Abstract
The hypothalamic paraventricular nucleus (PVN) and rostral ventrolateral medulla (RVLM) are key components of a neural network that generates and regulates sympathetic nerve activity (SNA). Although each region has been extensively studied, little is presently known about the in vivo discharge properties of individual PVN neurons that directly innervate the RVLM. Here extracellular recording was performed in anesthetized rats, and antidromic stimulation was used to identify single PVN neurons with axonal projections to the RVLM (n = 94). Neurons were divided into two groups that had either unbranched axons terminating in the RVLM (i.e., PVN-RVLM neurons, n = 65) or collateralized axons targeting both the RVLM and spinal cord [i.e., PVN-RVLM/intermediolateral cell column (IML) neurons, n = 29]. Many PVN-RVLM (32/65, 49%) and PVN-RVLM/IML (17/29, 59%) neurons were spontaneously active. The average firing frequency was not different across groups. Spike-triggered averaging revealed that spontaneous discharge of most neurons was temporally correlated with renal SNA (PVN-RVLM: 12/21, 57%; PVN-RVLM/IML: 6/9, 67%). Time histograms triggered by the electrocardiogram (ECG) R-wave indicated that discharge of most cells was also cardiac rhythmic (PVN-RVLM: 25/32, 78%; PVN-RVLM/IML: 10/17, 59%). Raising and lowering arterial blood pressure to increase and decrease arterial baroreceptor input caused a corresponding decrease and increase in firing frequency among cells of both groups (PVN-RVLM: 9/13, 69%; PVN-RVLM/IML: 4/4, 100%). These results indicate that PVN-RVLM and PVN-RVLM/IML neurons are both capable of contributing to basal sympathetic activity and its baroreflex modulation.
INTRODUCTION
Neurons of the hypothalamic paraventricular nucleus (PVN) control many important homeostatic functions, including release of pituitary hormones (Swanson 1991; Swanson and Sawchenko 1980; Vigas 1989) and regulation of ingestive behavior/metabolism (Konturek et al. 2005; Valassi et al. 2008). Over the past several years, studies have increasingly focused on the role of PVN neurons in regulating sympathetic nerve activity (SNA) (Akine et al. 2003; Allen 2002; Coote et al. 1998; Li and Pan 2007; Li et al. 2006; Lovick and Coote 1988b; Porter and Brody 1985; Stern 2004; Toney et al. 2003). Early studies showed that stimulation of the PVN reduces visceral organ blood flow and increases arterial blood pressure (ABP) (Martin and Haywood 1992; Porter and Brody 1985, 1986), whereas disinhibition of the PVN by GABAA receptor blockade increases ABP, heart rate, and plasma norepinephrine concentration (Martin and Haywood 1993; Martin et al. 1991). Collectively, these observations indicate that PVN neurons can increase SNA, although ongoing synaptic inhibition limits their influence on basal SNA.
In spite of receiving strong tonic inhibitory input (Chen and Toney 2003b; Chen et al. 2003; Kenney et al. 2003; Li et al. 2006; Martin et al. 1991), PVN neurons nevertheless do contribute to ongoing SNA. In anesthetized rats, for example, studies have shown that acute inhibition of PVN neuronal activity or blockade of excitatory inputs reduces ongoing renal SNA (RSNA) (Akine et al. 2003; Allen 2002; Stocker et al. 2004b, 2005), lumbar SNA (Stocker et al. 2005), and ABP (Akine et al. 2003; Allen 2002; Freeman and Brooks 2007; Stocker et al. 2004b, 2005). It is noteworthy that reductions of SNA in response to PVN inhibition are more pronounced in water-deprived (Freeman and Brooks 2007; Stocker et al. 2004b, 2005) and hypertensive (Akine et al. 2003; Allen 2002; Li and Pan 2007) rats, suggesting an increased contribution of PVN neuronal activity to the maintenance of resting SNA.
Anatomical studies have identified several groups of sympathetic-regulatory PVN neurons. One group monosynaptically targets the spinal intermediolateral cell column (IML; PVN-IML), the location of sympathetic preganglionic neurons (Saper et al. 1976; Swanson and Sawchenko 1980). Another group innervates presympathetic neurons in the rostral ventrolateral medulla (RVLM; PVN-RVLM) (Pyner and Coote 2000; Shafton et al. 1998; Stocker et al. 2006; Swanson and Sawchenko 1980). A third and more recently identified group has branched axons that innervate both the RVLM and IML (PVN-RVLM/IML) (Pyner and Coote 2000; Shafton et al. 1998; Stocker et al. 2004a).
In vivo electrophysiological studies performed to date have largely focused on the discharge behavior of PVN-IML neurons (Bains and Ferguson 1995; Bains et al. 1992; Chen and Toney 2003a; Lovick and Coote 1988a,b). They indicate that many are quiescent in anesthetized rats and those with spontaneous activity most often fire slowly (≤1.5 spike/s) at rest (Bains and Ferguson 1995; Bains et al. 1992; Chen and Toney 2003a; Lovick and Coote 1988a,b). In response to local application of various neurotransmitters (Bains and Ferguson 1995; Cato and Toney 2005; Chen and Pan 2006; Chen et al. 2006; Lee et al. 2008; Li et al. 2004; Lovick and Coote 1988a) and inputs activated by circulating hormones (e.g., angiotensin II, ANP) (Bains and Ferguson 1995; Bains et al. 1992; Cato and Toney 2005; Lovick and Coote 1988a), high-frequency discharge can be evoked. There is also evidence that some spontaneously active PVN-IML neurons are targeted by inhibitory inputs from arterial (Bains and Ferguson 1995; Chen and Toney 2003a; Lovick and Coote 1988b) and cardiopulmonary baroreceptors (Lovick and Coote 1988a,b). However, these inhibitory inputs do not appear to account fully for the lack of tonic discharge exhibited by many PVN-IML neurons (Chen and Toney 2003a; Lovick and Coote 1988a,b).
In marked contrast to PVN-IML neurons, few data are available concerning the in vivo discharge properties of PVN neurons that target the RVLM (Barman 1990). This is surprising given that sympathoexcitatory challenges such as water deprivation (Stocker et al. 2004a, 2006) and hemorrhage (Badoer and Merolli 1998; Badoer et al. 1993) have been shown to induce c-fos expression in PVN-RVLM neurons. Moreover, hemorrhage increases RSNA by stimulating ionotropic glutamate and vasopressin V1a receptors in the spinal cord (Yang and Coote 2006), possibly via recruitment of PVN-RVLM/IML neurons. Finally, PVN-RVLM neurons are largely glutamatergic (Stocker et al. 2006) and activation of the PVN increases the discharge of RVLM vasomotor neurons (Yang et al. 2001). Here extracellular single-unit recordings were performed in anesthetized rats to test the hypothesis that the patterning of discharge of PVN-RVLM and PVN-RVLM/IML neurons is consistent with their contributing to both the generation of resting SNA and its reflex modulation.
Individual PVN-RVLM and PVN-RVLM/IML neurons were identified using antidromic activation. To establish a possible functional link between discharge and control of ongoing SNA, spike-triggered averaging was used to determine the temporal relationship between spontaneous firing and RSNA. To further investigate whether PVN-RVLM and PVN-RVLM/IML neurons have the capacity to regulate SNA and ABP, the temporal relationship between spontaneous discharge and the cardiac cycle was determined using electrocardiogram (ECG) R-wave-triggered time histogram analysis. Finally, a role for PVN neurons in reflex control of ABP was evaluated by recording neuronal responses to loading and unloading of arterial baroreceptors.
METHODS
General procedures
Experiments were performed on 57 male Sprague-Dawley rats (350–450 g) (Charles River Laboratories) that were housed in a temperature-controlled room (22–23°C) with a 14:10 h light-dark cycle (lights on at 07:00 h). Tap water and laboratory chow (Harlan Teklad LM-485, 0.3% NaCl) were available ad libitum. Rats were anesthetized with a mixture of α-chloralose (80 mg/kg ip) and urethan (800 mg/kg ip) and catheters (PE-50 tubing) were placed in a femoral artery and vein to record ABP and administer drugs. Heart rate (HR) was obtained from an ECG (lead I). After tracheal cannulation, rats were artificially ventilated with oxygen-enriched room air and end-tidal pCO2 was monitored and maintained between 40 and 50 mmHg. Rats were then paralyzed with gallamine triethiodide (5 mg·kg−1·h−1 iv). Before paralysis, an adequate depth of anesthesia was assessed by the absence of limb withdrawal in response to noxious pinching of the foot. Thereafter adequacy of anesthesia was indicated by the lack of a pressor/tachycardic response to the foot pinch. Supplemental anesthetic (10% of initial dose) was given when indicated. Body temperature was maintained at 37 ± 1°C. All surgical and experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio.
