Abstract
Activity-dependent changes in excitatory synaptic transmission in the CNS have been shown to depend on the regulation of α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (AMPARs). In particular, several lines of evidence suggest that reversible phosphorylation of AMPAR subunit glutamate receptor 1 (GluR1, also referred to as GluA1 or GluR-A) plays a role in long-term potentiation (LTP) and long-term depression (LTD). We previously reported that regulation of serines (S) 831 and 845 on the GluR1 subunit may play a critical role in bidirectional synaptic plasticity in the Schaffer collateral inputs to CA1. Specifically, gene knockin mice lacking both S831 and S845 phosphorylation sites (“double phosphomutants”), where both serine residues were replaced by alanines (A), showed a faster decaying LTP and a deficit in LTD. To determine which of the two phosphorylation sites was responsible for the phenotype, we have now generated two lines of gene knockin mice: one that specifically lacks S831 (S831A mutants) and another that lacks only S845 (S845A mutants). We found that S831A mutants display normal LTP and LTD, whereas S845A mutants show a specific deficit in LTD. Taken together with our previous results from the “double phosphomutants,” our data suggest that either S831 or S845 alone may support LTP, whereas the S845 site is critical for LTD expression.
INTRODUCTION
Regulation of α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (AMPARs) plays a key role in altering excitatory synaptic transmission in the CNS. Interestingly, the regulatory mechanisms differ between distinct subunits of AMPAR, which range from glutamate receptor 1 (GluR1) to GluR4 (also referred to as GluA1–4 or GluR-A to -D). For example, subunits with a long intracellular carboxy terminus (i.e., GluR1, GluR2L, and GluR4) are involved in activity-dependent synaptic targeting of AMPAR, whereas those with a shorter carboxy terminus (i.e., GluR2, GluR3, and GluR4s) seem to maintain basal synaptic transmission (Kolleker et al. 2003; Lee SH et al. 2004; Shi et al. 2001; Zhu et al. 2000). However, the role of the GluR2 carboxy terminus in maintaining synaptic AMPAR is debated (Panicker et al. 2008). Among the four subunits, the role of GluR1 in synaptic plasticity paradigms, such as long-term potentiation (LTP) and long-term depression (LTD), has been extensively studied. GluR1 knockout mice lack LTP in the CA1 region of adult hippocampus (Jensen et al. 2003; Zamanillo et al. 1999), suggesting a critical role. The observation that GluR2/GluR3 double knockouts can express LTD (Meng et al. 2003) implies that GluR1 regulatory mechanisms may act independently to support LTD.
GluR1 has several identified phosphorylation sites on the intracellular carboxy terminus: serine (S) 818 (Boehm et al. 2006), S831 (Barria et al. 1997a; Mammen et al. 1997; Roche et al. 1996), threonine (T) 840 (Lee H-K et al. 2007), and S845 (Roche et al. 1996). Many of these sites have been demonstrated to play a role in synaptic AMPAR regulation and synaptic plasticity. The first to receive attention was S831, a site phosphorylated by protein kinase C (PKC) (Roche et al. 1996) and CaMKII (Barria et al. 1997a; Mammen et al. 1997), which increases its phosphorylation following LTP (Barria et al. 1997b; Lee H-K et al. 2000). Subsequently, activity-dependent synaptic trafficking of GluR1 was shown to depend on S845 (Esteban et al. 2003), a protein kinase A (PKA) site (Roche et al. 1996), and S818, a PKC site (Boehm et al. 2006). The requirement for S845 seems to be for targeting GluR1 to the plasma membrane and thus is suggested to “prime” AMPARs for synaptic trafficking (Derkach et al. 2007; Oh et al. 2006; Seol et al. 2007). On the other hand, S818 seems to act in conjunction with S831 and S845 to increase the GluR1 content at synapses (Boehm et al. 2006). Dephosphorylation of the S845 site on the GluR1 subunit has been correlated with LTD (Lee H-K et al. 1998, 2000) and down-regulation of cell surface GluR1 by a brief N-methyl-d-aspartate (NMDA) treatment (Holman et al. 2007; Lee H-K et al. 2003; Man et al. 2007).
We have previously shown that S831 and S845 are critical for LTP and LTD, using a gene knockin mouse that specifically lacks both of these phosphorylation sites on the GluR1 subunit (“double phosphomutants”) (Lee H-K et al. 2003). Specifically, we found that LTP in adults, but not in young, are reduced and decayed faster in the “double phosphomutants,” whereas LTD was absent in both young and adults. To further determine which of the two phosphorylation sites was responsible for the phenotype, we have now characterized two lines of knockin mice: one specifically lacking S831 (S831A mutants) and another lacking S845 (S845A mutants).
METHODS
Generation of GluR1–S831A and GluR1–S845A mutant mouse lines
Mutant mice carrying a single mutation at GluR1 serine 831 or serine 845 were generated as described previously (Lee H-K et al. 2003). Amino acid substitutions to alanine at each site were introduced by polymerase chain reaction mutagenesis in each targeting vector. Linearized targeting vectors were electroporated into R1 embryonic stem (ES) cells (Dr. A. Nagy, Mount Sinai Hospital, Toronto). Recombinant clones of correct homologous recombination, confirmed by Southern blot analysis, were injected into C57BL/6 blastocyst followed by chimera mice production at the Transgenic Facility of Johns Hopkins University School of Medicine. After germ line transmission, heterozygote mice were bred to CMV (cytomegalovirus promoter)-Cre mice to delete the neor cassette, using the Cre-loxP system (provided by Dr. A. Nagy), and the Cre gene was bred out in the next generation. Homozygous and wildtype (WT) mice were obtained by intercrossing of heterozygous mice. All procedures relating to animal care and treatment conformed to institutional and National Institutes of Health guidelines.
Quantitative immunoblots
Hippocampi dissected from WT, heterozygous, and homozygous mice were quickly frozen on dry ice. The samples were homogenized in ice-cold lysis buffer (in mM: 20 Na3PO4, 150 NaCl, 10 EDTA, 10 EGTA, 10 Na4P2O7, 50 NaF, and 1 Na3VO4, pH 7.4; 1 μM okadaic acid; 10 U/ml aprotinin) and crude membranes were prepared as previously described (Lee H-K et al. 2000). The primary antibodies were diluted in blocking buffer (1% bovine serum albumin and 0.1% Tween-20 in phosphate-buffered saline [PBS]), and alkaline phosphatase-linked second antibodies were used. The blots were developed using enhanced chemifluorescence substrate (ECF substrate; GE Healthcare Bio-Sciences, Waukesha, WI) and the resulting fluorescence signal was captured using VersaDoc3000 (Bio-Rad) and quantified using Quantity One software (Bio-Rad). For each gel, a control sample was loaded at four different concentrations to determine the linear range of signal for quantification. Only the samples that produced signal within the linear range were used for analysis. Signals from each sample were normalized to the average of WT samples run on the same blot and expressed as a percentage of average WT. The % average WT values were then averaged across blots to obtain the final mean data for all the samples. Statistics were run using the % of average WT values.
Histology and immunohistochemistry
The brain was perfused, fixed in 4% paraformaldehyde, and transferred to a 30% sucrose solution. Coronal sections were cut at 40-μm thickness and collected into cold 0.1 M PBS. Sections were stained with cresyl ciolet and observed under a light microscope or processed for immunohistochemistry using anti-GluR1 C-terminal Ab or anti-GluR2 C-terminal Ab.
