Abstract
Systemic administration of μ-opioids at clinical doses for analgesia typically slows respiratory rate. Mu-opioid receptors (MORs) on pre-Bötzinger Complex (pre-BötC) respiratory neurons, the putative kernel of respiratory rhythmogenesis, are potential targets. The purpose of this study was to determine the contribution of pre-BötC MORs to the bradypnea produced in vivo by intravenous administration of clinically relevant infusion rates of remifentanil (remi), a short-acting, potent μ-opioid analgesic. In decerebrate dogs, multibarrel micropipettes were used to record pre-BötC neuronal activity and to eject the opioid antagonist naloxone (NAL, 0.5 mM), the glutamate agonist d-homocysteic acid (DLH, 20 mM), or the MOR agonist [d-Ala2, N-Me-Phe4, gly-ol5]-enkephalin (DAMGO, 100 μM). Inspiratory and expiratory durations (TI and TE) and peak phrenic nerve activity (PPA) were measured from the phrenic neurogram. The pre-BötC was functionally identified by its rate altering response (typically tachypnea) to DLH microinjection. During intravenous remi-induced bradypnea (∼60% decrease in central breathing frequency, fB), bilateral injections of NAL in the pre-BötC did not change TI, TE, fB, and PPA. Also, NAL picoejected onto single pre-BötC neurons depressed by intravenous remi had no effect on their discharge. In contrast, ∼60 μg/kg of intravenous NAL rapidly reversed all remi-induced effects. In a separate group of dogs, microinjections of DAMGO in the pre-BötC increased fB by 44%, while subsequent intravenous remi infusion more than offset this DAMGO induced tachypnea. These results indicate that μ-opioids at plasma concentrations that cause profound analgesia produce their bradypneic effect via MORs located outside the pre-BötC region.
INTRODUCTION
Systemic administration of μ-opioids at clinical doses for analgesia typically produces bradypnea during sedation and sleep (Lalley 2008; Pattinson 2008). Mu-opioid receptors (MORs) on pre-Bötzinger Complex (pre-BötC) neurons, the putative kernel of respiratory rhythmogenesis, are potential targets (Gray et al. 1999). Studies in brain slices that contain the pre-BötC show that μ-opioids markedly slow the burst rate of respiratory-related output (Gray et al. 1999, Johnson et al. 1996) and produce slowing that has been characterized as quantal in neonatal rat brain stem-spinal cord preparations (Mellen et al. 2003). Perturbations of neuronal function within the pre-BötC of adult animals severely disrupt breathing (Gray et al. 2001; Krolo et al. 2005; Monnier et al. 2003; Pierrefiche et al. 1998; Solomon et al. 1999). In vivo studies using multibarrel micropipettes and microiontophoresis have shown that localized application of μ-opioids to medullary respiratory neurons causes a decrease in neuronal discharge and membrane hyperpolarization, demonstrating the presence of functional MORs on these neurons (Haji et al. 2003a,b; Lalley 2003).
Further evidence in support of the pre-BötC region as the site of the opioid-induced depression of breathing rate is suggested by the coexpression of 5-HT4a and MOR receptors in the pre-BötC and the ability of a 5-HT4a agonist to reverse most of the opioid effect on breathing rate without reversing analgesia (Manzke et al. 2003). The underlying mechanism for this functional antagonism was hypothesized to act by counterbalancing the opioid-induced decrease in intracellular cyclic adenosine monophosphate (cAMP) via an increase in cAMP levels produced by activation of 5-HT4a receptors. The functional antagonism did not affect the antinociceptive action of opioids, presumably because 5-HT4a receptors are absent in the regions of the spinal cord involved in the processing of pain stimuli. Similarly, a study by Lalley (2005) demonstrated that selective D1-dopamine receptor agonists, which are known to activate the cAMP-protein kinase A (PKA) signaling pathway in a variety of neurons, restored phrenic nerve activity after it had been abolished by the selective MOR agonist fentanyl in anesthetized and unanesthetized decerebrate cats. Again it was suggested that the potential site of action could be on pre-BötC respiratory neurons.
In a recent study using the picoejection technique combined with extracellular single-unit recording (Stucke et al. 2008), we found that respiratory bulbospinal premotor neurons in decerebrate dogs are depressed by μ- and δ-opioids when applied directly to neurons in millimolar concentrations. However, a depression of these neurons of similar magnitude (50%) by intravenous remifentanil (remi), a short-acting, potent, selective MOR agonist used for clinical analgesia, which leads to effect site (brain) agonist concentrations in the nanomolar range, could not be reversed by picoejection of the opioid antagonist naloxone directly onto the neurons. Thus we concluded that the μ-opioid effects occurred at sites upstream (presynaptic) from the respiratory premotor neurons. Because it has been shown that pre-BötC respiratory neurons supply synaptic inputs to the premotor neurons, we hypothesized that clinically relevant opioid-induced effects may be mediated via MORs on pre-BötC neurons. Accordingly, the purpose of our study was to determine the contribution of pre-BötC MORs to the bradypnea produced in vivo by intravenous remi.
METHODS
This research was approved by the subcommittee on animal studies of the Zablocki VA Medical Center, Milwaukee, WI, in accordance with provisions of the Animal Welfare Act, the PHS Guide for the Care and Use of Laboratory Animals, and VA policy. Experiments were performed on mongrel dogs of either sex, weighing from 8 to 16 kg. Inhalational anesthesia was induced by mask and maintained with isoflurane at 1.5–2.5% end-tidal concentration. The animals were monitored for signs of inadequate anesthesia such as salivation, lacrimation, and increases in blood pressure and heart rate. If required, anesthetic depth was increased immediately.
Surgical procedures
The trachea of dogs was intubated with a cuffed endotracheal tube, and their lungs were mechanically ventilated with an air-O2-isoflurane mixture. The surgical procedures, monitoring, and maintenance of body homeostasis have been previously described in detail (Dogas et al. 1998). Briefly, after cannulating the femoral artery for blood pressure recording and blood gas sampling and femoral vein for continuous infusion of maintenance fluids and administration of drugs, a bilateral pneumothorax was performed to reduce motion artifacts. The animal was then decerebrated by midcollicular transection (Tonkovic-Capin et al. 1998), and isoflurane was discontinued. After decerebration the animal was ventilated with an air-O2 mixture and maintained in hyperoxic normocapnia (FiO2 > 0.6). The dorsal surface of the medulla oblongata was exposed by an occipital craniotomy for neuronal recording and microinjections. Phrenic nerve activity was recorded from the desheathed right C5 rootlet. The phrenic neurogram (PNG) was obtained from the moving-time average (100 ms) of the amplified phrenic nerve activity and was used to produce timing pulses corresponding to the beginning and end of the inspiratory phase for the measurement of inspiratory duration (TI) and expiratory duration (TE). Peak phrenic activity (PPA) was also obtained from the PNG. Continuous neuromuscular block was achieved with pancuronium (0.1 mg·kg−1·h−1) to reduce motion artifacts during neuronal recordings.
