Abstract
Real lines and illusory contours (ICs) have been reported to either interfere with or facilitate the perception of the other, depending on real line orientation and contrast. Here we investigate contextual effects of real lines on illusory contour perception. Curvature discrimination thresholds of Kanizsa-contours were measured for superimposed real lines of different sub- and suprathreshold contrasts. We find that parallel lines interfere with curvature discrimination at suprathreshold, whereas orthogonal lines interfere at subthreshold contrasts. We did not find stable facilitating effects of lines in any orientation or contrast. These results are discussed in relation to existing physiological and imaging data.
INTRODUCTION
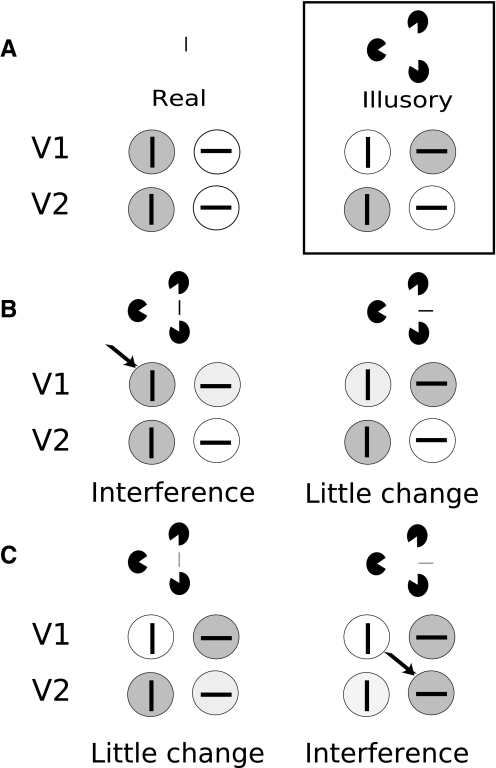
Illusory contour perception is influenced by real lines. Our perception of object shape is highly dependent on the perception of real and illusory contours. Although real lines are conveyed directly by physical luminance contrast, illusory contours (e.g., Kanizsa contours: Kanizsa 1976; abutting line contours: von der Heydt and Peterhans 1989; depth contrast contours: Heider et al. 2002; Hirsch et al. 1995) are induced by various contextual stimuli and are not based on luminance contrast stimulation at the location of the perceived contour. Kanizsa-type stimuli (Kanizsa 1976) contain partial figures that induce the outline of an apparent occluding shape (see curved triangle percept in Fig. 1A). Such illusory percepts can be influenced by the presence of real line cues. For example, the addition of orthogonal real line cues can enhance the illusory percept of a curved triangle (Fig. 1B), whereas the addition of real lines superimposed on the illusory contours can interfere with the coherence of the illusory triangle (Fig. 1C).
Fig. 1.
Influence of real lines on illusory contour perception. A: example of a Kanizsa figure (curved white triangle whose corners lay over 3 dark disks). B: addition of real lines orthogonal to the illusory contour enhances percept of curved triangle. C: addition of real lines parallel to the illusory contour degrades the coherence of the curved triangle.
Previous reports have suggested an orientation- and possibly contrast-dependent effect of real lines on illusory contour perception. Indeed, real elements in a scene can qualitatively change the perceptual strength of illusory contours depending on their orientation (Gillam and Chan 2002; Gillam and Nakayama 2002; Kanizsa 1976): Kanizsa-type triangles are perceptually strengthened by lines abutting the illusory outlines but weakened by lines superimposed on the illusory contours (Kanizsa 1976; Ringach and Shapley 1996).
This orientation dependency suggests that this real–illusory interaction may occur at lower cortical levels where neurons are known to be well-tuned to orientation. Neurophysiological studies in the macaque monkey have shown that, in early visual cortical areas, there are different classes of contour cells. Some cells (real contour cells) signal the orientation of only real contours, and others (general contour cells) signal the orientation of both real and illusory contours (both abutting line contours and occluded contours; Peterhans and von der Heydt 1989; von der Heydt and Peterhans 1989). Real contour cells are found in both areas V1 (primary visual cortex) and V2 (the second visual area), whereas illusory contour cells are found in V2 and not in V1. Thus neural processes recognizing illusory contours begin at the V2 stage or later (Merigan and Maunsell 1993).
One approach to studying the possible involvement of early visual cortical areas is to use low (subthreshold) contrasts (the real lines are invisible). In this way, we can test the perception of illusory contours without complications introduced by higher-order cognitive processes such as attention or motivation. Previous studies have not systemically studied the effect of real line contrast on illusory contour perception. Here, we studied the effect of adding real lines of different contrasts (either parallel or orthogonal to the illusory contour) on the perception of the illusory Kanizsa contour. We find that adding a high-contrast real line parallel to the illusory contour interferes with the illusory percept, whereas at low (subthreshold) contrasts, it has no consistent effect. For orthogonal lines, we find no consistent effects at high contrasts and interference at low contrast. These findings are discussed in the context of known V1 and V2 responses to real and illusory contours.
METHODS
Subjects
Four female subjects, between 20 and 27 yr old, participated in the experiments. Three of the subjects (research assistants) were naïve to the purpose of the experiments but understood the procedure and gave their consent to partake in the experiment. The fourth subject was one of the authors (S1). All subjects had normal or corrected to normal acuity.
Apparatus and basic experimental design
Experiments were programmed using OpenGL and C/C++ under Linux (Mandrake 9.2) on a Pentium computer. Stimuli were displayed on a 17-in CRT color monitor (Gateway 2000 Vivitron) with a refresh rate of 75 Hz. Luminance and contrast of the monitor were calibrated using a Minolta CS-100. Observers viewed the stimuli with both eyes in a dark room at a viewing distance of 57 cm to the monitor. The gray monitor background had a luminance of 25.6 cd/m2.
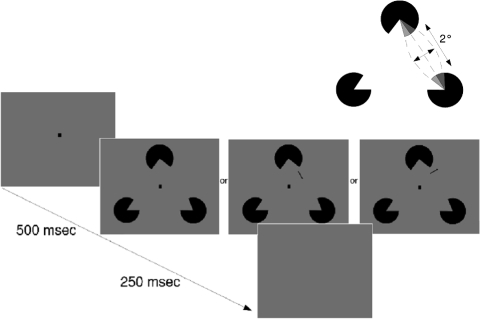
In all experiments, a square, black fixation spot (8 × 8 min of arc) was presented for 500 ms followed by a stimulus, which was shown for 250 ms (Figs. 2 and 3). The fixation spot remained visible throughout the stimulus presentation. After stimulus presentation, the fixation spot disappeared and the program awaited a subject's response provided by a press of either the left or right mouse button. No error feedback was provided. Subjects practiced the experiments for at least 3 days (i.e., ∼1,500 trials) before the results shown here were collected.
