Abstract
The septohippocampal system has been implicated in the cognitive deficits associated with ethanol consumption, but the cellular basis of ethanol action awaits full elucidation. In the medial septum/diagonal band of Broca (MS/DB), a muscarinic tone, reflective of firing activity of resident cholinergic neurons, regulates that of their noncholinergic, putatively GABAergic, counterparts. Here we tested the hypothesis that ethanol alters this muscarinic tone. The spontaneous firing activity of cholinergic and noncholinergic MS/DB neurons were monitored in acute MS/DB slices from C57Bl/6 mice. Exposing the entire slice to ethanol increased firing in both cholinergic and noncholinergic neurons. However, applying ethanol focally to individual MS/DB neurons increased firing only in cholinergic neurons. The differential outcome suggested different mechanisms of ethanol action on cholinergic and noncholinergic neurons. Indeed, with bath-perfused ethanol, the muscarinic antagonist methyl scopolamine prevented the increase in firing in noncholinergic, but not cholinergic, MS/DB neurons. Thus, the effect on noncholinergic neuronal firing was secondary to ethanol's direct action of acutely increasing muscarinic tone. We propose that the acute ethanol-induced elevation of muscarinic tone in the MS/DB contributes to the altered net flow of neuronal activity in the septohippocampal system that underlies compromised cognitive function.
INTRODUCTION
Ethanol (alcohol) is one of the most widely used and abused of psychoactive substances, exerting wide-ranging yet remarkably specific effects on myriad targets in the CNS (Crews et al. 1996; Valenzuela 1997). The septohippocampal system, connecting the medial septum/diagonal band of Broca (MS/DB) with the hippocampus via the fimbria-fornix, is one such prominent target. Hippocampus-dependent cognitive functions are particularly sensitive and vulnerable to the effects of ethanol consumption (Matthews and Silvers 2004; White et al. 2000). Thus, in humans and in animal models, both acute ethanol intoxication and chronic ethanol consumption impair hippocampus-dependent spatial learning and memory (Melia et al. 1996; Stokes et al. 1991; White et al. 1998). At the cellular level, the literature is replete with reports of ethanol exerting an impressive host of effects on neuronal excitability. In the hippocampus, ethanol alters not only the functional properties of resident neurons but also the activity of afferent inputs, notably the septohippocampal system, that contribute to orchestrating synchronized synaptic operations within its circuitry (Givens and McMahon 1995; Hendricson et al. 2002; Lima-Landman and Albuquerque 1989; Simson et al. 1993; Weiner et al. 1997).
The MS/DB has long been implicated in mnemonic functions (Dutar et al. 1995; Givens and Olton 1994; Olton et al. 1978; Poucet and Herrmann 1990). Memory impairments have been reported following experimental manipulations of the MS/DB and fimbria-fornix (Drachman and Sahakian 1979; Hepler et al. 1985; Kesner et al. 1986; Meck et al. 1987; Warburton 1972). Within the MS/DB, coordinated firing activity of cholinergic and noncholinergic, presumably GABAergic, neurons projecting to the hippocampus (Köhler et al. 1984; Lewis et al. 1967; Rye et al. 1984) underlies the generation of hippocampal theta rhythms and contribute to sustaining hippocampus-dependent forms of learning and memory (Colom 2006; Hasselmo 2005; O'Keefe 1993; Vertes and Kocsis 1997). There are in addition smaller contingents of peptidergic and glutamatergic projection neurons the functional roles of which remain to be elucidated (Colom et al. 2005; Gritti et al. 2006; Peterson and Shurlow 1992; Senut et al. 1989; Sotty et al. 2003).
An early series of studies demonstrated that acute administration of ethanol attenuated hippocampal theta rhythm and long-term potentiation, suppressed spontaneous firing and enhanced GABA-mediated inhibition in MS/DB neurons (Givens 1995; Givens and Breese 1990a,b; Givens and McMahon 1995). However, the acute effects of ethanol on identified neurons and circuit operations within the MS/DB remain outstanding. We and others have shown in rodents that firing rate is a reliable index for discriminating cholinergic versus noncholinergic MS/DB neurons and that the firing activity of the latter neuronal subpopulation is subject to constant regulation by a muscarinic tone that is sustained by the firing of cholinergic neurons (Alreja et al. 2000; Wu and Yeh 2005). Here we asked whether acute exposure to ethanol affected the firing activity of the cholinergic and noncholinergic neuronal subpopulations in the MS/DB and, if so, whether this could be attributed to an altered muscarinic tone.
METHODS
All procedures involving animals were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Dartmouth Medical School Institutional Animal Care and Use Committee.
This study used brain slices containing the MS/DB derived from postnatal day 10–22 C57Bl/6 mice when slice viability and recording conditions were optimal for stable and prolonged monitoring of spontaneous firing activity in MS/DB neurons. In rat, the septohippocampal system, including the MS/DB, acquires adult features around postnatal day 14 (Bender et al. 1996; Linke and Frotscher 1993, 1995; Milner et al. 1983). In mouse, information on the development of the MS/DB is limited (e.g., Ward and Hagg 1999, 2000). However, because the developmental schedule of the mouse CNS precedes that of the rat by ∼2 day (Clancy et al. 2001), the MS/DB in the slices used for our experiments are in a relatively mature state.