Microinjection technique
A minimum of 1 h was allowed for preparation stabilization before data collection. Extracellular neuronal recordings were obtained using multibarrel micropipettes (10–30 μm composite tip diameter) consisting of a recording barrel containing a 7-μm-thick carbon filament and three drug barrels. The micropipettes were used to locate pre-BötC neurons within the ventral respiratory column (VRC) prior to pressure microinjections of the glutamate agonist d-homocysteic acid (DLH, 1 mM) and the MOR antagonist naloxone (NAL, 500 μM and 5 mM) or the MOR agonist [d-Ala2, N-Me-Phe4, Gly-ol5]-enkephalin (DAMGO, 100 μM), which were dissolved in artificial cerebrospinal fluid (ACSF). Microinjections were made using an in-house, custom-built, four-channel microinjection system, which allowed independent control of ejection duration, repetition rate, and pressure 0–80 psi. The microinjected drug volumes were measured via height changes of the meniscus in the pipette barrel with a ×100 magnification microscope equipped with a reticule (resolution: ∼2 nl).
Method for locating the pre-BötC region
We used three criteria to locate the pre-BötC region: predetermined stereotaxic coordinates, presence of a mixture of respiratory neuron subtypes within the VRC, and PNG tachypneic response to d-homocysteic acid (DLH) microinjections (30–40 nl; 20 mM). Our previous studies showed that the canine pre-BötC can be found within a region in the VRC extending from ∼3 to ∼6 mm rostral to the obex. The region where the largest DLH-induced rate altering (typically tachypneic but see following text) responses were observed consisted of a heterogeneous mixture of propriobulbar I and E neuron subpopulations (e.g., Krolo et al. 2005), which is consistent with observations in other species (Chitravanshi and Sapru 1999; Connelly et al. 1992; Schwarzacher et al. 1995; Sun et al. 1998). The tachypneic response produced by DLH microinjection into the VRC has been accepted by many investigators as a functional marker of the pre-BötC region (Chitravanshi and Sapru 1999; Krolo et al. 2005; Monnier et al. 2003; Solomon et al. 1999; Wang et al. 2002) and is the reason we used it in this study as one of the main criteria. For the present study, starting at 3 mm rostral to obex and typically 4.5 mm lateral to the midline, the micropipette was advanced into the VRC, and the subtypes of neurons were noted from the dorsal to the ventral aspects of the VRC. The pipette was withdrawn to the midpoint, and DLH was then microinjected while monitoring the PNG response. The micropipette was then removed, the tip cleaned, and then reinserted 1 mm more rostral, and the procedure was repeated, continuing to 7 mm rostral. The site within this region of the VRC with the maximum tachypneic response to DLH microinjection (∼30 nl) was considered to represent the pre-BötC region (Krolo et al. 2005). If no tachypneic response was found within the VRC, then coordinates and neuron subtype composition were used to locate the pre-BötC region.
Remi infusion
The distinct advantages of using remi are its rapid onset and short latency to peak effect as well as its rapid recovery, which is independent of dose rate and length of infusion. These properties of remi are due to its rapid metabolism by nonspecific esterases in the blood and tissues. Because it is short acting, remi must be continuously infused, and the infusion rate can be adjusted to produce steady-state responses of various degrees. The context-sensitive half-life, the time to a 50% decrease of an effective site concentration after infusion is stopped, has been estimated to be ∼4 min for remi and is independent of infusion duration (Burkle et al. 1996). The effective dose, which reduces minimum aveolar concentration of volátile anesthetics by 50% to surgical stimuli in humans, was found to be ∼0.5 μg·kg−1·min−1 and appears to be similar in dogs (Michelsen and Hug 1996; Michelsen et al. 1996).
Protocols
BILATERAL MICROINJECTION OF NAL INTO THE PRE-BÖTC DURING INTRAVENOUS REMI-INDUCED BRADYPNEA.
After establishing a stable PNG baseline pattern, the pre-BötC region was located as previously described. Subsequently, remi was infused at an intravenous rate (∼0.1–1.0 μg·kg−1·min−1) that resulted in a marked steady-state bradypnea (∼60% decrease in central breathing frequency, fB). During continued steady-state remi infusion, a series of bilateral injections of NAL (500 μM solution, ∼120 nl each), three on each side, centered in the pre-BötC and 1 mm rostral and caudal, were performed to locally block MORs. TI, TE, and PPA were continuously monitored on-line with a PowerLab system (ADInstruments, Castle Hill, Australia, 16SP and Chart v5.5.6). Average values of these variables and fB were obtained for off-line analysis before remi infusion and from data 1 min before and after each NAL microinjection during remi infusion. The same concentration (500 μM) NAL solution was finally given intravenously (total dose: ∼60 μg/kg iv) following completion of all direct pre-BötC NAL microinjections to verify its continued effectiveness in reversing the intravenous remi effects.
PICOEJECTION OF NAL ONTO SINGLE PRE-BÖTC NEURONS DURING REMI-INDUCED BRADYPNEA.
After locating the pre-BötC region in a second set of animals, control recordings from single pre-BötC respiratory-related neurons were made before and during remi-induced bradypnea. During steady-state bradypnea, when pre-BötC neuronal discharge frequencies are typically depressed by 25–50%, NAL (500 μM) was continuously picoejected onto the depressed neuron at increasing picoejection rates up to a level, which was estimated to produce a NAL concentration at 200 μm from the pipette tip that approached the barrel concentration. Subsequently, the intravenous remi infusion was stopped, and neuronal activity was followed during the recovery phase from the effects of remi. The micropipette was then repositioned in a new location ≥500 μm distant from the previous track to avoid contamination from residual effects of intramedullary NAL, and the protocol was then repeated. In addition, at least once per animal, picoejection of the ACSF vehicle was used to verify that the ACSF constituents and ejected volumes were without effect on single neurons. A time-amplitude window discriminator was used to generate a standard logic pulse for each spike. Neuronal discharge frequency, Fn, was continuously determined on-line by the number of discharges in each 100-ms time increment. Neuronal and phrenic activity and the picoejection marker signals were recorded on a digital tape system (Model 3000A, A. R. Vetter, Rebersburg, PA) for further off-line analyses. Cycle-triggered histograms (CTHs; 50-ms bins) of the tape-recorded neuronal discharge were generated from template-discriminated spikes (Spike2, v6.04, Cambridge Electronic Design, Cambridge, UK). Peak and average discharge frequency were determined from the CTHs (15–20 cycle/CTH) and used to measure the remi and NAL effects.
MICROINJECTION OF DAMGO INTO THE PRE-BÖTC REGION: ASSESSMENT OF THE DIRECT MOR AGONIST EFFECT ON THE PHRENIC NERVE ACTIVITY AND BREATHING PATTERN.
After locating the pre-BötC region in the VRC in a separate third set of animals, DAMGO (100 μM) was microinjected into the pre-BötC region, and the effects on the PNG were measured. After completion of the DAMGO injections, once maximal DAMGO effects on the PNG were observed, remi was infused intravenously to measure additional changes in the PNG that would have resulted from systemic opioid effects on areas other than the pre-BötC. Then the remi infusion was stopped, and recovery from the remi-induced changes in fB was awaited. Because the effects of pre-BötC DAMGO microinjections are long-lasting and will outlast the effects of iv remi, the phrenic tachypnea recurred. This persistent DAMGO effect was then reversed by microinjection of a concentrated solution of NAL (5 mM) into the same site (pre-BötC). To confirm that all DAMGO effects were limited to the pre-BötC area, this was followed, 10–15 min later, by intravenous NAL (∼60 μg/kg), which would have antagonized any additional opioid effects on the brain stem. In a subset of four dogs, DAMGO was injected bilaterally into the pre-BötC to measure any additional direct effects.