Fig. 2.
A: Stimulus design: 2 of the inducers of a Kanizsa-type illusory triangle were changed (opening ±0–4°) to produce a percept of the illusory contour being curved inward or outward. The illusory contour's length was 2°. Appearance of inward and outward bending is exaggerated in this depiction. B: discrimination task. A fixation period of 500 ms was followed by a stimulus (250-ms duration), whose right side produced an illusionary contour bent outward or inward (only outward shown) that could also contain a parallel or orthogonal real line aligned with estimated illusory contour path. Subjects had to decide in a two-alternative forced choice procedure (2AFC) paradigm, to which side the illusory contour was bent. Inducer opening in this drawing is 10°.
Fig. 3.
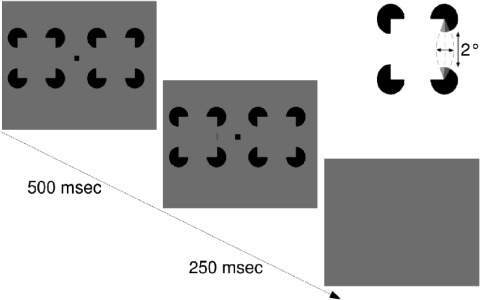
Detection task. During a fixation period of 500 ms, 2 illusory squares were presented to the left and right of the fixation spot. A real line was presented for 250 ms superimposed on 1 of the illusory contours next to the fixation spot. In a 2AFC paradigm, subjects had to indicate on which side they perceived the real line.
Illusory contour discrimination task
STIMULI.
The stimuli consisted of three black partial disks (0.5 cd/m2) with a radius of 37 min of arc. The discs induced a Kanizsa-type illusory triangle (see Fig. 2). We used a triangular stimulus, as angle discrimination thresholds around a base angle of 90 degree were found to be lower than thresholds around the base angle employed here (Heeley and Buchanan-Smith 1996). This triangular stimulus does not contain orthogonality cues that otherwise might interact with the perception of the overall form of the figure without the necessity to perceive an illusory contour. The illusory contour tested (right side of the illusory triangle) was about 2° long with a support ratio of 0.33. The opening angles of the partial disks inducing this contour were randomly changed to produce a percept of the contour being bent either outwards or inwards (cf. Ringach and Shapley 1996). Nine opening angle conditions were used to induce an illusory contour perceived as being straight (0°), curved inward (−1, −2, −3, −4°), or curved outwards (1, 2, 3, 4°).
For some stimuli, a short real contour was simultaneously presented with the illusory contour figure to test the effects of real lines on the perception of illusory contours. To test whether real line effects depend on orientation, real lines were presented either parallel (superimposed) or orthogonal to the illusory contour (Fig. 2).
The real contour was approximately one seventh of the illusory contour's length (18 min of arc). It was presented at a distance of 20 min of arc from the upper partial disc. This location corresponded to the middle of the upper half of the tested illusory contour. Because the distance between real and illusory stimuli has been shown to affect detectability of real components superimposed on illusory contours (McCourt and Paulson 1994), both positioning and length of the real contour were chosen to ensure minimal changes of distance between the real and illusory contour for each stimulus while positioning the real line not directly adjacent to the partial-disc inducer. The approximate relative positions of illusory and real contours are shown in Fig. 2.
To determine the interaction between real and illusory contours, we varied the contrasts of the real lines and presented oriented real lines at Weber contrasts that span the perceptual range from subthreshold to suprathreshold contrasts (3, 5, 10, 15, and 30%).
PARADIGM.
After a fixation period of 500 ms, the Kanizsa-type triangle was presented for 250 ms centered to the fixation spot (Fig. 2). Real lines were presented for the same duration as the illusory figure. Conditions of different real line contrasts were randomly interleaved with a baseline condition, in which no real line was presented. In a two-alternative forced choice procedure (2AFC) using the method of constant stimuli, subjects had to indicate with a computer mouse whether the illusory contour on the right side of the stimulus appeared curved to the left (left mouse button) or to the right (right mouse button).
We measured perceptual strength of illusory contour curvature at each of nine different settings (change of the partial disc openings of 0, 1, 2, 3, or 4° inward or outward). Stimuli were presented in a pseudo-random fashion. Analysis methods are described below in the Analysis section.
Real contour detection task
To measure real line detection thresholds in the presence of illusory contours, we conducted the following experiments.
STIMULI.
The stimulus consisted of two Kanizsa-type squares in a design comparable to Dresp and Bonnet (1995) and Ringach and Shapley (1996). Squares were presented to the left and right of the fixation spot (Fig. 3). The illusory contours had the same length and support ratio as the illusory contour tested in the illusory contour discrimination task. After 500 ms, real lines were superimposed on either the left or right Kanizsa-square. Real lines were 18 min of arc long (identical to the discrimination task) and were placed in the center of the illusory contour. Thus in contrast to the stimulus used by Dresp and Bonnet (1995), the real lines in our stimulus were not adjacent to the inducing partial discs and covered only a small fraction of the illusory contour. Both parallel and orthogonal real lines were tested. Contrasts were identical to contrasts used in the discrimination task.
PARADIGM.
To provide an estimate of the perceptual strength of real contours used in the discrimination task with illusory contours of varying curvature, separate sessions were conducted with illusory contours, which were straight, bent inward, or bent outward. For each subject, we measured contrast detection thresholds for real lines positioned either orthogonal or parallel to the illusory contour, using the method of constant stimuli. Two Kanizsa-type squares were presented side by side for 500 ms, with the subject holding fixation centered between them. A real line was presented on either the left or right virtual contour (close to fixation spot). Subjects had to report whether the real line had been presented to the left or the right of the fixation spot (2AFC procedure). Lines of different contrasts were presented in a pseudorandom fashion. Percent correct responses at each contrast were used to fit psychometric functions. Thresholds were calculated at 75% correct responses. Each threshold is based on ≥300 trials.
Detection thresholds of real lines were also measured in pretests (data not shown here) with one subject in a design more similar to the discrimination experiment. Only one triangular illusory figure was shown with the fixation spot located in the center of the figure. Real lines were shown on the right side illusory contour and could be either located closer to the upper or closer to the lower, inducing partial disc. The subject reported whether the real line had been presented on the upper or lower side of the illusory edge. Results in this experiment (data not shown) were comparable to those in the square configuration.
We designed the detection task (square) to resemble Dresp and Bonnet 1995 to test whether real lines superimposed on the illusory contour (IC) are detected more easily, even if they are not directly adjacent to the inducers as they were in the study of Dresp and Bonnet. The discrimination task is testing whether IC curvature is discriminated more easily with superimposed real lines. One possible argument against a test like this is the use of higher level cues such as orthogonality; we used a triangular Kanizsa figure to eliminate that potential cue.