Electrophysiology
C57Bl/6 mice (postnatal day 10–22) were asphyxiated by carbon dioxide and decapitated. The brain was quickly removed, blocked by removing the cerebellum and hindbrain, and immersed en bloc in ice-cold oxygenated artificial cerebral spinal fluid (ACSF) containing (in mM) 125 NaCl, 2.5 KCl, 2.0 CaCl2, 1.0 MgCl2, 1.25 NaH2PO3, 26 NaHCO3, and 10 glucose. All solutions were bubbled continuously with 5% CO2-95% O2. Coronal brain slices (200-μm) containing the MS/DB were prepared using an oscillating tissue slicer (Vibroslicer, World Precision Instruments, Sarasota, FL). The slices were incubated in a reservoir of ACSF at room temperature for ≥60 min prior to electrophysiological recording.
During electrophysiological recording, the brain slices were continuously perfused at rate of 0.5 ml/min with oxygenated ACSF maintained at 32–33°C by a heated stage fit onto an upright microscope (BXWI50, Olympus, Melville, NY). A CCD camera attached to a video frame grabber board (Integral Technologies, Indianapolis, IN) facilitated real-time visualization of cells under Hoffman Modulation Optics and placement of recording electrodes and drug pipettes (Fig. 1B). Putative cholinergic and noncholinergic MS/DB neurons were identified on-line by firing rate in the whole cell current-clamp mode (Wu and Yeh 2005); cholinergic cells were confirmed off-line by immunohistochemistry (Fig. 1, E–G).
Fig. 1.
Cholinergic and noncholinergic medial septum/diagonal band of Broca (MS/DB) neurons display different rates of spontaneous firing. A: coronal brain slice (200 μm) from a postnatal day 13 C57Bl/6 mouse with MS/DB outlined. B: a magnified view of the recording setup with a patch recording pipette (right) on the neuron being recorded and a multibarrel pipette assembly (left) used to deliver drugs positioned ∼10 μm from the cell. C: example of an MS/DB neuron filled with Lucifer yellow during recording (C1) displaying a slow firing rate (C2). D: example of an MS/DB neuron filled with Lucifer yellow during recording (D1) displaying a fast firing rate (D2). Scale bars apply to both C and D: vertical = 20 mV; horizontal = 1 s. E–G: the slice was fixed after electrophysiological recording and processed immunohistochemically with an antibody against choline acetyltransferase (ChAT). The cells illustrated in C and D were recovered and are denoted (→, *) in E and F, respectively. The overlay in F indicates that the slow-firing cell illustrated in C, but not the fast-firing cell illustrated in D, is ChAT immunopositive.
Recording pipettes were filled with internal solution containing (in mM) 140 KCl, 1.8 CaCl2, 1.0 MgCl2, and 5.0 HEPES (pH 7.4), 3.0 Mg2+-ATP, and either Lucifer yellow or biocytin (0.1%). Whole cell current and voltage-clamp recordings were conducted using an AxoPatch 200A amplifier (Axon Instruments, Foster City, CA).
Bioelectric activity was amplified, digitized using Clampex v8.0 and analyzed with Clampfit v8.0 (Axon Instruments) and Mini Analysis software (Version 6.01, Synaptosoft, Decatur GA). Statistical analysis was performed using Sigmaplot 8.02 (SPSS, Chicago, IL). Data were reported as means ± SE.
Drugs were dissolved in ACSF and either bath-perfused or loaded into a six-barrel multibarrel pipette assembly and were delivered by means of regulated pressure (Picospritzer, General Valve, Fairfield, NJ). The ejection pressure was routinely adjusted to ≤3 psi to avoid mechanical artifacts due to bulk flow. The drug pipette was visually placed 5–10 μm from the cell being recorded. During control and recovery phases of each recording, ACSF solution was continually applied to control for an ethanol-induced effect and to facilitate wash-out of the drug. In experiments involving focal application, the concentration of ethanol (50 mM) and methyl scopolamine (10 μM) indicated throughout this study are those used to fill the drug barrels and represent the maximal limit to which a cell would have been exposed. With constant perfusion of ACSF during electrophysiological recording, the final concentrations are difficult to ascertain but would be expectedly lower than those in the drug barrels due to dilution factors on ejection.
Immunohistochemistry
Immediately following electrophysiological recording, the recorded brain slices were fixed overnight with 4% paraformaldehyde dissolved in 0.1 M phosphate buffer (PB), then washed with PB, permeabilized with 0.5% Triton X-100/PB and blocked using 10% normal goat serum/PB. The slices were then incubated for ≥48 h in PB containing anti-choline acetyltransferase (ChAT) antibody (1:1,000 dilution; Chemicon/Millipore). The fluorescent goat-anti-rabbit Alexa Fluor 568 (1:800) was used as secondary antibody for incubation overnight. Images were captured using a laser-scanning confocal microscope (FV300, Olympus), and edited using Photoshop 6.0 (Adobe System, San Jose, CA).