Data analysis
Statistical procedures were carried out using SigmaStat 3.5 (Systat Software,, Richmond, CA). A one-way repeated-measures ANOVA was used on data that were normalized relative to control values. A general linear model was automatically used to provide least-squares estimates of the means for cells with missing data and unbalanced data sets. The Holm-Sidak method was used for pairwise multiple comparisons with a family-wide error rate of 0.05. For all data sets, tests for normality of the normalized data (Kolmogorov-Smirnov test) were performed before parametric procedures were used. For data sets that failed the normality test, a Kruskal-Wallis one-way ANOVA on ranks was used with Dunn's method for pairwise multiple comparisons. Differences were considered significant for P < 0.05. Values are expressed as means ± SE.
RESULTS
We found that the tachypneic region associated with the pre-BötC region was located within the VRC at 4.3–6.8 mm rostral to obex, 4–5 mm lateral to the midline and 6–8 mm below the dorsal medullary surface. The area also contained a mixture of inspiratory and expiratory neuron subpopulations as has been previously shown by Krolo et al. (2005).
Protocol 1. Bilateral microinjections of NAL into the pre-BötC during intravenous remi-induced bradypnea
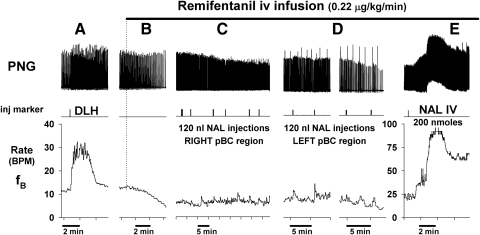
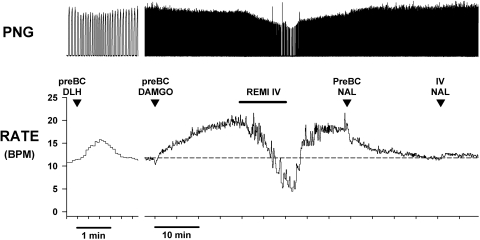
An example of this protocol is shown in Fig. 1, which shows traces of the PNG, injection markers, and breathing rate fB. DLH microinjection produced a strong tachypnea (from 12 to 30 breath/min; BPM), indicative of the pre-BötC region. Intravenous infusion of remi (0.22 μg·kg−1·min−1) decreased fB to ∼5 BPM. During steady-state remi-induced bradypnea, a series of NAL microinjections (120 nl each) were made on the right side and then the left side of the medulla centered in the pre-BötC region. Microinjections were placed at the center of the maximum tachypneic region and 1 mm rostral and 1 mm caudal to it. At each site two microinjections were made separated by ∼0.5 mm in depth. Note that none of the local NAL microinjections had any effects on fB. In Fig. 1D, augmented PNGs or sigh-like activity is apparent. Such sighs are seen at baseline and can become more frequent during intravenous remi-induced bradypnea in some but not all dogs with and without NAL microinjections.
Fig. 1.
Example of protocol 1: bilateral microinjections of naloxone into the pre-Bötzinger Complex (pre-BötC) region fail to antagonize intravenous remifentanil-induced bradypnea: Top: phrenic neurogram (PNG); middle: microinjection marker; bottom: phrenic breathing (burst) rate. A: d-homocysteic acid (DLH) microinjection induces tachypnea, which functionally identifies the pre-BötC location. B–E: intravenous remifentanil (IV REMI) infusion (upper horizontal bar) produces a gradual reduction in phrenic breathing rate during B. C and D: during steady-state IV REMI induced bradypnea, multiple microinjections of naloxone (NAL) into area of pre-BötC (pBC) in the right and left ventral respiratory columns fail to antagonize IV REMI-induced bradypnea. Of note, remifentanil (remi) can cause occasional sighs as visible in D. E: intravenous NAL (NAL) promptly reverses IV REMI bradypnea with overshoot tachypnea.
In contrast to pre-BötC NAL microinjections, intravenous NAL rapidly reversed the remi-induced bradypnea and depression of phrenic amplitude. This reversal tended to transiently overshoot beyond the control baseline levels to varying degrees (Figs. 1E and 2, NAL IV bars). A transient intravenous NAL-induced overshoot was also regularly seen in systemic blood pressure (data not shown). Note that due to drift in the central respiratory rate over time despite steady-state intravenous remi, fB had increased prior to NAL microinjections and was much higher after the final intravenous NAL bolus compared with the initial baseline rate. A slow, spontaneous increase in breathing frequency can be often seen in decerebrate dogs without as well as during steady-state remi infusions, but such slow increases occur over many hours so that they do not influence or negate assessment of the effects of interventions over shorter time spans (e.g., <30 min).
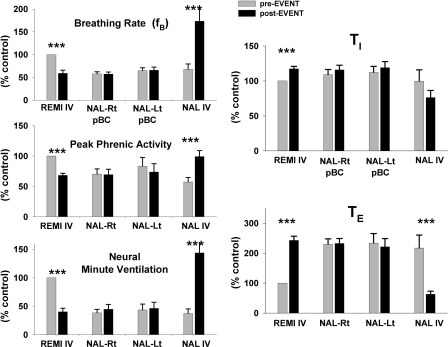
Fig. 2.
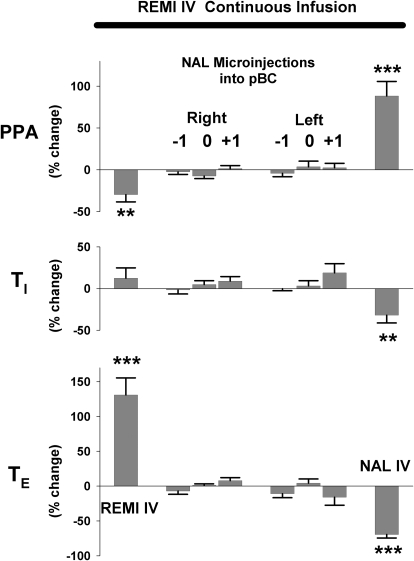
Summary data for protocol 1 from 15 dogs confirm that right sided (Rt)- and left-sided (Lt) microinjections of NAL into pre-BötC fail to reverse the intravenous remi (REMI IV) induced bradypnea, while intravenous NAL (NAL IV) promptly reverses REMI IV effects with overshoot tachypnea. Top left: phrenic (burst) breathing rate; middle left: peak phrenic activity; and bottom left: neural minute ventilation. Right: intravenous remi (REMI IV) induced a modest prolongation of inspiratory duration (TI) and marked prolongation of expiratory duration (TE), but bilateral microinjections of NAL into the pre-BötC failed to have any effect, whereas intravenous NAL (NAL IV) promptly reversed REMI IV effects. All values are normalized to control values, see text for details. ***, P < 0.001 for significant differences between preevents () and postevents (▪).
In 15 dogs, remi (0.50 ± 0–13 μg·kg−1·min−1) decreased fB by 41 ± 7% from 27.5 ± 4.6 to 14.5 ± 3.6 BPM, decreased PPA by 32 ± 3%, decreased neural minute ventilation by 60 ± 6% (Fig. 2, left), increased TI by 17 ± 4%, and increased TE by 143 ± 14% (Fig. 2, right). An average protocol took 2.6 h to complete. The time from the start of the remi infusion to the first NAL injection was 55 ± 10 min, and the average time between injection sites was 16 ± 2 min, which included cleaning the micropipette tip, insertion in the new location, locating neuronal activity in the VRC, placing two injections of ∼125 nl each and ∼0.5 mm apart in the dorsal and ventral regions of the VRC, and micropipette withdrawal. Based on a brain extracellular volume fraction of 0.21 [fraction of the total brain tissue volume (Nicholson 1985)], each 125 nl injection volume would have a spherical diameter of ∼1 mm and with subsequent diffusion would have an even larger effective volume diameter. Thus three injections that are 1 mm apart would allow the effects of NAL to extend over 3 mm in the rostral-caudal direction of VRC-pre-BötC region and well over 1 mm in the other directions. Despite the relative large projected volume of spread, Fig. 2 shows that the NAL injections had no effect on any of the phrenic respiratory timing and drive variables. To correct for the effects of time drift on respiratory rate (see preceding text) from the onset of the protocol, changes in the variables were always analyzed relative to their immediate local control values, that is, the value preceding each injection. As shown in Fig. 3 with such corrections, there were no changes in PPA, TI, and TE following the NAL injections. In stark contrast, intravenous NAL (60.7 ± 19 μg/kg) during the remi infusion promptly and fully reversed the remi-induced effects (Fig. 2, NAL IV).
