Abstract
The precedence effect (PE) is an auditory spatial illusion whereby two identical sounds presented from two separate locations with a delay between them are perceived as a fused single sound source whose position depends on the value of the delay. By training cats using operant conditioning to look at sound sources, we have previously shown that cats experience the PE similarly to humans. For delays less than ±400 μs, cats exhibit summing localization, the perception of a “phantom” sound located between the sources. Consistent with localization dominance, for delays from 400 μs to ∼10 ms, cats orient toward the leading source location only, with little influence of the lagging source. Finally, echo threshold was reached for delays >10 ms, where cats first began to orient to the lagging source. It has been hypothesized by some that the neural mechanisms that produce facets of the PE, such as localization dominance and echo threshold, must likely occur at cortical levels. To test this hypothesis, we measured both pinnae position, which were not under any behavioral constraint, and eye position in cats and found that the pinnae orientations to stimuli that produce each of the three phases of the PE illusion was similar to the gaze responses. Although both eye and pinnae movements behaved in a manner that reflected the PE, because the pinnae moved with strikingly short latencies (∼30 ms), these data suggest a subcortical basis for the PE and that the cortex is not likely to be directly involved.
INTRODUCTION
Sensory illusions have often been used to probe the operation of the nervous system. When perception is not congruent with what is expected based on only the physical stimulus, it can provide clues to how the brain encodes the stimuli (Parker and Newsome 1998). In some cases, the known physiological responses of neurons can help to provide insight into the illusory perception. Probably the most well known of such illusions are Mach bands, which are thought to be related to the inhibitory surrounds in the receptive fields of visual neurons from the retina to the cortex (Ratliff 1972). One problem with studying sensory illusions to deduce the operation of the nervous system, however, is that usually the illusions are experienced and documented in human subjects, whereas the physiological recordings are done in animals. It can be problematic to infer the perceptual judgments of the animals to illusory stimuli.
We have been studying the psychophysics and the physiology of a well-known auditory localization illusion known as the precedence effect (PE) in cats (Tollin and Yin 2003a; Tollin et al. 2004; Yin 1994). The PE occurs when two similar sounds are delivered to an observer in rapid succession from different spatial locations (Wallach et al. 1949). Depending on the interstimulus delay (ISD) between the sounds, human subjects report hearing one of three different localization effects. When the ISD is small (less than ∼1 ms), observers localize the sounds to a phantom source between the two sources and toward the leading source (summing localization); for longer ISDs (1 ms < ISD < 10 ms), observers report hearing a single fused sound corresponding to the direction of the leading sound source (localization dominance). For both summing localization and localization dominance, a single fused image is heard, but for long ISDs that exceed the so-called echo threshold (greater than ∼10 ms), there is breakdown of fusion: observers hear both sounds and can localize each to its respective location (Litovsky et al. 1999; Tollin and Yin 2003a). When ISD = 0 ms, the fused image is reported to be at the midpoint between the two sources and slightly elevated relative to the plane of the sources (Tollin and Yin 2003b). The term PE is often used to refer just to the ISDs corresponding to localization dominance because, for these ISDs, the spatial information in the leading sound seems to take precedence over that from the lagging one and the apparent location of these paired stimuli corresponds only to the leading location, as if the spatial information in the lagging source was perceptually suppressed. The PE is thought to be important for localizing the actual source of sound in a reverberant environment by suppressing the later-arriving echoes or reflections caused by near-by surfaces.
By training cats to look at sound sources and monitoring their eye movements, we are able to deduce their presumed perceived localization of the target. Their behavior is in good agreement with the perceptions of human subjects listening to similar stimuli (Populin and Yin 1998a; Tollin and Yin 2003b): with interstimulus delays in the localization dominance range, the cats look to the location of the leading sound, whereas delays in the summing localization range evoke saccades between the two speakers. We infer the approximate value of the echo threshold and ISD for fusion breakdown by the incidence of saccades toward the lagging sound source or saccades in both directions. Moreover, physiological recordings from cells in the inferior colliculus (IC) and auditory cortex of cats show suppression of the response to the lagging sounds when the interstimulus delays are in the range of the PE (Fitzpatrick et al. 1995, 1999; Litovsky and Yin 1998a,b; Mickey and Middlebrooks 2001, 2005; Reale and Brugge 2000; Tollin et al. 2004; Yin 1994). Dent et al. (2009) recently showed that the apparent sound location and the responses of neurons in the IC of cats were modulated similarly as a function of the relative spatial positions of the leading and lagging sources. Psychophysical correlates of the PE have also been described in other animals: in the midbrain of rats (Kelly 1974), barn owls (Nelson and Takahashi 2008; Spitzer et al. 2003, 2004), birds (Dent and Dooling 2003, 2004), and insects (Wyttenbach and Hoy 1993).
In the behavioral experiments described here, we used the eye position of the cats as an indication of the perceived (or “apparent”) spatial location of the sound source with the head held fixed (Populin and Yin 1998a). At the same time, we also monitored the position of the external ears, or pinnae, and found that pinna movements were also consistent and goal directed toward the auditory target even though there were no behavioral constraints placed on pinna position, i.e., the cats were rewarded based on their eye (or gaze) position not pinna position (Populin and Yin 1998b). Furthermore, consistent with our prior measurements (Populin and Yin 1998b; Tollin et al. 2009), pinna movements had very short latencies (30–40 ms) compared with latencies to eye or head movements, suggesting that pinna movements were generated at a fairly low level of the neuraxis. In support of the latter hypothesis, it has been shown that electrical stimulation of the IC (Syka and Straschill 1970) and the deep layers of the superior colliculus (Stein and Clamann 1981) can elicit short-latency movements of the contralateral pinna.
Neither the neural mechanisms that produce the PE phenomena nor the sites along the ascending auditory pathway where neural correlates of the PE emerge are known. The consistency and short latency of the sound-evoked pinna movements suggest that these pinna movements might provide another window into the neural mechanisms of the spatial location of auditory targets and that they might be useful in probing responses to the illusory stimuli that simulate the PE. Furthermore, the very short latencies of the pinna movements to these stimuli restrict the possible levels along the ascending auditory neuraxis where the neural correlate of the PE phenomena emerges. We therefore analyzed the pinna movements made by the cat while working in a sound localization task using stimuli that we have shown already to produce all aspects of the PE in cats (Dent et al. 2009; Tollin and Yin 2003a,b). We found that the orientations of the pinnae movements were very similar to the eye movements during both localization dominance and summing localization. Moreover, analysis of the latencies of the pinnae movements and the neuron responses to sounds suggests that the inferior colliculus may be the neural site at which the PE emerges.
METHODS
Three adult female cats were behaviorally trained to localize sound sources using operant conditioning procedures and food reward. Each cat was surgically fitted with a head post and eye and pinna coils made of fine wire (Cooner Wire, Chatsworth, CA). A detailed description of the surgical and behavioral procedures are given in Populin and Yin (1998a). The magnetic search coil technique was used to monitor eye and pinna position (Fuchs and Robinson 1966) by implanting eye coils around the eye ball and pinna coils subcutaneously behind the pinna as described earlier (Populin and Yin 1998b; Tollin et al. 2009). The eye coils were 18 to 19 mm in diameter, and the pinna coils were 10 mm in diameter. The head was restrained by a head post in these experiments. All procedures used were approved by the University of Wisconsin Animal Use and Care Committee and also complied with the National Institutes of Health guidelines for animal use.
Cats were food deprived 5–6 d/wk once training began. Body weight was monitored daily and maintained within 15% of pre-experimental weight. Water was provided ad libitum, and the animals were fed without restriction on nontesting days. Once accustomed to the head restraint, cats were trained to make saccades to locations of visual and acoustic stimuli in a dark, anechoic chamber. Stimuli were presented from a bank of speakers with light-emitting diodes (LEDs) secured in the center of each speaker. Acoustic stimuli were broadband (0.1–25 kHz) noise bursts and click trains and were generated by a custom-built digital stimulus system (see Populin and Yin 1998a) in the first experiments and later by a system developed around the Tucker Davis system III components. Speaker positions ranged between ±27° in azimuth and ±23° in elevation.