RESULTS
Cells in MS/DB slices were selected for recording, and Lucifer yellow or biocytin filled the cells while spontaneous firing rate was monitored. The recorded slices were then fixed and processed for ChAT immunohistochemistry. We found that expression of ChAT immunoreactivity was limited to the slow-firing (≤3 Hz) subpopulation of cells (Fig. 1). This is consistent with our previous study (Wu and Yeh 2005), which also demonstrated that the fast-firing subpopulation of MS/DB neurons typically are GABAergic because they expressed parvalbumin, calbindin, or calretinin. However, other peptidergic and glutamatergic neuronal subpopulations are present (Colom et al. 2005; Gritti et al. 2006; Peterson and Shurlow 1992; Senut et al. 1989; Sotty et al. 2003). Therefore the present study will refer to the slow-firing MS/DB neurons as cholinergic neurons and the fast-firing ones as “noncholinergic neurons” with the recognition that the majority of them are likely to be either GABAergic or glutamatergic neurons because they appear to be the predominant noncholinergic contingent in the MS/DB (Gritti et al. 2006; Sotty et al. 2003).
Bath perfusion of ethanol acutely increases firing in both cholinergic and noncholinergic MS/DB neurons
We first assessed the effect of acute ethanol exposure on the firing rates of cholinergic and noncholinergic MS/DB neurons. The spontaneous firing rates of MS/DB neurons were monitored under whole cell current-clamp conditions, and ethanol was included in the ACSF used to perfuse slices. Because the variability in firing rates precluded a systematic determination of ethanol concentration-response relationship (not shown), we chose to use 50 mM ethanol because this concentration produces acute intoxication and has been shown to affect synaptic and extrasynaptic neuronal activities (Jia et al. 2008). Figure 2 illustrates that exposing the entire slice to perfusion solution containing ethanol increased firing in both cholinergic and noncholinergic neurons. In the examples of ratemeter records taken from a cholinergic (Fig. 2A) and a noncholinergic MS/DB neuron (Fig. 2B), the firing rates increased gradually in response to a 5-min exposure to ethanol (solid line above the rate meter records). In both cholinergic and noncholinergic neurons (Fig. 2, A and B, respectively), a consistent observation was that recovery from the ethanol-induced effect was partial, as the increase in spontaneous firing rate persisted well beyond the termination of acute ethanol application and remained elevated over the duration of the recording (≥30 min). While incomplete wash-out of ethanol could not be ruled out entirely, two lines of evidence indicate that the slowly reversible ethanol-induced effect was not due to a continual run-up of firing activity typically encountered in injured neurons. First in control experiments in which spontaneous firing activity was monitored without exposure to ethanol in cholinergic and noncholinergic MS/DB neurons, the firing rate remained stable over the same length of time (Supplemental Fig. S1).1 Second, the firing activity stabilized, albeit to an elevated level of baseline firing.
Fig. 2.
Bath-perfusion of ethanol increases firing in both cholinergic and noncholinergic MS/DB neurons. A and B: examples of spontaneous firing activity in an individual cholinergic (A) and noncholinergic (B) neuron before, during, and after bath-perfusion of ethanol (50 mM). Insets: summary of firing rates recorded in cholinergic (A; n = 20) and noncholinergic (B; n = 16) neurons before and 5 min after onset of ethanol perfusion. Data are presented as mean firing rates ± SE. Statistics: ANOVA followed by Fisher's PLSD (protected least significant difference). C and D: scatter plot summarizing the change in firing rate in 20 cholinergic (C) and 16 noncholinergic (D) MS/DB neurons before and during exposure to 50 mM ethanol. Each pair of connected points represents data obtained 1 cell. E: time course of the increase in firing rate recorded in cholinergic (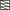 ) and noncholinergic (■) MS/DB neurons after ethanol perfusion (50 mM); ethanol increased firing in cholinergic neurons by 2 min and in noncholinergic neurons after 4 min. Data are presented as percentage of baseline firing, means ± SE, n = 8–12. Statistics: Student's t-test. *, statistically significant differences from baseline.
) and noncholinergic (■) MS/DB neurons after ethanol perfusion (50 mM); ethanol increased firing in cholinergic neurons by 2 min and in noncholinergic neurons after 4 min. Data are presented as percentage of baseline firing, means ± SE, n = 8–12. Statistics: Student's t-test. *, statistically significant differences from baseline.
The rates of spontaneous firing before and 5 min after the onset of ethanol perfusion were quantified, and data obtained from 11 cholinergic neurons and 16 noncholinergic neurons are summarized in Fig. 2, A (inset) and B (inset), respectively. As illustrated in the scatter plots (C and D), ethanol exposure increased the rate of spontaneous firing in 18 of 20 cholinergic neurons (Fig. 2C; 65 ± 15%; means ± SE; P < 0.01, Student's t-test) and in 11 of 16 noncholinergic neurons (D; 15 ± 1.7%; P < 0.01, Student's t-test). Figure 2 also reveals that ethanol increased firing to a greater extent in cholinergic than noncholinergic neurons and the onset of the increased firing differed temporally in the two populations of MS/DB neurons. As shown in Fig. 2C, the onset of ethanol-induced increase in firing was faster in cholinergic neurons than in noncholinergic neurons by ≥2 min. Because, in these experiments, the entire slice was uniformly exposed to ethanol, such differences prompted us to ask whether ethanol exerted its effects on the firing of cholinergic and noncholinergic neurons via different mechanisms. A subsequent set of experiments applying ethanol focally onto individual MS/DB neurons attempted to address this issue.