Fig. 3.
Summary data for protocol 1 from 15 dogs confirm that right- and left-sided microinjections of NAL into pre-BötC (pBC) region fail to reverse the intravenous remi (REMI IV) induced depression of phrenic amplitude (PPA), prolongation of inspiratory duration (Ti) and expiratory duration (TE) while intravenous NAL (NAL IV) promptly reversed all REMI IV effects. To correct for the effects of time drift on phrenic respiratory rate (see text for details) from the onset of the protocol, changes in the variables were always analyzed relative to their immediate local control values, that is the value preceding each injection (e.g., preevent in Fig. 2). **, P < 0.01; ***, P < 0.001 for significant differences from no change.
Protocol 2. Picoejection of NAL onto single neurons in or near the pre-BötC during remi-induced bradypnea
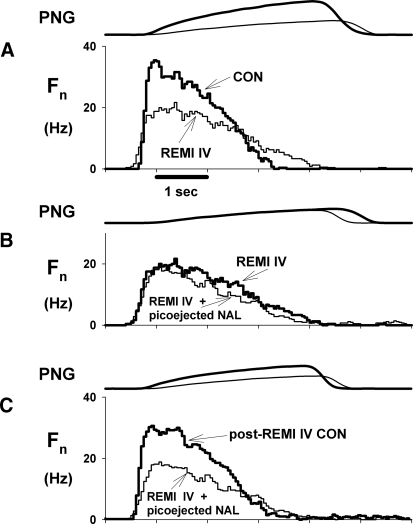
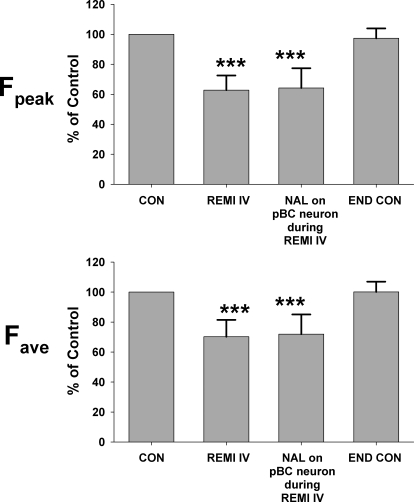
Figure 4 shows an example of protocol 2 for an inspiratory decrementing neuron in or near the pre-BötC region. Intravenous infusion of remi (0.16 μg·kg−1·min−1) decreased the peak discharge frequency (Fn) from 32.2 to 20.2 Hz (∼37%) and decreased the decrementing rate (Fig. 4A). Picoejection of NAL (500 μM at 2.9 nl/min or 1.45 pmol/min) onto this neuron had no effect on reversing the remi-induced depression (Fig. 4B), but the neuron's discharge pattern completely recovered following termination of the remi infusion (Fig. 4C). Pooled data from three inspiratory and three expiratory neurons show that highly localized application of NAL did not reverse the remi-induced depression of neuronal activity. Remi intravenous infusion (0.20 ± 0.03 μg·kg−1·min−1) produced a 37.2 ± 9.8% decrease in peak Fn and a 29.8 ± 11.2% decrease in the time-averaged Fn (Fig. 5, Fpeak and Fave). Picoejection of NAL (4.4 ± 1.2 pmol/min) had no effect, but neuronal activity completely recovered following termination of remi infusion. Even though we found that none of six neurons (0/6) respond to NAL, with this small sample, it is possible to miss neurons depressed by remi that would have been reversed by local NAL. Without prior knowledge of the proportion of the neuron population that is unresponsive, a 95% confidence interval analysis suggests that the proportion of neurons that did not respond to NAL lies between 0 and 46% (Motulsky 1995).
Fig. 4.
Example from an inspiratory pre-BötC neuron with a decrementing discharge pattern from protocol 2. Top trace of each panel shows effect on PNG; bottom trace of each panel shows effect on neuronal discharge pattern as a cycle triggered histogram of the discharge frequency. A: phrenic and neuronal activities were depressed by intravenous remi (REMI IV) from control (CON). B: picoejection of NAL (picoejected NAL) onto the neuron during remi-induced depression (REMI IV) did not reverse the decrease of the neuronal peak discharge. C: phrenic activity and neuronal discharge almost fully recovered after cessation of the intravenous remi infusion (post-REMI IV CON).
Fig. 5.
Pooled data for protocol 2 from 3 inspiratory and 3 expiratory neurons. Second bar of each panel show that intravenous remi infusion (REMI IV) decreased peak and time-averaged discharge frequency (Fpeak and Fave). Picoejection of NAL (NAL during REMI IV, 3rd bar) onto the neuron in the pre-BötC did not reverse the remi-induced depression of neuronal activity. Neuronal activity completely recovered following termination of remi infusion (END CON). ***, P < 0.001 for significant differences from control (100%).
Protocol 3. Microinjection of DAMGO into the pre-BötC region: assessment of the direct MOR agonist effect on the phrenic nerve activity and breathing pattern
Figure 6 shows an example of the protocol used to study the effects of microinjection of the MOR agonist DAMGO into the pre-BötC region on the breathing pattern. The pre-BötC region was functionally identified in the VRC, where I and E neurons were found, by the typical tachypneic response produced by DLH microinjection into the left VRC. Following recovery of the response to DLH, 109 nl 100 μM DAMGO was microinjected, and fB increased from ∼12 to ∼20 BPM (67% increase) over a 15-min period. In contrast, intravenous infusion of 0.8 μg·kg−1·min−1 remi after maximal DAMGO effects were reached produced a pronounced decrease in fB to ∼5 BPM. After recovery from remi-induced bradypneic effects (∼15 min), the prolonged residual DAMGO effect caused a return to relative tachypnea. At this point, microinjection of NAL (360 nl; 5 mM) into the same site where DAMGO was microinjected gradually reversed the DAMGO-induced tachypnea with return of respiratory rate to pre DAMGO baseline. Subsequent intravenous NAL (NAL IV; 79 μg/kg) produced no additional effect in this animal. Typically DAMGO-induced effects lasted >1 h before spontaneous resolution. The overall protocol took ∼90 min to complete.
Fig. 6.