Thus we tried to optimize each experiment design independently rather than aiming to compare between them. However, two aspects reconcile the configuration difference. First, Ringach and Shapley 1996 showed parallel line interference on IC shape discrimination in the square design comparable to our result in the triangular design. Second, we conducted the detection task in the triangular design in one subject and obtained similar results as in the square design. We therefore conclude that the results obtained in our experiments are not caused by the Kanizsa figure design but caused by the effects of real lines on illusory contours (discrimination) and effects of illusory contours on real lines (detection).
Analysis
For each subject, percent correct responses at each curvature setting and real line contrast were fitted to a psychometric function using the Mathematica Nonlinear Regress method. Mathematica's (Wolfram Research) Nonlinear Regress finds a least-squares fit in 40 iterations to the data for a given model. A cumulative Gaussian function was used to fit the psychometric function. Based on this, the contrast detection threshold (at 75% correct responses) and its asymptotic SE was calculated.
Because the absolute contrast detection thresholds for real lines differed across subjects, we pooled threshold values across subjects by first normalizing to each subject's detection threshold. This alignment allowed a comparison of results obtained with stimuli of perceptually similar contrasts (i.e., subthreshold versus suprathreshold contrasts).
For the curvature discrimination thresholds, percent correct responses at each curvature setting were fitted to a cumulative Gaussian function using psignifit version 2.5.6 (Wichmann and Hill 2001a). Thresholds at the 75% correct level and SD were derived from these fits, using the bootstrap method implemented in psignifit with 999 simulations (Wichmann and Hill 2001b). Each threshold presented here is based on ≥300 trials that were collected over several days.
We tested for significance between parallel and orthogonal real lines conditions in individual subject data by conducting a two-tailed Student's t-test. To test whether real lines of different orientations resulted in different performance across subjects, we conducted two-tailed paired t-test.
RESULTS
We present data that support both an orientation- and contrast-specific influence of real contours on illusory contour perception. First, we show data on subject-specific detection thresholds and their independence on illusory contour context. Second, we show baseline illusory contour perception estimates and the effect of adding real lines (both parallel and orthogonal) to illusory contour perception. Third, we present data showing the effect of real line contrast on illusory contour perception.
Real line detection thresholds
Our experimental design tests the influence of real lines on illusory contour perception. These tests involve the presentation of different configurations of real and illusory contours. First, we wanted to establish that, within each subject, detection thresholds remain reasonably stable for different stimulus configurations. Second, as different subjects have different thresholds, we wanted to tailor the analysis of stimuli of different contrasts to subject-specific thresholds.
We tested subjects on six different real/illusory configurations: two real line orientations (parallel or orthogonal to the illusory contour) superimposed on three curvatures of illusory contours (bent-inward, straight, or bent-outward). Real contours always remained in the same position: the parallel line was always superimposed on the straight virtual contour and the orthogonal line always abutted the straight virtual contour. Thus the orthogonal line slightly intersected the bent-outward illusory contour, abutted the straight illusory contour, and did not touch the bent-inward illusory contour. This difference in location of the real line relative to the illusory contour was minimized by positioning the real line very close to the inducers. At this location, the real lines are as close as possible to the illusory contour at any IC curvature, thereby reducing effects induced by relative location differences (McCourt and Paulson 1994), while not being directly adjacent to the inducers. Detection thresholds were measured in each condition using the method of constant stimuli with five levels of contrast, ranging from subthreshold to suprathreshold.
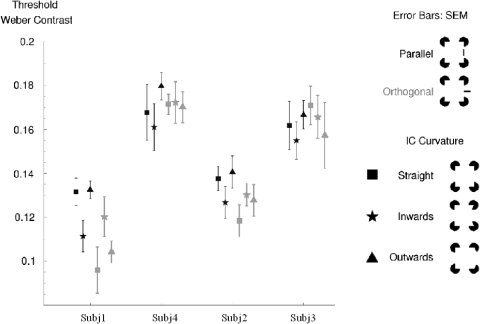
Real line detection thresholds for each of the four subjects are shown in Fig. 4. Detection thresholds were found to vary considerably between subjects but were fairly consistent within each subject (see McCourt and Paulson 1994 for comparison). As can be observed in Fig. 4, our results show lower detection thresholds over all testing conditions for subjects S1 (11.6 ± 0.6%) and S2 (13.0 ± 0.3%) in comparison to S3 (17.0 ± 0.2%) and S4 (16.3 ± 0.2%). These threshold data were used to define perceptually similar contrast categories across individuals.
Fig. 4.
Detection threshold of real contours superimposed on illusory contours. Shown are the individual contrast thresholds for 4 subjects. Gray data points correspond to orthogonal line detection; black data points correspond to parallel line detection. Symbols depict illusory contour shapes (box, straight; star, bent inward; triangle, bent outward). Error bars: SE.
Although real line detection thresholds varied across subjects, for each individual, detection thresholds were clustered across experimental conditions. The ranges of detection thresholds for subjects 1–4 were 12–14, 10–13, 16–18, and 16–17%, respectively. Consistent with the variability reported by McCourt and Paulson (1994), individual subjects showed variable thresholds across experimental conditions. In subjects S1 and S2, there is a tendency for lower detection thresholds for orthogonal (gray symbols) than for parallel (black symbols) real lines [parallel and orthogonal detection thresholds differences over all conditions are nonsignificant: S1, mean = 0.0184, SD = 0.024, t(2) = 1.34, 2-tail P > 0.1, S1, mean = 0.0095, SD = 0.012, t(2) = 1.41, 2-tail P > 0.1; yet for straight condition, S1: t(299) = 2.92, P < 0.01, S2: t(299) = 2.12, P < 0.05, and for outward condition, S1: t(299) = 4.44, P < 0.01]. However, subjects S3 and S4 showed no differences in thresholds for orthogonal and parallel real lines. Our data indicate in general similar detection thresholds across different illusory stimulus conditions. Because we found little difference across conditions within single subjects, we used a single averaged threshold value (averaged over all conditions) for each subject (S1: 11.6%, S2: 13.0%, S3: 16.3%, S4: 17.0%). This real line detection threshold value was used for the discrimination task (data below).
Perceptual strength of illusory contours
We tested the perceptual strength of illusory contours under three conditions: with a superimposed parallel real line, with a superimposed orthogonal line, and no line. Because different real–illusory contour interactions have been reported at both sub- and suprathreshold contrasts (Dresp and Bonnet 1995; Ringach and Shapley 1996), the influence of real components was tested at different subthreshold and suprathreshold real line contrasts. Tests with real lines at subthreshold contrasts furthermore allowed us to test whether real–illusory contour interactions are possible without subjects perceiving the interacting stimulus. We therefore aimed to test 1) whether real–illusory contour interaction is orientation-dependent, 2) whether real–illusory contour interaction depends on real line contrast, and 3) whether real–illusory interaction is evident at subthreshold contrasts.