Electrophysiological recording
Hippocampal slices (400 μm thick) were prepared from young (3-wk-old) and adult (≥3-mo-old) male mice as described previously (Lee H-K et al. 2003). All of the recordings were done “blind” to the genotype and mice were obtained from at least two different litters. In brief, hippocampi were dissected using oxygenated ice-cold dissection buffer (composition in mM: 212.7 sucrose; 2.6 KCl; 1.23 NaH2PO4; 26 NaHCO3; 10 dextrose; 3 MgCl2; and 1 CaCl2) and recovered at room temperature in artificial cerebrospinal fluid (ACSF, composition in mM: 124 NaCl; 5 KCl; 1.25 NaH2PO4; 26 NaHCO3; 10 dextrose; 1.5 MgCl2; and 2.5 CaCl2). All recordings were done in a submersion recording chamber perfused with ACSF (29–30°C, 2 ml/min) bubbled with 95% O2-5% CO2. Extracellular field potential responses were obtained by stimulating the Schaffer collaterals at about half-maximum intensity and recording from the dendritic field of CA1 using glass pipettes filled with ACSF. Synaptic responses were digitized and stored on-line using IGOR Pro software (WaveMetrics). LTP was induced using a theta burst stimulation [TBS: four trains, each consisting of ten 100-Hz bursts (four pulses) given at 5 Hz, repeated at 10-s intervals] (Larson et al. 1986) or a single-train TBS (1×TBS). LTD was elicited using either a 1-Hz protocol (1 Hz for 15 min) (Dudek and Bear 1992) or a paired-pulse 1-Hz protocol (PP-1-Hz: paired-pulses of 50-ms interstimulus interval [ISI] repeated at 1 Hz for 15 min) (Lee H-K et al. 2003). For measurement of paired-pulse facilitation (PPF), we used ISIs of 25, 50, 100, 200, 400, 800, and 1,000 ms.
Statistical analysis
The data are expressed as means ± SE. Unpaired Student's t-test was used for statistical analysis as specified in the text. A confidence interval of 95% was used to determine statistical significance. The P values, rounded to the closest values, are reported in the text.
RESULTS
Generation of the GluR1–S831A and the GluR1–S845A knockin mice
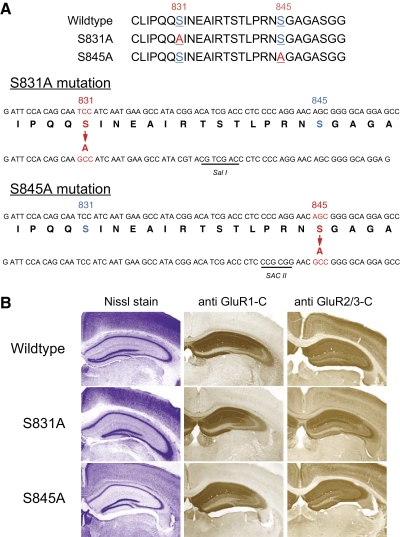
To determine the specific role of S831 and S845 on the GluR1 subunit, we generated two lines of gene knockin mice lacking either the S831 site (S831A mutants) or the S845 site (S845A mutants), using site-directed mutagenesis of the serine residue to an alanine (Fig. 1A). The resulting homozygous mice of S831A and S845A lines showed no gross abnormalities, including the cytoarchitecture of the hippocampus as visualized by Nissl stain (Fig. 1B), and the distribution of GluR1 and GluR2 in the hippocampal subfields, as shown using immunohistochemical labeling (Fig. 1B). To confirm the site-specific mutations, we performed immunoblot analysis on crude membrane fractions isolated from the hippocampus of WT and homozygotes of S831A and S845A mouse lines at two different ages (Fig. 2). In the S831A line, there was a specific loss of signal using the S831 phosphorylation site-specific antibody in the homozygotes, whereas S845 phosphorylation site-specific antibody and the GluR1 carboxy terminus recognizing antibody signals were still present (Fig. 2, A and B). Similarly, in the S845A line, only the signal from S845 phospho-antibody was missing in the homozygotes (Fig. 2, C and D). These results confirm the specific knockout of each phosphorylation site in the two knockin lines.
FIG. 1.
Generation of GluR1–S831A and GluR1–S845A mutant mice. A: schematics showing the specific mutations made to generate the GluR1–S831A targeting construct and the GluR1–S845A targeting constructs. Sal I or Sac II was included to each construct as depicted. B: normal gross cytoarchitecture of the hippocampus as revealed by Nissl staining (left column) and normal distribution of glutamate receptor 1 (GluR1, middle column) and GluR2/3 (right column) as shown using immunohistochemical labeling. Top rows: wildtype (WT) hippocampal sections. Middle rows: S831A homozygous hippocampal sections. Bottom rows: S845A homozygous hippocampal sections.
FIG. 2.
Quantitative analysis of phosphorylation of GluR1 on S831 and S845 in young and adult S831A and S845A mutants. A: no significant changes in the relative S845 phosphorylation or the total GluR1 levels in hippocampal samples obtained from young (3-wk-old) S831A mutants. Representative immunoblots are shown on the top panels. S831A mutant samples specifically lack signal when probed with S831 phosphospecific antibody (S831-p Ab). The relative S845 phosphorylation level (bottom left: S845 signal normalized to total GluR1 levels) and the total GluR1 level (bottom right) did not differ between WT and S831A. B: a significant reduction in the relative S845 phosphorylation (bottom left) and a significant increase in the total GluR1 level (bottom right) in hippocampal samples from adult (≥3-mo-old) S831A mutants. The observed decrease in the relative S845 phosphorylation level was largely due to the increase in the total GluR1 levels. *P < 0.001, t-test). Representative immunoblot images are shown in the top panel. S831A mutant samples specifically lack signal when probed with S831 phosphospecific antibody (S831-p Ab). C: no changes in the relative S831 phosphorylation (bottom left: S831 signal normalized to total GluR1 levels) and the total GluR1 levels (bottom right) in hippocampal samples of young (3-wk-old) S845A mutants. Representative immunoblot images are shown in the top panel. S845A mutant samples specifically lack signal when probed with S845 phosphospecific antibody (S845-p Ab). D: no significant changes in the relative S831 phosphorylation (bottom left) and GluR1 levels (bottom right) in hippocampal samples of adult (≥3-mo-old) S845A mutants. Representative immunoblot images are shown in the top panel. S845A mutant samples specifically lack signal when probed with S845 phosphospecific antibody (S845-p Ab).
Altered basal phosphorylation of S845 and GluR1 levels in the adult S831A mutants
The two phosphorylation sites are relatively close to one another; thus we sought to rule out the possibility that the absence of one site would affect the phosphorylation level of the other. To do this we performed quantitative immunoblot analysis of several mouse samples from the S831A WT and homozygous littermates and also from the S845A WT and homozygous littermates. We isolated crude membrane fractions from hippocampi of young (3-wk-old) and adult (≥3-mo-old) mice to compare the basal phosphorylation levels of the remaining phosphorylation site. In the young S831A line, we found that the S845 phosphorylation level normalized to the total GluR1 level was not significantly altered (WT: 100 ± 6.9% of average WT, n = 10 mice; S831A: 109 ± 11.2% of average WT, n = 9 mice; t-test: P = 0.49; Fig. 2A), neither was there a significant change in the total GluR1 level (WT: 100 ± 3.7% of average WT, n = 10 mice; S831A: 108 ± 14.0% of average WT, n = 9 mice; t-test: P = 0.60; Fig. 2A). However, the adult S831A mice showed a significant reduction in the relative fraction of GluR1 phosphorylated on the S845 site (WT: 100 ± 8.6% of average WT, n = 11 mice; S831A: 48 ± 8.7% of average WT, n = 10 mice; t-test: P = 0.0003; Fig. 2B), which was mainly due to a significant increase in the total GluR1 level (WT: 100 ± 4.2% of average WT, n = 11 mice; S831A: 133 ± 5.3% of average WT, n = 10; t-test: P = 0.0001; Fig. 2B). This indicates that there is some interaction between the two phosphorylation sites such that a chronic lack of S831 through adulthood increases the total level of GluR1 without a proportional increase in the S845 phosphorylation.