Focal application of ethanol increases firing only in cholinergic MS/DB neurons
When acutely and focally applied onto individual neurons, ethanol increased spontaneous firing rate only in cholinergic neurons, leaving that of noncholinergic neurons unaffected. Figure 3 illustrates examples of the outcome of an acute, focal exposure to ethanol (50 mM) for a cholinergic (A) and a noncholinergic neuron (B). Data derived from the all of the cholinergic and noncholinergic MS/DB neurons tested are summarized in Fig. 3, A (inset) and B (inset), respectively. Although the degree to which ethanol increased the firing rate of cholinergic neurons varied, the effect was consistently seen in all such neurons examined (11 of 11 cases). The ethanol-induced increase in firing was slowly reversible, typically returning to baseline (preethanol exposure) levels longer than 30 min after the termination of focal ethanol application (not shown). By contrast, focal exposure to ethanol did not affect firing rate in any of the noncholinergic neurons tested (13 of 13 cases).
Fig. 3.
Focal application of ethanol (50 mM) increases firing only in cholinergic MS/DB neurons. Spontaneous firing activity in a cholinergic (A) and noncholinergic (B) neuron before, during, and after focal application of ethanol (50 mM). Insets: summary of firing rate recorded in cholinergic (A; n = 11) and noncholinergic (B; n = 13) neurons before and 2 min after onset of focal ethanol application. Data are presented as mean firing rate ± SE. Statistics: ANOVA followed by Fisher's PLSD. *, statistically significant difference.
Ethanol increases muscarinic tone in the MS/DB
While acute exposure to ethanol could increase firing in both the cholinergic and noncholinergic MS/DB neuronal populations, pending the mode of ethanol exposure, our results prompted us to test the hypothesis that ethanol acts directly on cholinergic neurons but indirectly on the noncholinergic neurons. We postulated that the increase in firing seen in noncholinergic neurons following bath perfusion of ethanol is due to increased muscarinic tone brought about by increased firing activity in cholinergic neurons. If so, then the increase in noncholinergic neuronal firing induced by bath perfusion of ethanol should be mediated by muscarinic receptors and thus blocked in the presence of methyl scopolamine. Indeed this was the experimental outcome. As summarized in Fig. 4, focal application of methyl scopolamine (10 μM) did not block the ethanol-induced increase in firing recorded in cholinergic neurons (A) but blocked that in noncholinergic neurons (B).
Fig. 4.
Methyl scopolamine prevents the acute ethanol-induced increase in spontaneous firing in noncholinergic, but not cholinergic, neurons. Focal application of methyl scopolamine (10 μM) with ethanol (50 mM) bath perfused on cholinergic cells (A) and noncholinergic (B) MS/DB neurons. A: comparisonp of baseline rate of spontaneous firing in cholinergic neurons with firing rates monitored after exposure to methyl scopolamine only, then to methyl scopolamine plus ethanol, and then to ethanol alone. A statistically insignificant trend for methyl scopolamine to increase cholinergic neuronal firing is evident. However, concomitant exposure to methyl scopolamine did not affect the ethanol-induced increase in firing (P = 0.737). B: prevention of the ethanol-induced effect on increasing spontaneous firing in noncholinergic MS/DB neurons by methyl scopolamine (10 μM). Data are derived from a minimum of 8 neurons for each condition and presented as percentage baseline firing, means ± SE, n = 8 −14. Statistics: Student's t-test. *, statistically significant differences.
It should be noted that methyl scopolamine, by itself, tended to increase firing rate in cholinergic MS/DB neurons (Fig. 4A). Although this did not reach statistical significance (P = 0.082, Student's t-test), an issue that arose was whether such an increase might alter ethanol's effect in increasing the firing rate of cholinergic neurons. To address this, the baseline spontaneous firing rates of cholinergic MS/DB neurons (n = 8) were compared with those recorded after exposure to methyl scopolamine alone, methyl scopolamine plus ethanol, and ethanol alone. As summarized in Fig. 4A, muscarinic receptor blockade did not affect the magnitude of the ethanol-induced increase in the firing rate of cholinergic neurons. Importantly, Fig. 4B illustrates that exposure to methyl scopolamine alone suppressed baseline firing in noncholinergic neurons and prevented the increased firing activity normally seen in these cells following exposure to ethanol. Importantly, Fig. 4B illustrates that even a 10-min exposure to methyl scopolamine alone suppressed baseline firing. This is consistent with the notion that the muscarinic receptor antagonist effectively blocked the muscarinic tone that normally maintains firing in noncholinergic MS/DB neurons, thus precluding the effect of bath-perfused ethanol.
DISCUSSION
This study examined the acute effect of ethanol on the firing activity of neurons in the MS/DB. Monitoring spontaneous firing from cholinergic and noncholinergic MS/DB neurons allowed us to interpret our data in light of an ethanol-induced effect on muscarinic tone. The main conclusions of our study are that 1) the firing of both cholinergic and noncholinergic MS/DB neurons are increased on acute exposure to ethanol. 2) Ethanol elevates muscarinic tone by acting directly on cholinergic neurons to increase their firing. 3) The firing of noncholinergic neurons, on the other hand, is itself unaffected by ethanol but increases in response to the elevated muscarinic tone. The idea that MS/DB neurons are not uniformly sensitive to the effects of ethanol is reminiscent of early in vivo studies by Givens and Breese reporting site- and cell-type-specific effects of acute ethanol administration in the medial and lateral septum (Givens and Breese 1990a,b).