We measured perceptual thresholds for the illusory contours by asking subjects whether the illusory contour was bent inward or outward.
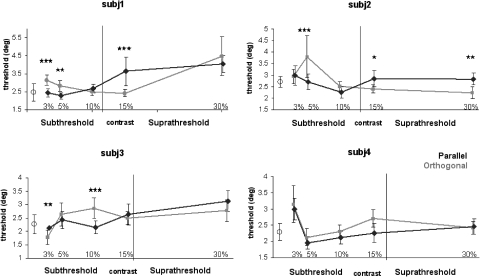
In Fig. 5, data from all four subjects are shown, exhibiting similar results, as will be described in the following sections. Thresholds are plotted in degrees of inducer angle difference over different contrasts of the superimposed real line. The black vertical line indicates each subject's average detection threshold for real lines (cf. Fig. 4). Shown are the thresholds from three conditions: superimposed parallel real line (black diamonds), orthogonal real line (gray squares), and the illusory contour alone (single open circle).
Fig. 5.
Discrimination thresholds of illusory contour shape at different contrasts for all subjects. Three conditions were tested: superimposed parallel line (black diamond), orthogonal line (gray square), and no line (open circle); error bars show SD. Vertical lines indicate individual real line detection thresholds. Stars indicate significance levels of difference between parallel and orthogonal line conditions (Student's t-test: 3×, P < 0.01; 2×, P < 0.05; 1×, P < 0.1).
Subject data for inward and outward percepts without superimposed real lines showed no consistent differences (consistent with Ringach and Shapley 1996), so these two conditions are pooled. Because “no line” conditions were identical in every block, it is expected that performance on this task would not change across “real line” blocks of different contrast. As expected, SD of average “no line” thresholds are smaller than the variability of thresholds across real line conditions (see Fig. 5, ○). We found that illusory contours are similar in their perceptual strength across subjects. Inducer opening has to be changed by 2.4 ± 0.2° for subjects to clearly perceive whether the illusory contour is bent outward or inward.
Influence of real lines on illusory contour perception
Comparing all subjects (Fig. 5), we find an interaction between real line contrast and illusory contour perception thresholds. Orthogonal real lines (gray squares) affect illusory contour perception differently from parallel real lines (black diamonds) most prominently at subthreshold contrasts, with the orthogonal line condition thresholds being larger than thresholds in the no-line condition, i.e., interfering with illusory contour perception. At different subthreshold contrasts, mean differences between orthogonal and parallel line threshold were significantly different from zero [S1, 3%: mean = 0.71, SD = 0.25, t(297) = 2.83, 2-tail P < 0.01, 5%: mean = 0.55, SD = 0.23, t(297) = 2.39, 2-tail P < 0.05; S2, 5%: mean = 1.09, SD = 0.21, t(297) = 3.98, 2-tail P < 0.01; S3, 10%: mean = 0.71, SD = 0.23, t(297) = 3.06, 2-tail P < 0.01]. S4 did not show any significant difference, but the mean difference between orthogonal and parallel line thresholds showed a trend to be different from zero at subthreshold contrast of 10% [mean = 0.45, SD = 0.23, t(297) = 1.97, 2-tail P < 0.1]. The orthogonal threshold at that contrast is significantly larger than the no-line condition threshold [mean = 0.42, SD = 0.17, t(297) = 2.42, 2-tailed P < 0.05]. Thus all subjects exhibited threshold differences between subthreshold orthogonal and parallel lines, with orthogonal lines resulting in larger thresholds than obtained in the no-line condition.
Two subjects exhibit additional results that divert from this general pattern: at subthreshold (3% contrast), S3's mean difference between orthogonal and parallel line threshold were significantly different from zero [mean = −0.36, SD = 0.17, t(297) = −2.1, 2-tail P < 0.05]. At this contrast, thresholds with the orthogonal line and in the no-line condition are significantly different [mean = −0.49, SD = 0.12, t(297) = −4.2, 2-tail P < 0.01], suggesting facilitation by orthogonal real lines. S4, on the other hand, has higher thresholds with both parallel and orthogonal real lines at the lowest contrast (3%). Note that these two subjects have high detection thresholds around 16–18%; thus the 3% contrast real lines are clearly not perceived.
At suprathreshold, results are less consistent. Most subjects show increased thresholds with both parallel and orthogonal real lines compared with the no line condition. However, only two subjects exhibit differential effects of parallel and orthogonal real lines, with mean differences between orthogonal and parallel line threshold being significantly different from zero for S1 at 15% contrast [mean = −1.21, SD = 0.298, t(297) = 4.07, 2-tail P < 0.01]. For S2, at 15% contrast, the mean difference between orthogonal and parallel line thresholds showed a trend to be different from zero [mean = −0.45, SD = 0.24, t(297) = −1.88, 2-tail P < 0.1], and, at the highest suprathreshold contrast (30%), orthogonal and parallel line thresholds were significantly different [mean = 0.59, SD = 0.23, t(297) = 2.58, 2-tail P < 0.05]. In all these cases, parallel lines result in decreased performance compared with orthogonal.
Thus the individual data sets show similar real line effects on illusory contour perception. Orthogonal real lines interfere with the illusory percept at low contrasts, whereas parallel real lines interfere at higher contrasts. The orientation reversal of real–illusory interaction occurs at a contrast near the individual's detection threshold.
Across all subjects
METHODOLOGY.
Because contrast perception can vary considerably between subjects, averaging across subjects at the same absolute contrast level may inappropriately combine data that reflect perceptually very different stimuli. In particular, given the observed real/illusory interaction reversal near individual contrast thresholds, we thought it was worthwhile to attempt to normalize data with respect to subject-specific thresholds.
We thus used a method that averages data from perceptually comparable conditions. We used each subject's detection threshold for real lines as a zero point for comparison across subjects. Data from each subject were categorized into four different subthreshold levels (Sub 1–4; S2 only had Sub 2–4) and two suprathreshold levels (Supra 1–2). In this way, individual data were aligned to permit averaging of data with similar perceptual strength. Two subjects, S1 and S4, had low detection thresholds (between 10 and 15%), and two subjects, S2 and S3, had detection thresholds >15%. We aligned the data to take these differences into account: for subjects S1 and S4, contrast levels 3, 5, and 10% were subthreshold, whereas, for subjects S2 and S3, contrast levels 3, 5, 10, and 15% were subthreshold.