In the S845A line, on the other hand, the relative fraction of GluR1 phosphorylated on S831 was normal in both young (WT: 100 ± 20.0% of average WT, n = 5 mice; S845A: 104 ± 9.6% of average WT, n = 9 mice; t-test: P = 0.87; Fig. 2C) and adults (WT: 100 ± 12% of averaged WT, n = 8 mice; S845A: 78 ± 12.8% of average WT, n = 8 mice; t-test: P = 0.22; Fig. 2D). In addition, the total GluR1 level was normal in both young (WT: 100 ± 8.1% of average WT, n = 5 mice; S845A: 93 ± 4.1% of average WT, n = 9 mice; t-test: P = 0.49; Fig. 2C) and adults (WT: 100 ± 6.9% of average WT, n = 8 mice; S845A: 87 ± 11.0% of average WT, n = 8 mice; t-test: P = 0.32; Fig. 2D). These results suggest that lacking the S845 site does not influence the relative phosphorylation of the remaining S831 site or the total level of GluR1.
Normal basal synaptic transmission and plasticity in the young S831A mutants
To determine whether lacking the S831 phosphorylation site affects basal synaptic transmission in young (3-wk-old) mice, we generated input–output (I-O) curves by plotting the slope of each extracellular field potential against the amplitude of associated presynaptic fiber volley at various stimulation intensities. We found no difference in the I-O curve between S831A homozygotes and WT littermates (Fig. 3A), suggesting that basal synaptic transmission, which is mainly mediated by AMPARs, is normal in the absence of the S831 site. In addition, S831A homozygotes showed no differences in PPF ratio, a measure of presynaptic function (Fig. 3B), compared with that of their WT littermates.
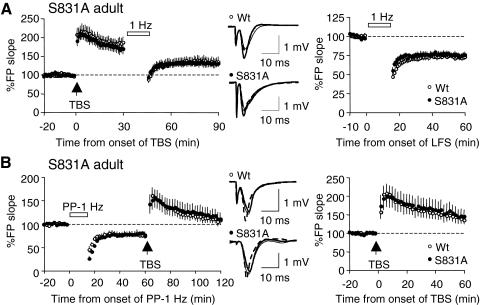
FIG. 3.
Characterization of basal synaptic transmission and plasticity in the Schaffer collateral synapses onto CA1 in the young (3-wk-old) S831A mutants. A: normal basal synaptic transmission in the young S831A mutants. Input–output (I-O) curves were generated by increasing stimulation intensities. Measured field potential (FP) slope was plotted against the amplitude of presynaptic fiber volley. There was no difference in the I-O curve of S831A mutants (closed symbols) and that of WT littermates (open symbols). B: normal paired-pulse facilitation (PPF) in the young S831A mutants. PPF ratios measured at different interstimulus intervals (ISIs) did not differ between S831A mutants (closed symbols) and WT littermates (open symbols). C: GluR1–S831A mutants exhibit normal long-term potentiation (LTP) induced by delivering 4 trains of theta burst stimulation (TBS). WTs: open symbols; S831A mutants: closed symbols. Representative traces taken right before (thin line) and 2 h after LTP induction (thick line) are shown to the right. D: the average magnitude of long-term depression (LTD) induced by a 15-min train of 1 Hz was slightly larger in the S831A mutants (closed symbols), but was not statistically significant from that of WT littermates (open symbols) at 1 h. S831A mutants displayed a significantly larger initial depression (average of the first 10 min post-1 Hz). Representative traces taken right before (thin line) and 1 h after the onset of 1-Hz stimulation (thick line) are shown to the right.
Next we examined LTP and LTD in the young S831A mutants. Similar to our previous results from the young “double phosphomutants” (Lee H-K et al. 2003), LTP induced by four trains of theta burst stimulation (TBS) was normal in the young S831A homozygotes (WT: 132 ± 6.0% of baseline at 2 h post-TBS, n = 8 from five mice; S831A: 131 ± 10.0% of baseline at 2 h post-TBS, n = 6 from four mice; t-test: P = 0.99; Fig. 3C). This is consistent with previous reports that the GluR1 subunit is not essential for LTP in juveniles (Jensen et al. 2003), although it is necessary for LTP in adults (Zamanillo et al. 1999). There was no statistically significant difference in the magnitude of LTD induced by a train of 1-Hz (15-min) stimulation in the S831A mutants, when measured 1 h after the onset of 1-Hz stimulation (WT: 86 ± 3.1% of baseline at 1 h postonset of 1 Hz, n = 12 from five mice; S831A: 77 ± 3.9% of baseline at 1 h postonset of 1 Hz, n = 18 from six mice; t-test: P = 0.07; Fig. 3D). However, we found a significantly larger initial synaptic depression in the S831A mutants when we compared the magnitude of synaptic depression immediately following the 1-Hz stimulation (when comparing the averages of the first 10 min post-1 Hz) (WT: 73 ± 3.0% of baseline at 10 min post-1 Hz, n = 12 from five mice; S831A: 63 ± 2.6% of baseline at 10 min post-1 Hz; n = 18 from six mice; Student's t-test: P = 0.02; Fig. 3D). These results suggest that the S831 site is not necessary for LTD expression, but the lack of this site can exaggerate the magnitude of the initial synaptic depression.
Normal basal synaptic transmission and plasticity in the adult S831A mutants
We previously found that in the GluR1 “double phosphomutants,” LTP was reduced only in the adults (≥3 mo old) (Lee H-K et al. 2003). Considering previous reports that S831 phosphorylation increases following LTP (Barria et al. 1997b; Lee H-K et al. 2000), we hypothesized that the LTP phenotype seen in the adult “double phosphomutants” was due to lacking the S831 site. Before testing the specific role of S831 in LTP, we first examined the properties of basal synaptic transmission in the adult S831A mutants compared with those of their WT littermates. We found that AMPAR function, as determined by I-O curve of field potential slopes (Fig. 4A), was normal in the adult S831A mutants. This suggests that the observed increase in total GluR1 in these mice (Fig. 2B) likely represents an increase in extrasynaptic and/or intracellular population. Presynaptic function, as measured by PPF ratio (Fig. 4B), was also normal in the adult S831A mutants. However, to our surprise, LTP induced by four trains of TBS was also normal in the adult S831A mutants (WT: 167 ± 10.3% of baseline at 2 h post-TBS, n = 10 from five mice; S831A: 168 ± 16.2% of baseline at 2 h post-TBS, n = 9 from six mice; t-test: P = 0.90; Fig. 4C). The LTP induced in the adult S831A mutants was dependent on N-methyl-d-aspartate receptors (NMDARs), as in WTs (Supplemental Fig. S1).1 Because the four-train TBS protocol used for inducing LTP is known to produce maximal LTP (Lee H-K et al. 1998), we decided to test whether a weaker induction protocol may detect abnormalities in the mutants. The magnitude of LTP induced in the adult S831A mutants by a single train of TBS (1×TBS) was also similar to WT levels (WT: 118 ± 4.6% of baseline at 2 h post1×TBS, n = 9 from four mice; S831A: 114 ± 5.9% of baseline at 2 h post-1×TBS, n = 9 from four mice; t-test: P = 0.92; Fig. 4D). These data suggest that the S831 phosphorylation site is not critical for LTP expression.
FIG. 4.
