Abstract
Stretch reflexes contribute to arm impedance and longer-latency stretch reflexes exhibit increased sensitivity during interactions with compliant or unstable environments. This increased sensitivity is consistent with a regulation of arm impedance to compensate for decreased stability of the environment, but the specificity of this modulation has yet to be investigated. Many tasks, such as tool use, compromise arm stability along specific directions, and stretch reflexes tuned to those directions could present an efficient mechanism for regulating arm impedance in a task-appropriate manner. To be effective, such tuning should adapt not only to the mechanical properties of the environment but to those properties in relation to the arm, which also has directionally specific mechanical properties. The purpose of this study was to investigate the specificity of stretch reflex modulation during interactions with mechanical environments that challenge arm stability. The tested environments were unstable, having the characteristics of a negative stiffness spring. These were either aligned or orthogonal to the direction of maximal endpoint stiffness for each subject. Our results demonstrate preferential increases in reflexes, elicited within 50–100 ms of perturbation onset, to perturbations applied specifically along the direction of the destabilizing environments. This increase occurred only when the magnitude of the environmental instability exceeded endpoint stiffness along the same direction. These results are consistent with task-specific reflex modulation tuned to the mechanical properties of the environment relative to those of the human arm. They demonstrate a highly adaptable, involuntary mechanism that may be used to modulate limb impedance along specific directions.
INTRODUCTION
The human arm is routinely used to perform postural tasks, such as pushing against a tool, that compromise limb stability (Rancourt and Hogan 2001). To complete these tasks, the neuromotor system must adapt the mechanical properties of the arm so that the coupled mechanics of the arm and the environment to which it is attached remain stable. When posture is constrained, this adaptation must occur through changes in muscle activity, mediated by some combination of feedforward and feedback pathways. Feedforward motor commands can be refined through learning (Shadmehr and Mussa-Ivaldi 1994), and it has been suggested that changes in feedforward planning contribute to the task-appropriate regulation of arm mechanics during movements through unstable environments (Burdet et al. 2001; Franklin et al. 2003). Specifically, it has been demonstrated that changes in feedforward activation can preferentially modulate the mechanical properties of the arm along the direction needed to compensate for the environmental instabilities. When compensating for unstable loads, these changes in feedforward activation correspond to the specific patterns or cocontraction needed to stabilize the arm. Presently, it is unclear if the same capacity for directional specificity exists in the reflex pathways providing feedback control, although this has long been a topic of interest and debate (Franklin et al. 2007; Hogan 1985; Lacquaniti et al. 1993).
Strong evidence suggests that stretch reflexes contribute to the regulation of limb mechanics and stability. They contribute to the stiffness of individual muscles (Nichols and Houk 1976) and joints (Kearney et al. 1997; Sinkjaer et al. 1988; Zhang and Rymer 1997). They also can coordinate the actions of muscles acting on different degrees of freedom within the same joint or spanning multiple joints, possibly countering mechanical interactions between these degrees of freedom (Gielen et al. 1988; Kurtzer et al. 2008, 2009; Lacquaniti and Soechting 1986; Smeets and Erkelens 1991). Furthermore, reflex sensitivity is not fixed. Longer-latency stretch reflexes, which appear to be mediated at least in part by the cortex (Matthews 1991), increase to compensate for single joint perturbations during interactions with compliant or unstable environments (Akazawa et al. 1983; Dietz et al. 1994; Doemges and Rack 1992b). We recently reported similar modulation throughout the arm in response to multijoint perturbations (Perreault et al. 2008). However, the mechanical properties of the environment used in that multijoint study were not found to alter the patterns of reflex coordination across muscles. Instead global increases in reflex sensitivity were found throughout the arm when subjects interacted with compliant environments. It is important to note that the mechanical environments tested in that previous study were isotropic and stable. Hence the observed lack of changes in reflex coordination may have resulted from the fact that the tested environments did not sufficiently compromise arm stability. Exerting large forces against the environment can compromise arm stability along specific directions (McIntyre et al. 1996). Franklin et al. (2003) demonstrated that stiffness contributions from biarticular muscles increase to compensate for such instabilities. However, the relative contributions of reflex pathways could not be estimated directly in that study because measures of muscle activity were not made.
To understand how the mechanical properties of the environment compromise arm stability, it is necessary to quantify the mechanical properties of the arm as well as those of the environment. This can be done through estimates of endpoint stiffness (Hogan 1985; Mussa-Ivaldi et al. 1985), which characterizes the static properties of the limb as seen at the point of contact with the environment. The stiffness of a multijoint system is directional, having an orientation along which the limb is most resistant to perturbations of posture. If stretch reflexes contribute to the mechanical properties of the arm and also compensate for changes in the mechanical properties of the environment, the specificity of their modulation may best be observed when considering the coupled mechanics of the arm and environment.
The goal of this study was to investigate the specificity of stretch reflex modulation in the human arm. This was achieved by quantifying how interactions with destabilizing haptic environments influenced reflex modulation. Importantly, the magnitude and orientation of the tested haptic environments were selected according to the endpoint stiffness estimated for each subject. We hypothesized that longer-latency stretch reflexes throughout the arm would be preferentially modulated to compensate for the mechanical properties of the environment relative to those of the arm. Such specificity would provide the first evidence that reflex responses throughout the arm can be coordinated to compensate for directionally specific environmental instabilities such as those that arise with tool use.
METHODS
Subjects
Ten right-handed subjects, 26–41 yr (8 males and 2 females), participated in this study. None had a history of neurological impairment or orthopedic limitations in the upper limbs. Subjects gave written informed consent and were free to withdraw at any time. All protocols were approved by the Northwestern University Institutional Review Board (IRB 1322-001). All 10 subjects participated in a preliminary experiment to measure limb impedance and in the primary reflex experiment. Five of these subjects also participated in two subsequent control experiments.
Equipment
Details of the equipment have been provided previously (Perreault et al. 2008; Trumbower et al. 2009). In summary, subjects interacted with a 3 degree of freedom (df) robotic manipulator (HapticMaster; FCS Control Systems; Fig. 1) during all experiments. The robot uses an admittance control algorithm, allowing it to simulate a range of haptic environments (Van der Linde et al. 2002). It was programmed as a stiff (50 kN/m) position servo to stochastically perturb the limb during the preliminary experiments for estimating endpoint impedance. In all reflex experiments, the robot was used both to simulate the unstable haptic environments and to transiently perturb arm posture as was needed to assess stretch reflex sensitivity. It was able to switch between the destabilizing haptic environments and the stiff position servo mode needed to perturb the arm in <1 ms (Perreault et al. 2008). The robot was instrumented to measure endpoint displacements and forces, both of which were recorded at 1.25 kHz.
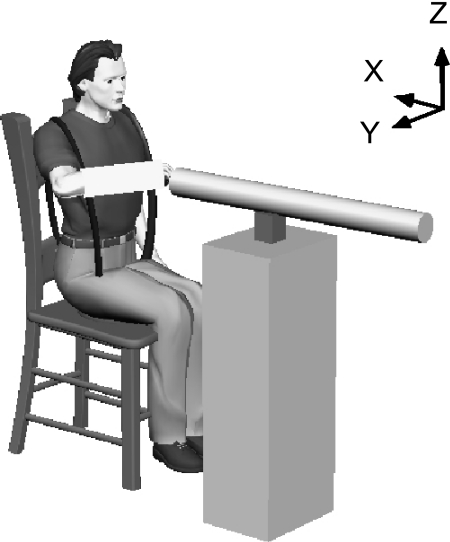
Fig. 1.
Experimental setup. Subjects used the arm to interact with a 3-dimensional (3D) robot while seated and secured to a rigid chair.
Subjects were rigidly attached to the robot using a custom-fitted fiberglass cast. The cast extended ∼1/3 of the distance from the wrist to the elbow, fixing the wrist in a slightly pronated position. The cast was mounted to a low-mass custom gimbal attached to the end of the manipulator, allowing the application of pure endpoint forces and no moments to the arm. The gimbal was instrumented with potentiometers that were used to provide subjects with visual feedback of arm posture so that a fixed posture could be maintained throughout each trial. The gimbal's center was positioned along the axis of the forearm, under the third metacarpophalangeal joint, which we defined as the endpoint of the limb for these experiments. Subjects were seated during these experiments and harnessed at the shoulders and waist to an immobile chair.