Recording RSNA
Rats were placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA) and a flank incision was made. A left renal nerve bundle was isolated and activity was recorded as previously described (Chen and Toney 2003a,b; Stocker et al. 2005). Nerve signals were acquired using a high-impendence head-stage connected to an AC amplifier equipped with half-amplitude frequency filters (band-pass: 30–1,000 Hz) and a 60-Hz notch filter. Signals were amplified (10,000- to 20,000-fold), full-wave rectified, integrated (time constant: 0.3–3.0 s), and digitized at a frequency of 1.0 kHz. Spike2 software (v5.16, Cambridge Electronic Design, Cambridge, UK) was used to display and analyze digitized RSNA.
Extracellular single-unit recording
With the skull leveled between bregma and lambda, a craniotomy was performed to gain access to the PVN. Microelectrodes were pulled from borosilicate glass capillaries (1.2 mm OD, 0.86 mm ID) and filled with 0.15 M NaCl containing 2% Chicago sky blue dye. Electrode resistance was measured in vivo and averaged 25–35 MΩ. Electrodes were lowered vertically into the PVN at the following stereotaxic coordinates (in mm): −1.6 to −2.3 mm from bregma, 0.2–0.8 mm lateral to midline, and 7.2–mm ventral to the brain surface (Chen and Toney 2001, 2003a,b; Chen et al. 2003). The PVN was probed for single-unit activity by advancing the recording electrode in 2-μm vertical steps with a piezoelectric micropositioner (EXFO-Burleigh, Quebec, Quebec, Canada). Recordings were made using an Axoclamp 2B amplifier in bridge mode. Signals were passed to a differential AC amplifier (Grass, P15D) and band-pass filtered between 0.3 and 3.0 kHz. Processed signals were led to an audio monitor, oscilloscope, and window discriminator. Each spike that crossed the window discriminator level (set to exclude background noise) generated a single TTL pulse. Pulses were used to construct rate-meter records and time histograms of cell discharge and to generate spike-triggered averages of RSNA. Data were digitized (1.0–5.0 kHz), and analyzed using Spike2 software (v5.16, Cambridge Electronic Design, Cambridge, UK).
Antidromic stimulation
To identify RVLM projecting PVN neurons in vivo, antidromic activation was used. A burr hole was made in the occipital bone and a concentric bipolar stimulating electrode (500 μm OD, tip tapered at 60°; FHC, Bowdoin, ME) was positioned in the RVLM at the following stereotaxic coordinates (in mm): −12.5 to −12.9 from bregma, 1.6–1.9 lateral to midline, and 8.5–9.2 ventral to the surface of cerebellum. The final position of the electrode was the site from which a significant increase of RSNA and a 15- to 20-mmHg increase of mean ABP (MAP) were recorded in response to microinjection of l-glutamate (0.1 nmol in 50 nl).
To identify PVN neurons with axons projecting to the spinal cord, a dorsal laminectomy was performed to expose the C2 spinal segment and an array of three monopolar electrodes, each separated by ∼0.5 mm, was positioned to span the entire dorsolateral funiculus on the side ipsilateral to the PVN recording site (the indifferent pole of the electrode was attached to the trachealis muscle). The electrode assembly was inserted to a depth of 0.5–0.7 mm.
Because the focus of these experiments was on RVLM projecting PVN neurons, standard tests (Chen and Toney 2003a; Lipski 1981; Stocker and Toney 2005, 2007) were first used to assess the antidromic nature of spikes evoked by stimulating the ipsilateral RVLM. Units were considered to be antidromically activated when each stimulus pulse (0.1 ms, 1 Hz, 100 – 850 μA) evoked a single constant-latency spike at a discrete stimulus threshold (i.e., all-or-none response). Antidromicity was confirmed in many cases when a single spike was recorded for each pulse in a high-frequency stimulus train (i.e., 200–300 Hz, 3 pulses). For spontaneously active neurons, evoked spikes were tested for cancellation by collision with spontaneous action potentials (i.e., the collision test). Once an antidromic response was recorded, RVLM stimulus intensity was increased gradually above threshold to determine if a discontinuous decrease (>1 ms) in the antidromic onset latency could be detected. Such antidromic latency “jumps” occur because of local axon branching and indicate the presence of a terminal arbor in the vicinity of the RVLM stimulating electrode.
Tests were then performed to determine if an antidromic response could be recorded from the same cell by stimulating the spinal cord. To guard against classification errors, large-amplitude stimuli (up to ∼1.5 mA) were applied to each electrode in the spinal array to minimize the possibility that spinal axons, although present, would fail to be activated. Neurons that had an antidromic response to stimulation of the ipsilateral RVLM but not the IML were initially classified as PVN-RVLM neurons. Those that had an antidromic response to stimulation of the RVLM and spinal cord were classified as having branched axons targeting both the RVLM and IML. These were designated PVN-RVLM/IML neurons. Reciprocal collision testing (Amri et al. 1990; Klemfuss et al. 1987; Lipski 1981) was performed in some cases to confirm correct classification of PVN-RVLM/IML neurons. Neurons that had maximum reciprocal collision intervals exceeding the collision interval predicted for a single unbranched axon segment were formally classified as PVN-RVLM/IML neurons.
Sympathetic-related discharge
Spike-triggered averaging was used as previously described (Barman 1990; Barman and Gebber 1982; Chen and Toney 2003a; Stocker and Toney 2005, 2007) to determine the temporal correlation between spontaneous action potentials and postganglionic RSNA. For each recorded neuron, the spike-triggered average of simultaneously recorded RSNA was compared with an average constructed from the same RSNA data but triggered by randomly generated TTL pulses. The number and frequency of TTL triggers were equal to the number and frequency of spontaneous spikes used to generate each corresponding spike-triggered RSNA average.
Cardiac-related discharge
To determine the temporal relationship between ongoing PVN neuronal discharge and the cardiac cycle, ECG R-wave triggered time histogram analysis was used as previously described (Chen and Toney 2003a; Stocker and Toney 2005, 2007). Correlated discharge occurred after the R-wave trigger, which was set to time (bin) zero. A post R-wave increase or decrease in activity ≥20% was considered to indicate cardiac-related activity.
Barosensitivity
To test the response of individual PVN-RVLM and PVN-RVLM/IML neurons to changes in ABP, we recorded discharge responses to increasing and decreasing ABP with phenylephrine (5–10 μg iv) and sodium nitroprusside (10–20 μg iv), respectively.
Histology
The location of each recorded PVN neuron was marked by iontophoretic deposition of Chicago sky blue dye (−5 μA, 10 min), which produced a spheroid spot ∼50–75 μm in diameter. After each experiment the brain was removed and placed in 4% paraformaldehyde for several days. The hypothalamus containing the PVN was then cut into 40-μm-thick coronal sections. The site of each unit recording was determined under brightfield microscopy and plotted on plates of a rat brain atlas (Paxinos 1998) that corresponded to the correct rostro-caudal plane.
Data analysis
Spontaneous firing frequency of each recorded neuron was determined from ≥60 s of data from the rate-meter record (bin size: 1.0 s). Axonal conduction velocity (CV; ms-1) was calculated as the ratio of the distance between the recording and stimulation sites and the antidromic onset latency (axonal refractory period was not taken into account). To evaluate reciprocal collision test data for PVN-RVLM/IML neurons, the collision interval predicted for an unbranched axon segment was estimated by dividing the linear distance between the RVLM and IML stimulation sites by the average axonal CV. The axon of a given neuron was deemed to collateralize and innervate the RVLM and spinal cord when collision of RVLM- and spinal-cord-evoked antidromic spikes occurred at stimulus intervals greater than that estimated for an unbranched axon.
To evaluate barosensitivity of unit discharge, firing rate achieved following each increase or decrease of ABP was compared with 30 s of baseline discharge recorded just prior to the change of ABP. Response data were compared using the paired Student's t-test. Changes in firing rate in response to increases and decreases of ABP were compared across groups of neurons using the unpaired Student's t-test.