Eye and pinna coil calibration
The eye coils to measure eye position were calibrated behaviorally using standard techniques relying on the assumption that the cat would naturally look at a small illuminated LED in a dark room (Populin and Yin 1998a). By having the cat fixate a series of LEDs positioned along the vertical and horizontal meridian while sampling the voltage output from the coil, we calculated linear regression coefficients for the horizontal and vertical components of eye position. We used a spherical coordinate system specified by angles in azimuth and elevation (azimuth, elevation) from the straight ahead position with positive angles corresponding to rightward in azimuth and upward in elevation. In this particular set of experiments, the data are taken only from those speakers located on either the horizontal or vertical meridian. Within the range of eye movements of the cat (approximately ±30°), the relationship between coil voltage and source azimuth or elevation was linear with high correlation coefficients (R2 for all cats was always >0.96).
Calibration of the pinna coils, however, required particular care because there were no behavioral constraints placed on pinnae position, and the external pinna has more degrees of freedom than the eyeball. As described earlier (Populin and Yin 1998b), we exploited the observation that when the cat was working in the behavioral task, it would usually bring its pinnae to a very consistent “ready” position as the trial was about to begin, and it anticipated the LED located straight ahead coming on. When the cat was fixating straight ahead and the pinnae were in the “ready” position, we observed the cat from behind and carefully bent a wire made of malleable copper so it was parallel to the orientation of the pinna coil, which could be seen underneath the thin skin behind the pinna. The orientation of the copper wire was estimated by two or three independent observers, and the results were averaged. The cat was carefully removed from the chamber without disturbing the copper wire, and the orientation and position of thie copper wire was measured. A coil of similar size and turns to the one in the pinna of the cat being measured was placed at the position where the middle of the head of the cat would be and in the same orientation as the cat's pinna coil when the cat was in the “ready” position. The coil was rotated in yaw and pitch through 5–10° increments spanning ±40–50° while measuring at each increment the voltage output of the coil at each position. As with eye coil calibration, the relation between the pinna coil voltage and horizontal and vertical position was linear (R2 typically >0.95). Eye and pinna movement data were sampled at 500 Hz.
Psychophysical procedure and reward contingencies
A small amount of food reward consisting of a paste made of wet and pulverized dry cat food was given by a peristaltic pump for correct behavior. Correct trials were determined by the cat's eye position, which had to be maintained within electronic spatial and temporal windows specified by the experimenter under computer control during fixation of and saccades to the targets (see Tollin and Yin 2003a). At the beginning of training, spatial temporal windows were set to be large and were gradually decreased as the cat's performance improved. Usually the spatial windows for acoustic targets were between ±8 and ±16° and remained large so we were measuring the animal's perceived localization and not conditioning the cat to any specific locations. Pinna position was sampled and stored but was not associated with behavioral rewards.
Each session of behavioral training included tasks of fixation, saccades, and delayed saccades (Populin and Yin 1998a) to visual and acoustic targets randomly presented with a range of target locations. Most of the data presented here were obtained using a simple saccade task where an LED that the cat must fixate was presented from straight ahead (0, 0°) for a duration ranging from 500 to 1,000 ms. At the end of this initial fixation, the LED went off and at the same time a target was presented from 1 of 15 possible LEDs or speakers. The cat had to make a saccade to the perceived location of the stimulus and maintain eye position within an electronic window around the target to receive the reward. The acoustic stimuli were broadband noise bursts or click trains.
After the cats had learned the behavioral task using broadband noise targets, usually after several months of training, we introduced stimuli mimicking the PE as acoustic targets. A train of 10-ms duration broadband noise bursts was presented at a rate of 5 Hz for 1 s (Tollin and Yin 2003a). The PE stimuli consisted of delivery of a pair of these stimuli one to a “leading” speaker followed by an identical stimulus delivered to a spatially separated “lagging” location with an interstimulus delay (ISD) between the two stimuli. The ISDs ranged from 50 μs to 20 ms. We found that the cats were much more likely to respond to a train of these noise bursts than a single burst, so the bursts were delivered as a train. For the stimuli mimicking the PE, we chose a pair of source locations varying either in azimuth or elevation and more or less centered on the midline. For horizontal sources, the speakers were located at (+18, 0°) and (−18, 0°), whereas for vertical sources, they were located at (0, 18°) and (0, −14°). For each pair of speakers, several ISDs were used randomly throughout any given session.
Because the PE is an illusion for which there is no “correct” localization response, we rewarded the cat on every PE trial, regardless of where the cat looked. To keep the cats working diligently, the PE stimuli constituted only a low percentage (∼5–10%) of total trials for any particular day and were randomly intermingled with the usual array of single source visual and auditory targets. Thus the PE stimuli discussed in this study represent only a small percentage of the total number of tasks that the cat was presented with on any given day.
Analysis of eye and pinnae position
Eye and pinna movements were analyzed using the methods discussed in Populin and Yin (1998a). Movement onset was identified as the point where the velocity of movement exceeded ±2 SD of zero velocity sampled between 125 ms before the onset of the stimulus and 5 ms after the stimulus when the eye and pinna were stationary. Final eye and pinna position was measured when the velocity of pinna movement returned to within 2 SD of zero velocity. Horizontal and vertical components were computed separately. Latency measurements were taken as the shortest latency of the two components. Note that this latency really signals the end of fixation rather than the saccade onset as usually measured. Both eye and pinna movements were analyzed in the same manner. For movement analysis all trials, including those in which the animal was not rewarded, were included so the size of the reward window played no role in the computation of the animal's response.
RESULTS
Pinna movement responses to single source stimuli
Results are based on the eye and pinnae movement responses to stimuli that produce the PE in three cats. Some of the eye movement data for these three cats has been published (Tollin and Yin 2003a). An important assumption underlying these experiments is that the movements of the pinna were goal-directed, consistent, and reproducible. If this were true, we hypothesize that pinna movements can accurately reflect the cat's ultimate perception of the spatial location of the stimulus. Our previous studies (Populin and Yin 1998b; Tollin et al. 2009) support this assumption at least within the context of our behavioral conditions of testing the cats under controlled laboratory conditions with hundreds of trials per day. It is important to keep in mind that all of the trials using stimuli that mimic the PE constituted only a small subset of the total trials that the cat performed. The majority of trials used a variety of acoustic stimuli (noise, clicks) delivered from single sources. For all of the pinna position traces shown in this study, a pair of traces is shown: one panel (top) reflecting the vertical component of pinna movement (up designates upward movement) and the other panel (bottom) reflecting the horizontal component (up designates rightward movement). In addition, for all of the trials, the movements are derived from our so-called saccade task in which the initial fixation LED was presented from straight ahead (0, 0°). All traces are synchronized (t = 0 ms) to the time at which the LED was turned off and the acoustic target turned on.
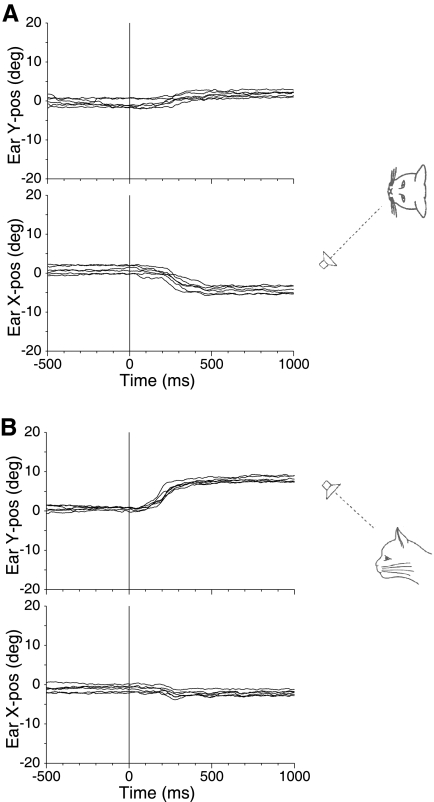
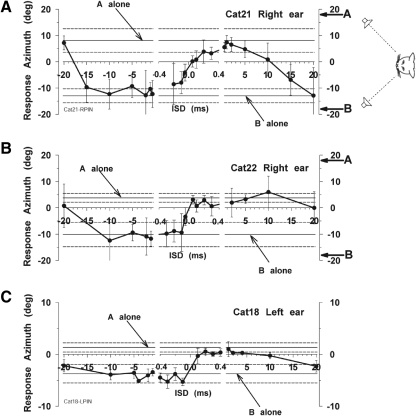
To show the consistency of pinna movements to sounds, Fig. 1 shows typical movements of a cat (cat 21) working in the saccade task and responding to a single sound source 18° to the left (Fig. 1A) and another 18° above the horizontal meridian in the midsagital plane (Fig. 1B). For the target 18° to the left (Fig. 1A), the mean final left pinna position was −4.5° leftward, with a small upward movement as well. Likewise, the pinna movements for the upward target (Fig. 1B) showed a consistent upward movement with a mean final pinna position of +7.3° upward. These results are in accord with our earlier observations and show the consistency of the pinna movements in both head-fixed (Populin and Yin 1998b) and head unrestrained (Tollin et al. 2009) conditions. As we observed with eye saccades to these stimuli (Tollin and Yin 2003a), the pinna movements undershoot the actual target location.