Basal synaptic transmission and plasticity in the Schaffer collateral synapses onto CA1 in the adult (≥3-mo-old) S831A mutants. A: normal basal synaptic transmission in the adult S831A mutants. I-O curves were generated by plotting the FP slope against the amplitude of presynaptic fiber volley. WTs: open symbols; S831A mutants: closed symbols. B: PPF ratios measured at different ISIs did not differ between S831A mutants (closed symbols) and WT littermates (open symbols). C: adult GluR1–S831A mutants exhibited normal LTP induced by delivering 4 trains of TBS. WTs: open symbols; S831A mutants: closed symbols. Representative traces taken right before (thin line) and 2 h after LTP induction (thick line) are shown to the right. D: adult GluR1–S831A mutants display normal LTP induced by a one-train TBS (1×TBS) protocol. WTs: open symbols; S831A mutants: closed symbols. Superimposed representative traces taken right before (thin line) and 2 h after LTP (thick line) are shown to the right. E: the magnitude of LTD induced by a 15-min train of paired-pulse 1 Hz (PP-1-Hz, ISI = 50 ms) was not significantly different at 1 h between adult S831A mutants (closed symbols) and WT littermates (open symbols). S831A mutants displayed a significantly larger initial depression (average of the first 10 min post PP-1-Hz). Representative traces taken right before (thin line) and 1 h after the onset of PP-1-Hz stimulation (thick line) are shown to the right.
To examine LTD, we used a paired-pulse 1-Hz (PP-1-Hz, 15 min) protocol, which we previously characterized as being effective at inducing NMDAR-dependent LTD in adult mice (Lee H-K et al. 2003). This differs from rats, where PP-1-Hz stimulation recruits both NMDAR- and mGluR-dependent LTD in adults (Lee H-K et al. 2005). The reason for using PP-1 Hz is because the conventional 1-Hz protocol, which is effective in young mice (<4 wk old), fails to induce LTD in older mice (unpublished observation). Similar to the results from young S831A, there was a significantly larger initial synaptic depression in adult S831A mutants compared with that of the WT littermates (WT: 58 ± 2.6% of baseline at 10 min post-PP-1 Hz, n = 16 from six mice; S831A: 48 ± 2.2% of baseline at 10 min post-PP-1 Hz, n = 20 from nine mice; t-test: P = 0.006; Fig. 4E) without significant differences in the magnitude of LTD (WT: 78 ± 3.3% of baseline at 1 h postonset of PP-1 Hz, n = 16 from six mice; S831A: 76 ± 3.6% of baseline at 1 h postonset of PP-1 Hz, n = 20 from nine mice; t-test: P = 0.58; Fig. 4E). Collectively, these results suggest that S831 phosphorylation is not necessary for LTP or LTD in the adults, but the lack of this site enhances the initial synaptic depression following the LTD-inducing stimulus train.
Since both LTP and LTD were normal in the S831A mutants, we next determined whether the reversal of LTP (depotentiation [DeP]) and/or LTD (dedepression [DeD]) is affected by the mutation. DeP was induced by delivery of a 1-Hz (15-min) stimulation train 30 min after LTP induction by a TBS. We found no significant difference in the magnitude of depotentiation between WT and S831A mutants (WT: 73 ± 3.3% of renormalized baseline, n = 9 from five mice; S831A: 75 ± 2.7% of renormalized baseline, n = 8 from four mice; t-test: P = 0.56; Fig. 5A). In addition, there was no statistically significant difference in the initial depression between WT and S831A, when we compared the average magnitude of field potential slopes during the first 10 min immediately following the 1-Hz stimulation (WT: 65 ± 3.2% of renormalized baseline, n = 9 from five mice; S831A: 69 ± 2.6% of renormalized baseline, n = 8 from four mice; t-test: P = 0.34; Fig. 5A).
FIG. 5.
Normal depotentiation and dedepression in the adult S831A mutants. A: depotentiation was induced by delivering a train of 1 Hz (15 min) at 30 min following LTP induction by 4 trains of TBS. Left: the entire experiment normalized to the pre-LTP baseline. Middle: superimposed representative traces taken right before LTP induction (thin line), 30 min after LTP (thick solid line), and 1 h after the onset of 1-Hz stimulation (thick dashed line). Right: the magnitude of depotentiation when renormalized to the pre-1-Hz baseline. B: dedepression was induced 1 h following the onset of PP-1-Hz stimulation by delivering 4 trains of TBS. Left: the entire experiment normalized to the pre-LTD baseline. Middle: superimposed representative traces taken right before LTD induction (thin line), 1 h after LTD (thick solid line), and 1 h after TBS (thick dashed line). Right: the magnitude of depotentiation when renormalized to the pre-TBS baseline.
Dedepression (DeD) was induced by delivering four trains of TBS following LTD. There was no significant difference in the average magnitude of DeD between the S831A mutants and the WTs (WT: 142 ± 11.9% of renormalized baseline, n = 7 from three mice; S831A: 148 ± 19.7% of renormalized baseline, n = 8 from three mice; t-test: P = 0.78; Fig. 5B). These results suggest that both DeP and DeD can occur in the absence of the S831 phosphorylation site.
Young S845A mutants display a specific deficit in LTD
Next we examined the role of S845 phosphorylation in basal synaptic transmission and plasticity by using the S845A mutants. We found that in young (3-wk-old) S845A mutants, basal AMPAR-mediated synaptic transmission (Fig. 6A) and presynaptic function, as measured by PPF ratio, were both indistinguishable from those of the WT littermates (Fig. 6B). Furthermore, the average magnitude of LTP induced by four trains of TBS in young S845A mutants was not different from that of WT littermates (WT: 153 ± 11.1% of baseline at 2 h post-TBS, n = 8 from five mice; S845A: 144 ± 7.2% of baseline at 2 h post-TBS, n = 7 from five mice; t-test: P = 0.53; Fig. 6C). This result together with the intact LTP observed in the young S831A mutants (Fig. 3C) is in line with the previous observations from the GluR1 knockout (Jensen et al. 2003) and the “double phosphomutant” (Lee H-K et al. 2003) that LTP at this age does not require GluR1 or phosphorylation of S831 and S845.
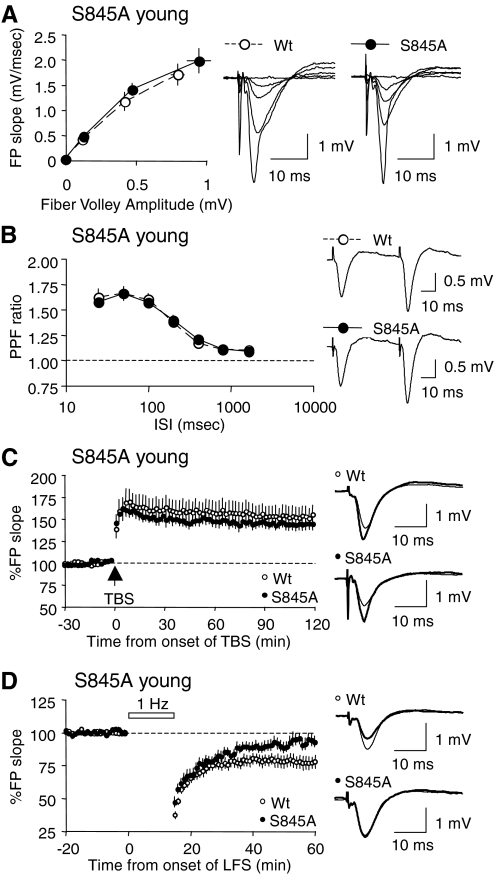
FIG. 6.
Young (3-wk-old) S845A mutants display a specific deficit in LTD. A: I-O curves generated by plotting FP slope against the amplitude of presynaptic fiber volley were not different between S845A mutants (closed symbols) and WT littermates (open symbols). B: PPF ratios measured at various ISIs did not differ between S845A mutants (closed symbols) and WT littermates (open symbols). C: LTP induced by delivering 4 trains of TBS was normal in the young S845A mutants. WTs: open symbols; S845A mutants: closed symbols. Representative traces taken right before (thin line) and 2 h after LTP induction (thick line) are shown to the right. D: LTD induced by a 15-min train of 1 Hz was virtually abolished in the young S845A mutants (closed symbols), but was normal in the WT littermates (open symbols). Representative traces taken right before (thin line) and 1 h after the onset of 1-Hz stimulation (thick line) are shown to the right.