During the impedance estimation experiments, endpoint displacement was redundantly measured with an optical motion analysis system (Optotrak 3020; Northern Digital, Waterloo, Ontario, Canada) with a resolution of 0.1 mm. The optical tracking data were used to correct for small errors in the endpoint displacement measures obtained from the robot due to compliance between the robot's end effector and its displacement sensors. The Optotrak tracks the motion of infrared light-emitting diodes (LEDs), which were mounted on a rigid body attached to a wrist cast and used to monitor endpoint location. All optical data were collected at 250 Hz.
During all reflex experiments, surface electromyograms (EMGs) were used to record muscle activity. These were used to monitor voluntary muscle activity before each perturbation and the involuntary muscle activity elicited by the perturbations. Eight of the dominant muscles crossing the elbow and shoulder were monitored. These were the anterior deltoid (AD), medial deltoid (MD), posterior deltoid (PD), clavicular head of pectoralis major (PECTCLAV), biceps brachii (BI), long head of triceps brachii (TRILONG), lateral head of the tricpes brachii (TRILAT), and brachioradialis (BRD). Conventional skin-preparation techniques were performed before applying disposable dual electrodes (Noraxon USA) to the skin. EMG signals were amplified with a Bortec AMT-16 EMG measurement system (Bortec Biomedical, Calgary, Alberta, Canada) that has high- and low-pass cut-off frequencies of 10 and 1,000 Hz, respectively. All EMG signals were anti-alias filtered using custom fifth-order, low-pass Bessel filters with a cutoff frequency of 500 Hz. These data were then sampled at 1.25 kHz by a 32-channel, 18-bit data-acquisition system (NI 6289; National Instruments, Austin, TX). A common clock was used to synchronize data from the robotic, motion analysis, and EMG systems. All of these signals were resampled at 1 kHz before further processing.
Protocols
All experiments were performed on the right arm, which was maintained at a fixed posture. This posture corresponded to the shoulder in ∼70° of abduction and ∼45° of horizontal flexion; the elbow was flexed at ∼90°, positioning the hand directly in front of the glenohumeral joint.
ESTIMATING ENDPOINT STIFFNESS.
The purpose of the preliminary experiment was to estimate endpoint impedance for each subject. For this purpose, the robot was configured as a stiff position servo and used to apply three-dimensional (3D), stochastic perturbations to the endpoint of the arm. The perturbations (Fig. 2A) were similar to those we have used previously (Perreault et al. 2001, 2004; Trumbower et al. 2009) having a SD of 3.0 mm and frequency spectrum that was flat ≤5 Hz, beyond which it decayed at a rate of 40 dB/decade.
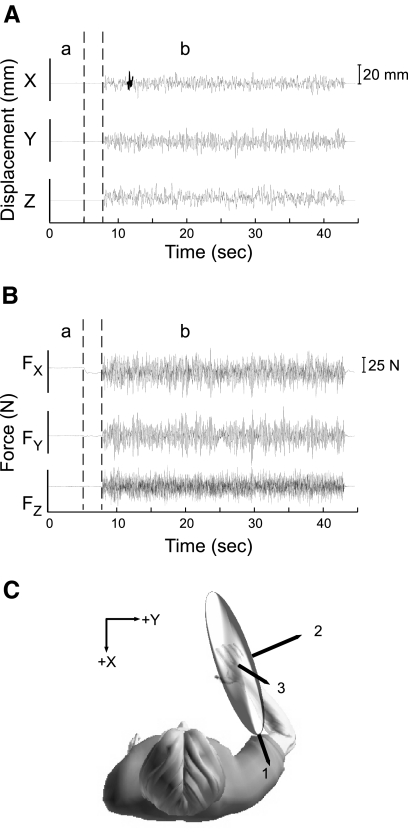
Fig. 2.
Endpoint impedance estimation using stochastic perturbations. A: stochastic displacement perturbations used to estimate endpoint impedance. B: corresponding endpoint forces measured in a single trial. C: 3 principal axes of the endpoint stiffness ellipse estimated using data in A and B; stiffness is the static component of impedance.
The subjects' task was to maintain a fixed arm posture while exerting a constant endpoint force but not reacting to the perturbation. The endpoint force targets were ±10 N along each of the measurement axes shown in Fig. 1. These magnitudes were chosen to match those used in the subsequent reflex experiments. It should be noted that the muscle activation required to reach these force targets was in addition to that required to support the arm against gravity. Real-time visual feedback of endpoint force and posture was provided using a computer display (Trumbower et al. 2009). During each trial, subjects first supported the arm's weight against gravity for 5 s (Fig. 2, A and B, a), after which a visual cue corresponding to the target force was presented. Once the target force was reached and held steady (within ±1 N) for 0.7 s, the robot applied a stochastic perturbation that lasted for 35 s (Fig. 2, A and B, b). Only the final 30 s of each trial were analyzed to avoid the small nonstationary corrective movements occasionally observed immediately following perturbation onset. Four trials were conducted for each of the six target force directions.
The recorded force and displacement data were used to estimate the 3D endpoint stiffness, the static component of endpoint impedance, as described in the following text. Endpoint stiffness is directional. These directional characteristics can be observed when stiffness is displayed graphically using an ellipsoid (Mussa-Ivaldi et al. 1985) (Fig. 2C). This directionality was used to create subject-specific haptic environments for each of the following experiments.
ASSESSMENT OF REFLEX MODULATION.
At the beginning of each reflex experiment, a series of maximum voluntary contractions (MVCs) were performed. These data were used to normalize EMGs recorded from each muscle. Standard muscle testing procedures were used to isolate the activity of each target muscle during the MVCs (Delagi et al. 1980); a separate isometric contraction was performed for each muscle. Each contraction lasted for ∼2 s and two repetitions were performed.
During each experiment, subjects interacted with two destabilizing haptic environments, each primarily simulating a negative-stiffness spring, acting along a line in 3D space. As subjects moved the position of their hand away from the neutral position of the haptic spring, the robot pushed the hand further with a force proportional to the distance between the hand and the neutral point; the proportionality constant was the magnitude of the simulated negative stiffness. All movements were constrained to the direction of the haptic instability by virtual walls with a simulated stiffness of 50 kN/m. These haptic instabilities were oriented relative to the endpoint stiffness measured for each subject in the preliminary experiment. The instability of the first environment (Fig. 3A) was aligned along the direction of maximal endpoint stiffness. The second environment was aligned along an orthogonal direction in which the arm was substantially less stiff. This chosen direction (Fig. 3B) was along the second principle axis of endpoint stiffness. Subjects interacted with each environment for half of the experiment in sequential order. The order of exposure to both environments was randomized across subjects. Finally, the haptic environment had a gravity-compensated, simulated mass of 5.0 kg and was slightly under damped in all directions; virtual stops were located at a distance of ±100 mm from the neutral position to ensure the safety of subjects.
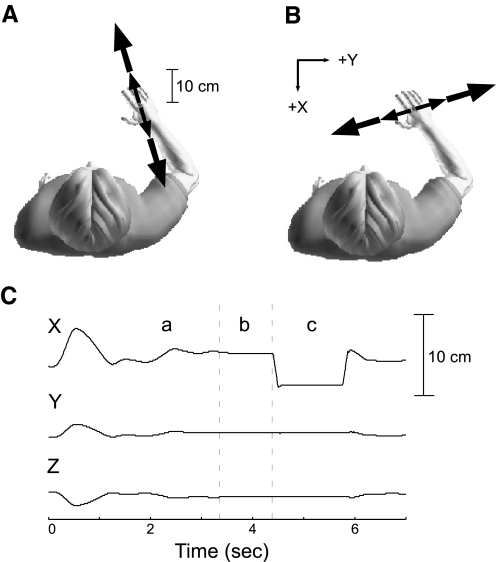
Fig. 3.