Example of a protocol 3 run shows that microinjections of the mu-opioid receptor (MOR) agonist [d-Ala2, N-Me-Phe4, gly-ol5]-enkephalin (DAMGO) into the pre-BötC region increased the phrenic burst rate. Top: the PNG and bottom: the fictive respiratory rate derived from the phrenic neurogram. From left to right: DLH microinjection (44 nl: 20 mM) induces tachypnea, which locates pre-BötC. Subsequent unilateral microinjection of DAMGO (109 nl; 100 μM) at the same site increased respiratory rate with only minor effects on peak phrenic activity. Intravenous remi (REMI IV; 0.8 μg·kg−1·min−1) overcame the effects of DAMGO and, on the contrary, produced a profound bradypnea and depression of peak phrenic activity. Following recovery of respiratory rate and peak phrenic activity after cessation of intravenous remi, microinjection of NAL (360 nl; 5 mM) into the same site where DAMGO was microinjected, gradually reversed the DAMGO-induced tachypnea. Subsequent intravenous NAL (NAL IV; 79 μg/kg) produced little or no additional effect.
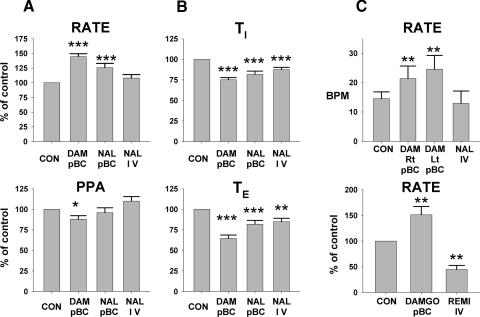
The pooled data (n = 16, Fig. 7) show that unilateral microinjection of 100 μM DAMGO (143 ± 16 nl) into the right pre-BötC region increased fB by 44.4 ± 5.2% from the baseline fB of 17.7 ± 2.9 BPM. This was due to a 25 ± 3% decrease in TI and a 35 ± 4% decrease in TE (Fig. 7B), where baseline TI and TE values were 1.64 ± 0.20 and 3.35 ± 0.64 s, respectively. PPA was decreased by 12 ± 4%. Subsequent microinjection of NAL (211 ± 39 nl, 5 mM) at the same location as the DAMGO microinjections could only partially antagonize the DAMGO-induced tachypnea, reducing fB to 25.7 ± 7.3% above control, but restored PPA to control levels (Fig. 7A, NAL pBC). However, intravenous administration of NAL (76 ± 5 μg/kg iv) produced additional and nearly complete antagonism of the DAMGO effects in the pre-BötC region. In 4 of the 16 dogs, bilateral DAMGO microinjections in the pre-BötC regions were studied to determine the magnitude of the additional DAMGO injection. The pooled data (Fig. 7C, top) show that the right-sided microinjections increased fB from 14.5 ± 2.3 to 21.4 ± 4.3 BPM or ∼48%. Additional left-sided DAMGO microinjections increased fB to 24.6 BPM or a further 22%. Subsequent intravenous NAL returned fB to control levels.
Fig. 7.
Summary of the normalized pooled data for protocol 3 (n = 16) shows that unilateral microinjection of DAMGO into the right pre-BötC region increased breathing rate (A, top, 2nd bar, DAM pBC). This was due to decrease in TI (B, top, 2nd bar, DAM pBC) and in TE (B, bottom, 2nd bar, DAM pBC). PPA was only modestly decreased (A, bottom, 2nd bar, DAM pBC). Subsequent microinjection of NAL (NAL pBC) at the same location as the DAMGO microinjections only partially antagonized the DAMGO-induced tachypnea (A, top, 3rd bar) but restored PPA to control levels (A, bottom, 3rd bar). However, intravenous administration of NAL (NAL IV) produced complete antagonism of the DAMGO effects in the pre-BötC region. C, top: summary of the pooled data from 4 of the 16 dogs with sequential bilateral DAMGO microinjections in the pre-BötC regions. Additional left-sided DAMGO (DAM Lt pBC) microinjections increased phrenic respiratory rate further, but the difference from the right-sided DAMGO (DAM Rt pBC) microinjections was not statistically significant. Subsequent intravenous NAL (NAL IV) reversed the DAMGO effects and returned the rate to control levels. C, bottom: summary data from 7 of 16 dogs, in which remi (REMI IV) was infused intravenously after the DAMGO microinjections to compare systemic vs. local μ-opioid effects on phrenic respiratory rate. Unilateral microinjection of DAMGO (DAMGO pBC) increased respiratory rate, whereas intravenous remi (REMI IV) decreased the respiratory rate relative to control. The 2 effects acted in opposite directions. *, P < 0.05; **, P < 0.01; ***, P < 0.001 for significant differences from control.
In 7 of the 16 dogs, remi (1.0 ± 0.1 μg·kg−1·min−1) was infused intravenously after the DAMGO microinjections, i.e., while the DAMGO effects persisted, to compare systemic versus local μ-opioid effects on fB. In these studies, unilateral microinjection of DAMGO increased fB by 51 ± 16%, whereas intravenous remi decreased fB by 55 ± 8% relative to control (Fig. 7C, bottom). Only after complete recovery from the remi effects were local NAL microinjections given, followed by intravenous NAL injections.
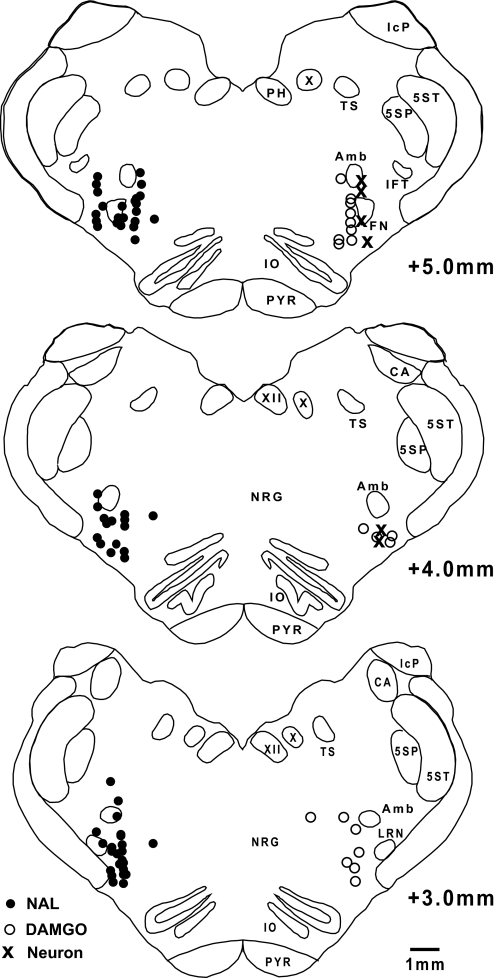
The anatomical sites of the NAL and DAMGO microinjections and studied neurons as determined by the stereotaxic coordinates relative to the obex, midline, and dorsal surface are shown in Fig. 8. The microinjection sites were always in a region of respiratory neuronal activity, presumably the VRC.
Fig. 8.
Coronal sections of the brain stem in the area of the pre-BötC show the anatomical sites of the NAL (•) and DAMGO (○) microinjections and neurons (×) relative to the obex, midline, and dorsal surface. For clarity, NAL and DAMGO injection sites are shown on separate sides.
DISCUSSION
The main finding of this study is that the bradypnea produced by systemically administered μ-opioids at clinically relevant doses for analgesia is not caused by direct MOR activation in the pre-BötC. Rather, direct activation of pre-BötC MORs with the selective agonist DAMGO consistently produced tachypnea. This conclusion is also supported by the lack of a reversing effect of NAL on single pre-BötC neurons that were depressed during intravenous remi infusion, within the limits imposed by the small sample size (n = 6). These findings suggest that MORs on neurons presynaptic to the pre-BötC neurons must be responsible for the bradypneic response. In addition, although there are functional MORs in the pre-BötC region, their in vivo role in the control of breathing is not clear. It is clear that high local opioid agonist concentrations in the micro- to millimolar range are required to directly depress respiratory neurons in the pre-BötC, similar to our findings for VRC premotor neurons (Stucke et al. 2008). Such high concentrations may be reached by synaptic release of endomorphins, i.e., pre-BötC MORs may be synaptically activated by endogenous peptides (Martin-Schild et al. 1999; Zadina et al. 1999). It is known from other neurotransmitters that their transient synaptic concentrations can reach the millimolar range (Bruns et al. 2000; Clements 1996).