Fig. 1.
Examples of left pinna movements of 1 cat (cat 21) during 1 day of testing in response to single-source sounds. A: the sound source was located at (−18, 0°), i.e., 18° to the left on the horizontal midline as diagrammed on the right. In our convention, positive directions correspond to rightward or upward. The pinna movement traces show the vertical (top) and horizontal (bottom) components synchronized to the time at which the target was turned on (t = 0). Before target onset, the cat was fixating an LED straight ahead (0, 0°). B: sound source was located at (0, +18°), i.e., above the cat on the vertical midline. For both horizontally and vertically displaced targets, the pinna moved consistently with the appropriate component (leftward in A and upward in B) with short latency toward the target.
Pinna movement responses to PE stimuli: summing localization
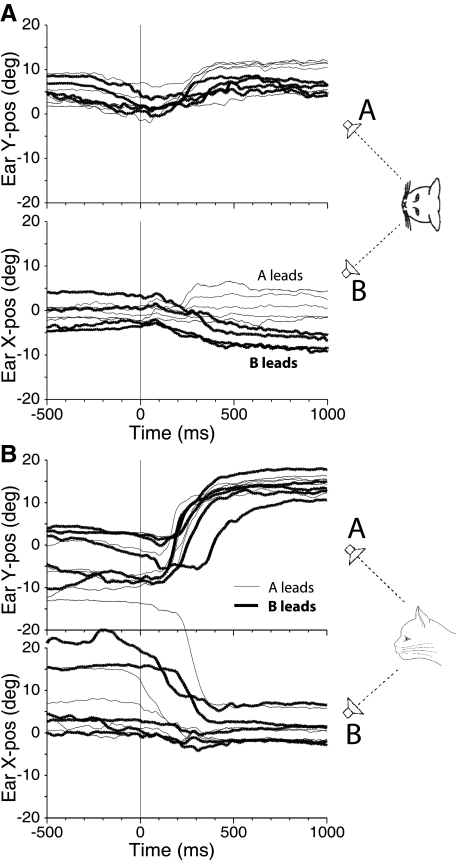
In three trained cats, we intermixed PE stimuli while the cat was working with the usual assortment of visual and single source auditory stimuli. The stimuli were presented to two speakers at two different spatial locations, displaced either in azimuth on the horizontal meridian or in elevation along the vertical meridian. Figure 2 shows movement responses of the right pinna in one cat (cat 21) to such stimuli when the interstimulus delay (ISD) was set to 100 μs in the range of summing localization. Results from responses to PE stimuli originating from two speakers separated in azimuth along the horizontal meridian (speaker A at +18° to the right and speaker B at −18° to the left) are shown in Fig. 2A. Bold traces show results when the stimulus to speaker B to the left was leading and thinner traces when speaker A was leading. The horizontal component of the pinna movement (Fig. 2A, bottom) shows that the pinna moved toward the leading speaker in virtually all trials. The mean rightward movement when the stimulus to speaker A to the right was leading was 1.0°, whereas the mean leftward movement when speaker B to the left was leading was −7.0°. There was also a consistent upward movement for both configurations of leading and lagging sources. This surprising upward movement is consistent with the behavioral response to look upward for these same stimuli with ISDs in the summing localization range (Tollin and Yin 2003a,b).
Fig. 2.
Pinna movements (right pinna, cat 21) during 1 day of testing in response to paired-source stimuli with interstimulus delays (ISDs) in the summing localization range. A: speakers were located along the horizontal meridian at (−18, 0°) and (+18, 0°) with ISDs = +100 μs (speaker A leading, thin traces) and ISDs = −100 μs (speaker B leading, thick traces). B: speakers were located along the vertical meridian at (0, +18°) and (0, −23°) with ISDs = +100 μs (speaker A leading, thin traces) and ISD + −100 μs (speaker B leading, thick traces).
Figure 2B shows right pinna movements when the speakers were separated in elevation along the vertical meridian with a 100-μs ISD. There are several surprising aspects of these results. First is that the pinna made consistent and large movements in the upward direction (top) regardless of which speaker was leading (mean 14.3° for A leading and 14.0° for B leading). For almost all other conditions, the pinna moved toward the leading sound source. Thus the pinna movements match the result that cats do not show the same type of summing localization for vertically displaced targets as they do for horizontal targets as assessed by eye movement, and this anomalous localization can be based on the spectral cues that result because of paired-source stimuli (Tollin and Yin 2003a,b). Second, the magnitude of the upward movement was large, even for these ISDs that were close to 0 μs. For example the mean magnitude of the horizontal movement in Fig. 2A (bottom) was 4°, whereas the mean magnitude of the vertical movement in Fig. 2B (top) was 14.1°. All three of our subjects showed this strong upward-movement bias for ISDs in the summing localization range.
Another aspect of the results is shown in Fig. 2B (bottom). Because the behavioral constraint was based on eye position, the position of the pinna during the fixation period can vary considerably from trial to trial without influencing the behavioral reward. In the trials shown in Fig. 2B (bottom) particularly, the pinna position varied over 10° in elevation and over 30° in azimuth during the period of LED fixation (−500 ms < t <0 ms) but quickly moved to a consistent final position after the PE stimuli were turned on (t > +300 ms). In general, the final pinna position following the target presentation was more consistent in response to an auditory stimulus than the position during the visual fixation of the LED at (0 ,0°). Although not examined in detail, the results suggest that the localization of sound sources by eye saccade remains accurate regardless of the initial position of the pinnae on the head.
Pinna movement responses to PE stimuli: localization dominance
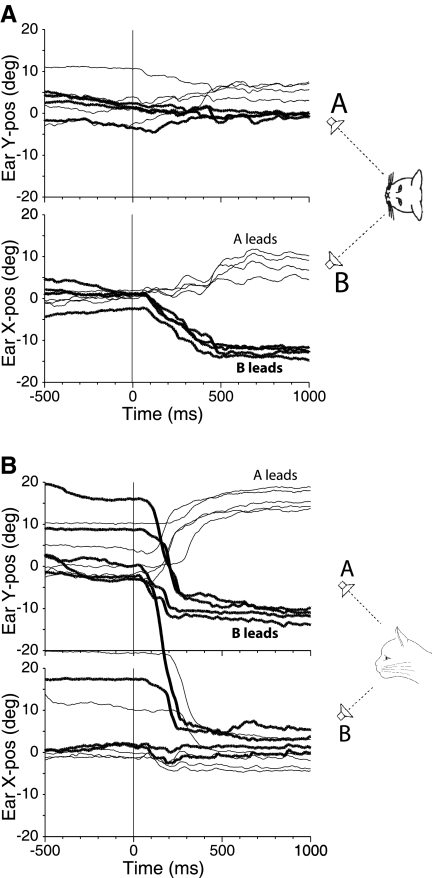
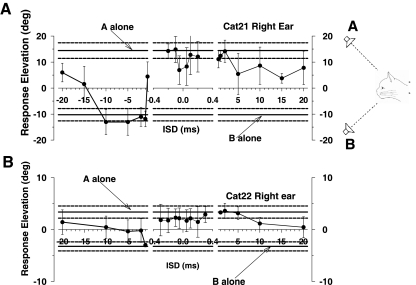
Figure 3 shows results from PE stimuli with ISDs of 1 ms in one cat (cat 21). A 1-ms ISD is sufficient to evoke localization dominance with these stimuli: cats invariably look at the leading speaker (Tollin and Yin 2003a). In these cases, the right pinna also consistently moved toward the leading speaker on the left (mean of −12.8°) and on the right (mean 7.8°; Fig. 3A, bottom). The magnitude of the pinna movements for ISD = 1 ms was considerably larger than those to ISD = 100 μs (Fig. 2A, bottom), which is congruent with the perception during the precedence effect that the sound is emanating just from the leading speaker. The pinna movements for vertically displaced speakers in Fig. 3B with ISDs in the localization dominance range were similar to those for horizontally displaced speakers: the pinna consistently made large movements toward the leading speaker locations (means of −12° with speaker B leading and 16.1° with speaker A leading).