The disposition of cholinergic MS/DB neurons in acute and chronic states of ethanol consumption has commanded intense experimental attention in part because much of the muscarinic receptor-mediated activities implicated in learning and memory has been attributed to the activity of cholinergic neurons in the MS/DB and their efferent projections to the hippocampus. Comparatively little is known about how ethanol affects the noncholinergic, notably the GABAergic, contingent, despite the fact that the latter has also been implicated in playing prominent roles in learning and memory and constitutes >30% of the total number of cells in the MS/DB (compared with ∼5% for cholinergic neurons) (Gritti et al. 2006). Muscarinic tone, determined by fluctuations in the firing activity of cholinergic MS/DB neurons and reflecting the ambient level of acetylcholine release, can be assayed by monitoring changes in the firing of GABAergic MS/DB neurons (Alreja et al. 2000; Wu and Yeh 2005). Thus by focusing on muscarinic tone, the present study took into account of not only the activity of cholinergic neurons but also that of the noncholinergic, including the GABAergic neurons that constitute a major component within the neurocircuitry of the MS/DB.
We employed slices derived from peri-weanling mice to optimize the viability of slices for prolonged and stable recording. In this light, despite the fact that the cholinergic and GABAergic neuronal populations are already established in the MS/DB by this time (Bender et al. 1996), this approach did not account for any possible age-dependent differences in sensitivity to ethanol. Indeed numerous studies have compared in human and rodent models the sensitivity of behavioral and neurochemical responses to acute ethanol between various stages of adolescence and adulthood (DeWit et al. 2000; Grant and Dawson 1997; Monti et al. 2005; Spear and Varlinskaya 2005). Overall, the outcomes of these studies have in common the theme that major differences in behavioral sensitivity to acute ethanol between adolescents and adults may be responsible for the young developing the propensity for increased ethanol consumption not only during adolescence but also later in adulthood. Future studies need to examine systematically whether adolescent and adult mice are differentially sensitive to the physiological effects of ethanol consumption in the MS/DB, including those that have been implicated in impaired cognitive and mnemonic functions.
The outcome of the experiments involving bath perfusion or focal application of ethanol proved revealing. With bath perfusion of ethanol, there was an overall increase in MS/DB neuronal firing. However, the time course for the firing increase in noncholinergic neurons lagged behind that of the cholinergic neurons, and this provided the first indication that different mechanisms underlying the ethanol-induced alterations in firing activity might be at play. The results indicated that ethanol acutely increased firing in cholinergic neurons, independent of the mode of delivery. By contrast, noncholinergic neurons increased firing only when whole slices were perfused and exposed to ethanol, and this effect was blocked by methyl scopolamine. These findings, in toto, pointed to two cell type-specific modes of acute ethanol action: a direct effect on cholinergic neurons and an indirect muscarinic receptor-mediated mechanism in noncholinergic neurons that is manifested through an elevated ambient muscarinic tone. In this light, noncholinergic neurons are circuitously influenced by acute ethanol in the MS/DB.
Figure 5 summarizes the results of the present study and proposes a mechanism by which ethanol may acutely elevate muscarinic tone in the MS/DB. Under control conditions (left), intraseptal release of acetylcholine from axon collaterals of hippocampal projecting cholinergic neurons (1) maintains a muscarinic tone that regulates the firing activity of noncholinergic neurons in the MS/DB (2). The noncholinergic neurons (3), in turn, feedback onto the cholinergic neurons to regulate their firing activity (4). Acute ethanol exposure (right) rapidly increases firing in cholinergic neurons (1), which, in turn, enhances acetylcholine release and elevates muscarinic tone (2). The heightened muscarinic tone increases firing in noncholinergic neurons (3), notably the GABAergic and glutamatergic neurons, both of which receive cholinergic innervation (Bialowas and Frotscher 1987; Colom et al. 2005; Van der Zee and Luiten 1994). Within the circuitry of the MS/DB, these findings invoke the following predictions. First the increased firing in noncholinergic excitatory glutamatergic neurons would feed forward to maintain or further elevate firing activity in cholinergic neurons. Second, as depicted in Fig. 5, the increased firing in noncholinergic inhibitory GABAergic neurons would suppress firing activity of cholinergic neurons and muscarinic tone. However, preliminary observations (Yeh, unpublished data) suggest that acute ethanol attenuates GABAA receptor-mediated responses in the postsynaptic cholinergic neurons (hypothetically depicted in Fig. 5 as decreased number of GABAA receptors), and this may offset any effect of acute ethanol exposure on facilitating presynaptic GABA release (4). A muted postsynaptic GABA response thus mitigates the normal complement of inhibitory drive onto the cholinergic MS/DB neurons, resulting in a net disinhibition that increases cholinergic neuronal firing.
Fig. 5.