To assess cardiac cycle-related activity from ECG R-wave triggered time histograms, we first constructed an interval histogram of R-wave trigger events. From this we determine the average duration (in ms) of the cardiac cycle. Spike data over the same post R-wave period in time histograms were then analyzed. For this purpose, spike data were placed in bins (10 ms), and those bins with spike values that exceeded by ≥20% the average spikes/bin for the entire cardiac cycle were considered to represent cardiac cycle-related activity. For statistical comparison using the paired Student's t-test, spike data from time histograms were expressed as spike/s.
To determine the temporal correlation between cell discharge and RSNA, the peak amplitude of RSNA in spike triggered averages was compared with the voltage at the same latency in randomly triggered “dummy” averages (see preceding text). Triggered SNA averages were constructed from RSNA recorded from 500 ms before to 500 ms after the occurrence of each trigger event (cell discharge or random TTL pulse). The occurrence of each trigger event was defined as time 0 in the assembled average. Correlated activity was taken as the posttrigger data segment that exceeded the mean value of the corresponding dummy average by ≥3-fold over the same posttrigger time interval. Statistical differences between these values were compared using the paired Student's t-test.
To compare between-group (PVN-RVLM vs. PVN-RVLM/IML) differences in recorded variables and unit responses, a nonparametric Wilcoxon test was used. The proportion of PVN-RVLM and PVN-RVLM/IML neurons that exhibited sympathetic- and/or cardiac-related spontaneous discharge or a response to changes in baroreceptor input were compared using Fisher's exact test. Mean differences were considered significant at a critical value of P < 0.05. Data are report as the means ± SE.
RESULTS
RVLM stimulation
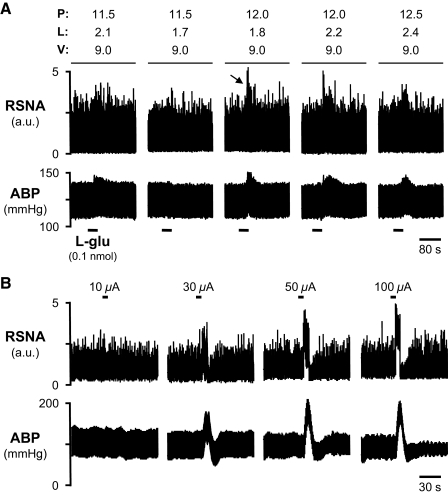
The location of RVLM was determined in each animal as the site from which microinjection of l-glutamate (Fig. 1A) elicited large-amplitude increases of RSNA and ABP (middle). Increases of mean ABP (MAP) ≥15 mmHg were considered to identify the RVLM. The microinjection pipette was withdrawn and replaced with an electrode for antidromic stimulation. From this location, graded intensities of high-frequency electrical stimulation evoked short-latency, graded increases of ABP (Fig. 1B).
Fig. 1.
Determination of the antidromic stimulation site in rostral ventrolateral medulla (RVLM). A: data traces of renal sympathetic nerve activity (RSNA, top) and arterial blood pressure (ABP, bottom) responses to l-glutamate microinjection (0.1 nmol in 50 nl) at specific stereotaxic coordinates within the RVLM region. In this animal, the largest responses (→, middle) occurred at stereotaxic coordinates of 12.0 mm posterior to bregma (P), 1.8 mm lateral to midline (L), and 9.0 mm ventral to the brain surface (V). B: in the same animal, graded RSNA and ABP responses were evoked by graded electrical stimulation (≤100 μA, 1 ms, 50 Hz, 5 s) of RVLM at the coordinates indicated in A (middle).
Identification of PVN-RVLM neurons
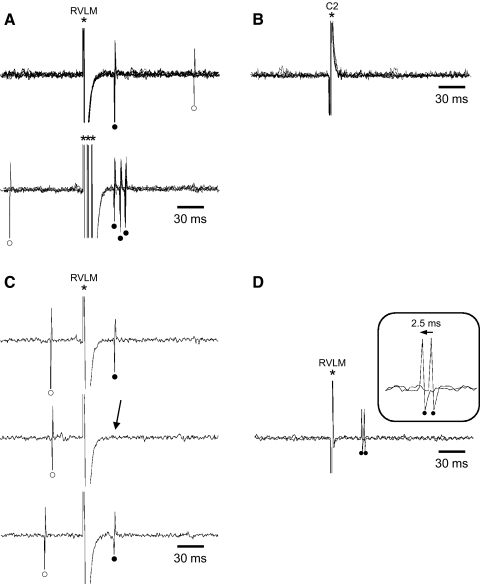
PVN-RVLM neurons were identified by their antidromic response to stimulation of the ipsilateral RVLM and by their lack of antidromic response to stimulation of the ipsilateral spinal cord. An example of antidromic identification of a single PVN-RVLM neuron is shown in Fig. 2. In A, five superimposed sweeps of data are shown to illustrate that each RVLM stimulus evoked a single spike with invariant onset latency (top). This neuron also responded to high-frequency RVLM stimulation – each of three stimulus pulses separated by 3 ms evoked a single spike (bottom). B shows that even large-amplitude (∼1.5 mA) stimulation of the C2 spinal segment failed to evoke a response from this neuron. C shows the timed collision test for this neuron. Note that the RVLM-evoked antidromic spike (top and bottom) was canceled by collision with a spontaneous action potential (middle). D illustrates the antidromic latency “jump” test. As the intensity of RVLM stimulation was gradually increased, the antidromic onset latency suddenly decreased by ∼2.5 ms. This indicates that a faster conducting (larger diameter) axon branch was activated in the vicinity of the RVLM stimulating electrode and suggests that the axon formed a terminal arbor in the RVLM.
Fig. 2.
Antidromic identification of paraventricular nucleus (PVN)-RVLM neurons. A: 5 superimposed sweeps of cell activity showing constant latency (39 ms) antidromic spikes evoked by single pulse stimulation of RVLM (top). All-or-none responses were consistently elicited above the stimulus threshold (320 μA). Five superimposed sweeps of data show that this neuron consistently responded with a single spike of invariant onset latency to each of 3 RVLM stimuli delivered in a high-frequency train (333 Hz; bottom). B: in contrast to RVLM stimulation, even large-amplitude stimulation (≤1.5 mA) of the C2 spinal segment failed to evoke antidromic spikes. C: for the same cell as in A, RVLM stimulation evoked an antidromic spike (top) that was cancelled (→, middle) by collision with a spontaneous action potential. Note that collision occurred when the interval between the spontaneous action potential and RVLM stimulus was reduced to <38 ms—the critical collision period. D: 2 superimposed sweeps of cell activity show that an abrupt decrease (2.5 ms) of the antidromic onset latency occurred (→) as RVLM stimulation intensity was gradually increased from ∼700 to 710 μA. Such “latency jumps” indicate local axon branching and are consistent with terminal arborization near the stimulation site. *, stimulus artifacts; •, antidromic spikes; ○, spontaneous action potentials
Among 65 identified PVN-RVLM neurons, antidromic responses to RVLM stimulation occurred at an average stimulus threshold of 626 ± 70 μA. Among these neurons, 32 (49%) were spontaneously active and antidromic spikes were observed to undergo cancellation by collision with spontaneous action potentials. While gradually increasing suprathreshold stimulus intensity, antidromic latency “jumps” were observed in 23 of 44 neurons tested (52%). The average latency reduction was 2.4 ± 0.6 ms and occurred as stimulus intensity was increased over a range of 15 ± 2.6 μA.
Identification of PVN-RVLM/IML neurons
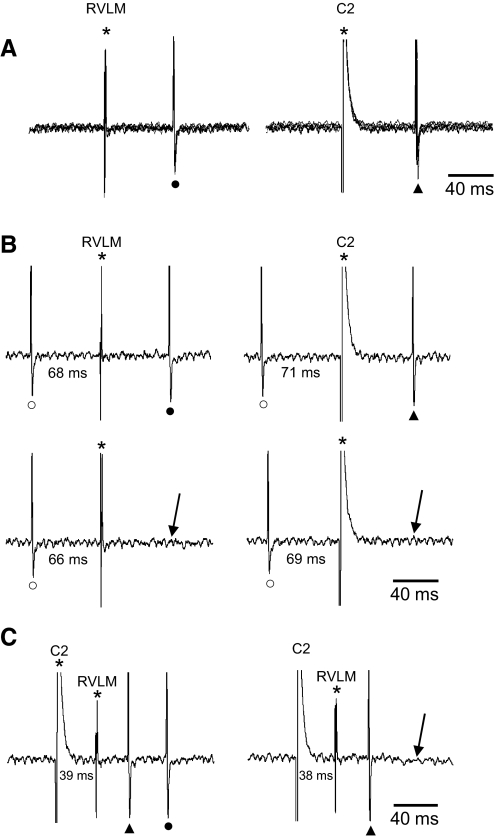
Neurons exhibiting an antidromic response to stimulation of the ipsilateral RVLM that were subsequently observed to also have an antidromic response to stimulation of the spinal cord were classified as PVN-RVLM/IML neurons. Figure 3 illustrates identification of one such neuron. In A, five superimposed data sweeps illustrate that each RVLM (left) and C2 spinal (right) stimulus evoked a single spike with little onset latency variation. B shows timed collision tests for this neuron. Note that antidromic spikes evoked from the RVLM (left, top) and the C2 spinal segment (right, top) each underwent cancellation by collision with spontaneous action potentials. C illustrates the reciprocal collision test for this neuron. When spinal and RVLM stimuli were separated by 39 ms, an antidromic response to each stimulus was recorded. When the interval between stimuli was reduced to 38 ms, the response to RVLM stimulation was cancelled by collision with the C2-evoked antidromic spike. This collision interval exceeded that predicted for an unbranched axon (3 ms) by 35 ms. Therefore this neuron was considered to have a branched axon that targeted both the RVLM and spinal cord.