Fig. 3.
Pinna movements (right pinna, cat 21) during 1 day of testing in response to paired source stimuli with ISDs in the localization dominance range. A: speakers were located along the horizontal meridian at (−18, 0°) and (+18, 0°) with ISDs = +1 ms (speaker A leading, thin traces) and ISDs = −1 ms (speaker B leading, thick traces). B: speakers were located along the vertical meridian at (0, +18°) and (0, −23°) with ISDs = +1 ms (speaker A leading, thin traces) and ISD = −1 ms (speaker B leading, thick traces).
Pinna movement responses to fusion breakdown: echo threshold
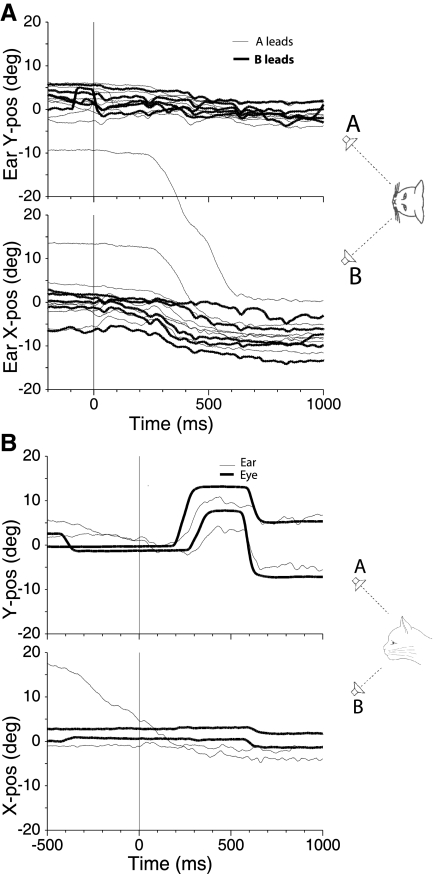
Behaviorally for these particular stimuli, when the ISDs were greater than ∼10 ms, the cats responded in a more erratic and inconsistent manner that suggested the ISDs were beyond the echo threshold; that is, they heard two distinct sound sources and were confused about which source to localize (Tollin and Yin 2003b). This period has been called fusion breakdown, because subjects no longer hear a fused image (Litovsky et al. 1999). Figure 4 shows two different examples of the apparent confusion. Figure 4A (bottom) shows the horizontal component of pinna movements to paired stimuli with ISDs of 20 ms when the speakers were on the horizontal meridian. When speaker B was leading, the pinna movements are consistently directed toward the leading source. However, when speaker A was leading, most of the pinna movements were also leftward, toward the lagging speaker B. For sound sources in the horizontal plane, it was very unusual to have pinna movements toward the lagging sound source unless the ISD was large. Note also the long latency (>200 ms) of some of the pinna movements and the larger variability in final pinna position compared with the pinna movements in response to ISDs in the localization dominance range (Fig. 3).
Fig. 4.
Pinna movements (right pinna, cat 21) (A) and eye and pinna movements (B) during 1 day of testing in response to paired source stimuli with ISDs beyond the echo threshold range. A: speakers were located along the horizontal meridian at (−18, 0°) and (+18, 0°) with ISDs = +20 ms (speaker A leading, thin traces) and ISDs = −20 ms (speaker B leading, thick traces). B: speakers were located along the vertical meridian at (0, +18°) and (0, −23°) with an ISD = +20 ms (upward speaker A leading). Two trials in which the eye movement (thick traces) and pinna movements (thin traces) showed the cat orienting 1st to the upward leading speaker and then to the downward lagging speaker.
Another indication that the cat heard both the leading and lagging sound was that, in some trials with such long ISDs, the cats would sometimes look toward both sounds (Tollin and Yin 2003a). Figure 4B shows both eye and right pinna movements for one cat (cat 21) for two trials with ISD = +20 ms with speakers above and below the horizontal meridian. In the example, speaker A was leading speaker B. In both trials, the eye first makes an upward saccade toward the leading upward speaker, followed 200 to 300 ms later by a saccade toward the lagging downward speaker. The pinna movements mirror the eye movements in their direction though they are not as smooth as the eye movements. In this example, the latency of pinna movement was nearly the same or slightly shorter than the eye movement. Even the duration between the upward and downward saccades apparent in the eye movements is evident in the pinna movements.
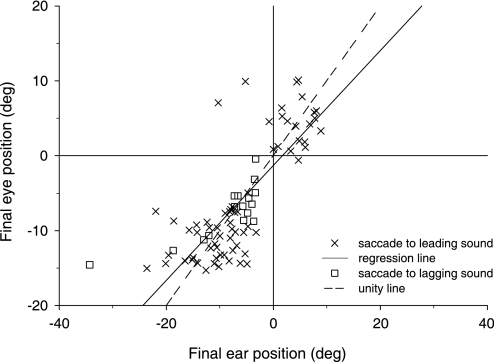
The close correlation between the eye and pinna movements in the example in Fig. 4B and the data as a whole suggest that the two measures, eye and pinna position, can be related on a trial-by-trial basis. To explore this possibility, we examined the eye and pinnae responses to PE stimuli with long ISDs where an eye saccade in only one direction was made. As noted in Fig. 4A, on some trials, the cat looked to the leading sound and on others to the lagging sound. Figure 5 shows a scatter plot of final eye position as a function of final pinna position for PE stimuli with ISDs = 10, 15, and 20 ms for the one cat (cat 21) tested most extensively. Trials in which the cat looked to the direction of the leading sound are plotted separately (X) from trials in which the cat looked to the lagging sound (squares). The fact that almost all points fall in either the upper right or lower left quadrant indicates that the eye and pinna movements were made in the same direction, even when the cat made the unusual response of looking toward the lagging sound for ISDs greater than the echo threshold. A linear regression analysis of these data showed that final eye position was related to final pinna position by the following equation (y = 0.69x – 1.72, r = 0.77). The regression analysis indicates that the pinna movements are predictive of the subsequent eye movements. Although not shown, a similar relation was found for eye and pinna movments to single sound sources (see also Populin and Yin 1998b and Tollin et al. 2009).
Fig. 5.
Scatter plot of final horizontal pinna position vs. eye position for large ISDs (10, 15, and 20 ms) in the fusion breakdown range for speakers at (±18, 0°) for the 1 cat tested most extensively (cat 21, right pinna). Saccadic eye movements made to the leading sound are plotted as X, whereas those to the lagging sound are plotted as open squares.
Average pinna position as a function of ISD
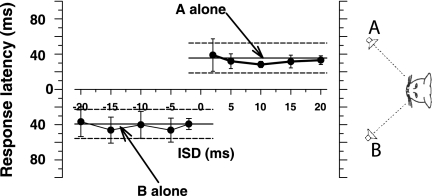
Comparison of the eye movements in Tollin and Yin (2003a) with the pinna movements in this study clearly shows that there is more variability in the pinna movements. It is difficult to capture the variability in the single trials of pinna movements shown above. Furthermore, there is also considerable variability between subjects that can be seen by examining responses of averaged pinna movements. Figure 6 summarizes results from three cats for PE stimuli by plotting the across-trial mean horizontal pinna position as a function of ISD. In this condition, the two speakers were positioned at ±18° along the azimuth and at 0° elevation. Positive ISDs correspond to cases where the speaker A on the right was leading in time. The horizontal components of final pinna position are shown in Fig. 6 along with the mean (±SD) responses of the pinna movements to the same stimuli when delivered from single sources at the same locations by the horizontal (and dashed) lines labeled “A alone” and “B alone.” Results from the right pinna of two cats are shown in Fig. 6, A and B, whereas results from the left pinna of cat 18 are shown in Fig. 6C. The results from the three cats show the range of variability in responses seen in our data. For example the pinna movements in cats 21 and 22 were substantially larger than those in cat 18. However, the global patterns and time courses of the resulting pinnae movements were consistent across cats.