Model of acute ethanol action on muscarinic tone in the MS/DB. A: simplified schematic illustration of the interplay between cholinergic and noncholinergic neurons. Ambient acetylcholine released from axon collaterals of hippocampal projecting cholinergic neurons (1) maintains a muscarinic tone that regulates firing activity in muscarinic receptor-expressing noncholinergic neurons in the MS/DB (2). The muscarinic receptors are depicted as filled oval figures. The noncholinergic neurons, in turn, innervate cholinergic neurons, suppressing (-) or facilitating (+) firing via GABA or glutamate, respectively. The GABAergic and glutamatergic receptors are depicted as open oval figures. B: On acute ethanol exposure, firing activity in cholinergic neurons increases (1), leading to heightened ambient acetylcholine (muscarinic tone) (2), elevated firing in noncholinergic neurons (3), and enhanced feedback regulation of cholinergic firing activity (4). ?, issues to be addressed in future investigations.
Indeed a key outstanding issue is how ethanol acutely increases the firing activity in cholinergic MS/DB neurons. Our working model (Fig. 5) favored an involvement of the GABAergic system because it is particularly sensitive to modulation by physiologically relevant concentrations of ethanol and has been implicated as a key target for many of ethanol's pre- and postsynaptic actions in the CNS (Grobin et al. 1998; Siggins et al. 2005; Weiner and Valenzuela 2006). In addition, in the MS/DB, cholinergic neurons are innervated by endogenous GABAergic neurons as well as by extra-septal afferents from the hippocampus (Leranth and Frotscher 1989; Tøth et al. 1993). Furthermore, ethanol has been reported to modulate GABA-mediated inhibition in the MS/DB (Givens and Breese 1990a,b; Soldo et al. 1994). Clearly, ethanol influences myriad ion channels, neurotransmitter systems and signaling mechanisms in the CNS. Whether and how these and other related factors come into play to confer ethanol's influence neuronal activity within the operations of the MS/DB circuitry and the septohippocampal system awaits experimental elucidation.
GRANTS
This work was supported by National Institute on Alcohol Abuse and Alcoholism Grant RO1 AA-014698.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Verginia Cuzon for critical reading and critique of the manuscript. P. Yeh provided excellent technical assistance.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Aguayo LG, Peoples RW, Yeh HH, Yevenes GE. GABAA receptors as molecular sites of ethanol action. Direct or indirect actions? Curr Top Med Chem 2: 869–885, 2002 [DOI] [PubMed] [Google Scholar]
- Alreja M, Wu M, Liu W, Atkins JB, Leranth C, Shanabrough M. Muscarinic tone sustains impulse flow in the septohippocampal GABA but not cholinergic pathway: implication for learning and memory. J Neurosci 20: 8103–8110, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender R, Plaschke M, Naumann T, Wahle P, Frotscher M. Development of cholinergic and GABAergic neurons in the rat medial septum: different onset of choline acetyltrasferase and glutamate decarboxylase mRNA expression. J Comp Neurol 372: 204–214, 1996 [DOI] [PubMed] [Google Scholar]
- Bialowas J, Frotscher M. Choline acetyltransferase-immunoreactive neurons and terminals in the rat septal complex: a combined light & electron microscopic study. J Comp Neurol 259: 298–307, 1987 [DOI] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience 105: 7–17, 2001 [DOI] [PubMed] [Google Scholar]
- Colom LV. Septal networks: relevance to theta rhythm, epilepsy, and Alzheimer's disease. J Neurochem 96: 609–624, 2006 [DOI] [PubMed] [Google Scholar]
- Colom LV, Castañeda MT, Reyna T, Hernández S, Garrido-Sanabria E. Characterization of medial septal glutamatergic neurons and their projection to the hippocampus. Synapse 58: 151–164, 2005 [DOI] [PubMed] [Google Scholar]
- Crews F, Morrow AL, Criswell H, Breese G. Effects of ethanol on ion channels. Int Rev Neurobiol 39: 283–367, 1996 [DOI] [PubMed] [Google Scholar]
- DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry 157: 745–750, 2000 [DOI] [PubMed] [Google Scholar]
- Drachman DA, Sahakian BJ. Effects of cholinergic agents on human learning and memory. In: Nutrition and the Brain, edited by Barbeau A, Growden JH, Wurtman RJ. New York: Raven, 1979, vol. 5, p. 351–366 [Google Scholar]
- Dutar P, Bassant MH, Senut MC, Lamour Y. The septohippocampal pathway: structure and function of a central cholinergic system. Physiol Rev 75: 393–427, 1995 [DOI] [PubMed] [Google Scholar]
- Givens BS. Low doses of ethanol impair spatial working memory and reduce hippocampal theta. Alcohol Clin Exp Res 19: 763–767, 1995 [DOI] [PubMed] [Google Scholar]
- Givens BS, Breese GR. Electrophysiological evidence that ethanol alters function of medial septal area without affecting lateral septal function. J Pharmacol Exp Therap 253: 95–103, 1990a [PMC free article] [PubMed] [Google Scholar]