Fig. 3.
Antidromic identification of PVN-RVLM/intermediolateral cell column (IML) neurons. A: 5 superimposed sweeps of cell activity showing constant latency antidromic spikes evoked by single pulse stimulation of RVLM (left: latency = 67 ms) and the C2 spinal segment (right: latency = 70 ms). All-or-none responses were consistently elicited from the RVLM and spinal cord at stimulus thresholds of 140 and 630 μA, respectively. B: antidromic spikes evoked by stimulating the RVLM (left, top) and the spinal cord (right, top) each underwent collision with spontaneous action potentials that occurred during their respective critical collision periods (↓, bottom, left and right sweeps). C: reciprocal collision testing was used to demonstrate that axons activated by antidromic stimulation of the RVLM and spinal cord arose from the same PVN cell soma. Single pulse stimuli directed to the C2 spinal segment and RVLM at an interval of 39 ms each induced an antidromic spike (left). When the interval was reduced to 38 ms (right), the RVLM antidromic spike was cancelled (↓). The reciprocal collision interval of 38 ms was considerably greater than predicted for a single axon segment (70-67 = 3 ms), thereby suggesting that the branch point that gave rise to the RVLM and IML axon near the cell soma. This cell also exhibited an abrupt decrease of antidromic onset latency (i.e., a latency “jump”) of ∼2 ms during graded stimulation of RVLM (data not shown). *, stimulus artifacts; ▴, antidromic spike from C2; •, antidromic spike from RVLM
Among 29 identified PVN-RVLM/IML neurons, 17 (59%) were spontaneously active and antidromic spikes evoked by RVLM and C2 spinal stimulation underwent timed collision with spontaneous action potentials. Reciprocal collisions were observed in 20 (∼69%) PVN-RVLM/IML neurons. The maximum reciprocal collision interval averaged 21 ± 2.1 ms, which was considerably longer than the collision interval predicted for unbranched axons (12 ± 1.5 ms). The large discrepancy between predicted and actually reciprocal collision intervals suggests that axons of PVN-RVLM/IML neurons collateralized mid-way between the PVN and the RVLM/spinal C2 segment (assuming each axon branch had a similar average CV).
The presence of axon collaterals to the RVLM and IML was affirmed in 13 of 20 neurons that underwent reciprocal collision (65%) by demonstrating an abrupt decrease in antidromic onset latency when RVLM stimulus intensity was gradually increased over a range of 32 ± 10 μA. The average latency jump was of 3.9 ± 1 ms. Recordings were lost prior to competing reciprocal collision testing in nine other putative PVN-RVLM/IML neurons. In these cells, however, an antidromic latency jump of 3.3 ± 1.1 ms was observed when RVLM stimulus intensity was increased over a range of 12 ± 2 μA. Again this argues against the axon being “en passage” and favors the conclusion that a terminal arbor was present in the RVLM and that an axon collateral extended to the spinal cord. In four other neurons, reciprocal collision testing revealed a maximum reciprocal collision interval that was very similar to the value predicted for a single axon segment. These neurons were all quiescent and had slow axonal CV (mean: 0.86 m/s) consistent with being unmyelinated. Consequently, these were deemed to be PVN-IML neurons the axons of which passed through RVLM without branching. Data from such neurons have been previously reported (Bains et al. 1992; Chen and Toney 2003a; Lovick and Coote 1988a) and therefore were excluded from further analysis.
Distribution of neurons in the PVN
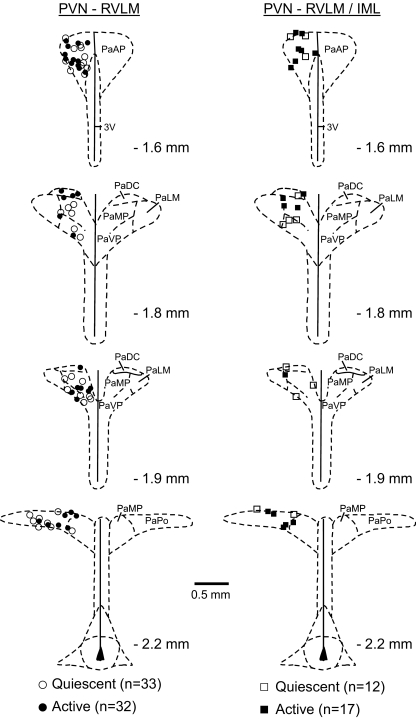
Consistent with retrograde tracing studies (Pyner and Coote 2000; Shafton et al. 1998; Stocker et al. 2004a), identified neurons were distributed throughout the rostral-caudal PVN and in near equal proportions in the anterior subnucleus/dorsal cap (PVN-RVLM: 41%; PVN-RVLM/IML: 45%) and the medial and ventral subnuclei (PVN-RVLM: 40%; PVN-RVLM/IML: 34%). A smaller proportion of neurons in each group was located in the posterior subnucleus (PVN-RVLM: 19%; PVN-RVLM/IML: 21%). Spontaneously active and quiescent PVN-RVLM and PVN-RVLM/IML neurons were present in nearly equal proportions and, again, were similarly distributed (Fig. 4).
Fig. 4.
Distribution of single-unit recording sites in the PVN. The location of PVN-RVLM and of PVN-RVLM/IML neurons is indicated (respectively, left, • and ○, n = 65; and right, ▪ and □, n = 29). • and ▪, cells with spontaneous discharge; ○ and □, quiescent neurons. PVN-RVLM and PVN-RVLM/IML neurons were distributed throughout the rostral-caudal PVN and were mostly located in the anterior subnucleus and dorsal cap as well as in the medial and ventral parvocellular subnuclei. PaAP, anterior parvocellular region; PaDC, dorsal cap; PaMP, medial parvocellular region; PaVP, ventral parvocellular region; PaLM, lateral magnocellular region; PaPo, posterior parvocellular region; 3V, third cerebral ventricle
Properties of PVN-RVLM and PVN-RVLM/IML neurons
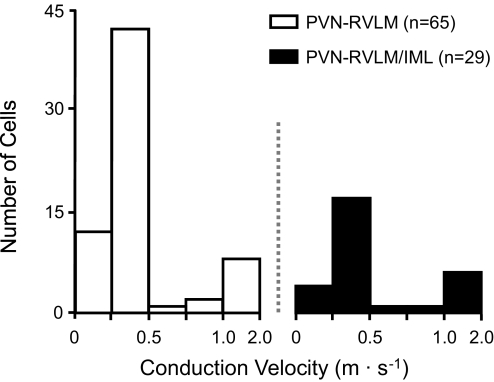
As noted in the preceding text, recording procedures were designed to select for neurons projecting to the RVLM. These were then further defined as having an IML projection or not. Among identified neurons, 69% (n = 65/94) had axons projecting to the RVLM, and the remaining 31% (n = 29/94) had collateral axons innervating both the RVLM and spinal cord. Axonal CV among PVN-RVLM (0.34 ± 0.03 m/s) and PVN-RVLM/IML (0.41 ± 0.04 m/s) neurons was similar (Fig. 5). Among PVN-RVLM/IML neurons, PVN-to-RVLM and PVN-to-spinal segments had nearly identical axonal CV. Collectively, these data indicate that neurons in each group had small diameter, unmyelinated axons. Consistent with this conclusion, the threshold stimulus intensity for antidromic responses evoked from the RVLM and C2 spinal segment (611 ± 46 μA) did not differ across groups. Spontaneous activity was recorded among 32 of 65 PVN-RVLM neurons (49%) and 17 of 29 PVN-RVLM/IML neurons (59%). The frequency of discharge did not differ across groups (PVN/RLVM: 3.9 ± 1.1, PVN-RVLM/IML: 2.8 ± 1.2 spike/s). No differences in axonal CV, antidromic threshold, or basal firing rate were noted among neurons located in different PVN subnuclei.