Fig. 6.
Summary plots for 3 cats of the final horizontal pinna position as a function of ISDs for speakers along the horizontal meridian at (±18, 0°) with stimuli mimicking the precedence effect. The middle section of each plot represents ISDs in the summing localization range. The 2 horizontal lines and dashed lines indicate the mean response ± SD to the A and B speakers when delivered as a single source target.
All of the cats showed some common features in their pinna movements to stimuli known to produce the PE in the horizontal plane. With ISDs in the summing localization range (|ISD| < ∼0.4 ms; represented by the middle segment of the graphs in Fig. 6, the pinna moved to an intermediate position corresponding to a phantom source between the two speakers, with a bias toward the leading speaker that was dependent on the magnitude of the ISD. This resulted in a sigmoidal relationship between pinna position and ISD in this range, consistent with summing localization (Tollin and Yin 2003b). In general, the pinna moved to a position near 0°, i.e., the position it assumed when the cat was fixating straight ahead, when the ISD = 0 ms, and moved to the left when speaker B was leading and to the right when speaker A was leading.
For ISDs in the range of localization dominance (ISDs from ∼0.5 to 10 ms), for all three of our subjects, the across-trial mean pinna position was nearly equal, or at least in the range of the variance, to that when the leading speaker was delivered in isolation (solid horizontal lines). That is, for these ISDs, the pinna movements behaved as if only a single stimulus was delivered from the leading sound location in isolation and the spatial information about the stimulus from the lagging location was somehow suppressed or inhibited. This is the time period of the localization dominance phase of the PE where the leading sound takes precedence for the perceived localization of sounds delivered from two different spatial locations.
Finally, for ISDs greater than ∼10 ms for these stimuli, the echo threshold has been reached. Here, the across-trial mean pinna position no longer remained at the leading speaker location but rather moved toward the lagging one as the ISD was increased beyond 10–15 ms. We have previously shown that the eye movements of cats presented with stimuli with ISDs >10 ms reflected an apparent uncertainty of the animal as to which speaker to fixate; on some trials they look toward the leading location, on other trials toward the lagging locations, and in some trials they looked toward the location of both speakers in quick succession (Fig. 4; see also Tollin and Yin 2003b). Averaging these behavioral responses result in the across-trial mean response azimuth to tend toward 0° azimuth with a concommittant increase in the SD of responses (Tollin and Yin 2003a). A comparable result was seen in the pinna movements: the across-trial mean azimuth was no longer near the leading speaker, but tended toward 0° or even crossing 0° azimuth, and the response variances were much larger as the pinnae oriented to the leading and lagging speaker on different trials reflecting the cat's perceptual uncertainty and breakdown of fusion.
Figure 7 shows the vertical component of the pinna movements obtained from the same trials as in Fig. 6. In one animal (Fig. 7A), there was a substantial departure from zero elevation for stimuli in the summing localization range even though the speakers themselves were located on the horizontal meridian at zero elevation. However, the pinna movements of the other two cats did not consistently show this effect on all trials.
Fig. 7.
Same data as in Fig. 6 except that the vertical component of pinna position is plotted as a function of ISDs.
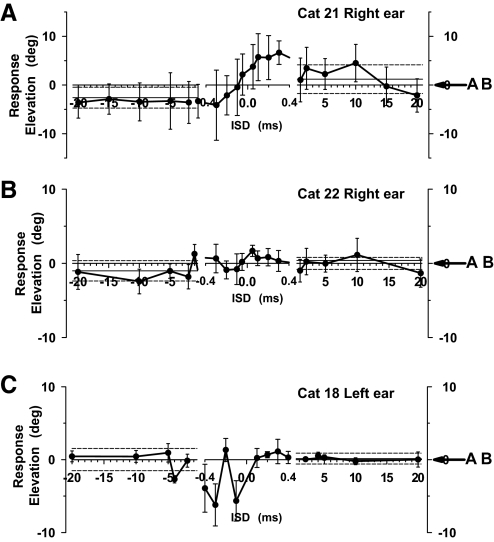
Finally, Fig. 8 shows the vertical component of pinna movements as a function of ISD for stimuli in which PE was elicited in elevation by placing the two speakers on the midsagittal plane varying in the vertical dimension. Two of the three cats showed a correlate of localization dominance in the movements of the pinna for ISDs >1.0 ms. Two of the three cats also showed vertical pinna movements for |ISDs| < 0.4 ms that were consistently elevated above the response expected for a stimulus presented on the horizontal plane (0° elevation). For example, the pinna movements of cat 21 in Fig. 8A were consistently upward during the entire period of summing localization, even when the downward speaker led by 0.4 ms, with a sudden transition toward the downward speaker when the ISD was −1 ms. Although the magnitude of the pinnae movements varied across the cats, the patterns of the movements with changes in ISDs was largely consistent.
Fig. 8.
Summary plots for 2 cats of the final vertical pinna position as a function of ISDs for speakers along the vertical meridian at (0, +18°) and (0, −23°) with stimuli mimicking the precedence effect. The middle section of each plot represents ISDs in the summing localization range.
Laterality of pinna movements
In earlier studies of pinna movements, Populin and Yin (1998b) showed movements of the pinna in both directions for targets at small eccentricities (±18° or less) but a clear laterality at larger eccentricities where the pinna ipsilateral to the sound source responded while the contralateral pinna was largely unresponsive. We recently reported a similar finding in head-unrestrained cats (Tollin et al. 2009). In these experiments, because the speakers were limited to ±18° in azimuth, the pinnae responded with movements to both the ipsilateral and contralateral side, consistent with our earlier observations. There was some variability between the three cats that were studied; for example, the magnitude of cat 18's pinna movements in azimuth were substantially smaller than those of the other two cats (Fig. 6). However, the patterns of pinnae movements with changes in ISD were comparable across cats.
Pinna-movement latencies to single and paired source stimuli
A striking aspect of the responses of the pinnae to these stimuli that elicit the auditory illusions of the PE was the generally short response latencies compared with the eye or head movments. Figure 9 shows a plot of pinna latency of the horizontal component versus ISD for PE stimuli varying on the horizontal plane for one cat (cat 21, right ear) for which we had collected considerable data. The mean latencies were very short, with a mean of 37.4 ± 16.9 (SD) ms (n = 170 trials). By comparison, the latencies for eye movements for the same cat to the same PE stimuli were much longer on average, 200–300 ms, and tended to be much more variable (Tollin and Yin 2003a); the eye saccade latencies to these stimuli ranged from 66 to 357 ms. The large differences in the magnitudes of the eye and pinna latencies may be explained by the stimuli themselves. Recall that the stimuli used here were trains of 10-ms duration noise bursts presented five times with a period of 200 ms (for a total duration of 1 s). Consistently with these stimuli, if the cats did not initiate an eye saccade to the initial stimulus in the train, they seemed to wait until sometime after the presentation of the second stimulus in the train to respond. The latter was more often the case. Thus latencies for eye saccades were on average >200 ms (1 period of the stimulus) in all cats. This was not the case for the pinna, which typically responded in a goal-directed manner to the very first stimulus in the train, resulting in lower latencies than the eye saccades. Interestingly, both the pinna and eye latencies were generally independent of the ISD, as shown in Fig. 9 for paired source (see Tollin and Yin 2003a for eye movement latencies).
Fig. 9.
Summary plot of pinna response latency for the right pinna for 1 of our cats (cat 21) for the precedence effect (PE) stimuli plotted as a function of ISD. The 2 horizontal lines and dashed lines indicate the mean pinna latencies ± SD to the A and B speakers when delivered as a single source target.
