnell Center for Higher Brain Function, and The M. Bauer Foundation.
ACKNOWLEDGMENTS
We thank members of the Lukasiewicz laboratory and Drs. J.Y. Sebe and V. Kefalov for helpful discussion and comments on this manuscript and J. Debrecht for technical assistance.
REFERENCES
- Ashmore JF, Copenhagen DR. Different postsynaptic events in two types of retinal bipolar cell. Nature 288: 84–86, 1980 [DOI] [PubMed] [Google Scholar]
- Barlow HB, Derrington AM, Harris LR, Lennie P. The effects of remote retinal stimulation on the responses of cat retinal ganglion cells. J Physiol 269: 177–194, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB, Levick WR. Threshold setting by the surround of cat retinal ganglion cells. J Physiol 259: 737–757, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez AE, Singer JH, Diamond JS. Fast neurotransmitter release triggered by Ca influx through AMPA-type glutamate receptors. Nature 443: 705–708, 2006 [DOI] [PubMed] [Google Scholar]
- Chichilnisky EJ, Kalmar RS. Functional asymmetries in on and off ganglion cells of primate retina. J Neurosci 22: 2737–2747, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ED. Interactions of inhibition and excitation in the light-evoked currents of X type retinal ganglion cells. J Neurophysiol 80: 2975–2990, 1998 [DOI] [PubMed] [Google Scholar]
- Cook PB, Lukasiewicz PD, McReynolds JS. Action potentials are required for the lateral transmission of glycinergic transient inhibition in the amphibian retina. J. Neurosci 18: 2301–2308, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook PB, McReynolds JS. Lateral inhibition in the inner retina is important for spatial tuning of ganglion cells. Nat Neurosci 1: 714–719, 1998 [DOI] [PubMed] [Google Scholar]
- DeVries SH. Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron 28: 847–856, 2000 [DOI] [PubMed] [Google Scholar]
- Distler PG, Gruber C, Boeckh J. Synaptic connections between GABA-immunoreactive neurons and uniglomerular projection neurons within the antennal lobe of the cockroach, Periplaneta americana. Synapse 29: 1–13, 1998 [DOI] [PubMed] [Google Scholar]
- Dong CJ, Werblin FS. Temporal contrast enhancement via GABAC feedback at bipolar terminals in the tiger salamander retina. J Neurophysiol 79: 2171–2180, 1998 [DOI] [PubMed] [Google Scholar]
- Dowling JE, Boycott BB. Organization of the primate retina: electron microscopy. Proc Roy Soc Lond B Biol Sci 166: 80–111, 1966 [DOI] [PubMed] [Google Scholar]
- Dowling JE, Werblin FS. Organization of retina of the mudpuppy Inecturus maculosu). I. Synaptic structure. J Neurophysiol 32: 315–338, 1969 [DOI] [PubMed] [Google Scholar]
- Eckhorn R, Wilms M, Schanze T, Eger M, Hesse L, Eysel UT, Kisvarday ZF, Zrenner E, Gekeler F, Schwahn H, Shinoda K, Sachs H, Walter P. Visual resolution with retinal implants estimated from recordings in cat visual cortex. Vision Res 46: 2675–2690, 2006 [DOI] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. GABA(A), GABA(C) and glycine receptor-mediated inhibition differentially affects light-evoked signalling from mouse retinal rod bipolar cells. J Physiol 572: 215–225, 2006a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. Receptor and transmitter release properties set the time course of retinal inhibition. J Neurosci 26: 9413–9425, 2006b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, McCall MA, Lukasiewicz PD. Presynaptic inhibition differentially shapes transmission in distinct circuits in the mouse retina. J Physiol 582: 569–582, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C, Lennie P. The control of retinal ganglion cell discharge by receptive field surrounds. J Physiol 247: 551–578, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enz R, Brandstätter JH, Wässle H, Bormann J. Immunocytochemical localization of the GABAc recptor rho subunits in the mammalian retina. J Neurosci 16: 4479–4490, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Masland RH. Light-evoked responses of bipolar cells in mammalian retina. J Neurophysiol 83: 1817–1829, 2000 [DOI] [PubMed] [Google Scholar]
- Euler T, Wässle H. Different contributions of GABAA and GABAC receptors to rod and cone bipolar cells in a rat retinal slice preparation. J Neurophysiol 79: 1384–1395, 1998 [DOI] [PubMed] [Google Scholar]
- Fletcher EL, Wässle H. Indoleamine-accumulating amacrine cells are presynaptic to rod bipolar cells through GABAC receptors. J Comp Neurol 413: 155–167, 1999 [DOI] [PubMed] [Google Scholar]