Fig. 5.
Axonal conduction velocities of PVN-RVLM and PVN-RVLM/IML neurons. Consistent with having fine diameter unmyelinated fibers, axonal conduction velocities of PVN-RVLM (□; n = 65) and PVN-RVLM/IML neurons (▪; n = 29) were consistently ≤2.0 m/s, with most being ≤0.5 m/s.
Cardiac- and sympathetic-related discharge
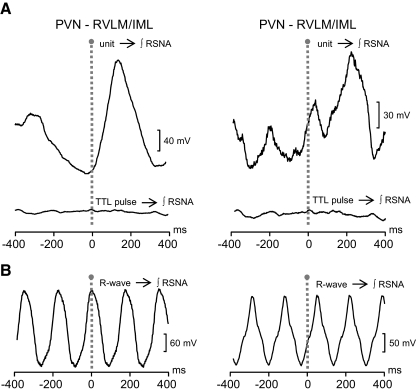
The temporal relationship between spontaneous cell firing and SNA was assessed by spike-triggered averaging of RSNA. In Fig. 6A, the correlation between the discharge of a PVN-RVLM neuron (left) and a PVN-RVLM/IML neuron (right) is shown. Note that in each, the peak of correlated RSNA occurred between ∼150 and ∼220 ms after spike occurrence (time 0). Bottom traces in each panel demonstrate that the same RSNA data showed no correlation when averages were triggered by randomly occurring events (TTL pulse → ƒRSNA). Overall, correlation was detected in similar proportions of PVN-RVLM (12 of 21, 57%) and PVN-RVLM/IML (6 of 9, 67%) neurons. The number of spikes used to construct RSNA averages for PVN-RVLM neurons (784 ± 132) and PVN-RVLM/IML neurons (614 ± 41) was not statistically different. Across groups of neurons, the latency-to-peak correlation (PVN-RVLM: 137 ± 9 ms, PVN-RVLM/IML: 143 ± 30 ms) and the duration of correlated RSNA (PVN-RVLM: 293 ± 13 ms, PVN-RVLM/IML: 301 ± 55) were also similar. The same RSNA data as used for PVN spike triggered averages were also used to construct ECG R-wave-triggered averages. Figure 6B shows that there was a consistently strong correlation between RSNA and cardiac cycle rhythm in these anesthetized, arterial baroreceptor intact rats.
Fig. 6.
Sympathetic-related spontaneous discharge of PVN neurons. A: spike-triggered RSNA averages (top, unit→f RSNA) constructed from spontaneous spikes recorded from a PVN-RVLM neuron (left) and a PVN-RVLM/IML neuron (right). RSNA correlated with discharge recorded from the PVN-RVLM and PVN-RVLM/IML neurons occurred at peak latencies of 179 and 206 ms, respectively. Bottom traces (TTL pulse→f RSNA) are “dummy” RSNA averages constructed from the same RSNA data used for constructing the corresponding spike-triggered averages (top). The number and average frequency of square-wave (TTL) pulses used to construct dummy averages were equal to the number of spikes and the frequency of cell activity used for constructing the corresponding PVN-RVLM (571 spikes at 2.2 Hz) and PVN-RVLM/IML (729 spikes at 2.1 Hz) neuron spike-triggered averages (top). Note that dummy averages show no correlated RSNA, indicating that the correlation between cell discharge and RSNA in the top traces is not due to phase-locking of harmonic frequencies. B: traces show ECG R-wave-triggered RSNA averages. Note that in each case, RSNA was tightly coupled to the frequency of the cardiac cycle. RSNA data used for constructing R-wave triggered averages was the same as used for each spike-triggered RSNA average in A.
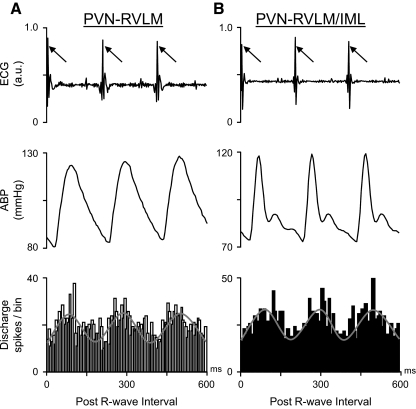
To assess whether cell firing was also cardiac rhythmic, ECG R-wave triggered time histograms of unit activity were constructed. Figure 7, A and B, shows ECG (top) and ABP (middle) data across three representative cardiac cycles from two different rats. Simultaneously recorded cell discharge was used to construct R-wave-triggered time histograms of unit discharge (bottom) for a PVN-RVLM neuron (Fig. 7A) and a PVN-RVLM/IML neuron (B). Summary data indicate that discharge was correlated with the ECG R-wave in 25 of 32 (78%) PVN-RVLM neurons and 10 of 17 (59%) PVN-RVLM/IML neurons. Time histograms of discharge for cells in each group were constructed from similar numbers of R-wave events (899 ± 117) and unit discharges (593 ± 96). Collectively, these findings indicate that some PVN-RVLM and PVN-RVLM/IML neurons are capable of participating in the ongoing (i.e., tonic) control of sympathetic outflow.
Fig. 7.
Cardiac-related spontaneous discharge of PVN neurons. ECG data (top) are and simultaneously recorded ABP (middle) are shown for 3 representative cardiac cycles. The post ECG R-wave-triggered time histogram (bin width: 10 ms) of spontaneous discharge (bottom) recorded from a PVN-RVLM neuron (A) and PVN-RVLM/IML neuron (B). Time histograms of both neurons show that discharge was periodic and synchronized with the cardiac cycle. The number of PVN-RVLM and PVN-RVLM/IML cell discharges used to accumulate each time histogram was 571 and 523, respectively.
Barosensitive discharge
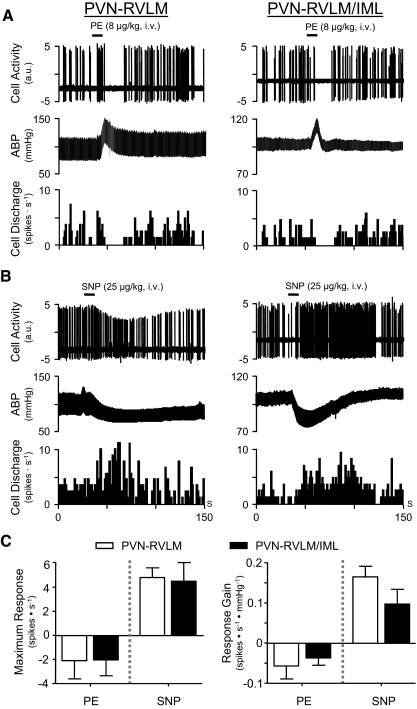
The presence of cardiac cycle-related spontaneous firing suggests that discharge might be entrained by arterial baroreceptor inputs that are active at the prevailing level of ABP. To further assess the influence of baroreceptor input on the discharge of PVN-RVLM and PVN-RVLM/IML neurons, changes in discharge of neurons with cardiac-related activity were recorded in response to raising and lowering ABP with phenylephrine (PE) and sodium nitroprusside (SNP), respectively. Figure 8A shows representative data from a single PVN-RVLM neuron (left) and a single PVN-RVLM/IML neuron (right). Note that both cells underwent a rapid cessation of discharge in response to an acute increase of ABP with PE. Figure 8B shows that discharge of the same PVN-RVLM neuron (left) and PVN-RVLM/IML neuron (right) increased during an acute reduction of ABP with SNP.
Fig. 8.