Alignment resulted in an asymmetric pooling of the data, with Sub1 and Supra2 category containing data from only two subjects. Sub2, Sub3, Sub4, and Supra1 are averages of data from all four subjects. This approach has the intuitive advantage of treating perceptually different contrast levels as categorically distinct. For the statistical assessment, we used only categories containing data from all subjects.
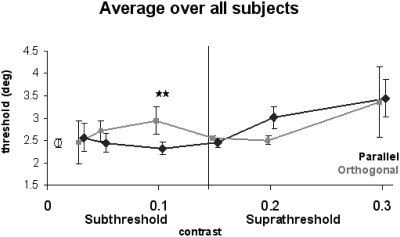
Figure 6 shows the average of aligned data from all four subjects. The open circle indicates the average no line threshold across all four subjects. Our first observation is that, in general, taking all points into account, the addition of real lines (both orthogonal and parallel) has either little effect on illusory contour perception or tends to interfere with illusory contour perception (all thresholds are equal to or higher than open circle).
Fig. 6.
Average over all subjects at perceptually similar contrasts. Thresholds in the orthogonal (gray square) and parallel (black diamond) conditions are shown over contrast categories relative to the average detection threshold (vertical line). The “no line” threshold is shown as an open circle. Error bars indicate SE. Statistical significance was assessed using a t-test over mean differences between parallel and orthogonal line conditions (P < 0.05).
EFFECTS OF PARALLEL LINES.
To assess whether adding real lines resulted in a changed performance of the subjects in the illusory contour discrimination task, we conducted mean difference tests between thresholds in different real line conditions and thresholds in the no line condition. At subthreshold contrasts and, in particular, close to detection threshold (Sub4), parallel lines (black diamonds) tend to have little effect on illusory contour perception.
At suprathreshold contrasts, on the other hand, perceptual thresholds with parallel real lines were not significantly different from those with orthogonal lines {mean = 0.51, SD = 0.499, n = 4, mean difference greater than zero [t(3) = 2.05, 2-tail P = 0.13], but parallel lines tend to interfere compared with the no-line condition [mean = 0.58, SD = 0.506, n = 4, t(3) = 2.29, 2-tail P = 0.11]}.
EFFECTS OF ORTHOGONAL LINES.
The effect of orthogonal real lines (gray stars) on illusory contour perception differs from that of parallel real lines. At subthreshold contrast Sub3, perceptual thresholds with orthogonal real lines were significantly larger than those with parallel lines (mean = 0.64, SD = 0.37, n = 4), with the mean difference being significantly greater than zero (3.41, 2-tail P = 0.04). At contrast levels above detection threshold, no orthogonal effects were observed.
Note that, consistent with the single subject data shown in Fig. 5, there is a crossover point between orthogonal and parallel line effects at near-threshold contrast levels. Thus these data show that the average effects are dependent on the perceptual strength of the real line stimuli, indicating a fine balance between real line strength and its interaction with the illusory contour percept.
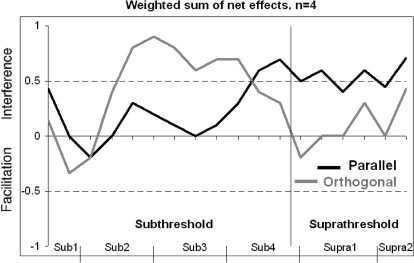
To test whether the effects found in the averaged data result from a few strong effects in the individual data combined with no or very weak effects in other data points, we aligned individual data to subject-specific detection thresholds and examined them without regard to the strength of the effect. Each data point was classified as interference (1), no effect (0), and facilitation (−1), respectively, with threshold differences (real line condition minus no line) <0.17 (2 times the SE of threshold differences) being classified as no effect. Data were aligned to individual's detection thresholds by shifting contrast values (x-axis) by the difference between individual and average detection threshold. We calculated the weighted sum of the each data point as one half of the nearest neighbors and one quarter of the second neighbors. Sums were normalized through dividing by 2.5 (maximum). Indices between −0.5 and 0.5 indicate no or inconsistent effects at the respective condition and contrast range. Indices >0.5 (interference) or lower than −0.5 (facilitation) indicate that at least one half of the neighbor effects are the same. Data analyzed in this manner are shown in Fig. 7.
Fig. 7.
Weighted sums of the data of 4 subjects. Interference was initially set to 1 and facilitation to −1. At every contrast, the individual effect was summed with the weighted neighbor effects. Parallel line effects are shown in black and orthogonal in gray. The average detection threshold is depicted as vertical line between sub- and suprathreshold contrasts. The dotted lines indicate the ranges (<−0.5 or >0.5) at which effects are consistent over different subjects at perceptually similar contrasts. Contrast ranges are indicated on the x-axes, with ticks indicating single weighted sums.
Similar effects as observed in Figs. 5 and 6 are seen. Real lines influence illusory contour perception in an orientation-dependent and contrast-dependent manner. In general, across most contrasts, real lines (both parallel, black, and orthogonal, light gray) either have no effect or interfere (average indices greater than −0.5 and majority >0.5) with illusory contour perception. Parallel lines have little consistent effect at subthreshold contrasts close to detection threshold but interfere close to detection threshold and at suprathreshold contrasts. Orthogonal lines interfere below detection threshold but not at higher contrasts. Consistent with the other methods of analysis, there is a crossover point from orthogonal interference to parallel line interference near the detection threshold. The effects are consistent across contrasts and subjects and thus are not biased by single individuals.
The results thus support our prediction that influence of real lines on illusory contour perception depends on orientation and contrast range. We find that parallel lines interfere at suprathreshold contrasts (similar to a previous report, Ringach and Shapley 1996), and surprisingly, that orthogonal lines interfere at subthreshold contrasts.
DISCUSSION
We measured the effect of superimposed short, low-contrast real lines on the discrimination of an illusory contour, as well as the detection of these real lines superimposed on an illusory contour. We designed the experiments to largely resemble previous studies in which detection of real lines (Dresp and Bonnet 1995) and curvature discrimination of illusory contours (Ringach and Shapley 1996) were measured. Unlike previous studies, we interpreted the data based on subject-specific real line detection thresholds. Thus our study used a perceptual threshold as criterion rather than any fixed luminance contrast. We found that the strength of the illusory contour depends both on the orientation and the contrast of the real line.
No subthreshold summation between real and illusory contours
In contrast to a previous study (Dresp and Bonnet 1995), we found no evidence for improved detectability of subthreshold parallel real lines superimposed on illusory contours. In fact, in neither task, the real line detection task nor the illusory contour discrimination task, did we find any evidence for contrast summation between parallel real and illusory contours. Instead, we found high variability of real–illusory contour interactions in real line detection. This is consistent with McCourt and Paulson (1994), who also reported high variability, and with a more recent report on lack of subthreshold summation using the Ehrenstein figure (Salvano-Pardieu et al. 2006).