Destabilizing environments used to examine reflex modulation. Subjects interacted with destabilizing environments that were either aligned (A) or orthogonal (B) to maximum endpoint stiffness. C: during each trial, subjects had to voluntarily stabilize the arm to hold the nominal posture and apply the target force (a). After the target force and posture were held for a random amount of time (b) a ramp-hold-and-return perturbation was applied (c).
The subjects' task was to maintain a constant endpoint force and fixed arm posture. This ensured that during interactions with both environments posture, endpoint position, and endpoint force were matched. Target forces were 0, and 5, and 10 N away from the body (along the primary axis of stiffness) and to the right (along the secondary axis). The target force magnitudes were chosen to be well below a level likely to result in fatigue (Rohmert 1960). Finally, recording reflexes at a range of voluntary endpoint forces allowed us to match background EMG within each muscle across the different haptic environments. During all reflex assessments, subjects were instructed to not react to the imposed perturbation. These explicit instructions were used because it has been well documented that stretch reflexes are sensitive to changes in the instructions given to subjects (Crago et al. 1976; Hammond 1956).
After moving to the target position and force (Fig. 3C, a), then holding this position and force for a uniformly distributed random period of time between 0.5 and 1.5 s (Fig. 3C, b), a single ramp- hold perturbation was applied to the arm (Fig. 3C, c). Following each perturbation, the hand was returned to the neutral position so that all perturbations were applied from this point. Each perturbation had a velocity of 400 mm/s and duration of 100 ms, sufficient to elicit the long-latency component of the stretch reflex (Lewis et al. 2005). All perturbations applied in the same direction were identical during interactions with both environments (Perreault et al. 2008). Approximately 10 perturbations were repeated for each perturbation direction and target force level. The presentation order of the target forces and perturbation directions was randomized.
During experiment 1, subjects interacted with two destabilizing haptic environments of equal strength. This strength, KENV, was initially set to have a magnitude greater than the maximum magnitude of arm stiffness along the secondary axis of stiffness but less than the maximum magnitude of stiffness along the primary axis (Table 1, 1st column), as estimated in the preliminary experiment. Next, KENV was adjusted to the largest value at which the subject could perform the postural maintenance task while interacting with the environment orthogonal to maximal stiffness. For only one subject (3), it was necessary to decrease KENV below the maximum magnitude of the second axis of stiffness. Perturbations were applied in three orthogonal directions, along the coordinate axes shown in Fig. 1, and were similar to those we have used previously (Perreault et al. 2008). This allowed us to compare reflex coordination in these experiments to that estimated in our previous study.
Table 1.
Strength of the negative-stiffness environments used during reflex experiments and maximal endpoint stiffness magnitude along the primary and secondary principal axes of endpoint stiffness
| Field Strength, N/m |
Maximal Endpoint Stiffness, N/m |
||||
|---|---|---|---|---|---|
| Subject | Experiment 1 | Experiment 2 | Experiment 3 | Primary Axis | Secondary Axis |
| 1 | −500 | −1650 | 1462 | 227 | |
| 2 | −600 | −600 | −1800 | 622 | 165 |
| 3 | −300 | −300 | 1154 | 425 | |
| 4 | −600 | −600 | −1850 | 1994 | 455 |
| 5 | −500 | −500 | −1550 | 1138 | 314 |
| 6 | −250 | −250 | 938 | 217 | |
| 7 | −500 | −1600 | 751 | 198 | |
| 8 | −500 | 1319 | 197 | ||
| 9 | −500 | 795 | 168 | ||
| 10 | −400 | 742 | 181 | ||
In experiment 2, perturbations were applied along the primary and secondary axes of stiffness rather than the measurement coordinate axes used in experiment 1 (Fig. 1). This change was made in response to the observation that the reflexes elicited in experiment 1 appeared to be tuned not only to the orientation of limb mechanics relative to the environment but also to the orientation of the perturbations relative the environment. In experiment 2, subjects applied a restricted number of target forces: 0 N, 10 N away from the body, and 10 N to the right. The nonzero forces were oriented along the primary and secondary axes of endpoint stiffness. All other aspects of the experiment were matched to experiment 1 (Table 1, 2nd column).
A third experiment (experiment 3) was performed to examine the influence of the strength of the destabilizing environments on reflex modulation. This was tested because the modulation observed in experiments 1 and 2 was such that reflexes were always increased during interactions with the environment orthogonal to maximal stiffness. We hypothesized that this might be due to the fact that the values of KENV used in the first two experiments typically exceeded arm stiffness along the secondary axis but never along the primary axis. Hence we sought to examine reflex modulation in an experiment in which the magnitude of each of the two destabilizing haptic environments was different. The strength of the haptic environments was selected based on endpoint stiffness magnitude along that same direction. This was accomplished by setting KENV for the haptic environment aligned to the direction of maximal endpoint stiffness to the maximum value at which subjects could complete the postural maintenance task. For one subject (4), the magnitude of the haptic environment, aligned to the direction of maximal endpoint stiffness, had to be decreased slightly below the magnitude of maximum arm stiffness. All other aspects of this experiment were matched to the second experiment, including the strength of the haptic environment orthogonal to the direction of maximal endpoint stiffness.
Analysis
ENDPOINT STIFFNESS.
Endpoint impedance completely describes the dynamic relationship between displacements applied to the hand and the forces generated in response. Endpoint stiffness is the static component of impedance and can be obtained from these more general estimates. Endpoint impedance was calculated from the endpoint position and force data collected during the preliminary experiments using linear, nonparametric system identification to estimate the transfer functions between displacements along each measurement axis and the corresponding forces as described in detail previously (Perreault et al. 1999; Trumbower et al. 2009). Force data were utilized as recorded by the robot. The redundant displacements measured with the motion analysis system were used to account for the small compliance between the robot's displacement sensors and the endpoint (Trumbower et al. 2009).
Three measures were used to evaluate the quality of the estimated endpoint impedance. First, we evaluated the nonparametric fit for each trial using the multiple correlation coefficient, R2, to characterize the relationship between the predicted and measured endpoint forces. Next multiple coherence was used to determine the range of frequencies for which the linear transfer functions were appropriate (Bendat and Piersol 2000). Finally, inertial (I), viscous (B), and elastic (K) parameter matrices were fit to the nonparametric models. The quality of these fits was again quantified using R2. The principal axes of the ellipsoids describing the I, B, and K parameter matrices were calculated using singular value decomposition, as described by Gomi and Osu (1998).
REFLEX SENSITIVITY WITHIN MUSCLES.
EMGs were used to quantify the magnitude of the stretch reflex elicited in each muscle. The mean was removed from all EMG recordings, which were then rectified before further processing. EMGs for each muscle were normalized by the mean rectified value (0.5-s average) recorded during the MVCs performed at the start of each experiment. Endpoint displacement and EMG data were aligned to the start of each perturbation. Responses for each perturbation and experimental condition were averaged. Reflexes were quantified by the average rectified response within 50–100 ms following perturbation onset, which is where the majority of the reflex activity was located and still prior to the onset of voluntary activity.
To compare reflex sensitivity during interactions with the different environments, it was necessary to ensure that comparisons for each subject were made at matched levels of voluntary EMG (Matthews 1986; Pruszynski et al. 2009; Smeets and Erkelens 1991). This was accomplished using the linear interpolation technique we have described previously (Perreault et al. 2008). In summary, reflex EMG for each muscle was modeled as a linear function of the voluntary EMG within that muscle. Reflex sensitivity, defined as the change in reflex amplitude in response to a specific perturbation, was compared only when there was overlap in the voluntary EMG recorded in both environments.
PATTERNS OF REFLEX COORDINATION ACROSS MUSCLES.
An additional goal of this study was to examine if any reflex modulation that was observed within muscles was the result of coordinated changes in the groups of activated muscles between environments or if there were changes in the relative activations of similar groups of muscles between environments. To accomplish this, we used PCA/ICA, a two-stage dimensionality reduction technique shown to produce robust estimates of muscle coordination (Tresch et al. 2006) and which we have used previously for this purpose (Perreault et al. 2008). Briefly, we approximated the reflex EMG across all muscles using a specific number (N) of eight-muscle coordination patterns (ICs). The reflex EMGs in all muscles could be predicted by multiplying these ICs by N time-varying activation coefficients, and summing the results. The dimensionality of this reduced system, N, was chosen to be the least number of ICs that accounted for >90% of the data variance. The weighting of the components within each IC represented the relative activation of each muscle. Confidence intervals for the relative activation of each muscle were determined by applying a bootstrap analysis with 100 repetitions (Press et al. 1986). Similarity between sets of N ICs was estimated using the multiple correlation coefficient, R2, to quantify the amount of variance in one dataset that could be accounted for by the ICs estimated from another.