Baro-sensitivity of spontaneous discharge. A: raw cell activity (top), ABP (middle), and discharge frequency (bottom) of an individual PVN-RVLM neuron (left) and PVN-RVLM/IML neuron (right). In response to intravenous administration of phenylephrine (PE), both cell types responded similarly. An abrupt cessation of discharge was observed, with recovery of discharge as ABP returned to baseline. B: raw cell activity (top), ABP (middle), and discharge frequency (bottom) of the same PVN-RVLM neuron (left) and PVN-RVLM/IML neuron (right) shown in A. In response to intravenous administration of sodium nitroprusside (SNP), both cell types responded with a similar increase of discharge. Note that the reduction in spike amplitude of the PVN-RVLM neuron (left) likely reflects a transient increase in the distance between the neuron and recording electrode due to mechanical instability during the SNP-induced depressor response. C: summary data indicate that maximum changes in discharge frequency (left graph) to PE (left bars) were similar for PVN-RVLM (open bars; n = 4) and PVN-RVLM/IML (▪; n = 4) neurons. Likewise, maximum changes in discharge to SNP (right bars) were similar across groups (PVN-RVLM: □, n = 8; PVN-RVLM/IML: ▪, n = 4). The gain (sensitivity) of baroreflex modulation of discharge (right graph) was similar for PVN-RVLM (n = 4 to 8) and PVN-RVLM/IML neurons (n = 4).
Among 13 PVN-RVLM neurons and 4 PVN-RVLM/IML neurons with cardiac cycle-related spontaneous discharge, 9 of the former (69%) and 4 (100%) of the latter were tested for their response to PE. Among PVN-RVLM neurons, one increased discharge, four were unaffected, and another four responded with a reduction of discharge. Among PVN-RVLM/IML neurons, all four showed a reduction of discharge in response to PE. Effects of PE on MAP were consistent for all cells tested, with MAP rising from a baseline of 92 ± 6 to 131 ± 8 mmHg. In response to SNP, discharge frequency increased in eight of eight (100%) PVN-RVLM neurons and four of four (100%) PVN-RVLM/IML. Effects of SNP on MAP were also consistent across all cells tested, with MAP falling from a baseline of 94 ± 5 to 50 ± 2 mmHg. Across groups of PVN-RVLM (n = 7) and PVN-RVLM/IML (n = 4) neurons exposed to both PE and SNP, maximum discharge responses were not different (Fig. 8C, left). Likewise, the gain of the discharge response (i.e., arterial pressure sensitivity) did not differ across groups (Fig. 8C, right). For responses to PE, neither the level of MAP at which discharge was silenced (i.e., MAP cut-off) nor the duration of cell quiescence differed across groups of PVN-RVLM (n = 4) and PVN-RVLM/IML (n = 4) neurons. For responses to SNP, discharge of PVN-RVLM neurons and PVN-RVLM/IML neurons increased rapidly and most often (7 of 8 PVN-RVLM neurons; 3 of 4 PVN-RVLM/IML neurons) returned gradually toward baseline with restoration of ABP. An example of this response pattern is shown in Fig. 8B, left. Infrequently (1 of 8 PVN-RVLM neurons; 1 of 4 PVN-RVLM/IML neurons), elevated discharge persisted as ABP returned toward baseline. An example of such a response is shown in Fig. 8B, right. Differences in the time course of discharge recovery to SNP do not appear to depend on the nature of the depressor response because neither the magnitude nor the duration of depressor responses was different for neurons exhibiting rapid recovery compared with those with more prolonged responses.
In contrast to neurons with cardiac-related discharge, those that were quiescent (PVN-RVLM, n = 9; PVN-RVLM/IML, n = 3) or lacked cardiac rhythmic discharge (PVN-RVLM, n = 4; PVN-RVLM/IML, n = 4) consistently failed to respond to either PE or SNP (data not shown).
DISCUSSION
Our goals for this study were to record the in vivo discharge of PVN neurons with axons projecting to the RVLM and to test the hypothesis that they have patterns of spontaneous activity related to cardiovascular function. Neurons were classified as having unbranched axons to the RVLM (PVN-RVLM) or branched axons targeting the RVLM and spinal IML (PVN-RVLM/IML). We determined that PVN-RVLM and PVN-RVLM/IML neurons had similar axonal conduction velocities, and a similar fraction of neurons in each group had slow spontaneous discharge that was temporally correlated with the cardiac cycle and RSNA. The response of PVN-RVLM and PVN-RVLM/IML neurons to acute increases and decreases of arterial baroreceptor input was also quantitatively similarly. Our findings suggest that these two neuronal populations serve similar functions related to the generation and baroreflex modulation of sympathetic vasomotor tone.
Technical considerations
Antidromic activation was used to RVLM-projecting PVN neurons. Because PVN neurons terminate over a large rostral-to-caudal portion of the ventrolateral medulla, including the caudal ventrolateral medulla (CVLM) (Hardy 2001; Krukoff et al. 1997), we were careful to place antidromic stimulating electrodes in the RVLM at sites from which large-amplitude increases of ABP were elicited by injection of l-glutamate (see Fig. 1A). This is important because activation of the PVN has been shown to directly influence the discharge of barosensitive neurons in both the RVLM (Yang and Coote 1998) and CVLM (Yang and Coote 1999). Graded intensities of stimulation were used to insure activation of axons in the RVLM and spinal dorsolateral funiculus. By also using a three-electrode array to stimulate the upper spinal cord, our design increased the likelihood of recording antidromic responses and thereby reduced the probability of making a classification error. Use of antidromic latency “jump” tests to determine if axons underwent terminal branching within the RVLM (Klemfuss et al. 1987; Lipski 1981) and reciprocal collision tests to identify branched axons innervating both the RVLM and spinal cord (Lipski 1981) further guarded against classification errors.
PVN-RVLM and PVN-RVLM/IML neurons and support of sympathetic vasomotor tone
Acute inhibition of PVN neuronal activity has been reported to reduce ongoing RSNA (Akine et al. 2003; Stocker et al. 2004b, 2005), lumbar SNA (Allen 2002; Stocker et al. 2005), and ABP (Akine et al. 2003; Allen 2002; Freeman and Brooks 2007; Stocker et al. 2004b, 2005) in anesthetized rats. Moreover, reductions of SNA in response to PVN inhibition or blockade of excitatory inputs are more pronounced in water deprived (Freeman and Brooks 2007; Stocker et al. 2004b, 2005) and hypertensive (Allen 2002; Li and Pan 2007) rats. These findings indicate that PVN neuronal activity contributes to both the maintenance of resting SNA and to the elevation of SNA under physiologic and disease conditions.
In a previous study, we reported that two functionally distinct groups of paraventriculo-spinal (PVN-IML) neurons can be identified based on having distinct electrophysiological and discharge properties (Chen and Toney 2003a)—group I neurons have relatively fast conducting spinal axons and group II neurons have slower conducting axons like those of PVN-IML neurons described by others (Lovick and Coote 1988a,b). Based on the patterning of their spontaneous discharge, group I neurons appear to contribute to the generation and baroreflex regulation of ongoing sympathetic vasomotor tone. In contrast, group II neurons more likely contribute to regulation of nonvasomotor components of sympathetic outflow (Chen and Toney 2003a).
In the present study, PVN-RVLM and PVN-RVLM/IML neurons both had sympathetic- and cardiac-rhythmic spontaneous discharge that was modulated by arterial baroreceptor input. These features closely resemble group I PVN-IML neurons from our previous study and suggest that RVLM-projecting PVN neurons might also contribute to the generation and regulation of vasomotor tone. However, RVLM-projecting PVN neurons also differ from group I PVN-IML neurons in that they have relatively slow axonal CV. This feature more closely resembles group II PVN-IML neurons from our previous study and studies by the Coote laboratory (Lovick and Coote 1988a,b). Thus RVLM-projecting PVN neurons appear to have characteristics unlike either group I or group II PVN-IML neurons.
Because anatomical studies have shown that 15–20% of RVLM-projecting PVN neurons have branched axons that also target the IML (Pyner and Coote 2000; Shafton et al. 1998; Stocker et al. 2006), one would expect that a reasonably large sample of cells antidromically activated from the spinal cord would include at least some neurons with branched axons that also innervate the RVLM. This being the case, one would expect that some group I or group II neurons from our previous study should have had properties nearly identical to those of PVN-RVLM/IML neurons in the present study. As noted in the preceding text, however, this was not observed. The reason for this apparent discrepancy is not clear at present, one possibility is that the percentage of axons in the PVN-spinal pathway that arise from branching PVN-RVLM/IML neurons may be exceeding small. If this is the case, then by chance alone our previous study may have failed to identify any PVN-RVLM/IML neurons. Another factor that could have contributed to the failure of our previous study to identify PVN-IML neurons with properties resembling PVN-RVLM/IML neurons is that our earlier work was performed using high resistance recording electrodes (30–50 MΩ) and triangular “pulses” to stimulate the spinal cord. These methods, separately or combined, might have prevented detection of neurons with behaviors resembling those in the present study.