DISCUSSION
Here we showed that the pinna movements of cats to stimuli that are known to produce all facets of the PE, summing localization, localization dominance, and fusion breakdown (Tollin and Yin 2003b), are similar to the corresponding movements of the eyes. The pinna movements were a reliable and significant predictor of the eye movements in response to stimuli that evoked the PE (Fig. 5). This correlation was not unexpected for stimuli evoking summing localization and localization dominance because these are very powerful and convincing illusions. However, the correlation was most striking, and unexpected, for stimuli with ISDs exceeding echo threshold and in the unusual instances when the cat looked at the lagging sound after having first looked at the leading one (Figs. 4B and 5). It is important to keep in mind that we have trained and rewarded the cats to localize sounds based on their eye movements and not pinna movements; they were free to move their pinnae in any fashion and still got rewarded on all trials. The results presented here show a close association of eye and pinna movements to both single and paired sources that elicit the PE. The short latency response of pinna movements, however, suggests that the neural mechanisms for the PE are likely present at the level of the auditory brain stem and are not generated exclusively at the cortical level.
Pinnae movements to stimuli evoking summing localization
For speakers on the horizontal plane, all cats consistently looked to a point between the two speakers that was biased toward the leading speaker for ISDs (±400 μs) in the summing localization range (Tollin and Yin 2003b). We found that the pinna movements were also congruent with the eye saccade behavior: the final pinna position was dependent on ISD and consistently fell between the mean pinna positions measured in response to stimuli delivered from each of the speakers in isolation (Fig. 2).
For speakers located on the horizontal plane, the cats systematically perceived the phantom source to be above the horizontal meridian. Tollin and Yin (2003b) hypothesized that the upward localization reflected a spectral feature of the stimuli that corresponded to a spectral notch in the head-related transfer function (HRTF) for vertical targets. These spectral notch cues, which are generated by direction-dependent acoustic interactions of the propagating sound waves with the pinna (Tollin and Koka 2009), are hypothesized to be used for the perception of sound source elevation (Rice et al. 1992). The orienting responses of the pinna to these same stimuli showed a similar upward deviation for ISDs in this range in one cat (Fig. 7A) but not in the other two (Fig. 7, B and C).
When the sound sources were separated along the vertical dimension with ISDs in the summing localization range, Tollin and Yin (2003a) found that cats did not show the same graded, semilinear response between the speakers but tended to look upward. The explanation for this unexpected upward bias in behavioral localization response was the same as that for the horizontally placed paired sources described above. Here the spectral cues that result from paired stimuli delivered from two sources on the midsagittal plane indicate an elevated sound source and not one near the midline. This was still considered summing localization by Tollin and Yin (2003a) because it is the localization cues that result from the interaction of the stimuli delivered from the two source locations. Our results show that the pinna movements also exhibited the sudden transition from one speaker to the other with no graded responses (Fig. 8), consistent with that found with eye saccades. For two cats (cats 21 and 22), the pinna consistently moved toward the upward speaker throughout the summing localization range, like the eye movements.
Pinnae movements to stimuli evoking localization dominance
For ISDs in the range of localization dominance (0.5 to ∼10 ms), both the eye and pinna movements were consistently directed only toward the leading sound source. This is the range of ISDs that constitute the classical aspect of the PE: that is, the leading sound source takes precedence in localizing the paired stimulus. This was true for sound sources placed along the horizontal meridian for all of our subjects for either left leading or right leading sounds (Fig. 6). In the vertical dimension, the results were not as consistent though on average the same results were found (Fig. 8). The perceptual phenomena associated with localization dominance represents a spatial illusion because the sound location of the leading source is preserved in the face of a conflicting simulated reflection, which would be easily locatable if presented on its own. Moreover, the perception of a single source is not consistent with the physical reality that another source (i.e., the reflection) is also present. The localization dominance aspect of the PE is largely a spatial phenomenon because psychophysical studies in humans (Divenyi 1992; Freyman et al. 1998; Gaskell and Henning 1999; Zurek 1980) and owls (Spitzer et al. 2003) have consistently shown that the PE does not result from a peripheral masking of the lagging stimulus by the leading stimulus, because many nonspatial attributes of the lag can still be discriminated. That is, the spatial information from the lagging source is suppressed, but not necessarily other aspects.
Pinnae movements to stimuli evoking fusion breakdown
At ISDs greater than ∼10 ms, the eye movements were compatible with the hypothesis that the cats no longer heard a fused image but rather heard both sources and could localize them; however, they were uncertain as to which source to localize and which was the leading one (Tollin and Yin 2003a). This is consistent with previous reports in human observers showing that, with PE stimuli not unlike those presented here, although two distinct sounds can be heard near their locations, it can be difficult to accurately determine which one was actually the lead and which was the lag because of a confusion about the temporal order of the stimuli (Stellmack et al. 1999). The pinna movements to PE stimuli with large ISDs behaved similarly and, like the eye position data, were also much more variable, and the SD was large (Fig. 6). If the cats heard and localized both sources, it is not surprising that they would be confused about where to look. Two observations in these experiments reinforce the hypothesis that these ISDs were beyond the echo threshold. Perhaps the most compelling is the trials in which the cat looked at both sources in quick succession. Figure 4B shows that, when the eyes look to both sources, the pinna also moves in similar fashion. Second, at these long ISDs, it was sometimes common for the cat to look at the lagging source, which almost never happened at ISDs <10 ms, in the localization dominance range. Figure 5 shows that, on those trials in which the eye was directed to the lagging source, the pinna also deviated toward the lagging source. Similarly, when the pinnae oriented toward the leading source, invariably the eyes also did as well. These observations with stimuli that evoked the PE are consistent with our prior reports that the movements of the pinnae to sounds in general are correlated with the eye or gaze movements (Populin and Yin 1998b; Tollin et al. 2009).
Response latencies for pinna movements and neural responses to PE stimuli suggest a subcortical bases for the mechanisms producing the PE
Given the close similarities in the magnitude, direction, and time course of the pinnae and eye saccades to the PE stimuli, we hypothesized that the same neural mechanisms mediate both of these goal-directed orienting responses. We believe that the latencies to initiate these different orienting responses can be used to constrain the possible sites along the ascending auditory neuraxis where the neural correlates of the PE, and possibly sound location encoding more generally, emerges. One important and useful discrepancy in the correspondence between eye and pinna movements noted above was the disparity in the response latencies. The latency for pinna movements was much shorter than for eye movements. One of the likely reasons, discussed in the results, had to do with the stimuli, which were trains of brief noise bursts with a period of 200 ms. The cats generally appeared to respond via eye saccade after the second stimulus in the train (occurring 200 ms after stimulus onset) while the pinnae invariably responded to the initial stimulus. However, even in those cases where the eye saccade appeared to be in response to the initial stimulus with latencies <200 ms, the very shortest latency recorded across all cats tested was 66 ms, nearly twice the mean pinna latency. Given the concordance of pinna movements with behavior, the short latency of pinna responses to acoustic stimuli suggests that the spatial representation of PE sounds must be dictated by a circuit from the cochlea back to the pinna muscles that has a correspondingly short activation time. Neuronal response latencies can be useful to estimate the possible anatomical pathway.
Given the short latency of pinna movements, we can infer some constraints on a likely anatomical pathway for activation of the muscles in response to acoustic stimuli by tracing backward from the pinna muscles. The motoneurons innervating the muscles of the pinna lie in the facial nucleus (Populin and Yin 1995). Electrical stimulation of the deep layers of the superior colliculus can elicit movements of the contralateral pinna (Stein and Clamann 1981) by way of a circuit that traverses the paralemniscal zone of the lateral midbrain formation (Henkel and Edwards 1978). In the bat electrical stimulation of the deep layers of the SC elicits pinna movements with latencies of 16–21 ms (Valentine et al. 2002) or an average of ∼18.5 ms. Recordings from cells in the deep and intermediate layers of the SC of unanesthetized cats to acoustic stimuli show a mean latency of 17.7 (Populin and Yin 2002) or 17.6 ms (Wallace et al. 1998). If this is indeed the circuit mediating sound-elicited pinnae movements, the total expected mean latency from sound onset to pinna movement should be predictable by the sum of these two latencies (18.5 + 17.7 ms), which results in 36.2 ms. The predicted latency falls squarely in the middle of our emprically measured pinna latencies to single and paired-source acoustic stimuli of 30–40 ms (Fig. 9).