- Givens BS, Breese GR. Site-specific enhancement of γ-aminobutyric acid-mediated inhibition of neural activity by ethanol in the rat medial septal area. J Pharmacol Exp Therap 254: 528–538, 1990b [PMC free article] [PubMed] [Google Scholar]
- Givens B, McMahon K. Ethanol suppresses the induction of long-term potentiation in vivo. Brain Res 688: 27–33, 1995 [DOI] [PubMed] [Google Scholar]
- Givens B, Olton DS. Local modulation of basal forebrain: effects on working and reference memory. J Neurosci 14: 3578–3587, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Ages at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse 9: 103–110, 1997 [DOI] [PubMed] [Google Scholar]
- Gritti I, Henny P, Galloni F, Mainville L, Mariotti M, Jones BE. Stereological estimates of the basal forebrain cell population in the rat, including neurons containing choline acetyltransferase, glutamic acid decarboxylase or phosphate-activated glutaminase and colocalizing vesicular glutamate transporters. Neuroscience 143: 1051–1064, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, Morrow L. The role of GABAA receptors in the acute and chronic effects of ethanol. Psychopharmacology 139: 2–19, 1998 [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. What is the function of hippocampal theta rhythm? Linking behavioral data to phasic properties of field potential and unit recording data. Hippocampus 15: 936–949, 2005 [DOI] [PubMed] [Google Scholar]
- Hendricson AW, Alek Miao CL, Lippmann MJ, Morrisett RA. Ifenprodil and ethanol enhance NMDA receptor-dependent long-term depression. J Pharmacol Exp Therap 301: 938–944, 2002 [DOI] [PubMed] [Google Scholar]
- Hepler DJ, Olton DS, Wenk GL, Coyle JT. Lesions in nucleus basalis magnocellularis and medial septal area of rats produce qualitatively similar memory impairments. J Neurosci 5: 866–873, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F, Chandra D, Homanics DE, Harrison NL. Ethanol modulates synaptic and extrasynaptic GABAA receptors in the thalamus. J Pharacol Exp Therap 326: 475–482, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Crutcher KA, Measom MO. Medial septal and nucleus basalis magnocellularis lesions produce order memory deficits in rats which mimic symptomatology of Alzheimer's disease. Neurobiol Aging 7: 287–295, 1986 [DOI] [PubMed] [Google Scholar]
- Knapp JA, Morris NP, Henderson Z, Matthews RT. Electrophysiological characteristics of non-bursting, glutamate decarboxylase messenger RNA-positive neurons of the medial septum/diagonal band nuclei of guinea pig and rat. Neuroscience 98: 661–668, 2000 [DOI] [PubMed] [Google Scholar]
- Köhler C, Chan-Palay V, Wu J-Y. Septal neurons containing glutamic acid decarboxylase immunoreactivity project to the hippocampal region in the rat brain. Anat Embryol 169: 41–44, 1984 [DOI] [PubMed] [Google Scholar]
- Leranth C, Frotscher M. Organization of the septal region in the rat brain: cholinergic-GABAergic interconnections and the termination of hippocampo-septal fibers. J Comp Neurol 289: 304–314, 1989 [DOI] [PubMed] [Google Scholar]
- Lewis PR, Shute CCD, Silver A. Confirmation from choline acetylase analyses of a massive cholinergic innervation to the rat hippocampus. J Physiol 191: 215–224, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima-Landman MT, Albuquerque EX. Ethanol potentiates and blocks NMDA-activated single-channel currents in rat hippocampal pyramidal cells. FEBS Lett 247: 61–67, 1989 [DOI] [PubMed] [Google Scholar]
- Linke R, Frotscher M. Development of the rat septohippocampal projection: tracing with DiI and electron microscopy of identified growth cones. J Comp Neurol 332: 69–88, 1993 [DOI] [PubMed] [Google Scholar]
- Linke R, Pabst T, Frotscher M. Development of the hippocamposeptal projection in the rat. J Comp Neurol 351: 602–616, 1995 [DOI] [PubMed] [Google Scholar]
- Markram H, Segal M. Electrophysiological characteristics of cholinergic and non-cholinergic neurons in the medial septum-diagonal band complex. Brain Res 513: 171–174, 1990 [DOI] [PubMed] [Google Scholar]
- Matthews DB, Silvers JR. The use of acute ethanol administration as a tool to investigate multiple memory systems. Neurobiol Learn Mem 82: 299–308, 2004 [DOI] [PubMed] [Google Scholar]
- Meck WH, Church RM, Wenk GL, Olton DS. Nucleus basalis magnocellularis and medial septal area lesions differential impair temporal memory. J Neurosci 7: 3505–3511, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melia KR, Ryabinin AE, Corodimas KP, Wilson MC, Ledoux JE. Hippocampal-dependent learning and experience-dependent activation of hippocampus are preferentially disrupted by ethanol. Neuroscience 74: 313–322, 1996 [DOI] [PubMed] [Google Scholar]
- Milner TA, Loy R, Amaral DG. An anatomical study of the development of the septo-hippocampal projection in the rat. Dev Brain Res 8: 343–371, 1983 [DOI] [PubMed] [Google Scholar]