Baroreflex modulation of SNA by RVLM-projecting PVN neurons
In earlier work, ∼50% of PVN-IML neurons were reported to receive inhibitory input from arterial baroreceptors (Bains and Ferguson 1995; Chen and Toney 2003a; Lovick and Coote 1988a,b). Here baroreceptor inputs targeted a similar proportion of PVN-RVLM and PVN-RVLM/IML neurons and caused abrupt silencing of neuronal discharge. In this regard, RVLM-projecting PVN neurons resemble RVLM vasomotor neurons (Guyenet 2006). Although baroreceptor inhibition of RVLM neuronal firing is known to be mediated by GABAergic inputs from the CVLM (Guyenet 2006), the neurotransmitter(s) mediating inhibition of RVLM-projecting PVN neurons is not known. Because discharge inhibition was abrupt and spontaneous discharge of most barosensitive PVN-RVLM and PVN-RVLM/IML neurons was also cardiac rhythmic, a fast synaptic transmitter such as GABA is likely to be involved.
If GABA were the principle mediator of baroreceptor inhibition of RVLM-projecting PVN neurons, then a recent study by Stern and co-workers is of interest (Park et al. 2009). They showed that in vitro discharge of PVN-RVLM neurons is suppressed by a tonic GABA current. Whereas one would not expect a tonic GABA current to underlie cardiac rhythmic discharge (i.e., baroreceptor entrainment) or abrupt baroreceptor inhibition of discharge, a tonic GABA current could help to set the overall level of activity/excitability of PVN sympathetic-regulatory neurons. If extracellular levels of GABA in the PVN rise and fall in proportion to the level of arterial baroreceptor input, then a reduction of GABA availability could possibly contribute to the gradual and more sustained increases of discharge observed in some neurons when ABP was reduced with SNP (see Fig. 8B, right).
An alternative, but perhaps less likely, mediator of baroreceptor inhibition of RVLM-projecting PVN neurons is norepinephrine. Not only do CVLM GABAergic neurons project to the RVLM, but they also target nearby A1 noradrenergic neurons as well (Chan and Sawchenko 1998). The latter have been reported to facilitate the discharge of nearby neurosecretory neurons in the PVN (Day et al. 1984), but whether A1 neurons are uniformly inhibited by baroreceptor inputs and ascend to directly synapse on RVLM-projecting PVN neurons is not known, although an A1-to-PVN pathway has been reported (Caverson and Ciriello 1984).
Concluding remarks
The present study used in vivo electrophysiological methods to identify PVN neurons with axons projecting to the RVLM, including a population of neurons with axon collaterals that innervate the spinal cord. Consistent with evidence that the PVN is strongly inhibited by GABAergic inputs in vivo (Li et al. 2006), ∼50% of PVN-RVLM and PVN-RVLM/IML neurons lacked spontaneous discharge. Remaining neurons had slow spontaneous discharge that was barosensitive and temporally correlated with the cardiac cycle and RSNA. These activity patterns suggest that RVLM-projecting PVN neurons are capable of contributing to both the generation of basal SNA as well as its baroreflex modulation.
GRANTS
Support for Q. H. Chen was provided by a postdoctoral fellowship from the American Heart Association, Texas Affiliate. This study was funded by National Heart, Lung, and Blood Institute Grants HL-71645, HL-76312 and was performed during the tenure of an American Heart Association Established Investigator Grant (EIG 014016N) to G. M. Toney.
ACKNOWLEDGMENTS
We gratefully acknowledge B. Guler and M. Cato for excellent technical assistance.
REFERENCES
- Akine A, Montanaro M, Allen AM. Hypothalamic paraventricular nucleus inhibition decreases renal sympathetic nerve activity in hypertensive and normotensive rats. Auton Neurosci 108: 17–21, 2003 [DOI] [PubMed] [Google Scholar]
- Allen AM. Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension 39: 275–280, 2002 [DOI] [PubMed] [Google Scholar]
- Amri M, Car A, Roman C. Axonal branching of medullary swallowing neurons projecting on the trigeminal and hypoglossal motor nuclei: demonstration by electrophysiological and fluorescent double labeling techniques. Exp Brain Res 81: 384–390, 1990 [DOI] [PubMed] [Google Scholar]
- Badoer E, McKinley MJ, Oldfield BJ, McAllen RM. A comparison of hypotensive and non-hypotensive hemorrhage on Fos expression in spinally projecting neurons of the paraventricular nucleus and rostral ventrolateral medulla. Brain Res 610: 216–223, 1993 [DOI] [PubMed] [Google Scholar]
- Badoer E, Merolli J. Neurons in the hypothalamic paraventricular nucleus that project to the rostral ventrolateral medulla are activated by hemorrhage. Brain Res 791: 317–320, 1998 [DOI] [PubMed] [Google Scholar]
- Bains JS, Ferguson AV. Paraventricular nucleus neurons projecting to the spinal cord receive excitatory input from the subfornical organ. Am J Physiol Regulatory Integrative Comp Physiol 268: R625–633, 1995 [DOI] [PubMed] [Google Scholar]
- Bains JS, Potyok A, Ferguson AV. Angiotensin II actions in paraventricular nucleus: functional evidence for neurotransmitter role in efferents originating in subfornical organ. Brain Res 599: 223–229, 1992 [DOI] [PubMed] [Google Scholar]
- Barman SM. Descending projections of hypothalamic neurons with sympathetic nerve-related activity. J Neurophysiol 64: 1019–1032, 1990 [DOI] [PubMed] [Google Scholar]
- Barman SM, Gebber GL. Hypothalamic neurons with activity patterns related to sympathetic nerve discharge. Am J Physiol Regulatory Integrative Comp Physiol 242: R34–43, 1982 [DOI] [PubMed] [Google Scholar]
- Cato MJ, Toney GM. Angiotensin II excites paraventricular nucleus neurons that innervate the rostral ventrolateral medulla: an in vitro patch-clamp study in brain slices. J Neurophysiol 93: 403–413, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caverson MM, Ciriello J. Electrophysiological identification of neurons in ventrolateral medulla sending collateral axons to paraventricular and supraoptic nuclei in the cat. Brain Res 305: 375–379, 1984 [DOI] [PubMed] [Google Scholar]
- Chan RK, Sawchenko PE. Organization and transmitter specificity of medullary neurons activated by sustained hypertension: implications for understanding baroreceptor reflex circuitry. J Neurosci 18: 371–387, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Li DP, Pan HL. Presynaptic alpha1 adrenergic receptors differentially regulate synaptic glutamate and GABA release to hypothalamic presympathetic neurons. J Pharmacol Exp Ther 316: 733–742, 2006 [DOI] [PubMed] [Google Scholar]
- Chen Q, Pan HL. Regulation of synaptic input to hypothalamic presympathetic neurons by GABA(B) receptors. Neuroscience 142: 595–606, 2006 [DOI] [PubMed] [Google Scholar]
- Chen QH, Haywood JR, Toney GM. Sympathoexcitation by PVN-injected bicuculline requires activation of excitatory amino acid receptors. Hypertension 42: 725–731, 2003 [DOI] [PubMed] [Google Scholar]
- Chen QH, Toney GM. AT(1)-receptor blockade in the hypothalamic PVN reduces central hyperosmolality-induced renal sympathoexcitation. Am J Physiol Regulatory Integrative Comp Physiol 281: R1844–1853, 2001 [DOI] [PubMed] [Google Scholar]
- Chen QH, Toney GM. Identification and characterization of two functionally distinct groups of spinal cord-projecting paraventricular nucleus neurons with sympathetic-related activity. Neuroscience 118: 797–807, 2003a [DOI] [PubMed] [Google Scholar]
- Chen QH, Toney GM. Responses to GABA-A receptor blockade in the hypothalamic PVN are attenuated by local AT1 receptor antagonism. Am J Physiol Regulatory Integrative Comp Physiol 285: R1231–1239, 2003b [DOI] [PubMed] [Google Scholar]
- Coote JH, Yang Z, Pyner S, Deering J. Control of sympathetic outflows by the hypothalamic paraventricular nucleus. Clin Exp Pharmacol Physiol 25: 461–463, 1998 [DOI] [PubMed] [Google Scholar]