We suggest two factors that may have contributed to these differences. In Dresp and Bonnet (1995), the real line spanned the entire gap between the collinear Kanizsa inducers, potentially creating a collinear facilitation (Kapadia et al. 1995; Wehrhahn and Dresp 1998). In our paradigm, in an effort to reduce this confounding factor, we used short real lines that did not span the gap and did not come into direct contact with inducers (Williams and Hess 1998). A second factor is the duration of stimulus presentation. We were concerned that long presentation and processing times might engage higher-level processes or slow horizontal connections, leading to involvement of association fields in contour completion (Field et al. 1993; Poom 2001). Previous studies have shown illusory contour masking at SOAs of ≤120 ms for local, presumably induction, processes, and ≤250 ms for global, or form, processes (Dillenburger and Wehrhahn 2005; Guttman and Kellman 2004; Ringach and Shapley 1996; Westheimer and Li 1996). Therefore to reduce engagement of association fields and higher-order processes, we shortened presentation times to 250 ms (in comparison to 350 ms in Dresp and Bonnet 1995 and 1,000 ms in Poom 2001). Note that these psychophysical estimates are consistent with illusory contour responses in areas V2 (70–90 ms) and V1 (superficial layers at 100 ms, deep layers at >190 ms; Lee and Nguyen 2001).
Thus we made efforts in our experiments to minimize confounding factors of collinear facilitation and higher-order processes. We suggest that there is little evidence for real and illusory contour summation.
Asymmetry of real-illusory contour interactions
As previously shown by Paradiso et al. (1989) in a tilt-aftereffect study, effects of real lines on illusory contours may differ from effects of illusory contours on real lines. We showed here that real line detection in illusory contour stimuli is highly variable across subjects and conditions but that real lines have consistent effects on illusory contour discrimination depending on contrast and orientation. Factors contributing to such asymmetries may include saliency differences of real and illusory contours (Westheimer and Li 1997; Westheimer and Wehrhahn 1997). Also, the direction of attention in a task has been shown to modulate early cortical processing (Ito and Gilbert 1999; Motter 1993), thus possibly biasing effects depending on the perceptual task. Finally, the asymmetry in real–illusory contour interactions may point toward differences in the feedforward versus feedback relationships between V1 and V2 (see discussion below; Ramsden et al. 2001) or to the recruitment of different neural pools in illusory contour versus real contour processing (Paradiso et al. 1989).
Possible feedforward-feedback competition?
ROLE OF V2.
There is large agreement that visual area V2 is the first processing stage that plays a central role in encoding illusory contours across a broad range of inducing cues (2nd-order envelope cues: Mareschal and Baker 1999; abutting line cues: Sheth et al. 1996; von der Heydt and Peterhans 1989; Kanizsa cues: Peterhans and von der Heydt 1989; motion border cues: Lu et al. 2007; Marcar et al. 2000; depth-induced border cues: Chen et al. 2008; Qiu and von der Heydt 2005). Consistent with V2's role in contour generalization, optically imaged orientation maps in V2 obtained in response to oriented real lines appear very similar to those obtained in response to oriented illusory (abutting line) contours (Ramsden et al. 2001; Sheth et al. 1996; Zhan and Baker 2006), indicating the presence of generalized contour domains in V2; such domains are absent in V1 (Lu et al. 2007, 2009; Ramsden et al. 2001). The role of V2 in illusory contour perception is further underscored by behavioral studies showing the loss of illusory contour perception after lesions of V2 (Merigan and Maunsell 1993).
ROLE OF V1.
Although there may be some orientation-biased responses in the retina and lateral geniculate nucleus, it is generally agreed that V1 is the first stage at which strong orientation selectivity is generated (Hubel and Wiesel 1968). With respect to illusory contour processing, however, the role of V1 is less clear. Some studies have shown a lack of response to illusory contours in V1 (Peterhans and von der Heydt 1989; Sheth et al. 1996). Others have shown an “orientation reversal” response where neurons tuned to the orientation of the illusory contour are relatively suppressed, whereas those selective for orthogonal orientations are relatively increased (Ramsden et al. 2001). Other studies have shown more complex response patterns that are dependent on the orientation, motion, and spatial frequency content of the stimulus (Basole et al. 2003; Song and Baker 2006). Thus response of V1 seems to be more dependent on the specific cues generating the contour.
ROLE OF V2-V1 FEEDBACK.
Because V2 is a stage where the earliest generalized illusory contour responses arise, we suggest that it is a possible source of influence of V1 response to illusory contours. This hypothesis is supported by neurophysiological and psychophysical data. Whereas responses to real contours arise first in V1 (primary visual cortex), followed by V2 (the 2nd visual area) (Givre et al. 1995; Nowak et al. 1995; Schmolesky et al. 1998), illusory contours (such as flashed Kanizsa figures) produce V1 responses that are delayed relative to V2 activation (Lee and Nguyen 2001). Moreover, psychophysical experiments examining the timing of real line interference with illusory contour perception suggest that this feedback, or the process leading from inducing stimuli representation to illusory contour representation, occurs at a latency of ∼100 ms (Dillenburger and Wehrhahn 2005; Dillenburger et al. 2004; Guttmann and Kellman 2004; Reynolds 1981; Ringach and Shapley 1996). This delayed response in V1 raises the possibility that feedback from V2 influences V1 response during illusory contour processing.
FEEDFORWARD VERSUS FEEDBACK COMPETITION MODEL.
Based on the ideas that V1 is the first stage where orientation selectivity is generated and V2 is the first stage for generalized contour orientation recognition, we propose that contour processing in early visual cortex may comprise a competitive balance between feedforward V1-V2 and feedback V2-V1 influences. Although illusory contours can be interpreted as a high-order percept involving many cortical stages (Halgren et al. 2003; Merigan and Maunsell 1993; Murray et al. 2002; Ritzl et al. 2003; Seghier et al. 2000), we believe that the subthreshold stimuli used in our experiments may preferentially inform about processes at early cortical stages. We propose that, during real contour processing, the feedforward signals from V1 to V2 dominate and activate matching orientation domains in V2 (Fig. 8 A, left). During illusory contour processing, feedback signals originating from V2 gain prominence and evoke a reversed activation pattern in V1 (Ramsden et al. 2001) (Fig. 8A, right). The model proposes a competitive process between real (feedforward) and illusory (feedback) signals during processing of visual contours. The perceived contour (either real or illusory) would result from a competitive balance between these feedforward and feedback influences.
Fig. 8.