RESULTS
Estimates of endpoint stiffness
As reported previously, linear, nonparametric transfer functions were appropriate for characterizing the 3D impedance of the human arm. Across all subjects and bias forces, the average R2 value was 92.0 ± 0.4% (mean ± SE). These nonparametric transfer functions were characterized well by second-order models. These parametric descriptions had an average R2 of 78.9 ± 1.5% over the frequency range from 0 to 10 Hz, above which limb inertia dominates the endpoint forces measured in response to imposed displacements (Perreault et al. 2004).
Estimates of the orientation of maximal endpoint stiffness for each subject were repeatable across the tested force levels. This was determined by comparing the orientation of the primary axis of endpoint stiffness (in 3D space) across the six tested force levels for each subject. On average, the orientation of maximal endpoint stiffness differed in magnitude by only 6.5 ± 1.0°. The orientation of the secondary principal axis of stiffness varied more with an average magnitude difference of 25.5 ± 3.0°. The goal of these initial experiments was to identify the direction of maximal endpoint stiffness. Because this varied little across the tested voluntary forces, we selected to average the stiffness estimated across all force levels to provide an average estimate of endpoint stiffness orientation that could be used throughout the rest of the experiment. All references to endpoint stiffness orientation in the remainder of the manuscript refer to this average.
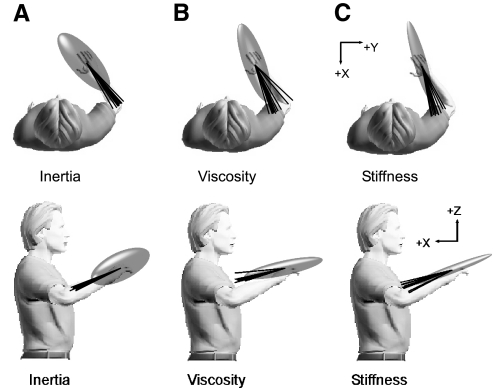
Across subjects, the direction of maximal endpoint stiffness was oriented along a 3D line extending approximately from the hand to the center of the humerus. This can be seen in Fig. 4C, which displays the average endpoint stiffness ellipsoid for a single subject. The average inertia and viscosity ellipsoids for the same subject are also presented (Fig. 4, A and B). For this subject, the orientation of maximal endpoint viscosity was co-aligned with that of maximal stiffness, whereas the orientation of maximal inertia was more closely aligned to the axis of the forearm. These orientations were largely consistent across subjects, as indicated by the lines in each part of Fig. 4. Because the destabilizing environments were aligned to the primary and secondary axes of endpoint stiffness, subjects interacted with similarly oriented environments.
Fig. 4.
Consistency of estimated endpoint impedance. Average endpoint inertia (A), viscosity (B), and stiffness (C) ellipsoids for a single subject. Each ellipsoid has been normalized to its maximal eigenvalue, thereby providing an indication of orientation but not magnitude. —, the orientations of the primary axes estimated for each subject. These provide an indication of the variability in the orientation of each component of endpoint impedance across all subjects.
Reflex modulation within muscles
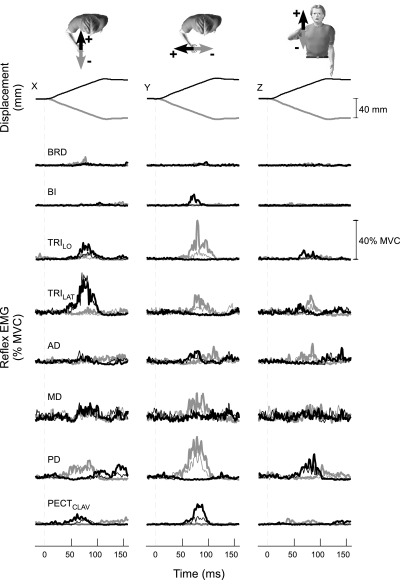
Stretch reflexes were elicited throughout the tested muscles as subjects interacted with each environment. Typical reflex modulation is shown in Fig. 5. The elicited reflexes were modulated according to the orientation of the environment with which subjects interacted. Identical perturbations elicited reflexes that were larger during interactions with the environment orthogonal to maximal endpoint stiffness (thick lines), relative to those elicited during interactions with the environment aligned to maximal stiffness (thin lines). For this subject, reflexes elicited by ±Y perturbations were increased in seven of the eight tested muscles, excluding AD. The response to ±X perturbations was increased in TRILO and PECTCLAV. The response to ±Z perturbations was increased in TRILO and TRILAT. For all subjects, increased reflexes were observed for most muscles in the response to ±Y perturbations but for fewer muscles in the response to ±X and ±Z perturbations.
Fig. 5.
Typical average stretch reflexes obtained during interactions with the destabilizing environments used in experiment 1. Responses were obtained from a subject who was applying 10 N laterally, along the secondary axis of endpoint stiffness. Top: the applied perturbations; perturbations applied during interactions with both environments are overlaid but are indistinguishable. The characters at the top of the figure indicate the direction of the applied perturbations; the colors of the arrows are matched to the perturbation traces and the reflex electromyograms (EMGs) below. Thin and thick traces correspond to data recorded during interactions with the environment aligned and orthogonal to endpoint stiffness, respectively. Identical perturbations elicited responses of different magnitude, depending on the environment with which the subject interacted.
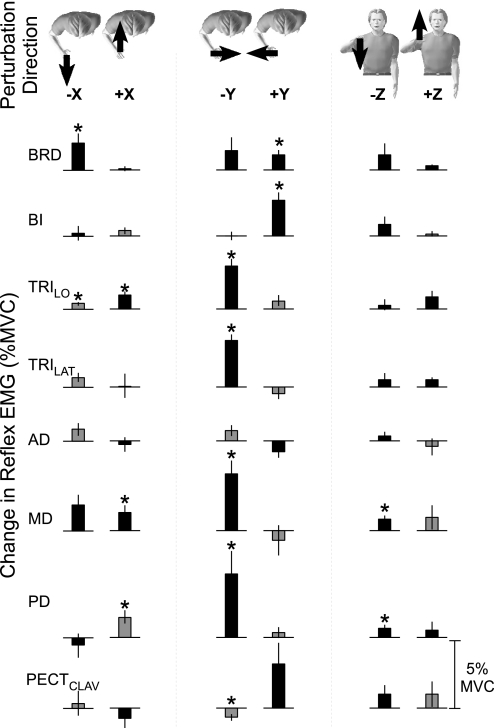
The observed reflex modulation was due to changes in reflex sensitivity not differences in voluntary muscle activity prior to the perturbation. This was determined by comparing the reflexes elicited during interactions with each environment at matched levels of background muscle activity. We computed the difference between the reflex EMGs (at matched voluntary EMG) recorded during interactions with the environment orthogonal to maximal stiffness and those recorded during interactions with the environment aligned to maximal stiffness. Statistical significance across environments was assessed using a paired t-test. Group results are shown in Fig. 6. Significant modulation was observed for all muscles except AD. Most of the observed significant reflex modulation corresponded to increased reflex excitation during interactions with the orthogonal environment. Significantly increased inhibition (reduced EMG activity relative to baseline) during interactions with the orthogonal environment was noted during shortening of the PECCLAV (−Y perturbations). Only two muscles showed increased inhibition during interactions with the aligned environment. These were the TRILO and PD, in response to –X and +X perturbations, respectively.
Fig. 6.