- Day TA, Ferguson AV, Renaud LP. Facilitatory influence of noradrenergic afferents on the excitability of rat paraventricular nucleus neurosecretory cells. J Physiol 355: 237–249, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman KL, Brooks VL. AT(1) and glutamatergic receptors in paraventricular nucleus support blood pressure during water deprivation. Am J Physiol Regulatory Integrative Comp Physiol 292: R1675–1682, 2007 [DOI] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006 [DOI] [PubMed] [Google Scholar]
- Hardy SG. Hypothalamic projections to cardiovascular centers of the medulla. Brain Res 894: 233–240, 2001 [DOI] [PubMed] [Google Scholar]
- Kenney MJ, Weiss ML, Mendes T, Wang Y, Fels RJ. Role of paraventricular nucleus in regulation of sympathetic nerve frequency components. Am J Physiol Heart Circ Physiol 284: H1710–1720, 2003 [DOI] [PubMed] [Google Scholar]
- Klemfuss H, Young SJ, Groves PM. Do antidromic latency jumps indicate axonal branching in nigrostriatal and hypothalamo-neurohypophysial neurons? Brain Res 409: 197–203, 1987 [DOI] [PubMed] [Google Scholar]
- Konturek PC, Konturek JW, Czesnikiewicz-Guzik M, Brzozowski T, Sito E, Konturek SJ. Neuro-hormonal control of food intake: basic mechanisms and clinical implications. J Physiol Pharmacol 56, Suppl 6: 5–25, 2005 [PubMed] [Google Scholar]
- Krukoff TL, Mactavish D, Jhamandas JH. Activation by hypotension of neurons in the hypothalamic paraventricular nucleus that project to the brain stem. J Comp Neurol 385: 285–296, 1997 [DOI] [PubMed] [Google Scholar]
- Lee KS, Han TH, Jo JY, Kang G, Lee SY, Ryu PD, Im JH, Jeon BH, Park JB. Serotonin inhibits GABA synaptic transmission in presympathetic paraventricular nucleus neurons. Neurosci Lett 439: 138–142, 2008 [DOI] [PubMed] [Google Scholar]
- Li DP, Chen SR, Pan HL. VR1 receptor activation induces glutamate release and postsynaptic firing in the paraventricular nucleus. J Neurophysiol 92: 1807–1816, 2004 [DOI] [PubMed] [Google Scholar]
- Li DP, Pan HL. Glutamatergic inputs in the hypothalamic paraventricular nucleus maintain sympathetic vasomotor tone in hypertension. Hypertension 49: 916–925, 2007 [DOI] [PubMed] [Google Scholar]
- Li YF, Jackson KL, Stern JE, Rabeler B, Patel KP. Interaction between glutamate and GABA systems in the integration of sympathetic outflow by the paraventricular nucleus of the hypothalamus. Am J Physiol Heart Circ Physiol 291: H2847–2856, 2006 [DOI] [PubMed] [Google Scholar]
- Lipski J. Antidromic activation of neurons as an analytic tool in the study of the central nervous system. J Neurosci Methods 4: 1–32, 1981 [DOI] [PubMed] [Google Scholar]
- Lovick TA, Coote JH. Effects of volume loading on paraventriculo-spinal neurones in the rat. J Auton Nerv Syst 25: 135–140, 1988a [DOI] [PubMed] [Google Scholar]
- Lovick TA, Coote JH. Electrophysiological properties of paraventriculo-spinal neurones in the rat. Brain Res 454: 123–130, 1988b [DOI] [PubMed] [Google Scholar]
- Martin DS, Haywood JR. Sympathetic nervous system activation by glutamate injections into the paraventricular nucleus. Brain Res 577: 261–267, 1992 [DOI] [PubMed] [Google Scholar]
- Martin DS, Haywood JR. Hemodynamic responses to paraventricular nucleus disinhibition with bicuculline in conscious rats. Am J Physiol Heart Circ Physiol 265: H1727–1733, 1993 [DOI] [PubMed] [Google Scholar]
- Martin DS, Segura T, Haywood JR. Cardiovascular responses to bicuculline in the paraventricular nucleus of the rat. Hypertension 18: 48–55, 1991 [DOI] [PubMed] [Google Scholar]
- Park JB, Jo JY, Zheng H, Patel KP, Stern JE. Regulation of tonic GABA inhibitory function, presympathetic neuronal activity and sympathetic outflow from the PVN by astroglial GABA transporter. J Physiol 587: 4645–4660, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos GW. (Ed.) The Rat Brain in Stereotaxic Coordinates San Diego, CA: Academic, 1998 [Google Scholar]
- Porter JP, Brody MJ. Neural projections from paraventricular nucleus that subserve vasomotor functions. Am J Physiol Regulatory Comp Physiol 248: R271–281, 1985 [DOI] [PubMed] [Google Scholar]
- Pyner S, Coote JH. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience 100: 549–556, 2000 [DOI] [PubMed] [Google Scholar]
- Saper CB, Loewy AD, Swanson LW, Cowan WM. Direct hypothalamo-autonomic connections. Brain Res 117: 305–312, 1976 [DOI] [PubMed] [Google Scholar]
- Shafton AD, Ryan A, Badoer E. Neurons in the hypothalamic paraventricular nucleus send collaterals to the spinal cord and to the rostral ventrolateral medulla in the rat. Brain Res 801: 239–243, 1998 [DOI] [PubMed] [Google Scholar]
- Stern JE. Nitric oxide and homeostatic control: an intercellular signalling molecule contributing to autonomic and neuroendocrine integration? Prog Biophys Mol Biol 84: 197–215, 2004 [DOI] [PubMed] [Google Scholar]
- Stocker SD, Cunningham JT, Toney GM. Water deprivation increases Fos immunoreactivity in PVN autonomic neurons with projections to the spinal cord and rostral ventrolateral medulla. Am J Physiol Regulatory Integrative Comp Physiol 287: R1172–1183, 2004a [DOI] [PubMed] [Google Scholar]
- Stocker SD, Hunwick KJ, Toney GM. Hypothalamic paraventricular nucleus differentially supports lumbar and renal sympathetic outflow in water-deprived rats. J Physiol 563: 249–263, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker SD, Keith KJ, Toney GM. Acute inhibition of the hypothalamic paraventricular nucleus decreases renal sympathetic nerve activity and arterial blood pressure in water-deprived rats. Am J Physiol Regulatory Integrative Comp Physiol 286: R719–725, 2004b [DOI] [PubMed] [Google Scholar]
- Stocker SD, Simmons JR, Stornetta RL, Toney GM, Guyenet PG. Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventrolateral medulla. J Comp Neurol 494: 673–685, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker SD, Toney GM. Median preoptic neurones projecting to the hypothalamic paraventricular nucleus respond to osmotic, circulating Ang II and baroreceptor input in the rat. J Physiol 568: 599–615, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker SD, Toney GM. Vagal afferent input alters the discharge of osmotic and ANG II-responsive median preoptic neurons projecting to the hypothalamic paraventricular nucleus. Brain Res 1131: 118–128, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. Biochemical switching in hypothalamic circuits mediating responses to stress. Prog Brain Res 87: 181–200, 1991 [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology 31: 410–417, 1980 [DOI] [PubMed] [Google Scholar]
- Toney GM, Chen QH, Cato MJ, Stocker SD. Central osmotic regulation of sympathetic nerve activity. Acta Physiol Scand 177: 43–55, 2003 [DOI] [PubMed] [Google Scholar]
- Valassi E, Scacchi M, Cavagnini F. Neuroendocrine control of food intake. Nutr Metab Cardiovasc Dis 18: 158–168, 2008 [DOI] [PubMed] [Google Scholar]
- Vigas M. Central regulation of adenohypophyseal function. Vnitr Lek 35: 242–248, 1989 [PubMed] [Google Scholar]
- Yang Z, Bertram D, Coote JH. The role of glutamate and vasopressin in the excitation of RVL neurons by paraventricular neurons. Brain Res 908: 99–103, 2001 [DOI] [PubMed] [Google Scholar]
- Yang Z, Coote JH. Influence of the hypothalamic paraventricular nucleus on cardiovascular neurons in the rostral ventrolateral medulla of the rat. J Physiol 513: 521–530, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Coote JH. The influence of the paraventricular nucleus on baroreceptor dependent caudal ventrolateral medullary neurones of the rat. Pfluegers 438: 47–52, 1999 [DOI] [PubMed] [Google Scholar]
- Yang Z, Coote JH. The role of supraspinal vasopressin and glutamate neurons in an increase in renal sympathetic activity in response to mild haemorrhage in the rat. Exp Physiol 91: 791–797, 2006 [DOI] [PubMed] [Google Scholar]