In a minority of animals, mostly those with low baseline respiratory rates, we observed an increase in respiratory rate with remi rather than a slowing. These animals were not used for the study protocols. This discrepancy to the clinical experience with patients is likely due to our particular experimental setup. Vagotomy may eliminate a direct opioid effect on vagal inputs and thus reduce slowing. Also the increase in arterial CO2 that results from opioid application in patients can cause slowing of the respiratory rate, whereas in our animals, ventilation was controlled and CO2 remained unchanged.
Methodological considerations
We used three criteria to locate the pre-BötC region: predetermined stereotaxic coordinates, presence of a mixture of respiratory neuron subtypes within the VRC, and PNG tachypneic response to DLH microinjections (30–40 nl; 20 mM). Our previous studies showed that the canine pre-BötC can be found within a region in the VRC extending from ∼3 to ∼6 mm rostral to the obex. The region where the largest DLH-induced rate altering (typically tachypneic) responses were observed consisted of a heterogeneous mixture of propriobulbar I and E neuron subpopulations (e.g., Krolo et al. 2005), which is consistent with observations in other species (Chitravanshi and Sapru 1999; Connelly et al. 1992; Schwarzacher et al. 1995; Sun et al. 1998). The tachypneic response produced by DLH microinjection into the VRC has been accepted by many investigators as a functional marker of the pre-BötC region (Chitravanshi and Sapru 1999; Krolo et al. 2005; Monnier et al. 2003; Solomon et al. 1999; Wang et al. 2002) and is the reason we used it in this study as one of the main criteria. The pre-BötC region in the dog consists of a heterogeneous mixture of propriobulbar inspiratory and expiratory neuron subpopulations and is located within the VRC at 4.3–6.8 mm rostral to obex (Krolo et al. 2005). We are fairly certain that our local microinjections were sufficient to achieve maximal drug effects on all neurons of the pre-BötC. The size and spread of the multiple NAL injections (∼125 nl, >3 mm in the rostral-caudal direction, centered at the point of maximum tachypneic response and >1 mm in the medial-lateral and dorsal-ventral aspects of the VRC) probably exceeded the boundaries of the pre-BötC region. Microinjections were only made in the ventrolateral medulla where respiratory neuronal activity was found. In addition, the concentration of microinjected NAL (500 μM) was >1,000 times greater than the plasma concentration that was required for reversal of remi-induced effects by intravenous NAL. Furthermore, because diffusion occurs with NAL microinjections, the effective volume of MOR antagonism by local NAL would be much greater than the injected volume. Finally, microinjections of DAMGO into the same region produced a reproducible tachypneic response consistent with direct activation of MORs on neurons in the pre-BötC region. On the other hand, the fact that NAL microinjections did not have any effects on the PNG suggests that diffusion of the drug was limited to the VRC and did not affect other areas of respiratory control.
Our conclusions are based on whole phrenic nerve recordings during microinjections into a network of neurons; however, these conclusions are also supported at the cellular level. Picoejection of NAL on single pre-BötC neurons (n = 6) failed to reverse any of the neuronal depression induced by intravenous remi. Similarly, a previous study found that none of the 18 (95% confidence interval: 0–18.5%) bulbospinal respiratory premotor neurons studied were affected by NAL picoejection during intravenous remi-induced depression even though these neurons have been shown to have μ- and ∂-opioid receptors using selective agonists (Stucke et al. 2008).
A possible explanation for the lack of a direct effect on pre-BötC MORs by systemic administration of remi is the low plasma and thus brain concentration levels that are attained. The typical remi infusion rates we used (∼0.5 μg·kg−1·min−1) are expected to result in plasma concentrations of ∼10 ng/ml or 24 nM in dogs (Michelsen et al. 1996), which is ∼4,000 times less than the concentration of microinjected DAMGO (100 μM), which produced a tachypneic response. An IC50 of 35 ± 9 nM for DAMGO inhibition of cAMP production was found in cells expressing cloned MORs (Gharagozlou et al. 2003). In mice, the unbound brain EC50 concentrations for remi related opioids, fentanyl, alfentanil, and sufentanil, have been found to be very similar to the unbound EC50 serum concentrations and to the in vitro affinity (Ki) (Kalvass et al. 2007). Because remi is about half as potent as fentanyl (Michelsen et al. 1996), the estimated affinity Ki for remi would be between 5 and 10 nM. This may explain why the targeted ∼24 nM remi plasma concentration we used in the current study induced the observed, marked bradypneic responses, assuming that highly opioid-sensitive sites responsible for respiratory rate control were affected.
In our previous study on VRC bulbospinal premotor neurons, where intravenous remi had only upstream effects but no direct depressant effects at the premotor neuronal level, picoejection of remi at concentrations >20 times larger than reported peak plasma concentrations had no effect on the discharge of single neurons. A similar scenario may take place in the more rostral VRC pre-BötC region. Thus it seems likely that the remi-induced depression of breathing rate occurs at respiratory-related neurons that are presynaptic to the pre-BötC neurons, which may express MORs that respond to low nanomolar opioid concentrations that are typical during clinical analgesia and respiratory depression. The sensitivity of neurons to low concentrations of opioids may not only be related to the number or density of MORs but may also depend on the location of the MORs on the neuron. For example, if the receptors are strategically located on axon terminals, similar to the primary afferent fibers in the dorsal horn of the spinal cord that appear to inhibit the release of neurotransmitters via inhibition of voltage-dependent Ca2+ channels, the opioid effect could be markedly enhanced compared with a location on or near the soma.
DAMGO-induced tachypnea
Microinjections of DAMGO into the pre-BötC region produced an increase in respiratory rate, which was opposite to the slowing response produced by intravenous remi and also in in vitro studies. In brain stem slices containing the pre-BötC, rhythm generation appears to rely on pacemaker neuron activity (15) (Ballanyi et al. 1999; Butera et al. 1999a,b). Opioid-induced membrane hyperpolarizations of pacemaker neurons produce slowing of the burst rate whereas depolarizations increase burst rate (Gray et al. 1999). Bath application of DAMGO in brain stem-spinal cord preparations (Mellen et al. 2003) and systemic fentanyl in juvenile rats (P7–P13; 16–32 g) (Janczewski and Feldman 2006) produce quantal slowing of inspiratory burst activity, while the burst rate of expiratory related activity is unchanged. We have not observed quantal slowing in our in vivo dog model but rather a gradual dose-dependent increase in TI and TE with intravenous remi. In addition, the discharge of canine expiratory neurons in the pre-BötC region is prolonged with remi-induced increases in TE and remains coupled to inspiratory neuronal activity in a 1:1 manner (Zuperku, personal observation).