Reflex modulation at matched levels of voluntary EMG. Data are group responses. Characters at the top of the figure indicate the direction of the applied perturbation. Each plot indicates the difference between average reflex EMGs (50–100 ms after perturbation onset) recorded during interactions with the environments orthogonal and aligned to maximal endpoint stiffness. Each row indicates the reflexes elicited from a single muscle. The horizontal lines indicate (0) no change in reflex EMG. The color of the bars indicates whether the elicited response is excitatory (black) or inhibitory (gray). Black bars above the horizontal line indicate increased reflex EMG in the environment orthogonal to maximal endpoint stiffness; black bars below the horizontal line indicate decreased reflex EMG during interactions with the environment orthogonal to maximal stiffness. Gray bars below the horizontal line indicate increased inhibition during interactions with the environment orthogonal to maximal stiffness; gray bars above the horizontal line indicate decreased inhibition during interactions with the environment orthogonal to maximal stiffness. Error bars indicate standard errors. Asterisks indicate P < 0.05.
The observed modulation was perturbation specific. The number of muscles exhibiting increased excitation during responses to ±Y perturbations was greater than for ±X and ±Z perturbations. For the group, increased sensitivity to ±Y perturbations was observed in six of eight tested muscles, but for only three muscles for ±X perturbations and two muscles for ±Z perturbations. Finally, the strongest increase in reflex sensitivity relative to background muscle activity was observed in the responses to ±Y perturbations. This was quantified by dividing the difference in reflex EMG between environments (as seen in Fig. 6) by the background EMG at which the reflexes were compared. Across all muscles exhibiting significant excitatory responses, reflex sensitivity increased by 102.6 ± 25.4% for ±Y perturbations but only by 33.5 ± 8.6% for ±Z perturbations and 16.7 ± 13.6% for ±X perturbations.
Patterns of reflex coordination
Four reflex coordination patterns were sufficient to describe >90% of the reflex data across all subjects and experimental conditions. The average R2 using four ICs for each subject was 93 ± 1%, similar to that reported previously for multijoint reflexes (Perreault et al. 2008). Hence four ICs were used for the remainder of the analysis.
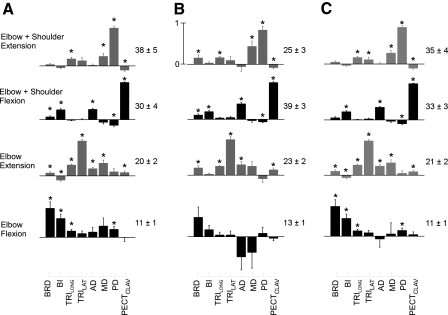
The patterns of reflex coordination estimated during interactions with both environments were similar. This can be seen in Fig. 7, A and B, which display the four ICs estimated during interactions with each environment; data are pooled across subjects. The ICs are named according to the actions of the dominant muscles in each. These correspond to “elbow + shoulder extension,” “elbow + shoulder flexion,” “elbow extension,” and “elbow flexion.” To quantify similarity between the ICs estimated for each environment, we determined if the ICs estimated from one environment described a similar amount of variance in the data from that environment and the other environment. We randomly selected 50% of the data from each environment to estimate two sets of ICs; each was used to predict the remaining data from that environment (self-prediction) and the other (cross-prediction). This was repeated 100 times to produce an average R2 for the self- and cross-predictions for each subject. Across subjects, the average R2 for self-predictions was 0.91 ± 0.01 and 0.88 ± 0.01 for cross-predictions. Although these values were statistically different (paired t-test, t = 6.02, P < 0.001), the differences were small especially when compared with the observed differences in reflex magnitude reported in the preceding text. These results suggest that the environment-dependent changes in reflex magnitude were driven largely by changes in the activation of a common set of reflex patterns rather than by coordinated changes in the pattern of reflex activity.
Fig. 7.
Reflex coordination patterns (ICs). ICs were estimated from data pooled across subjects. A: ICs estimated during interactions with the environment orthogonal to the orientation of maximal endpoint stiffness. B: ICs estimated during interactions with the environment aligned to the orientation of maximal endpoint stiffness. C: ICs estimated from data pooled across environments. Each IC was named according to the actions of the most prominent muscles. Data were collected using the protocols of experiment 1. Muscles in each coordination pattern are indicated at the bottom of the figure. The magnitude of the vector described by each 8-muscle pattern was normalized to unity. Numbers to the right of each pattern indicate percentages of the relative variance described by that pattern. Only data remaining after the initial PCA reduction are considered in the relative variance calculation. Error bars indicate SE. *, components that are significant at a level of P < 0.05.
The activation coefficients for the estimated ICs differed according to the environment with which subjects interacted. This was determined by pooling the reflex data across subjects and environments; we estimated a single set of ICs (Fig. 7C), then compared the activations of these ICs for each subject across environments using paired t-test (Table 2). Increased activations were observed during interactions with the environment orthogonal to maximal stiffness, relative to the environment aligned to maximal stiffness. Perturbations that elicited stretch responses in muscles that were the primary contributors to each IC resulted in increased activation coefficients for that IC. Moreover, the extent to which the activation coefficients were modulated was highly perturbation specific. For example, the activations of all ICs in response to perturbations applied in the ±Y direction were increased during interactions with the environment orthogonal to maximum stiffness. Specifically, the activation coefficients for the elbow + shoulder extension and elbow extension ICs were increased in response to –Y perturbations, whereas the activations of elbow + shoulder flexion and elbow flexion ICs were increased in the response to +Y perturbations. Three of these four ICs showed reciprocal changes in activation, exhibiting enhanced inhibition during perturbations that shortened the primary muscles in each IC. Less modulation was observed for perturbations in the ±X and ±Z directions. The elbow + shoulder flexion IC exhibited enhanced excitation and inhibition in response to perturbations along the z axis, while the elbow flexion and elbow extension ICs had increased activations in response to –X and +X perturbations, respectively. Note that all comparisons were performed on activation coefficients that were estimated without controlling for background EMG throughout the arm. Because of the difficulty associated with simultaneously controlling background activity across multiple muscles, the remaining data were analyzed only on an individual muscle basis.
Table 2.
Reflex coordination patterns (ICs) exhibiting significantly modulated activation
| Perturbation Direction |
||||||
|---|---|---|---|---|---|---|
| IC | −X | +X | −Y | +Y | −Z | +Z |
| Elbow+shoulder extension | −A | ++O | −−O | |||
| Elbow+shoulder flexion | −−O | ++O | ++O | −−O | ||
| Elbow extension | ++O | ++O | −−O | |||
| Elbow flexion | ++O | −−A | ++O | |||
O and A, in the environment (orthogonal or aligned) in which greater excitation or inhibition occurred. • P <0.05 and •• P <0.01, where • = + indicates enhanced excitation and • = − indicates enhanced inhibition.
Reflex responses to perturbations aligned with the environmental instabilities
Perturbations in the ±Y direction were roughly aligned to the destabilizing environment oriented orthogonal to maximal endpoint stiffness; thus the direction dependent modulation noted above suggests that the elicited reflexes may have been tuned specifically to perturbations oriented along the direction of the environmental instability. To examine this possibility, we performed a study in which perturbations were applied along the primary and secondary axes of endpoint stiffness (axes shown in Fig. 4C). Medial perturbations along the secondary axis of stiffness elicited excitatory responses in TRILO, TRILAT, MD, and PD, whereas lateral perturbations elicited responses in BRD, BI, AD, and PECTCLAV. Along the primary axis, perturbations in the posterior direction elicited excitatory responses in TRILO, TRILAT, AD, MD, PECTCLAV, and, in some cases, BI, whereas anterior perturbations elicited responses in BRD, MD, PD, and, in some cases, BI.