- Flores-Herr N, Protti DA, Wassle H. Synaptic currents generating the inhibitory surround of ganglion cells in the mammalian retina. J Neurosci 21: 4852–4863, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed MA, Smith RG, Sterling P. Timing of quantal release from the retinal bipolar terminal is regulated by a feedback circuit. Neuron 38: 89–101, 2003 [DOI] [PubMed] [Google Scholar]
- Geffen MN, de Vries SE, Meister M. Retinal ganglion cells can rapidly change polarity from off to on. PLoS Biol 5: e65, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh KK, Bujan S, Haverkamp S, Feigenspan A, Wässle H. Types of bipolar cells in the mouse retina. J Comp Neurol 469: 70–82, 2004 [DOI] [PubMed] [Google Scholar]
- Greferath U, Muller F, Wässle H, Shivers B, Seeburg P. Localization of GABA{-A} receptors in the rat retina. Vis Neurosci 10: 551–561, 1993 [DOI] [PubMed] [Google Scholar]
- Grunert U, Hughes TE. Immunohistochemical localization of GABAA receptors in the scotopic pathway of the cat retina. Cell Tissue Res 274: 267–277, 1993 [DOI] [PubMed] [Google Scholar]
- Hartveit E. Reciprocal synaptic interactions between rod bipolar cells and amacrine cells in the rat retina. J Neurophysiol 81: 2923–2936, 1999 [DOI] [PubMed] [Google Scholar]
- Hsueh HA, Molnar A, Werblin FS. Amacrine-to-amacrine cell inhibition in the rabbit retina. J Neurophysiol 100: 2077–2088, 2008 [DOI] [PubMed] [Google Scholar]
- Ichinose T, Lukasiewicz PD. Inner and outer retinal pathways both contribute to surround inhibition of salamander ganglion cells. J Physiol 565: 517–535, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova E, Muller U, Wässle H. Characterization of the glycinergic input to bipolar cells of the mouse retina. Eur J Neurosci 23: 350–364, 2006 [DOI] [PubMed] [Google Scholar]
- Kim IB, Lee MY, Oh S, Kim KY, Chun M. Double-labeling techniques demonstrate that rod bipolar cells are under GABAergic control in the inner plexiform layer of the rat retina. Cell Tissue Res 292: 17–25, 1998 [DOI] [PubMed] [Google Scholar]
- Kisvarday ZF, Beaulieu C, Eysel UT. Network of GABAergic large basket cells in cat visual cortex (area 18): implication for lateral disinhibition. J Comp Neurol 327: 398–415, 1993 [DOI] [PubMed] [Google Scholar]
- Klump KE, Zhang AJ, Wu SM, Marshak DW. Parvalbumin-immunoreactive amacrine cells of macaque retina. Vis Neurosci 26: 287–296, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulen P, Brandstatter JH, Enz R, Bormann J, Wassle H. Synaptic clustering of GABA(C) receptor rho-subunits in the rat retina. Eur J Neurosci 10: 115–127, 1998 [DOI] [PubMed] [Google Scholar]
- Li CY, Zhou YX, Pei X, Qiu FT, Tang CQ, Xu XZ. Extensive disinhibitory region beyond the classical receptive field of cat retinal ganglion cells. Vision Res 32: 219–228, 1992 [DOI] [PubMed] [Google Scholar]
- Lukasiewicz PD, Shields CR. Different combinations of GABAA and GABAC receptors confer distinct temporal properties to retinal synaptic responses. J Neurophysiol 79: 3157–3167, 1998 [DOI] [PubMed] [Google Scholar]
- Lukasiewicz PD, Werblin FS. A novel GABA receptor modulates synaptic transmission from bipolar to ganglion and amacrine cells in the tiger salamander retina. J Neurosci 14: 1213–1223, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland RH. The fundamental plan of the retina. Nat Neurosci 4: 877–886, 2001 [DOI] [PubMed] [Google Scholar]
- McCall MA, Lukasiewicz PD, Gregg RG, Peachey NS. Elimination of the ρ1 subunit abolishes GABAC receptor expression and alters visual processing in the mouse retina. J Neurosci 22: 4163–4174, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwain JT. Receptive fields of optic tract axons and lateral geniculate cells: peripheral extent and barbiturate sensitivity. J Neurophysiol 27: 1154–1173, 1964 [DOI] [PubMed] [Google Scholar]
- Menger N, Wässle H. Morphological and physical properties of the A17 amacrine cell of the rat retina. Vis Neurosci 17: 769–780, 2000 [DOI] [PubMed] [Google Scholar]
- Nelson R, Kolb H. A17: A broad-field amacrine cell in the rod system of the cat retina. J Neurophysiol 54: 592–614, 1985 [DOI] [PubMed] [Google Scholar]
- O'Brien BJ, Richardson RC, Berson DM. Inhibitory network properties shaping the light evoked responses of cat alpha retinal ganglion cells. Vis Neurosci 20: 351–361, 2003 [DOI] [PubMed] [Google Scholar]