Effect of adding real lines to illusory contour activation in V1 and V2. A: schematic of orientation domain activations during real (left) and illusory (right) contour processing in V1 and V2. Effect of adding suprathreshold (B) and subthreshold (C) real line to the illusory contour activation pattern (black box in A). Arrows indicate loci of strong activation. B: adding suprathreshold parallel line (left) activates vertical orientation domains in V1, thereby interfering with the illusory contour activation pattern. Adding a suprathreshold orthogonal line (right) activates horizontal V1 and V2 domains but also serves as abutting line inducer facilitating the reverse-oriented activation pattern. It therefore has an inconsistent effect on the illusory contour activation pattern. C: adding a subthreshold parallel line (left) does little to change the illusory activation pattern. Adding a subthreshold orthogonal line (right) adds to the reverse-oriented activation of horizontal V1 domains, possibly resulting in activation of horizontal V2 domains and thereby disrupting the illusory contour pattern.
In this context, our psychophysical data showed that adding a high-contrast real line parallel to the illusory contour interferes with the illusory contour percept, because high-contrast real lines bias the feedforward process and activate parallel domains in V1 and V2, thereby disrupting the illusory contour activation pattern (Fig. 8B, left). Adding a high-contrast orthogonal line (Fig. 8B, right) did not have a consistent effect on the illusory contour percept in our experiment (showed interference in 2 subjects at high contrast). Our interpretation is that the high-contrast orthogonal line drives a feedforward real (orthogonal) line process, thereby disrupting the parallel illusory contour process balance; at the same time, this abutting line inducer has a facilitating effect (see Fig. 1). These opposing effects of interference and facilitation may explain the inconsistent results across subjects. Thus interpretation of psychophysical effects at high contrast remain somewhat speculative. Considering subthreshold real lines, we argue that adding subthreshold signal to V1 domains has a differential effect depending on the orientation added. Adding a subthreshold parallel real line is not effective at driving parallel V1 domains and has little subsequent effect on V2 neurons; therefore it does not change the illusory contour balance (Fig. 8C, left). Because orthogonal domains in V1 are already potentiated by the illusory contour process, the addition of an orthogonal subthreshold real line leads to activation of orthogonal V2 domains, resulting in the disruption of the illusory contour pattern and perceptual interference (Fig. 8C, right). Indeed with respect to real line detection, this model suggests that subthreshold orthogonal lines in fact may be more readily detectable in illusory contour context. Although our detection data (Fig. 4) do not show clear evidence for this in all subjects, S1 and S2 indeed showed lower detection thresholds for orthogonal than for parallel lines when real lines were abutting or overlapping with the illusory contour.
One can argue that the positioning of the real lines relative to the illusory contour may be critical to understanding the mechanisms underlying their interaction. For example, lines truly abutting the illusory contour may be supportive, as shown in Fig. 1, whereas real lines crossing the illusory contour may even interfere with the percept. Such interference could be caused by high level perceptual and cognitive effects at high contrasts or by geometrical aspects of the inducer/contour relationship at low contrasts. Therefore our paradigm may lead to opposing effects, consistent with our psychophysical results at high contrast. However, the orthogonal interference at low but not at high contrast is inconsistent with the interpretation that orthogonal line position generally interferes with our illusory contour task—in that case, we would expect stronger interference with increasing contrast and thus stronger induction effects, which is opposite to our findings.
Thus we suggest that our perceptual results are consistent with the interpretation that illusory contour processing in V1 and V2 occurs in a reverse-oriented manner and that real line stimuli can interfere with this process in an orientation- and contrast-dependent manner. High-contrast real lines activate collinear domains in V1 and V2 in a feedforward direction, whereas subthreshold real lines can modulate activation in V1, thereby disrupting the illusory contour balance between V1 and V2.
GRANTS
This work was supported by National Eye Institute Grant EY11744, the Packard Foundation, the Center for Integrative and Cognitive Neuroscience, and Grant P30EY008126.
ACKNOWLEDGMENTS
We thank I. Kwong for assistance in data collection, S. Guttman and R. Blake for helpful discussions in the early stages of the experiment, K. Nielsen, L. Gu, R. Friedman, and C. Wehrhahn for valuable feedback and constructive critique on the manuscript, and anonymous reviewers for invaluable feedback.
Part of this work was presented at the VSS meeting in 2004 and is part of the dissertation of B. Dillenburger (Eberhard Karls University, Tuebingen, 2005).
REFERENCES
- Basole A, White LE, Fitzpatrick D. Mapping multiple features in the population response of visual cortex. Nature 423: 986–90, 2003 [DOI] [PubMed] [Google Scholar]
- Chen G, Lu HD, Roe AW. A map for horizontal disparity in monkey V2. Neuron 58: 442–450, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillenburger B, Kwong IW, Roe AW. Timing of illusory contour processing probed by real lines: support for V2-V1 feedback effects. Soc Neurosci Abstr 713 12, 2004 [Google Scholar]
- Dillenburger B, Wehrhahn C. Backward masks specifically interfere with illusory contours or their inducers dependent on timing. J Vision 5: 570a, 2005 [Google Scholar]
- Dresp B, Bonnet C. Subthreshold summation with illusory contours. Vision Res 35: 1071–1088, 1995 [DOI] [PubMed] [Google Scholar]
- Ffytche DH, Zeki S. Brain activity related to the perception of illusory contours. Neuroimage 3: 104–108, 1996 [DOI] [PubMed] [Google Scholar]
- Field DJ, Hayes A, Hess RF. Contour integration by the human visual system: evidence for a local ‘association field’. Vision Res 33: 173–193, 1993 [DOI] [PubMed] [Google Scholar]
- Gillam B, Chan WM. Grouping has a negative effect on both subjective contours and perceived occlusion at T-junctions. Psychol Sci 13: 279–283, 2002 [DOI] [PubMed] [Google Scholar]
- Gillam B, Nakayama K. Subjective contours at line terminations depend on scene layout analysis, not image processing. J Exp Psych: Hum Percept Perform 2002, 28: 43–53, 2002 [Google Scholar]