To compare LTD in young S845A mutants and WT littermates, we delivered a train of 1-Hz (15-min) stimulation. We found that the LTD induced in the young S845A homozygotes returned to near baseline levels after 1 h, unlike the WTs that displayed stable LTD (WT: 78 ± 4.8% of baseline at 1 h postonset of 1 Hz, n = 16 from six mice; S845A: 93 ± 4.1% of baseline at 1 h postonset of 1 Hz, n = 15 from six mice; t-test: P = 0.024; Fig. 6D). Our result suggests that the S845 phosphorylation site is critical for LTD expression. Taken together with the normal LTD observed in the S831A mutants (Fig. 3D), this result suggests that the absence of LTD reported in the “double phosphomutants” (Lee H-K et al. 2003) is due to the absence of the S845 site.
Adult S845A mutants also display a specific deficit in LTD
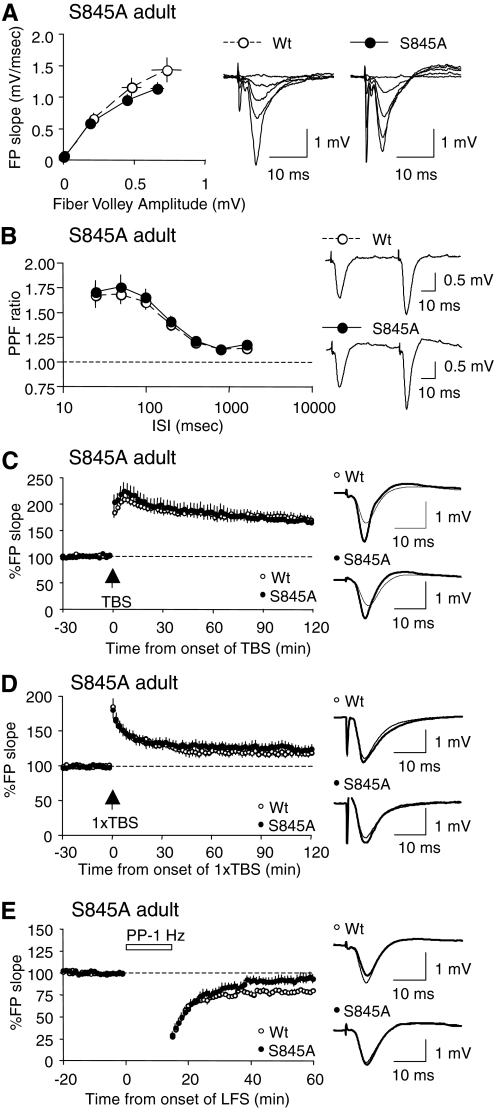
Next we examined synaptic transmission and plasticity of the adults (≥3 mo old) of S845A line. We found no abnormalities in the AMPAR-mediated basal synaptic transmission (Fig. 7A) or the presynaptic function, as monitored by measuring PPF ratios (Fig. 7B). Since we did not observe a deficit in LTP in the adult S831A mutants (Fig. 4C), whereas adult mice lacking both S831 and S845 sites (“double phosphomutants”) exhibit unstable LTP (Lee H-K et al. 2003), we expected that the S845 phosphorylation site might be necessary for stabilizing LTP in the adults. However, unlike our initial expectation we found that the magnitude of LTP induced by four trains of TBS (WT: 170 ± 7.2% of baseline at 2 h post-TBS, n = 11 from five mice; S845A: 167 ± 5.1% of baseline at 2 h post-TBS, n = 10 from five mice; t-test: P = 0.77; Fig. 7C), as well as that induced by one-train TBS (1×TBS, WT: 120 ± 4.0% of baseline at 2 h post-1×TBS, n = 9 from four mice; S845A: 125 ± 6.7% of baseline at 2 h post-1×TBS, n = 8 from four mice; t-test: P = 0.54; Fig. 7D), were indistinguishable between the S845A mutants and their WT littermates. The LTP induced in S845A mutants was dependent on NMDARs (Supplemental Fig. S1). Our results clearly suggest that NMDAR-dependent LTP can be induced in the absence of the S845 phosphorylation site on the GluR1 subunit of AMPARs. On the other hand, we found that the magnitude of LTD induced by a train of PP-1 Hz (15 min, ISI = 50 ms) was almost back to baseline levels by 1 h in the adult S845A mutants (WT: 79 ± 2.2% of baseline at 1 h postonset of PP-1 Hz, n = 11 from four mice; S845A: 92 ± 4.9% of baseline at 1 h postonset of PP-1 Hz, n = 15 from five mice; t-test: P = 0.02; Fig. 7E). This result suggests that the S845 site is necessary for LTD in the adults, similar to its requirement in the young.
FIG. 7.
Adult (≥3-mo-old) S845A mutants also display a specific deficit in LTD. A: I-O curves generated by plotting FP slope against the amplitude of presynaptic fiber volley did not differ between S845A mutants (closed symbols) and WT littermates (open symbols). B: PPF ratios measured at various ISIs were not different between S845A mutants (closed symbols) and WT littermates (open symbols). C: LTP induced by delivering 4 trains of TBS was normal in the adult S845A mutants. WTs: open symbols; S845A mutants: closed symbols. Representative traces taken right before (thin line) and 2 h after LTP induction (thick line) are shown to the right. D: one train TBS (1×TBS) induced LTP was also normal in the adult S845A mutants. WTs: open symbols; S845A mutants: closed symbols. Superimposed representative traces taken before (thin line) and 2 h after LTD induction (thick line) are shown to the right. E: a 15-min train of PP-1 Hz (ISI = 50 ms) failed to induce LTD in hippocampal slices from the adult S845A mutants (closed symbols), while producing normal LTD in the WT littermates (open symbols). Representative traces taken right before (thin line) and 1 h after the onset of PP-1-Hz stimulation (thick line) are shown to the right.
Taking advantage of the fact that LTP was normal in the adult S845A mutants, we examined whether the reversal of LTP (DeP) is affected by the mutation. We found that the magnitude of DeP induced by delivering a train of 1-Hz (15-min) stimuli at 30 min following LTP induction was similar in the adult S845A compared with that in WTs (WT: 72 ± 3.3% of renormalized baseline at 1 h postonset of 1 Hz, n = 10 from three mice; S845A: 66 ± 5.2% of renormalized baseline at 1 h postonset of 1 Hz, n = 11 from three mice; t-test: P = 0.31; Fig. 8). This suggests that DeP can be induced in the absence of the S845 site.
FIG. 8.
Normal depotentiation in the adult S845A mutants. Depotentiation induced by delivering a train of 1 Hz (15 min) at 30 min following LTP induction was normal in the S845A mutants (closed symbols) compared with WT littermates (open symbols). Left: the entire experiment normalized to the pre-LTP baseline. Middle: superimposed representative traces taken right before LTP induction (thin line), 30 min after LTP (thick solid line), and 1 h after the onset of 1-Hz stimulation (thick dashed line). Right: the magnitude of depotentiation when renormalized to the pre-1-Hz baseline.
DISCUSSION
Here we report that the two well-characterized phosphorylation sites on the GluR1 subunit—S831 and S845—not only may have distinct roles, but also may substitute for each other, in mediating synaptic plasticity in the Schaffer collateral synapses onto CA1. We found that basal synaptic transmission is quite normal in both S831A and S845A mutants. In S831A mutants all forms of synaptic plasticity tested (LTP, LTD, DeP, and DeD) were normal, whereas S845A mutants showed a specific deficit in LTD.