In developmentally mature in vivo preparations, rhythm generation appears to be the result of a network in which synaptic interactions among neurons play a key role (Richter and Spyer 2001). In such models that have been analyzed via computer simulation, the indispensable element is the reciprocal synaptic inhibition between I and E neurons with intrinsic adaptive properties that result in decrementing patterns (Rybak et al. 2008). Computer simulation of such a reduced network model consisting only of I and E decrementing neurons with inhibitory reciprocal interconnections shows that a reduction in tonic excitatory drive produces an increase in oscillatory rate, whereas an increase in excitation produces slowing due to increased synaptic inhibition (Stuth et al. 2005). Because many VRC neurons appear to express MORs (Manzke et al. 2003; Stucke et al. 2008), a possible explanation for the tachypnea produced by microinjection of DAMGO into the pre-BötC region is that a simultaneous postsynaptic depression of pre-BötC region neurons by DAMGO may result in decreased excitation and thus an increase in the rate of the oscillator and the respiratory rate. However, other mechanistic explanations are possible.
Possible sites for intravenous remi-induced effects
Immunohistochemical studies, using antibodies to cloned MORs have been used to examine the distribution of MORs in the rat brain. Within respiratory-related areas, the most intense MOR-like immunoreactivity (LI) was seen in the pontine lateral parabrachial nuclei (PBN), the locus coeruleus, rostral ambiguous nucleus, and the medial and commissural subnuclei of the solitary tract (NTS) (Ding et al. 1996). The NTS contained nerve fibers with intense MOR-LI, including the primary afferent fibers of the vagus nerve. Endomorphin types 1 and 2 (EM-1 and EM-2) are endogenous peptides that have high affinity and selectivity for MORs and potent analgesic activity. Immunoreactivity to EM-1 and EM-2 was used to determine the location of fibers and cell bodies of endomorphin-containing neurons (Martin-Schild et al. 1999). In these studies, dense EM-1 LI was seen in the PBN, locus coeruleus, and the NTS. Some of the endomorphin inputs to the PBN in rats arise from EM-1 and EM-2 LI neurons in the hypothalamus (Chen et al. 2004). Using dual labeling, immunocytochemistry combined with electron microscopy, Silverman et al. (2005) found EM-2 LI primarily in the unmyelinated axons and axon terminals in the NTS, including some vagal afferents, and MOR LI in many of the larger dendritic targets of the EM-2 LI terminals. In addition, there appear to be reciprocal connections of EM-1- and EM-2-containing neurons between the hypothalamus and the NTS in the rat (Hui et al. 2006).
It is well known that inputs to the pre-BötC region from the NTS and the PBN contribute to respiratory phase switching. Afferent inputs from pulmonary stretch receptors end in the NTS and mediate the Breuer-Hering inspiratory inhibitory and expiratory facilitatory reflexes (Kubin et al. 2006). Outputs from the neurons in the parabrachial complex region, including the Kölliker-Fuse nucleus and intertrigeminal region, project to the VRC, and chemical or electrical activation of these neurons profoundly alter respiratory phase timing (Chamberlin 2004; Cohen 1971). A recent network model, which is based on spike-train cross-correlation analysis and which assigns specific synaptic connections between pontine neurons and pre-BötC/Bötzinger neurons, is able to simulate experimentally observed responses to various perturbations (Rybak et al. 2008). Thus it appears that the presence of dense MORs in the PBN and NTS suggest that they could be potential sites where systemic opioids such as intravenous remi act to produce their effect on breathing rate. Another possible site may include the midline medullary raphe region where MOR LI has been seen (Ding et al. 1996). Zhang et al. (2007) found that the systemic DAMGO-induced inhibition of ventilation and of the carbon dioxide response was significantly reduced after pretreatment of the caudal raphe region with a selective MOR antagonist.
Summary
Even though pre-BötC neurons contain μ opioid receptors, it does not appear that clinical doses of intravenous opioids act directly on them to produce bradypnea, in vivo. We speculate that the effect of intravenous opioids, such as remi, may be on presynaptic neurons that project to the pre-BötC region and are involved with the control of respiratory phase timing.
GRANTS
This work was supported by the Department of Anesthesiology, Medical College of Wisconsin, Children's Hospital of Wisconsin and the Zablocki Veterans Administration Medical Center, Milwaukee, Wisconsin. This work also was supported by National Institute of General Medical Sciences Grant GM-59234-06A1 to E.A.E. Stuth and Veterans Affairs Medical Research Funds to E. J. Zuperku.
ACKNOWLEDGMENTS
The authors thank J. Tomlinson, biological laboratory technician, the Zablocki VA Medical Center, Milwaukee, WI, for excellent technical assistance.
REFERENCES
- Ballanyi K, Onimaru H, Homma I. Respiratory network function in the isolated brain stem-spinal cord of newborn rats. Prog Neurobiol 59: 583–634, 1999 [DOI] [PubMed] [Google Scholar]
- Bruns D, Riedel D, Klingauf J, Jahn R. Quantal release of serotonin. Neuron 28: 205–220, 2000 [DOI] [PubMed] [Google Scholar]
- Burkle H, Dunbar S, Van Aken H. Remifentanil: a novel, short-acting, mu-opioid. Anesth Analg 83: 646–651, 1996 [DOI] [PubMed] [Google Scholar]
- Butera RJ, Jr, Rinzel J, Smith JC. Models of respiratory rhythm generation in the pre-Bötzinger complex. I. bursting pacemaker neurons. J Neurophysiol 81: 382–397, 1999a [DOI] [PubMed] [Google Scholar]
- Butera RJ, Jr, Rinzel J, Smith JC. Models of respiratory rhythm generation in the pre-Bötzinger complex. II. populations of coupled pacemaker neurons. J Neurophysiol 81: 398–415, 1999b [DOI] [PubMed] [Google Scholar]
- Chamberlin NL. Functional organization of the parabrachial complex and intertrigeminal region in the control of breathing. Respir Physiol Neurobiol 143: 115–125, 2004 [DOI] [PubMed] [Google Scholar]
- Chen T, Hui R, Dong YX, Li YQ, Mizuno N. Endomorphin 1- and endomorphin 2-like immunoreactive neurons in the hypothalamus send axons to the parabrachial nucleus in the rat. Neurosci Lett 357: 139–142, 2004 [DOI] [PubMed] [Google Scholar]
- Chitravanshi VC, Sapru HN. Phrenic nerve responses to chemical stimulation of the subregions of ventral medullary respiratory neuronal group in the rat. Brain Res 821: 443–460, 1999 [DOI] [PubMed] [Google Scholar]
- Clements JD. Transmitter timecourse in the synaptic cleft: its role in central synaptic function. Trends Neurosci 19: 163–171, 1996 [DOI] [PubMed] [Google Scholar]
- Cohen MI. Switching of the respiratory phases and evoked phrenic responses produced by rostral pontine electrical stimulation. J Physiol 217: 133–158, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly CA, Dobbins EG, Feldman JL. Pre-Bötzinger complex in cats: respiratory neuronal discharge patterns. Brain Res 590: 337–340, 1992 [DOI] [PubMed] [Google Scholar]
- Ding YQ, Kaneko T, Nomura S, Mizuno N. Immunohistochemical localization of mu-opioid receptors in the central nervous system of the rat. J Comp Neurol 367: 375–402, 1996 [DOI] [PubMed] [Google Scholar]