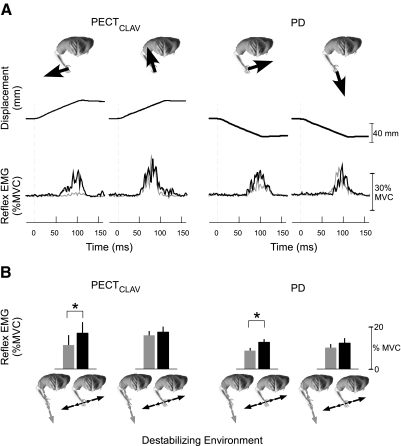
Stretch reflexes elicited by perturbations orthogonal to the direction of maximal endpoint stiffness were larger when subjects were interacting with a destabilizing haptic environment that also was aligned in this same direction. In contrast, the reflex responses to perturbations oriented along the direction of maximal endpoint stiffness were not significantly affected by the orientation of the haptic environment. Sample responses for typical muscles, PD and PECTCLAV, from a single subject are shown in Fig. 8A. Only responses to perturbations that stretched these muscles are shown. The corresponding group results for these two muscles are shown in Fig. 8B. For the group response, paired t-tests were used to assess the influence of the haptic environment on the stretch reflexes elicited by each perturbation direction. For these muscles, the orientation of the haptic environment modulated reflex sensitivity only for perturbations oriented orthogonal to the direction of maximal endpoint stiffness as shown in the first and third columns of Fig. 8B (tPECT = 8.55, pPECT = 0.003; tPD = 5.83, pPD< 0.001). In contrast, the orientation of the haptic environment with which the subject interacted did not have a statistically significant effect on the reflexes elicited by perturbations directed along the direction of maximal arm stiffness (tPECT = 0.85, pPECT = 0.434; tPD = 1.95, pPD = 0.092).
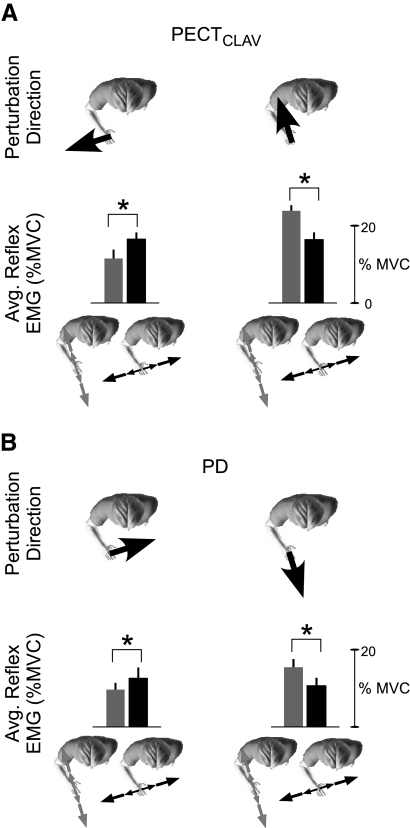
Fig. 8.
Modulation of reflexes elicited by perturbations applied along the primary and secondary axes of endpoint stiffness during interactions with equal strength environments (experiment 2). A: average stretch recorded from clavicular head of pectoralis major (PECTCLAV) and posterior deltoid (PD) in a single subject during interactions with each destabilizing environment. Perturbations were applied in the directions indicated by the black arrows at the top of each column. Gray traces are the reflexes elicited during interactions with the environment aligned to the direction of maximal endpoint stiffness and black traces correspond to reflexes elicited during interactions with the orthogonal environment. B: average reflex EMGs, at matched levels of background muscle activity, for the group. Perturbation directions are as indicated in A. Colors correspond to the environment with which subjects interacted, as indicated by the characters at the bottom of the figure. Error bars SE. Asterisks, P < 0.05.
Across all muscles, the responses elicited by perturbations oriented orthogonal to the direction of maximal endpoint stiffness were the most consistently and most strongly modulated by the haptic environments. This modulation was observed in seven of the eight muscles tested (Table 3). One muscle, MD, also exhibited significantly increased responses to perturbations that were aligned to the direction of maximal endpoint stiffness, although this modulation was small (10.2 ± 1.1%) relative to that observed in response to perturbations orthogonal to the direction of maximal endpoint stiffness (128.8 ± 31.0%). Increased inhibition during interactions with the haptic environment aligned to endpoint stiffness also was noted in three muscles, TRILO, TRILAT, and AD (−31.6 ± 19.2%).
Table 3.
Muscles exhibiting significantly modulated stretch reflexes at matched levels of background muscle activity
| Perturbation Direction |
||||
|---|---|---|---|---|
| Primary Axis |
Secondary Axis |
|||
| Muscle | Anterior | Posterior | Medial | Lateral |
| Experiment 2 | ||||
| BRD | ||||
| BI | ++O | |||
| TRILO | −A | ++O | −A | |
| TRILAT | ++O | −A | ||
| AD | −A | +O | ||
| MD | +O | +O | ++O | |
| PD | ++O | |||
| PECTCLAV | ++O | |||
| Experiment 3 | ||||
| BRD | ++A | |||
| BI | ||||
| TRILO | ++O | |||
| TRILAT | +A (0.052) | +O | ||
| AD | −−A | +A | ||
| MD | ||||
| PD | +A | −A | +O | |
| PECTCLAV | −−A | ++A | −O | ++O |
Number in parentheses indicates P value. BRD, brachioradalis; BI, biceps brachii; TRILO, long head of triceps brachii; TRILAT, lateral head of tricep brachii; AD, MD, and PD, anterior, medial, and posterior deltoid, respectively; PECTCLAV, clavicular head of pectoralis major.
Influence of destabilizing force field magnitude on elicited stretch reflexes
The preceding results demonstrate that perturbations oriented orthogonally to the direction of maximal endpoint stiffness elicit larger excitatory responses when subjects interact with destabilizing environments oriented along this same direction. Similar modulation was not observed during interactions with destabilizing environments aligned to maximal stiffness. In the first two experiments, the magnitude of the haptic environments was greater than the maximal magnitude of the secondary axis of endpoint stiffness (for 9 of 10 subjects) but less than the maximal magnitude of the primary axis of endpoint stiffness. To determine if a similar degree of modulation could be observed along the direction aligned to maximal stiffness, we performed a third experiment in which the strength of the environment aligned to maximal stiffness was greatly increased. The purpose was to determine if there would also be preferential increases in size of the reflexes elicited by perturbations applied in this direction.
Increasing the magnitude of the destabilizing environment oriented along the direction of maximal stiffness caused an increase in the size of the reflexes elicited by perturbations along the same direction. The magnitude of the reflex elicited in the PECTCLAV at matched levels of background EMG was increased when this perturbation was applied along the direction of the higher-strength environment aligned to maximal stiffness, relative to when an identical perturbation was applied and the subject was interacting with the environment orthogonal to maximal stiffness (Fig. 9A, right; paired t-test, t = 5.11, P = 0.001). Similar results were observed for the PD, an antagonist to PECTCLAV (Fig. 9B, right; paired t-test, t = 3.04, P = 0.023). Furthermore, perturbations oriented orthogonally to the direction of maximal stiffness still elicited larger responses while subjects interacted with destabilizing environments oriented along this same orthogonal direction. Again, these results were observed for the PECTCLAV (Fig. 9A, left; paired t-test, t = 3.83, P = 0.006) and the PD (Fig. 9B, left; paired t-test; t = 2.58, P = 0.036).
Fig. 9.
Modulation of reflexes elicited by perturbations applied along the primary and secondary axes of endpoint stiffness during interactions with environments scaled to the magnitude of endpoint stiffness along each direction (experiment 3). A: group results from PECTCLAV. B: group results from PD. Figures are formatted as described for Fig. 8B.
This preferential tuning of reflex sensitivity occurred throughout the tested arm muscles. Across the group of subjects, environment-specific modulation was observed in six of eight tested muscles (Table 3). In these muscles, perturbations oriented along the direction of maximal arm stiffness always elicited larger reflex responses when subjects were interacting with an unstable environment oriented along this same direction. Similarly, perturbations oriented orthogonal to the direction of maximal arm stiffness always resulted in larger reflex responses when subjects interacted with an unstable environment that also was oriented orthogonal to the direction of maximal arm stiffness. Again, these comparisons were made only at matched levels of background muscle activity.
DISCUSSION
This study examined whether stretch-sensitive reflexes elicited by multijoint perturbations are modulated according to the orientation of destabilizing haptic environments with which a subject interacts. The environments were either aligned or orthogonal to the direction of maximal endpoint stiffness of the arm. Our results demonstrated that stretch reflexes were tuned to the orientation and strength of the environments relative to the mechanical properties of the arm. There was a preferential increase in reflex sensitivity to perturbations applied along the direction of the destabilizing environments but only when the magnitude of the haptic instability exceeded the endpoint stiffness of the arm along the same direction. This is consistent with task-specific reflex modulation that could increase limb impedance in a task-appropriate direction.