The normal NMDAR-dependent LTP observed in the adult S831A mutants was quite unexpected, considering several studies implicating phosphorylation of this site in LTP. The role of GluR1–S831 in LTP was first suggested by the observation that LTP induction increases the phosphorylation of this site (Barria et al. 1997b; Lee H-K et al. 2000). Phosphorylation of S831 has been shown to increase the single-channel conductance of AMPARs (Derkach et al. 1999) and was thus thought to mediate the observed increase in single-channel conductance following LTP induction (Benke et al. 1998). However, the increase in single-channel conductance by S831 is restricted to homomeric GluR1 and is not seen when GluR1 is in a heteromeric complex with GluR2 (Oh and Derkach 2005). Considering that the majority of AMPARs in the hippocampus are GluR1/GluR2 heteromers (He et al. 2009; Lu et al. 2009; Wenthold et al. 1996), this suggests that the role of GluR1–S831 phosphorylation on channel conductance may be limited. A recent study suggested that GluR1 homomers appear at synapses immediately after LTP induction and are required for stabilizing LTP by promoting exchange with receptors containing GluR2 (Plant et al. 2006). This prompted the idea that phosphorylation of S831 on these new synaptic GluR1 homomers may aid in stabilizing LTP (Lee and Huganir 2008). However, there is controversy as to whether GluR1 homomers are present shortly after LTP induction and whether their activation contributes to LTP (Adesnik and Nicoll 2007). In addition, our data showing normal LTP in the GluR1–S831A mutants suggest that S831 is not necessary for LTP. This is in contrast to our previous reports showing a reduction in LTP in the GluR1 “double phosphomutants” (S831A, S845A) (Lee H-K et al. 2003) and the “penta phosphomutants” (S831A, T838A, S839A, T840A, S845A) (Lee H-K et al. 2007). We initially thought that perhaps the remaining S845 site is necessary for LTP; however, our data from the S845A mutants indicate that this is not the case. Considering that LTP is reduced when lacking both sites, but normal when lacking one or the other, we suggest that either S831 or S845 alone can support LTP, but lacking both compromises the stability of LTP. This implies that these two sites may substitute each other for supporting LTP. Taking into account recent data showing a critical role of another GluR1 phosphorylation site, S818, in LTP (Boehm et al. 2006), we surmise that S831 or S845 may act together with S818 to support LTP. However, S818 mutation to an aspartate (D) to mimic phosphorylation is able to place homomeric mutated GluR1 to synapses (as measured by changes in rectification of current), but it does not change the number of receptors at synapses (as measured by AMPAR current amplitude) (Boehm et al. 2006). It is only when S831 and S845 are also mutated to D does the AMPAR synaptic current increase (Boehm et al. 2006), suggesting that there is an interplay between different phosphorylation sites to mediate the increase in synaptic AMPARs following LTP. Interestingly, similar to our results on LTP, S831A, or S845A single mutants are able to support incentive learning, but “double mutants” lacking both sites were unable to do so (Crombag et al. 2008a).
In contrast to LTP, which can be supported by either S831 or S845, LTD seems to be critically dependent on the GluR1–S845 site, independent of the age of the animal. This is consistent with our previous results showing dephosphorylation of S845 following LTD (Lee H-K et al. 1998, 2000). In addition, our finding is in line with several studies showing a critical role of PKA and PKA anchoring protein in LTD. For example, a recent study showed that knockout mice lacking the PKA-RIIbeta subunit specifically lack LTD after the second postnatal week (Yang et al. 2009); moreover, several studies highlighted that PKA anchoring protein AKAP79/150 plays a critical role in regulating GluR1–S845 phosphorylation (Colledge et al. 2000; Tavalin et al. 2002) and LTD expression (Lu et al. 2008; Smith et al. 2006; Tavalin et al. 2002). Although our data from the S845A mutants are the same as those observed in the GluR1 “double phosphomutants” (Lee H-K et al. 2003), it is slightly different from the “penta phosphomutants,” which showed LTD deficits only in the adults (Lee H-K et al. 2007). Unlike the “double” or the “single” phosphomutants, the “penta” phosphomutants carry additional three mutations at amino acid residues, including a novel phosphorylation site, T840 (Delgado et al. 2007; Lee H-K et al. 2007). The lack of T840 apparently rescued the LTD phenotype in juveniles lacking both S831 and S845. We had interpreted this result to suggest that there may be an alternative LTD signaling pathway present in the juveniles that gets recruited in the absence of S831, S845, and T840 (Lee H-K et al. 2007). However, the absence of LTD in the juveniles of both S845A mutants (Fig. 6D) and the “double phosphomutants” (Lee H-K et al. 2003) suggests that the presence of the T840 site may prevent the recruitment of this alternative mechanism. We recently reported that the S845 site is involved in stabilizing perisynaptic GluR1 homomers and that LTD is associated with the removal of these receptors (He et al. 2009). Therefore the lack of LTD in S845A mutants may be due to the specific absence of perisynaptic GluR1 homomers.
Interestingly, S831A mutants showed a significantly larger initial depression but normal LTD at both ages (Figs. 3D and 4E). This cannot be attributed to alterations in the basal levels of GluR1–S845 phosphorylation or total GluR1 levels in these mice, which differed depending on the age (Fig. 2B). Neither can changes in presynaptic function explain this because we failed to see changes in the PPF ratio at any of the ages (Figs. 3B and 4B). At this point, we cannot rule out whether there is a deficit in activity-dependent regulation of S845 in the S831A mutants. In any case, this was quite specific to LTD, since we did not observe an increase in the initial depression following depotentiation (Fig. 5A). The larger initial depression without effects on the magnitude of LTD has also been reported in adult synGAP (synaptic GTPase activating protein) heterozygotes (Kim et al. 2003). These results imply that LTD and the initial synaptic depression may have distinct underlying mechanisms.
Many of the published works on LTD indicate that GluR2-dependent mechanisms are involved (Carroll et al. 2001). These include GluR2 phosphorylation on the S880 site (Kim et al. 2001; Seidenman et al. 2003), which then switches the interaction of GluR2 carboxy-tail from GRIP/ABP to PICK-1 (Chung et al. 2000; Matsuda et al. 1999). Interaction of GluR2 with GRIP/ABP and/or PICK-1 (Daw et al. 2000; Kim et al. 2001; Seidenman et al. 2003), as well as N-ethylmaleimide-sensitive factor (NSF) (Luthi et al. 1999) and/or AP2, the clathrin adaptor protein (Lee SH et al. 2002), have all been shown to be involved in LTD. Although GluR2-dependent mechanisms have been demonstrated to be critical for LTD in the cerebellum (Chung et al. 2003; Matsuda et al. 2000; Steinberg et al. 2004, 2006; Xia et al. 2000), both GluR2 knockouts and GluR2/3 double knockouts display normal LTD in the hippocampal CA1 region (Meng et al. 2003). Furthermore, activity-dependent endocytosis of AMPARs, which is thought to accompany LTD, is also reported to be independent of GluR2/3 (Biou et al. 2008). These findings collectively suggest that LTD in the CA1 can be supported by GluR2-independent mechanisms, one of which may involve GluR1–S845.
We previously reported a correlation between depotentiation and dephosphorylation of GluR1–S831 (Lee H-K et al. 2000). Therefore the normal depotentiation seen in the S831A mutants was unexpected. Although our data show that the S831 site is not necessary for LTP and depotentiation, they do not rule out the involvement of S831 in these processes. Our data showing a normal depotentiation in the S845A mutants, which lack LTD, provide additional support that these two forms of synaptic depression are likely mediated by distinct mechanisms. There are several indications that LTD and depotentiation are two distinct forms of synaptic depression. Whereas LTD is blocked by calcineurin inhibitors (Mulkey et al. 1994), depotentiation is not (Huang et al. 2001; Lu et al. 1996). Furthermore, LTD, but not depotentiation, is specifically absent in the forebrain-specific calcineurin knockout mouse (Zeng et al. 2001). Our results add that S845 is necessary for LTD, but not for depotentiation.