- Gharagozlou P, Demirci H, David Clark J, Lameh J. Activity of opioid ligands in cells expressing cloned mu opioid receptors. BMC Pharmacol 3: 1, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBotzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci 4: 927–930, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBötzinger complex. Science 286: 1566–1568, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haji A, Okazaki M, Ohi Y, Yamazaki H, Takeda R. Biphasic effects of morphine on bulbar respiratory neuronal activities in decerebrate cats. Neuropharmacology 45: 368–379, 2003a [DOI] [PubMed] [Google Scholar]
- Haji A, Yamazaki H, Ohi Y, Takeda R. Distribution of mu receptors in the ventral respiratory group neurons; immunohistochemical and pharmacological studies in decerebrate cats. Neurosci Lett 351: 37–40, 2003b [DOI] [PubMed] [Google Scholar]
- Hui R, Chen T, Li YQ. The reciprocal connections of endomorphin 1- and endomorphin 2-containing neurons between the hypothalamus and nucleus tractus solitarii in the rat. Neuroscience 138: 171–181, 2006 [DOI] [PubMed] [Google Scholar]
- Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol 570.2: 407–420, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SM, Smith JC, Feldman JL. Modulation of respiratory rhythm in vitro: role of Gi/o protein-mediated mechanisms. J Appl Physiol 80: 2120–2133, 1996 [DOI] [PubMed] [Google Scholar]
- Kalvass JC, Olson ER, Cassidy MP, Selley DE, Pollack GM. Pharmacokinetics and pharmacodynamics of seven opioids in P-glycoprotein-competent mice: assessment of unbound brain EC50, μ and correlation of in vitro, preclinical, and clinical data. J Pharmacol Exp Ther 323: 346–355, 2007 [DOI] [PubMed] [Google Scholar]
- Krolo M, Tonkovic-Capin V, Stucke A, Stuth E, Hopp F, Dean C, Zuperku E. Subtype composition and responses of respiratory neurons in the pre-Botzinger region to pulmonary afferent Inputs in dogs. J Neurophysiol 93: 2674–2687, 2005 [DOI] [PubMed] [Google Scholar]
- Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol 101: 618–627, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley PM. D1-dopamine receptor agonists prevent and reverse opiate depression of breathing but not antinociception in the cat. Am J Physiol Regualtory Integrative Comp Physiol 289: R45–51, 2005 [DOI] [PubMed] [Google Scholar]
- Lalley PM. Mu-opioid receptor agonist effects on medullary respiratory neurons in the cat: evidence for involvement in certain types of ventilatory disturbances. Am J Physiol Regulatory Integrative Comp Physiol 285: R1287–1304, 2003 [DOI] [PubMed] [Google Scholar]
- Lalley PM. Opioidergic and dopaminergic modulation of respiration. Respir Physiol Neurobiol , 164: 160–167, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzke T, Guenther U, Ponimaskin EG, Haller M, Dutschmann M, Schwarzacher S, Richter DW. 5-HT4. (a) receptors avert opioid-induced breathing depression without loss of analgesia. Science 301: 226–229, 2003 [DOI] [PubMed] [Google Scholar]
- Martin-Schild S, Gerall AA, Kastin AJ, Zadina JE. Differential distribution of endomorphin 1- and endomorphin 2-like immunoreactivities in the CNS of the rodent. J Comp Neurol 405: 450–471, 1999 [PubMed] [Google Scholar]
- Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron 37: 821–826, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelsen LG, Hug CC., Jr The pharmacokinetics of remifentanil. J Clin Anesth 8: 679–682, 1996 [DOI] [PubMed] [Google Scholar]
- Michelsen LG, Salmenpera M, Hug CC, Jr, Szlam F, VanderMeer D. Anesthetic potency of remifentanil in dogs. Anesthesiology 84: 865–872, 1996 [DOI] [PubMed] [Google Scholar]
- Monnier A, Alheid GF, McCrimmon DR. Defining ventral medullarly respiratory compartments with a glutamate receptor agonist in the rat. J Physiol 548. 3: 859–874, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motulsky H. Intuitive Biostatistics. New York: Oxford, 1995 [Google Scholar]
- Nicholson C. Diffusion from an injected volume of a substance in the brain tissue with arbitrary volume fraction and tortuosity. Brain Res 333: 325–329, 1985 [DOI] [PubMed] [Google Scholar]
- Pattinson KT. Opioids and the control of respiration. Br J Anaesth 100: 747–758, 2008 [DOI] [PubMed] [Google Scholar]
- Pierrefiche O, Schwarzacher SW, Bischoff AM, Richter DW. Blockade of synaptic inhibition within the pre-Bötzinger complex in the cat suppresses respiratory rhythm generation in vivo. J Physiol 509: 245–254, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter DW, Spyer KM. Studying rhythmogenesis of breathing: comparison of in vivo and in vitro models. Trends Neurosci 24: 464–472, 2001 [DOI] [PubMed] [Google Scholar]
- Rybak IA, O'Connor R, Ross A, Shevtsova NA, Nuding SC, Segers LS, Shannon R, Dick TE, Dunin-Barkowski WL, Orem JM, Solomon IC, Morris KF, Lindsey BG. Reconfiguration of the pontomedullary respiratory network: a computational modeling study with coordinated in vivo experiments. J Neurophysiol 100: 1770–1799, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzacher SW, Smith JC, Richter DW. Pre-Bötzinger complex in the cat. J Neurophysiol 73: 1452–1461, 1995 [DOI] [PubMed] [Google Scholar]
- Silverman MB, Hermes SM, Zadina JE, Aicher SA. Mu-opioid receptor is present in dendritic targets of endomorphin-2 axon terminals in the nuclei of the solitary tract. Neuroscience 135: 887–896, 2005 [DOI] [PubMed] [Google Scholar]
- Solomon IC, Edelman NH, Neubauer JA. Patterns of phrenic motor output evoked by chemical stimulation of neurons located in the pre-Bötzinger complex in vivo. J Neurophysiol 81: 1150–1161: 1999, 1999 [DOI] [PubMed] [Google Scholar]
- Stucke AG, Zuperku EJ, Sanchez A, Tonkovic-Capin M, Tonkovic-Capin V, Mustapic S, Stuth EA. Opioid receptors on bulbospinal respiratory neurons are not activated during neuronal depression by clinically relevant opioid concentrations. J Neurophysiol 100: 2878–2888, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuth EAE, Stucke AG, Zuperku EJ. Central effects of general anesthesia. In: Pharmacology and Pathophysiology of the Control of Breathing, edited by Ward DS, Dahan A, Teppema LJ. Boca Raton, FL: Taylor and Francis, 2005, chap 15, p. 571–652 [Google Scholar]
- Sun QJ, Goodchild AK, Chalmers JP, Pilowsky PM. The pre-Bötzinger complex and phase-spanning neurons in the adult rat. Brain Res 809: 204–213, 1998 [DOI] [PubMed] [Google Scholar]
- Tonkovic-Capin M, Krolo M, Stuth EAE, Hopp FA, Zuperku EJ. Improved method of canine decerebration. J Appl Physiol 85: 747–750, 1998 [DOI] [PubMed] [Google Scholar]
- Wang H, Germanson TP, Guyenet PG. Depressor and tachypneic responses to chemical stimulation of the ventral respiratory group are reduced by ablation of neurokinin-1 receptor-expressing neurons. J Neurosci 22: 3755–3764, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadina JE, Martin-Schild S, Gerall AA, Kastin AJ, Hackler L, Ge LJ, Zhang X. Endomorphins: novel endogenous mu-opiate receptor agonists in regions of high mu-opiate receptor density. Ann NY Acad Sci 897: 136–144, 1999 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Xu F, Zhang C, Liang X. Activation of opioid mu receptors in caudal medullary raphe region inhibits the ventilatory response to hypercapnia in anesthetized rats. Anesthesiology 107: 288–297, 2007 [DOI] [PubMed] [Google Scholar]