The pathway to the SC is likely to involve the IC, where we have recorded responses to the exact same PE stimuli used here in awake cats with mean latencies of 8.9 ms (Tollin et al. 2004). Electrical stimulation of the IC while recording from cells in the SC evokes responses in the short-latency group of cells with latencies of ∼5 ms as well as concommittant movements of the contralateral pinna with the lowest threshold of activation (Syka and Straschill 1970). The combined latency of 13.9 ms (8.9 + 5 ms) is comparable to the empirical SC latencies to acoustic stimulation cited above. At higher stimulation voltages in the IC joint eye movements and contralateral pinna movement could be evoked. The results based on both the empirical neural and the electrical stimulation response latencies suggest that the likely circuitry for evoking short latency pinna movements to sounds involves ascending projections to the IC, then to the SC, and then paralemniscal zone of the lateral midbrain formation and facial nucleus.
While the analysis here leaves open the possibility that there is an even shorter anatomical pathway between cochlea and facial nucleus, for which there is some evidence (Harrison and Irving 1966), it rules out longer pathways and mechanisms that would involve the auditory cortex. For example, neural latencies for auditory stimuli in the primary auditory cortex (A1) of anesthetized cats range from ∼10 to 30 ms (Phillips and Irvine 1982); similar latencies have been reported in unanesthetized cats (Mickey and Middlebrooks 2005). Although the latencies of A1 neurons are comparable to the latencies in the SC, the neural path from A1 back to the motor neurons that drive the pinnae, perhaps through the auditory field of the anterior ectosylvian sulcus (AES) (Clarey and Irvine 1986), which projects to the SC or the motor cortex, would involve substantial increases in response latencies. For example, latencies of AES neurons to auditory stimuli are longer (∼5 ms) than those in A1 (Clarey and Irvine 1986) and further latencies of ≤5 ms are incurred from AES to the SC (Wallace et al. 1993). Thus, although there may be some overlap with the longer pinna movement latencies, the bulk of the short-latency movements of the pinnae cannot likely be accounted for by a pathway through cortex. The data presented in this paper support the hypothesis of a subcortical origin, perhaps at the level of the IC, for the mechanisms responsible for the auditory illusions of the PE and sound localization in general.
Physiological correlates of the precedence effect
Physiological correlates of the PE has been studied at nearly all levels of the auditory system, including the auditory nerve (Parham et al. 1996), the cochlear nucleus (Fitzpatrick et al. 1995; Parham et al. 1998; Wickesberg 1996), superior olivary complex (Fitzpatrick et al. 1995), IC (Burger and Pollak 2001; Dent et al. 2009; Fitzpatrick et al. 1995; Litovsky and Yin 1998a,b; Nelson and Takahashi 2008; Spitzer et al. 2004; Tollin et al. 2004; Yin 1994), and auditory cortex (Fitzpatrick et al. 1999; Mickey and Middlebrooks 2001, 2005; Reale and Brugge 2000). At each stage, for small ISDs with transient or short-duration stimuli such as the 10-ms duration noise bursts used here, neuronal responses to the lag are substantially reduced compared with responses to the same stimulus presented in isolation from the same location, yet the responses to the lead are generally unchanged. With increasing ISD, the lag responses recover to levels comparable to the response elicited when the lagging source is presented in isolation. However, the rate of recovery with increasing ISD is highly dependent on where in the auditory system the neurons are being recorded. At the auditory nerve and cochlear nucleus, neurons can respond to the lead and the lag for ISDs as low as 1–2 ms. However, the behavioral responses of cats with such ISDs depend almost exclusively on the lead (Dent et al. 2009; Tollin and Yin 2003a). It is not until the level of the IC and the auditory cortex, where convincing neural correlates of the PE phenomena have been found: at short ISDs for which cats experience localization dominance, there is an accurate neural representation of the leading source, but the response to the lag is diminished or nonexistent (see above IC citations). As we argued above, the short latency of the pinna movements and their similarity to many aspects of behavior suggest that aspects of the PE phenomena, including summing localization, localization dominance, and the echo threshold, are probably mediated in large part at brain stem levels and are unlikely to depend greatly on cortical processing. However, there are some higher order aspects of the PE, such as the Clifton effect and the build-up and break-down of the PE (Clifton 1987; Clifton and Freyman 1989), which were not studied here that may be more likely to be under cortical control.
GRANTS
This work was supported by National Institutes of Deafness and Other Communicative Disorders (NIDCD) Grants DC-00116, DC-07177, and DC-02840 to T.C.T. Yin and an Individual National Research Service Award (NIDCD DC-000376) to D. J. Tollin.
ACKNOWLEDGMENTS
We acknowledge R. Kochhar and J. Sekulski for designing and implementing the analysis software and the data collection software, respectively, and J. Ruhland for assistance in training animals and data analysis.
REFERENCES
- Blauert J. Spatial Hearing. The Psychophysics of Human Sound Localization. Cambridge, MA: MIT Press, 1983 [Google Scholar]
- Burger RM, Pollak GD. Reversible inactivation of the dorsal nucleus of the lateral lemniscus reveals its role in the processing of multiple sound sources in the inferior colliculus of bats. J Neurosci 21: 4830–4843, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarey JC, Irvine DRF. Auditory response properties of neurons in the anterior ectosylvian sulcus of the cat. Brain Res 386: 12–19, 1986 [DOI] [PubMed] [Google Scholar]
- Clifton RK. Breakdown of echo suppression in the precedence effect. J Acoust Soc Am 82: 1834–1835, 1987 [DOI] [PubMed] [Google Scholar]
- Clifton RK, Freyman RL. Effect of click rate and delay on breakdown of the precedence effect. Percept Psychophys 46: 139–145, 1989 [DOI] [PubMed] [Google Scholar]
- Dent ML, Tollin DJ, Yin TCT. Influence of sound source location on the behavior and physiology of the precedence effect in cats. J Neurophysiol 102: 724–734, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent ML, Dooling RJ. Investigations of the precedence effect in budgerigars: effects of stimulus type, intensity, duration, and location. J Acoust Soc Am 113: 2146–2158, 2003 [DOI] [PubMed] [Google Scholar]
- Dent ML, Dooling RJ. The precedence effect in three species of birds (Melopsittacus undulatus, Serinus canaria, and Taeniopygia guttata). J Comp Psychol 118: 325–331, 2004 [DOI] [PubMed] [Google Scholar]
- Divenyi PL. Binaural suppression of nonechoes. J Acoust Soc Am 91: 1078–1084, 1992 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick DC, Kuwada S, Batra R, Trahiotis C. Neural responses to simple simulated echoes in the auditory brain stem of the unanesthetized rabbit. J Neurophysiol 74: 2469–2486, 1995 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick DC, Kuwada S, Kim DO, Parham K, Batra R. Responses of neurons to click-pairs as simulated echoes: auditory nerve to auditory cortex. J Acoust Soc Am 106: 3460–3472, 1999 [DOI] [PubMed] [Google Scholar]
- Freyman RL, McCall DD, Clifton RK. Intensity discrimination for precedence effect stimuli. J Acoust Soc Am 103: 2031–2041, 1998 [DOI] [PubMed] [Google Scholar]