Collectively, our data suggest that S831 and S845 phosphorylation sites on the GluR1 subunit may have specific functions in mediating synaptic plasticity in the Schaffer collateral synapses onto CA1 neurons. In addition, our work together with studies on other GluR1 phosphorylation sites underscore that several phosphorylation sites may compensate for or cooperate with each other in mediating synaptic plasticity. Recent studies suggest that regulation of GluR1 S831 and S845 phosphorylation is not only involved in LTP and LTD in the hippocampus, but is also important for enhancement of emotional learning (Hu et al. 2007) and various types of activity-dependent synaptic plasticity in the cortex (Goel et al. 2006; Heynen et al. 2003; Seol et al. 2007). Interestingly, the use of S831A and S845A mutants to study functions mediated by other forebrain structures have yielded slightly different results from what we have found in the hippocampus. For example, in the visual cortex, S845A mutants lack both spike-timing–dependent LTP and LTD, whereas S831A lack only spike-timing–dependent LTD (Seol et al. 2007). On the other hand, S831A, but not S845A, mutants display deficits in learning Pavlovian cue-based conditioned reinforcement, which is dependent on amygdala circuitry (Crombag et al. 2008b). Therefore the use of the single phosphomutants will allow for a better understanding of the critical function each individual phosphorylation site plays in mediating various brain functions and may also tease out compensatory mechanisms involved in synaptic plasticity.
GRANTS
This work was supported by a Sloan Foundation grant to H.-K. Lee, National Institutes of Health Grants R01-EY-014882 to H.-K. Lee and R01-NS-036715 to R. L. Huganir, and a Howard Hughes Medical Institute grant to R. L. Huganir.
Supplementary Material
ACKNOWLEDGMENTS
We thank A. MacArthur and J. Silva for providing assistance on some of the data collection.
Present address of K. Takamiya: Department of Integrative Physiology, University of Miyazaki Faculty of Medicine, Miyazaki 889-1692, Japan.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Adesnik H, Nicoll RA. Conservation of glutamate receptor 2-containing AMPA receptors during long-term potentiation. J Neurosci 27: 4598–4602, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Derkach V, Soderling T. Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate type gluatamate receptor. J Biol Chem 272: 32727–32730, 1997a [DOI] [PubMed] [Google Scholar]
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science 276: 2042–2045, 1997b [DOI] [PubMed] [Google Scholar]
- Benke TA, Luthi A, Isaac JT, Collingridge GL. Modulation of AMPA receptor unitary conductance by synaptic activity. Nature 393: 793–797, 1998 [DOI] [PubMed] [Google Scholar]
- Biou V, Bhattacharyya S, Malenka RC. Endocytosis and recycling of AMPA receptors lacking GluR2/3. Proc Natl Acad Sci USA 105: 1038–1043, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron 51: 213–225, 2006 [DOI] [PubMed] [Google Scholar]
- Carroll RC, Beattie EC, von Zastrow M, Malenka RC. Role of AMPA receptor endocytosis in synaptic plasticity. Nat Rev Neurosci 2: 315–324, 2001 [DOI] [PubMed] [Google Scholar]
- Chung HJ, Steinberg JP, Huganir RL, Linden DJ. Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long-term depression. Science 300: 1751–1755, 2003 [DOI] [PubMed] [Google Scholar]
- Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci 20: 7258–7267, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colledge M, Dean RA, Scott GK, Langeberg LK, Huganir RL, Scott JD. Targeting of PKA to glutamate receptors through a MAGUK-AKAP complex. Neuron 27: 107–119, 2000 [DOI] [PubMed] [Google Scholar]
- Crombag HS, Sutton JM, Takamiya K, Holland PC, Gallagher M, Huganir RL. A role for alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid GluR1 phosphorylation in the modulatory effects of appetitive reward cues on goal-directed behavior. Eur J Neurosci 27: 3284–3291, 2008a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Sutton JM, Takamiya K, Lee H-K, Holland PC, Gallagher M, Huganir RL. A necessary role for GluR1 serine 831 phosphorylation in appetitive incentive learning. Behav Brain Res 191: 178–183, 2008b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw MI, Chittajallu R, Bortolotto ZA, Dev KK, Duprat F, Henley JM, Collingridge GL, Isaac JT. PDZ proteins interacting with C-terminal GluR2/3 are involved in a PKC-dependent regulation of AMPA receptors at hippocampal synapses. Neuron 28: 873–886, 2000 [DOI] [PubMed] [Google Scholar]
- Delgado JY, Coba M, Anderson CN, Thompson KR, Gray EE, Heusner CL, Martin KC, Grant SG, O'Dell TJ. NMDA receptor activation dephosphorylates AMPA receptor glutamate receptor 1 subunits at threonine 840. J Neurosci 27: 13210–13221, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach V, Barria A, Soderling TR. Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc Natl Acad Sci USA 96: 3269–3274, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci 8: 101–113, 2007 [DOI] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and the effects of NMDA receptor blockade. Proc Natl Acad Sci USA 89: 4363–4367, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci 6: 136–143, 2003 [DOI] [PubMed] [Google Scholar]
- Goel A, Jiang B, Xu LW, Song L, Kirkwood A, Lee H-K. Cross-modal regulation of synaptic AMPA receptors in primary sensory cortices by visual experience. Nat Neurosci 9: 1001–1003, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K, Song L, Cummings LW, Goldman J, Huganir RL, Lee H-K. Stabilization of Ca2+-permeable AMPA receptors at perisynaptic sites by GluR1-S845 phosphorylation. Proc Natl Acad Sci USA ( November 5, 2009). doi: 10.1073/pnas.0910338106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heynen AJ, Yoon BJ, Liu CH, Chung HJ, Huganir RL, Bear MF. Molecular mechanism for loss of visual cortical responsiveness following brief monocular deprivation. Nat Neurosci 6: 854–862, 2003 [DOI] [PubMed] [Google Scholar]
- Holman D, Feligioni M, Henley JM. Differential redistribution of native AMPA receptor complexes following LTD induction in acute hippocampal slices. Neuropharmacology 52: 92–99, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Real E, Takamiya K, Kang MG, Ledoux J, Huganir RL, Malinow R. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell 131: 160–173, 2007 [DOI] [PubMed] [Google Scholar]
- Huang CC, Liang YC, Hsu KS. Characterization of the mechanism underlying the reversal of long term potentiation by low frequency stimulation at hippocampal CA1 synapses. J Biol Chem 276: 48108–48117, 2001 [DOI] [PubMed] [Google Scholar]
- Jensen V, Kaiser KM, Borchardt T, Adelmann G, Rozov A, Burnashev N, Brix C, Frotscher M, Andersen P, Hvalby O, Sakmann B, Seeburg PH, Sprengel R. A juvenile form of postsynaptic hippocampal long-term potentiation in mice deficient for the AMPA receptor subunit GluR-A. J Physiol 553: 843–856, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Chung HJ, Lee H-K, Huganir RL. Interaction of the AMPA receptor subunit GluR2/3 with PDZ domains regulates hippocampal long-term depression. Proc Natl Acad Sci USA 98: 11725–11730, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lee H-K, Takamiya K, Huganir RL. The role of synaptic GTPase-activating protein in neuronal development and synaptic plasticity. J Neurosci 23: 1119–1124, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolleker A, Zhu JJ, Schupp BJ, Qin Y, Mack V, Borchardt T, Kohr G, Malinow R, Seeburg PH, Osten P. Glutamatergic plasticity by synaptic delivery of GluR-B(long)-containing AMPA receptors. Neuron 40: 1199–1212, 2003 [DOI] [PubMed] [Google Scholar]