Preferential modulation of reflex sensitivity
The multijoint reflex modulation presented here is consistent with previous single-joint studies that suggested reflex gain is increased to enhance limb stability when that stability is not provided by the environment. This has been demonstrated for muscles spanning the thumb (Akazawa et al. 1983), index finger (Doemges and Rack 1992a), wrist (Doemges and Rack 1992b), and elbow (Dietz et al. 1994). The present results are also consistent with our recent study that demonstrated increased reflex sensitivity throughout the arm during interactions with isotropically compliant environments (Perreault et al. 2008). In each of these previous studies, only a general increase in reflex excitability was observed. The novel contribution of the present study is the demonstration that this modulation can be tuned to compensate for direction-specific properties of the mechanical environment with which a subject is interacting. This was accomplished using haptic instabilities, characterized by a negative stiffness spring. When the magnitude of this haptic instability exceeded the arm stiffness along the direction of the instability, stretch reflexes elicited by perturbations along the direction of the haptic instability were preferentially increased. These results suggest that stretch reflex pathways are tuned not only to the mechanical properties of the environments with which we interact but also to the directional characteristics of these environments and how those characteristics are oriented relative to the mechanical properties of the arm.
The observed reflex modulation may have resulted from a number of physiological mechanisms. The cortex may contribute to the observed behavior through modulation of long-loop pathways or by descending cortical control of spinal circuitry (Capaday et al. 1991; Evarts and Fromm 1978; Prochazka 1989). Evidence for modulation of long-loop reflexes is supported by recent work demonstrating that changes in reflex sensitivity linked to interactions with external forces can be altered by magnetic stimulation of motor cortex (Kimura et al. 2006). We recently (Shemmell et al. 2009) used similar techniques to demonstrate that the motor cortex also is involved in regulating changes in stretch reflex sensitivity that occur during interactions with different mechanical environments, such as those used in this work, but that it alone cannot account for the modulation reported in other tasks, such as those that require the subject to react to the perturbation (Lewis et al. 2006; MacKinnon et al. 2000). Supraspinal contributions from the cerebellum also are possible because this structure has been linked to the manipulation of objects with unstable dynamics (Milner et al. 2006) and may modulate the gain of feedback neuromotor pathways (MacKay and Murphy 1979; Strick 1983). Increased heteronymous reflexes mediated by spinal or supraspinal pathways also may have contributed to the observed modulation. Heteronymous pathways have been demonstrated to have increased excitability during interactions with compliant loads (Maluf et al. 2007). Their contributions also may have been enhanced by changes in background muscle activity during interactions with each environment. While comparisons of the reflexes elicited in each muscle only were made at matched levels of background activity within that muscle, it was not possible to control for the background activity of all muscles within the limb, and selective cocontraction is known to be an effective strategy for stabilizing the arm during interactions with unstable loads (Franklin and Milner 2003; Osu et al. 2003).
Patterns of reflex coordination
The reflex coordination patterns estimated during interactions with both destabilizing environments were similar. This suggests that these patterns were primarily linked to the direction of the imposed perturbations that were consistent and independent of the environment with which a subject interacted. This is in agreement with our previous study examining reflex modulation during interactions with isotropically rigid and compliant environments (Perreault et al. 2008). In contrast to our previous results, we found that the destabilizing environments used in this study significantly altered the strength with which these reflex coordination patterns were activated. This is consistent with studies reporting task-appropriate changes in the activations of fixed muscle patterns for the human (d'Avella et al. 2006), non-human primate (Overduin et al. 2008), cat (Torres-Oviedo et al. 2006), and frog (Cheung et al. 2005).
Functional implications
To guarantee arm stability during interactions with the robot, it was necessary for the net stiffness of the coupled robot and arm system to be positive (Colgate and Hogan 1988). This required our subjects to increase limb stiffness in the direction of the simulated instability. We found that reflex responses also were greatest in response to perturbations directed along this instability. Such a preferential tuning of the involuntary response to postural perturbations may represent an important mechanism that the neuromotor system may use to adapt endpoint stiffness to the functional constraints of a task.
In summary, this study demonstrates that multijoint reflex responses throughout the arm adapt to the directional characteristics of the environments with which we interact, the relative instability of these environments, and the relationship between the mechanical properties of these environments and those of the arm. The results suggest that stretch reflex pathways may contribute to the task-appropriate directional tuning of arm mechanics. These findings extend our previous studies of multijoint reflex adaptation by demonstrating the flexibility by which stretch reflexes can be tuned to more specialized tasks.
GRANTS
This work was supported by American Heart Association predoctoral fellowship 0615573Z and National Institutes of Health grants K25 HD-044720 and NS-053813.
ACKNOWLEDGMENTS
The authors thank T. Haswell for technical assistance and E. Krepkovich for help with manuscript preparation.
REFERENCES
- Akazawa K, Milner TE, Stein RB. Modulation of reflex EMG and stiffness in response to stretch of human finger muscle. J Neurophysiol 49: 16–27, 1983 [DOI] [PubMed] [Google Scholar]
- Bendat JS, Piersol AG. Random Data: Analysis and Measurement Procedures New York: Wiley-Interscience, 2000 [Google Scholar]
- Burdet E, Osu R, Franklin DW, Milner TE, Kawato M. The central nervous system stabilizes unstable dynamics by learning optimal impedance. Nature 414: 446–449, 2001 [DOI] [PubMed] [Google Scholar]
- Capaday C, Forget R, Fraser R, Lamarre Y. Evidence for a contribution of the motor cortex to the long-latency stretch reflex of the human thumb. J Physiol 440: 243–255, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung VC, d'Avella A, Tresch MC, Bizzi E. Central and sensory contributions to the activation and organization of muscle synergies during natural motor behaviors. J Neurosci 25: 6419–6434, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgate JE, Hogan N. Robust control of dynamically interacting systems. Int J Control 48: 65–88, 1988 [Google Scholar]
- Crago PE, Houk JC, Hasan Z. Regulatory actions of human stretch reflex. J Neurophysiol 39: 925–935, 1976 [DOI] [PubMed] [Google Scholar]
- d'Avella A, Portone A, Fernandez L, Lacquaniti F. Control of fast-reaching movements by muscle synergy combinations. J Neurosci 26: 7791–7810, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delagi EF, Iazzetti J, Perotto A, Morrison D. Anatomic Guide for the Electromyographer Springfield, IL: Thomas, 1980 [Google Scholar]
- Dietz V, Discher M, Trippel M. Task-dependent modulation of short- and long-latency electromyographic responses in upper limb muscles. Electroencephalogr Clin Neurophysiol 93: 49–56, 1994 [DOI] [PubMed] [Google Scholar]
- Doemges F, Rack PMH. Changes in the stretch reflex of the human first dorsal interosseous muscle during different tasks. J Physiol 447: 563–573, 1992a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doemges F, Rack PMH. Task-dependent changes in the response of human wrist joints to mechanical disturbance. J Physiol 447: 575–585, 1992b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evarts EV, Fromm C. The pyramidal tract neuron as summing point in a closed-loop control system in the monkey. In: Cerebral Motor Control in Man: Long Loop Mechanisms, edited by Desmedt JE. New York: Karger AG, 1978, vol. 4, p. 56–69 [Google Scholar]