- Fuchs A, Robinson D. A method for measuring horizontal and vertical eye movements chronically in the monkey. J Appl Physiol 21: 1068–1070, 1966 [DOI] [PubMed] [Google Scholar]
- Gaskell H, Henning GB. Forward and backward masking with brief impulsive stimuli. Hear Res 129: 92–100, 1999 [DOI] [PubMed] [Google Scholar]
- Haas H. Über den Einfluss eines Einfachechos auf die Horsamkeit von Sprache. Acoustica 1: 49–58, 1951 [Google Scholar]
- Harrison JM, Irving R. Visual and nonvisual auditory systems in mammals. Science 154: 738–743, 1966 [DOI] [PubMed] [Google Scholar]
- Henkel CK, Edwards SB. The superior colliculus control of pinna movements in the cat: possible anatomical connections. J Comp Neurol 182: 763–776, 1978 [DOI] [PubMed] [Google Scholar]
- Kelly J. Localization of paired sound sources in the rat: small time differences. J Acoust Soc Am 55: 1277–1284, 1974 [DOI] [PubMed] [Google Scholar]
- Litovsky RY, Colburn HS, Yost WA, Guzman SJ. The precedence effect. J Acoust Soc Am 106: 1633–1654, 1999 [DOI] [PubMed] [Google Scholar]
- Litovsky RY, Rakerd B, Yin TC, Hartmann WM. Psychophysical and physiological evidence for a precedence effect in the median sagittal plane. J Neurophysiol 77: 2223–2226, 1997 [DOI] [PubMed] [Google Scholar]
- Litovsky RY, Yin TC. Physiological studies of the precedence effect in the inferior colliculus of the cat. I. Correlates of psychophysics. J Neurophysiol 80: 1285–1301, 1998a [DOI] [PubMed] [Google Scholar]
- Litovsky RY, Yin TC. Physiological studies of the precedence effect in the inferior colliculus of the cat. II. Neural mechanisms. J Neurophysiol 80: 1302–1316, 1998b [DOI] [PubMed] [Google Scholar]
- Mickey BJ, Middlebrooks JC. Responses of auditory cortical neurons to pairs of sounds: correlates of fusion and localization. J Neurophysiol 86: 1333–1350, 2001 [DOI] [PubMed] [Google Scholar]
- Mickey BJ, Middlebrooks JC. Sensitivity of auditory cortical neurons to the locations of leading and lagging sounds. J Neurophysiol 94: 979–989, 2005 [DOI] [PubMed] [Google Scholar]
- Nelson BS, Takahashi TT.Independence of echo-threshold and echo-delay in the barn owl. PLoS ONE 3: e3598, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham K, Zhao HB, Kim DO. Responses of auditory nerve fibers of the unanesthetized decerebrate cat to click pairs as simulated echoes. J Neurophysiol 76: 17–29, 1996 [DOI] [PubMed] [Google Scholar]
- Parham K, Zhao HB, Ye Y, Kim DO. Responses of anteroventral cochlear nucleus neurons of the unanesthetized decerebrate cat to click pairs as simulated echoes. Hear Res 125: 131–146, 1998 [DOI] [PubMed] [Google Scholar]
- Parker AJ, Newsome WT. Sense and the single neuron: probing the physiology of perception. Annu Rev Neurosci 21: 227–277, 1998 [DOI] [PubMed] [Google Scholar]
- Phillips DP, Irvine DRF. Properties of single neurons in the anterior auditory field (AAF) of cat cerebral cortex. Brain Res 248: 237–244, 1982 [DOI] [PubMed] [Google Scholar]
- Populin LC, Yin TC. Topographical organization of the motoneuron pools that innervate the muscles of the pinna of the cat. J Comp Neurol 363: 600–614, 1995 [DOI] [PubMed] [Google Scholar]
- Populin LC, Yin TC. Behavioral studies of sound localization in the cat. J Neurosci 18: 2147–2160, 1998a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Populin LC, Yin TCT. Pinna movements of the cat during sound localization. J Neurosci 18: 4233–4243, 1998b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Populin LC, Yin TC. Bimodal interactions in the superior colliculus of the behaving cat. J Neurosci 22: 2826–2834, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratliff F. Contour and contrast. Sci Am 226: 91–101, 1972 [PubMed] [Google Scholar]
- Reale RA, Brugge JF. Directional sensitivity of neurons in the primary auditory (AI) cortex of the cat to successive sounds ordered in time and space. J Neurophysiol 84: 435–450, 2000 [DOI] [PubMed] [Google Scholar]
- Rhode WS. A digital system for auditory neurophysiological research. In: Current Computer Technology in Neurobiology, edited by Brown PB. Washington DC: Hemisphere, 1976, p. 543–567 [Google Scholar]
- Rice JJ, May BJ, Spirou GA, Young ED. Pinna-based spectral cues for sound localization in cat. Hear Res 58: 132–152, 1992 [DOI] [PubMed] [Google Scholar]
- Spitzer MW, Bala AD, Takahashi TT. Auditory spatial discrimination by barn owls in simulated echoic conditions. J Acoust Soc Am 113: 1631–1645, 2003 [DOI] [PubMed] [Google Scholar]
- Spitzer MW, Bala AD, Takahashi TT. A neuronal correlate of the precedence effect is associated with spatial selectivity in the barn owl's auditory midbrain. J Neurophysiol 92: 2051–2070, 2004 [DOI] [PubMed] [Google Scholar]
- Stein BE, Clamann HP. Control of pinna movements and sensorimotor register in cat superior colliculus. Brain Behav Evol 19: 180–192, 1981 [DOI] [PubMed] [Google Scholar]
- Stellmack MA, Dye RH, Jr, Guzman SJ. Observer weighting of interaural delays in source and echo clicks. J Acoust Soc Am 105: 377–387, 1999 [DOI] [PubMed] [Google Scholar]
- Syka J, Straschill M. Activation of superior colliculus neurons and motor responses after electrical stimulation of the inferior colliculus. Exp Neurol 28: 384–392, 1970 [DOI] [PubMed] [Google Scholar]
- Tollin DJ, Koka K. Postnatal development of sound pressure transformations by the head and pinnae of the cat: monaural characteristics. J Acoust Soc Am 125: 980–994, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollin DJ, Populin LC, Yin TC. Neural correlates of the precedence effect in the inferior colliculus of behaving cats. J Neurophysiol 92: 3286–3297, 2004 [DOI] [PubMed] [Google Scholar]
- Tollin DJ, Ruhland JL, Yin TCT. The vestibulo-auricular reflex. J Neurophysiol 101: 1258–1266, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollin DJ, Yin TC. Psychophysical investigation of an auditory spatial illusion in cats: the precedence effect. J Neurophysiol 90: 2149–2162, 2003a [DOI] [PubMed] [Google Scholar]
- Tollin DJ, Yin TC. Spectral cues explain illusory elevation effects with stereo sounds in cats. J Neurophysiol 90: 525–530, 2003b [DOI] [PubMed] [Google Scholar]
- Valentine DE, Sinha SR, Moss CF. Orienting responses and vocalizations produced by microstimulation in the superior colliculus of the echolocating bat, Eptesicus fuscus. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 188: 89–108, 2002 [DOI] [PubMed] [Google Scholar]
- Wallace MT, Meredith MA, Stein BE. Converging influences from visual, auditory, and somatosensory cortices onto output neurons of the superior colliculus. J Neurophysiol 69: 1797–1809, 1993 [DOI] [PubMed] [Google Scholar]
- Wallace MT, Meredith MA, Stein BE. Multisensory integration in the superior colliculus of the alert cat. J Neurophysiol 80: 1006–1010, 1998 [DOI] [PubMed] [Google Scholar]
- Wallach H, Newman E, Rosenzweig M. The precedence effect in sound localization. Amer J Psychol 57: 315–336, 1949 [PubMed] [Google Scholar]
- Wickesberg RE. Rapid inhibition in the cochlear nuclear complex of the chinchilla. J Acoust Soc Am 100: 1691–1702, 1996 [DOI] [PubMed] [Google Scholar]
- Wise LZ, Irvine DR. Auditory response properties of neurons in deep layers of cat superior colliculus. J Neurophysiol 49: 674–685, 1983 [DOI] [PubMed] [Google Scholar]
- Wyttenbach RA, Hoy RR. Demonstration of the precedence effect in an insect. J Acoust Soc Am 94: 777–784, 1993 [DOI] [PubMed] [Google Scholar]
- Yin TC. Physiological correlates of the precedence effect and summing localization in the inferior colliculus of the cat. J Neurosci 14: 5170–5186, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurek PM. The precedence effect and its possible role in the avoidance of interaural ambiguities. J Acoust Soc Am 67: 953–964, 1980 [DOI] [PubMed] [Google Scholar]