- Monti PM, Miranda R, Jr, Nixon K, Schwartzwelder HS, Tapert SF, White A, Crews FT. Adolescence: booze, brains, and behavior. Alcohol Clin Exp Res 29: 207–220, 2005 [DOI] [PubMed] [Google Scholar]
- O'Keefe J. Hippocampus, theta and spatial memory. Curr Opin Neurobiol 3: 917–924, 1993 [DOI] [PubMed] [Google Scholar]
- Olton DS. The function of septo-hippocampal connections in spatially organized behaviour. Ciba Found Symp 58: 327–349, 1977 [DOI] [PubMed] [Google Scholar]
- Olton DS, Walker JA, Gage FH. Hippocampal connections and spatial discrimination. Brain Res 139: 295–308, 1978 [DOI] [PubMed] [Google Scholar]
- Peterson GM, Shurlow CL. Morphological evidence for a substance P projection from medial septum to hippocampus. Peptides 13: 509–517, 1992 [DOI] [PubMed] [Google Scholar]
- Poucet B, Herrmann T. Septum and medial frontal cortex contribution to spatial problem-solving. Behav Brain Res 37: 269–280, 1990 [DOI] [PubMed] [Google Scholar]
- Rye DB, Wainer BH, Mesulam M-M, Mufson EJ, Saper CB. Cortical projections arising from the basal forebrain: a study of cholinergic and non-cholinergic components employing combined retrograde tracing and immunohistochemical localization of choline acetyltransferase. Neuroscience 13: 627–643, 1984 [DOI] [PubMed] [Google Scholar]
- Senut MC, Menetrey D, Lamour Y. Cholinergic and peptidergic projections from the medial septum and the nucleus of the diagonal band of Broca to dorsal hippocampus, cingulated cortex and olfactory bulb: a combined wheatgerm agglutinin-apohorseradish peroxidase-gold immunohistochemical study. Neuroscienc e30: 385–403, 1989 [DOI] [PubMed] [Google Scholar]
- Siggins GR, Roberto M, Nie Z. The tipsy terminal: presynaptic effects of ethanol. Pharmacol Ther 107: 80–98, 2005 [DOI] [PubMed] [Google Scholar]
- Simson PE, Criswell HE, Breese GR. Inhibition of NMDA-evoked electrophysiological activity by ethanol in selected brain regions: evidence for ethanol-sensitive and ethanol-insensitive NMDA-evoked responses. Brain Res 607: 9–16, 1993 [DOI] [PubMed] [Google Scholar]
- Soldo BL, Proctor WR, Dunwiddie TV. Ethanol differentially modulates GABAA receptor-mediated chloride currents in hippocampal, cortical, and setal neurons in rat brain slices. Synapse 18: 94–103, 1994 [DOI] [PubMed] [Google Scholar]
- Sotty F, Danik M, Manseau F, Laplante F, Quirion R, Williams S. Glutamatergic, cholinergic and GABAergic neurons contribute to the septohippocampal pathways and exhibit distinct electrophysiological properties: novel implications for hippocampal rhythmicity. J Physiol 551: 927–943, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Dev Alcohol 17: 143–159, 2005 [PubMed] [Google Scholar]
- Stokes A, Belger AF, Belger A, Banich MT, Taylor H. Effects of acute aspartame and acute ethanol ingestion upon the cognitive performance of pilots. Aviat Space Environ Med 62: 648–653, 1991 [PubMed] [Google Scholar]
- Tøth K, Borhegyi Z, Freund TF. Postsynaptic targets of GABAergic hippocampal neurons in the medial septum-diagonal band of Broca complex. J Neurosci 13: 3712–3724, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela CF. Alcohol and neurotransmitter interactions. Alcohol Health Res World 21: 144–148, 1997 [PMC free article] [PubMed] [Google Scholar]
- Van der Zee EA, Luiten PG. Cholinergic and GABAergic neurons in the rat medial septum express muscarinic acetylcholine receptors. Brain Res 652: 263–272, 1994 [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B. Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience 81: 893–926, 1997 [DOI] [PubMed] [Google Scholar]
- Ward NL, Hagg T. p75 (NGFR) and cholinergic neurons in the developing forebrain: a re-examination. Brain Res Dev Brain Res 118: 79–91, 1999 [DOI] [PubMed] [Google Scholar]
- Ward NL, Hagg T. BDNF in needed for postnatal maturation of basal forebrain and neostriatum cholinergic neurons. Exp Neurol 162: 297–310, 2000 [DOI] [PubMed] [Google Scholar]
- Warburton D. The cholinergic control of internal inhibition. In: Inhibition and Learning, edited by Boakes RA, Halliday MS. New York: Academic, 1972, p. 431–458 [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Therapy 111: 533–554, 2006 [DOI] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF, Watson PL, Frazier CJ, Dunwiddie TV. Elevation of basal protein kinase C activity increases ethanol sensitivity of GABAA receptors in rat hippocampal CA1 pyramidal neurons. J Neurochem 68: 1949–1959, 1997 [DOI] [PubMed] [Google Scholar]
- White AM, Elek TM, Beltz TL, Best PJ. Spatial performance is more sensitive to ethanol than nonspatial performance regardless of cue proximity. Alcohol Clin Exp Res 22: 2102–2107, 1998 [PubMed] [Google Scholar]
- White AM, Matthews DB, Best PJ. Ethanol, memory and hippocampal function: a review of recent findings. Hippocampus 10: 88–93, 2000 [DOI] [PubMed] [Google Scholar]
- Wu C-W, Yeh HH. Nerve growth factor rapidly increases muscarinic tone in mouse medium septum/diagonal band of Broca. J Neurosci 25: 4232–4242, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