- Pan Z-H, Lipton SA. Multiple GABA receptor subtypes mediate inhibition of calcium influx at rat retinal bipolar cell terminals. J Neurosci 15: 2668–2679, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passaglia CL, Enroth-Cugell C, Troy JB. Effects of remote stimulation on the mean firing rate of cat retinal ganglion cells. J Neurosci 21: 5794–5803, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roska B, Nemeth E, Werblin FS. Response to change is facilitated by a three-neuron disinhibitory pathway in the tiger salamander retina. J Neurosci 18: 3451–3459, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotolo TC, Dacheux RF. Two neuropharmacological types of rabbit on-alpha ganglion cells express GABAC receptors. Vis Neurosci 20: 373–384, 2003 [DOI] [PubMed] [Google Scholar]
- Sagdullaev BT, McCall MA. Stimulus size and intensity alter fundamental receptive-field properties of mouse retinal ganglion cells in vivo. Vis Neurosci 22: 649–659, 2005 [DOI] [PubMed] [Google Scholar]
- Sagdullaev BT, McCall MA, Lukasiewicz PD. Presynaptic inhibition modulates spillover, creating distinct dynamic response ranges of sensory output. Neuron 50: 923–935, 2006 [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives MV, Bal T, McCormick DA. Inhibitory interactions between perigeniculate GABAergic neurons. J Neurosci 17: 8894–8908, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell JH, Masland RH, Raviola E, Dacheux RF. Connections of indoleamine-accumulating cells in the rabbit retina. J Comp Neurol 283: 303–313, 1989 [DOI] [PubMed] [Google Scholar]
- Schmidt M, Boller M, Ozen G, Hall WC. Disinhibition in rat superior colliculus mediated by GABAc receptors. J Neurosci 21: 691–699, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapf JL, Copenhagen DR. Differences in the kinetics of rod and cone synaptic transmission. Nature 296: 862–864, 1982 [DOI] [PubMed] [Google Scholar]
- Shevelev IA, Lazareva NA, Saltykov KA, Novikova RV, Tikhomirov AS, Sharaev GA, Tsutskiridze DY. The time course of disinhibition of visual cortex neurons and sensitivity to cross-shaped figures. Neurosci Behav Physiol 36: 7–14, 2006 [DOI] [PubMed] [Google Scholar]
- Shields CR, Lukasiewicz PD. Spike-dependent GABA inputs to bipolar cell axon terminals contribute to lateral inhibition of retinal ganglion cells. J Neurophysiol 89: 2449–2458, 2003 [DOI] [PubMed] [Google Scholar]
- Vaughn JE, Famiglietti EV, Jr, Barber RP, Saito K, Roberts E, Ribak CE. GABAergic amacrine cells in rat retina: immunocytochemical identification and synaptic connectivity. J Comp Neurol 197: 113–127, 1981 [DOI] [PubMed] [Google Scholar]
- Vigh J, Li GL, Hull C, von Gersdorff H. Long-term plasticity mediated by mGluR1 at a retinal reciprocal synapse. Neuron 46: 469–482, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgyi B, Xin D, Bloomfield SA. Feedback inhibition in the inner plexiform layer underlies the surround-mediated responses of AII amacrine cells in the mammalian retina. J Physiol 539: 603–614, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassle H. Parallel processing in the mammalian retina. Nat Rev Neurosci 5: 747–757, 2004 [DOI] [PubMed] [Google Scholar]
- Wong-Riley MTT. Synaptic organization of the inner plexiform layer in the retina of the tiger salamander. J Neurocytol 1: 1–33, 1974 [DOI] [PubMed] [Google Scholar]
- Zhang AJ, Wu SM. Receptive fields of retinal bipolar cells are mediated by heterogeneous synaptic circuitry. J Neurosci 29: 789–797, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Chang-Sub J, Slaughter MM. Serial inhibitory synapses in retina. Vis Neurosci 14: 553–563, 1997 [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang HH, Yang CY. Synaptic organization of GABAergic amacrine cells in the salamander retina. Vis Neurosci 21: 817–825, 2004 [DOI] [PubMed] [Google Scholar]
- Zhou C, Dacheux RF. All amacrine cells in the rabbit retina possess AMPA-, NMDA-, GABA-, and glycine-activated currents. Vis Neurosci 21: 181–188, 2004 [DOI] [PubMed] [Google Scholar]
- Zhou ZJ, Fain GL. Neurotransmitter receptors of starburst amacrine cells in rabbit retinal slices. J Neurosci 15: 5334–5345, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, Lo FS. Three GABA receptor-mediated postsynaptic potentials in interneurons in the rat lateral geniculate nucleus. J Neurosci 19: 5721–5730, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]