- Franklin DW, Liaw G, Milner TE, Osu R, Burdet E, Kawato M. Endpoint stiffness of the arm is directionally tuned to instability in the environment. J Neurosci 27: 7705–7716, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin DW, Milner TE. Adaptive control of stiffness to stabilize hand position with large loads. Exp Brain Res 152: 211–220, 2003 [DOI] [PubMed] [Google Scholar]
- Franklin DW, Osu R, Burdet E, Kawato M, Milner TE. Adaptation to stable and unstable dynamics achieved by combined impedance control and inverse dynamics model. J. Neurophysiol 90: 3270–3282, 2003 [DOI] [PubMed] [Google Scholar]
- Gielen CCAM, Ramaekers L, van Zuylen EJ. Long-latency stretch reflexes as co-ordinated functional responses in man. J Physiol 407: 275–292, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomi H, Osu R. Task-dependent viscoelasticity of human multijoint arm and its spatial characteristics for interaction with environments. J Neurosci 18: 8965–8978, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond PH. The influence of prior instruction to the subject on an apparently involuntary neuro-muscular response. J Physiol 132: 17–18P, 1956 [PubMed] [Google Scholar]
- Hogan N. The mechanics of multi-joint posture and movement control. Biol Cybern 52: 315–331, 1985 [DOI] [PubMed] [Google Scholar]
- Kearney RE, Stein RB, Parameswaran L. Identification of intrinsic and reflex contributions to human ankle stiffness dynamics. IEEE Trans Biomed Eng 44: 493–504, 1997 [DOI] [PubMed] [Google Scholar]
- Kimura T, Haggard P, Gomi H. Transcranial magnetic stimulation over sensorimotor cortex disrupts anticipatory reflex gain modulation for skilled action. J Neurosci 26: 9272–9281, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzer IL, Pruszynski JA, Scott SH. Long-latency reflexes of the human arm reflect an internal model of limb dynamics. Curr Biol 18: 449–453, 2008 [DOI] [PubMed] [Google Scholar]
- Kurtzer IL, Pruszynski JA, Scott SH. Long-latency responses during reaching account for the mechanical interaction between the shoulder and elbow joints. J Neurophysiol 102: 3004–3015, 2009 [DOI] [PubMed] [Google Scholar]
- Lacquaniti F, Carrozzo M, Borghese NA. Time-varying mechanical behavior of multijointed arm in man. J Neurophysiol 69: 1443–1463, 1993 [DOI] [PubMed] [Google Scholar]
- Lacquaniti F, Soechting JF. EMG responses to load perturbations of the upper limb: effect of dynamic coupling between shoulder and elbow motion. Exp Brain Res 61: 482–496, 1986 [DOI] [PubMed] [Google Scholar]
- Lewis GN, Mackinnon CD, Perreault EJ. The effect of task instruction on the excitability of spinal and supraspinal reflex pathways projecting to the biceps muscle. Exp Brain Res 174: 413–425, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GN, Perreault EJ, Mackinnon CD. The influence of perturbation duration and velocity on the long-latency response to stretch in the biceps muscle. Exp Brain Res 163: 361–369, 2005 [DOI] [PubMed] [Google Scholar]
- MacKay WA, Murphy JT. Cerebellar modulation of reflex gain. Prog Neurobiol 13: 361–417, 1979 [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Verrier MC, Tatton WG. Motor cortical potentials precede long-latency EMG activity evoked by imposed displacements of the human wrist. Exp Brain Res 131: 477–490, 2000 [DOI] [PubMed] [Google Scholar]
- Maluf KS, Barry BK, Riley ZA, Enoka RM. Reflex responsiveness of a human hand muscle when controlling isometric force and joint position. Clin Neurophysiol 118: 2063–2071, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PB. Observations on the automatic compensation of reflex gain on varying the pre-existing level of motor discharge in man. J Physiol 374: 73–90, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PBC. The human stretch reflex and the motor cortex. Trends Neurosci 14: 87–91, 1991 [DOI] [PubMed] [Google Scholar]
- McIntyre J, Mussa-Ivaldi FA, Bizzi E. The control of stable arm postures in the multi-joint arm. Exp Brain Res 110: 248–264, 1996 [DOI] [PubMed] [Google Scholar]
- Milner TE, Franklin DW, Imamizu H, Kawato M. Central representation of dynamics when manipulating handheld objects. J Neurophysiol 95: 893–901, 2006 [DOI] [PubMed] [Google Scholar]
- Mussa-Ivaldi FA, Hogan N, Bizzi E. Neural, mechanical, and geometric factors subserving arm posture in humans. J Neurosci 5: 2732–2743, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TR, Houk JC. Improvement in linearity and regulation of stiffness that results from actions of stretch reflex. J Neurophysiol 39: 119–142, 1976 [DOI] [PubMed] [Google Scholar]
- Osu R, Burdet E, Franklin DW, Milner TE, Kawato M. Different mechanisms involved in adaptation to stable and unstable dynamics. J Neurophysiol 90: 3255–3269, 2003 [DOI] [PubMed] [Google Scholar]
- Overduin SA, d'Avella A, Roh J, Bizzi E. Modulation of muscle synergy recruitment in primate grasping. J Neurosci 28: 880–892, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault EJ, Chen K, Trumbower RD, Lewis G. Interactions with compliant loads alter stretch reflex gains but not intermuscular coordination. J Neurophysiol 99: 2101–2113, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault EJ, Kirsch RF, Acosta AM. Multiple-input, multiple-output system identification for the characterization of limb stiffness dynamics. Biol Cybern 80: 327–337, 1999 [DOI] [PubMed] [Google Scholar]
- Perreault EJ, Kirsch RF, Crago PE. Effects of voluntary force generation on the elastic components of endpoint stiffness. Exp Brain Res 141: 312–323, 2001 [DOI] [PubMed] [Google Scholar]
- Perreault EJ, Kirsch RF, Crago PE. Multijoint dynamics and postural stability of the human arm. Exp Brain Res 157: 507–517, 2004 [DOI] [PubMed] [Google Scholar]
- Press W, Flannery BP, Teukolsky SA, Vetterling WT. Numerical Recipes New York: Cambridge Univ. Press, 1986 [Google Scholar]
- Prochazka A. Sensorimotor gain control: a basic strategy of motor systems? Prog Neurobiol 33: 281–307, 1989 [DOI] [PubMed] [Google Scholar]
- Pruszynski JA, Kurtzer I, Lillicrap TP, Scott SH. Temporal evolution of “automatic gain-scaling.” J Neurophysiol 102: 992–1003, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancourt D, Hogan N. Stability in force-production tasks. J Mot Behav 33: 193–204, 2001 [DOI] [PubMed] [Google Scholar]
- Rohmert W. Ermittung von Erholung-spausen fur statische arbeit des menschen. Int Angew Physiol 18: 123–164, 1960 [PubMed] [Google Scholar]
- Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci 14: 3208–3224, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemmell JBH, An JH, Perreault EJ. The differential role of motor cortex in stretch reflex modulation induced by changes in environmental mechanics and verbal instruction. J Neurosci 29: 13255–13263, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkjaer T, Toft E, Andreassen S, Hornemann BC. Muscle stiffness in human ankle dorsiflexors: intrinsic and reflex components. J Neurophysiol 60: 1110–1121, 1988 [DOI] [PubMed] [Google Scholar]
- Smeets JB, Erkelens CJ. Dependence of autogenic and heterogenic stretch reflexes on pre-load activity in the human arm. J Physiol 440: 455–465, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick PL. The influence of motor preparation on the response of cerebellar neurons to limb displacements. J Neurosci 3: 2007–2020, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Oviedo G, Macpherson JM, Ting LH. Muscle synergy organization is robust across a variety of postural perturbations. J Neurophysiol 96: 1530–1546, 2006 [DOI] [PubMed] [Google Scholar]
- Tresch MC, Cheung VC, d'Avella A. Matrix factorization algorithms for the identification of muscle synergies: evaluation on simulated and experimental data sets J Neurophysiol 95: 2199–2212, 2006 [DOI] [PubMed] [Google Scholar]
- Trumbower RD, Krutky MA, Yang B-S, Perreault EJ. Use of self-selected postures to regulate multi-joint stiffness during unconstrained tasks. PLoS ONE 4: e5411, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Linde RQ, Lammertse P, Frederiksen E, Ruiter B. The HapticMaster, a new high-performance haptic interface. In: Proceedings Europtics Edinburgh, UK, 2002, p. 1–5 [Google Scholar]
- Zhang L-Q, Rymer WZ. Simultaneous and nonlinear identification of mechanical and reflex properties of human elbow joint muscles. IEEE Transn Biomed Eng 44: 1192–1209, 1997 [DOI] [PubMed] [Google Scholar]