- Larson J, Wong D, Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res 368: 347–350, 1986 [DOI] [PubMed] [Google Scholar]
- Lee H-K, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature 405: 955–959, 2000 [DOI] [PubMed] [Google Scholar]
- Lee H-K, Huganir RL. AMPA receptor regulation and the reversal of synaptic plasticity: LTP, LTD, depotentiation, and dedepression. In: Learning and Memory: A Comprehensive Reference, edited by Byrne JH. (volume editor: Sweatt JD.). San Diego, CA: Elsevier/Academic Press, 2008, vol. 4, p. 633–648 [Google Scholar]
- Lee H-K, Kameyama K, Huganir RL, Bear MF. NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus. Neuron 21: 1151–1162, 1998 [DOI] [PubMed] [Google Scholar]
- Lee H-K, Min SS, Gallagher M, Kirkwood A. NMDA receptor-independent long-term depression correlates with successful aging in rats. Nat Neurosci 8: 1657–1659, 2005 [DOI] [PubMed] [Google Scholar]
- Lee H-K, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, Ding L, He C, Petralia RS, Wenthold RJ, Gallagher M, Huganir RL. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell 112: 631–643, 2003 [DOI] [PubMed] [Google Scholar]
- Lee H-K, Takamiya K, Kameyama K, He K, Yu S, Rossetti L, Wilen D, Huganir RL. Identification and characterization of a novel phosphorylation site on the GluR1 subunit of AMPA receptors. Mol Cell Neurosci 36: 86–94, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Liu L, Wang YT, Sheng M. Clathrin adaptor AP2 and NSF interact with overlapping sites of GluR2 and play distinct roles in AMPA receptor trafficking and hippocampal LTD. Neuron 36: 661–674, 2002 [DOI] [PubMed] [Google Scholar]
- Lee SH, Simonetta A, Sheng M. Subunit rules governing the sorting of internalized AMPA receptors in hippocampal neurons. Neuron 43: 221–236, 2004 [DOI] [PubMed] [Google Scholar]
- Lu W, Shi Y, Jackson AC, Bjorgan K, During MJ, Sprengel R, Seeburg PH, Nicoll RA. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron 62: 254–268, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Zhang M, Lim IA, Hall DD, Allen M, Medvedeva Y, McKnight GS, Usachev YM, Hell JW. AKAP150-anchored PKA activity is important for LTD during its induction phase. J Physiol 586: 4155–4164, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YF, Hayashi Y, Moriwaki A, Tomizawa K, Matsui H. FK506, a Ca2+/calmodulin-dependent phosphatase inhibitor, inhibits the induction of long-term potentiation in the rat hippocampus. Neurosci Lett 205: 103–106, 1996 [DOI] [PubMed] [Google Scholar]
- Luthi A, Chittajallu R, Duprat F, Palmer MJ, Benke TA, Kidd FL, Henley JM, Isaac JT, Collingridge GL. Hippocampal LTD expression involves a pool of AMPARs regulated by the NSF-GluR2 interaction. Neuron 24: 389–399, 1999 [DOI] [PubMed] [Google Scholar]
- Mammen AL, Kameyame K, Roche KW, Huganir RL. Phosphorylation of the α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J Biol Chem 272: 32528–32533, 1997 [DOI] [PubMed] [Google Scholar]
- Man HY, Sekine-Aizawa Y, Huganir RL. Regulation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc Natl Acad Sci USA 104: 3579–3584, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S, Launey T, Mikawa S, Hirai H. Disruption of AMPA receptor GluR2 clusters following long-term depression induction in cerebellar Purkinje neurons. EMBO J 19: 2765–2774, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S, Mikawa S, Hirai H. Phosphorylation of serine-880 in GluR2 by protein kinase C prevents its C terminus from binding with glutamate receptor-interacting protein. J Neurochem 73: 1765–1768, 1999 [DOI] [PubMed] [Google Scholar]
- Meng Y, Zhang Y, Jia Z. Synaptic transmission and plasticity in the absence of AMPA glutamate receptor GluR2 and GluR3. Neuron 39: 163–176, 2003 [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature 369: 486–488, 1994 [DOI] [PubMed] [Google Scholar]
- Oh MC, Derkach VA. Dominant role of the GluR2 subunit in regulation of AMPA receptors by CaMKII. Nat Neurosci 8: 853–854, 2005 [DOI] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem 281: 752–758, 2006 [DOI] [PubMed] [Google Scholar]
- Panicker S, Brown K, Nicoll RA. Synaptic AMPA receptor subunit trafficking is independent of the C terminus in the GluR2-lacking mouse. Proc Natl Acad Sci USA 105: 1032–1037, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, McBain CJ, Collingridge GL, Isaac JT. Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat Neurosci 9: 602–604, 2006 [DOI] [PubMed] [Google Scholar]
- Roche KW, O'Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron 16: 1179–1188, 1996 [DOI] [PubMed] [Google Scholar]
- Seidenman KJ, Steinberg JP, Huganir R, Malinow R. Glutamate receptor subunit 2 serine 880 phosphorylation modulates synaptic transmission and mediates plasticity in CA1 pyramidal cells. J Neurosci 23: 9220–9228, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seol GH, Ziburkus J, Huang S, Song L, Kim IT, Takamiya K, Huganir RL, Lee H-K, Kirkwood A. Neuromodulators control the polarity of spike-timing-dependent synaptic plasticity. Neuron 55: 919–929, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 105: 331–343, 2001 [DOI] [PubMed] [Google Scholar]
- Smith KE, Gibson ES, Dell'Acqua ML. cAMP-dependent protein kinase postsynaptic localization regulated by NMDA receptor activation through translocation of an A-kinase anchoring protein scaffold protein. J Neurosci 26: 2391–2402, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg JP, Huganir RL, Linden DJ. N-Ethylmaleimide-sensitive factor is required for the synaptic incorporation and removal of AMPA receptors during cerebellar long-term depression. Proc Natl Acad Sci USA 101: 18212–18216, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg JP, Takamiya K, Shen Y, Xia J, Rubio ME, Yu S, Jin W, Thomas GM, Linden DJ, Huganir RL. Targeted in vivo mutations of the AMPA receptor subunit GluR2 and its interacting protein PICK1 eliminate cerebellar long-term depression. Neuron 49: 845–860, 2006 [DOI] [PubMed] [Google Scholar]
- Tavalin SJ, Colledge M, Hell JW, Langeberg LK, Huganir RL, Scott JD. Regulation of GluR1 by the A-kinase anchoring protein 79 (AKAP79) signaling complex shares properties with long-term depression. J Neurosci 22: 3044–3051, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthold RJ, Petralia RS, Blahos IIJ, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci 16: 1982–1989, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Chung HJ, Wihler C, Huganir RL, Linden DJ. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron 28: 499–510, 2000 [DOI] [PubMed] [Google Scholar]
- Yang Y, Takeuchi K, Rodenas-Ruano A, Takayasu Y, Bennett MV, Zukin RS. Developmental switch in requirement for PKA RIIbeta in NMDA-receptor-dependent synaptic plasticity at Schaffer collateral to CA1 pyramidal cell synapses. Neuropharmacology 56: 56–65, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanillo D, Sprengel R, Hvalby O, Jensen V, Brunashev N, Rozov A, Kaiser KM, Koster HJ, Borchardt T, Worley P, Lubke J, Frotscher M, Kelly PH, Sommer B, Andersen P, Seeburg PH, Sakmann B. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science 284: 1805–1811, 1999 [DOI] [PubMed] [Google Scholar]
- Zeng H, Chattarji S, Barbarosie M, Rondi-Reig L, Philpot BD, Miyakawa T, Bear MF, Tonegawa S. Forebrain-specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic-like memory. Cell 107: 617–629, 2001 [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Esteban JA, Hayashi Y, Malinow R. Postnatal synaptic potentiation: delivery of GluR4-containing AMPA receptors by spontaneous activity. Nat Neurosci 3: 1098–1106, 2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.