- Givre SJ, Arezzo JC, Schroeder CE. Effects of wavelength on the timing and laminar distribution of illuminance-evoked activity in macaque V1. Visual Neurosci 12: 229–239, 1995 [DOI] [PubMed] [Google Scholar]
- Guttman SE, Kellman PJ. Contour interpolation revealed by a dot localization paradigm. Vision Res 44: 1799–1815, 2004 [DOI] [PubMed] [Google Scholar]
- Halgren E, Mendola J, Chong CD, Dale AM. Cortical activation to illusory shapes as measured with magnetoencephalography. Neuroimage 18: 1001–1009, 2003 [DOI] [PubMed] [Google Scholar]
- Hawley SJ, Keeble DR. Tilt aftereffect for texture edges is larger than in matched subjective edges, but both are strong adaptors of luminance edges. J Vision 6: 37–52, 2006 [DOI] [PubMed] [Google Scholar]
- Heeley DW, Buchanan-Smith HM. Mechanisms specialized for the perception of image geometry. Vision Res 36: 3607–3627, 1996 [DOI] [PubMed] [Google Scholar]
- Heider B, Spillmann L, Peterhans E. Stereoscopic illusory contours - cortical neuron responses and human perception. J Cogn Neurosci 4: 1018–1029, 2002 [DOI] [PubMed] [Google Scholar]
- Hirsch J, DeLaPaz RL, Relkin NR, Victor J, Kim K, Li T, Borden P, Rubin N, Shapley R. Illusory contours activate specific regions in human visual cortex: Evidence from functional magnetic resonance imaging. Proc Natl Acad Sci USA 92: 6469–6473, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields and functional architecture of monkey striate cortex. J. Physiol 195: 215–243, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imber ML, Shapley RM, Rubin N. Differences in real and illusory shape perception revealed by backward masking. Vision Res 45: 91–102, 2005 [DOI] [PubMed] [Google Scholar]
- Ito M, Gilbert CD. Attention modulates contextual influences in the primary visual cortex of alert monkeys. Neuron 22: 593–604, 1999 [DOI] [PubMed] [Google Scholar]
- Julesz B, Schumer RA. Early visual perception. Ann Rev Physiol 32: 575–627, 1981 [DOI] [PubMed] [Google Scholar]
- Kanizsa G. Subjective contours. Sci Am 234: 48–52, 1976 [DOI] [PubMed] [Google Scholar]
- Kapadia MK, Ito M, Gilbert CD, Westheimer G. Improvement in visual sensitivity by changes in local context: parallel studies in human observers and in V1 of alert monkeys. Neuron 15: 843–856, 1995 [DOI] [PubMed] [Google Scholar]
- Kapadia MK, Westheimer G, Gilbert CD. Spatial distribution of contextual interactions in primary visual cortex and in visual perception. Proc Natl Acad Sci USA 96: 12073–12078, 2000. 10518578 [Google Scholar]
- Lamme VA. The neurophysiology of gure-ground segregation in primary visual cortex. J Neurosci 15: 1605–1615, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson J, Amunts K, Gulyas B, Malikovic A, Zilles K, Roland PE. Neuronal correlates of real and illusory contour perception: functional anatomy with PET. Eur J Neurosci 11: 4024–4036, 1999 [DOI] [PubMed] [Google Scholar]
- Lee TS, Nguyen M. Dynamics of subjective contour formation in the early visual cortex. Proc Natl Acad Sci USA 98: 1907–1911, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt JB, Lund JS. Contrast dependence of contextual effects in primate visual cortex. Nature 387: 73–76, 1997 [DOI] [PubMed] [Google Scholar]
- Li Z. Computational design and nonlinear dynamics of a recurrent network model of the primary visual cortex. Neural Comput 13: 1749–1780, 2001 [DOI] [PubMed] [Google Scholar]
- Lu HD, Chen G, Roe AW. A map for motion streaks in V1 and V2. Soc Neurosci Abstr 337.3, 2007 [Google Scholar]
- Lu HD, Chen G, Roe AW. Motion processing in the ventral pathway: evidence for direction maps in macaque V2 and V4. Vision Science Society, Naples, FL, May 2009, 743 [Google Scholar]
- Marcar VL, Raiguel SE, Xiao D, Orban GA. Processing of kinetically defined boundaries in areas V1 and V2 of the macaque monkey. J Neurophysiol 84: 2786–2798, 2000 [DOI] [PubMed] [Google Scholar]
- Mareschal I, Baker CL., Jr Cortical processing of second-order motion. Vis Neurosci 16: 527–540, 1999 [DOI] [PubMed] [Google Scholar]
- Mareschal I, Andrew HJ, Shapley RM. A psychophysical correlate of contrast dependent changes in receptive field properties. Vision Res 42: 1879–1887, 2002 [DOI] [PubMed] [Google Scholar]
- McCourt ME, Paulson K. The influence of illusory contours on the detection of luminance increments and decrements. Vision Res 34: 2469–2475, 1994 [DOI] [PubMed] [Google Scholar]
- Mendola JD, Dale AM, Fischi B, Liu AK, Tootell RBH. The representation of illusory and real contours in human cortical visual areas revealed by functional magnetic resonance imaging. J Neurosci 19: 8560–8572, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan WH, Maunsell JH. How parallel are the primate visual pathways? Annu Rev Neurosci 16: 369–402, 1993 [DOI] [PubMed] [Google Scholar]
- Montaser-Kouhsari L, Landy MS, Heeger DJ, Larsson J. Orientation-selective adaptation to illusory contours in human visual cortex. J Neurosci 27: 2186–2195, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motter B. Focal attention produces spatially selective processing in visual cortical areas V1, V2, and V4 in the presence of competing stimuli. J Neurophysiol 70: 909–919, 1993 [DOI] [PubMed] [Google Scholar]
- Murray RF, Sekuler AB, Bennett PJ. Time course of amodal completion revealed by a shape discrimination task. Psychonomic Bull Rev 8: 713–720, 2001 [DOI] [PubMed] [Google Scholar]
- Murray MM, Wylie GR, Higgings BA, Javitt DC, Schroeder CE, Foxe JJ. The spatiotemporal dynamics of illusory contour processing: combined high-density electrical mapping, source analysis, and functional magnetic resonance imaging. J Neurosci 22: 5055–5073, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak LG, Munk MH, Girard P, Bullier J. Visual latencies in areas V1 and V2 of the macaque monkey. Visual Neurosci 12: 371–384, 1995 [DOI] [PubMed] [Google Scholar]
- Paradiso MA, Shimojo S, Nakayama K. Subjective contours, tilt aftereffects, and visual cortical organization. Vision Res 29: 1205–1213, 1989 [DOI] [PubMed] [Google Scholar]
- Peterhans E, von der Heydt R. Mechanisms of contour perception in monkey visual cortex. ii. contours bridging gaps. J Neurosci 9: 1749–1763, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polat U, Mizobe K, Pettet MW, Kasamatsu T, Norcia AM. Collinear stimuli regulate visual response depending on cell's contrast threshold. Nature 391: 580–584, 1998 [DOI] [PubMed] [Google Scholar]
- Poom L. Visual summation of luminance lines and illusory contours induced by pictorial, motion, and disparity cues. Vision Res 41: 3805–3816, 2001 [DOI] [PubMed] [Google Scholar]
- Qiu FT, von der Heydt R. Figure and ground in the visual cortex: V2 combines stereoscopic cues with gestalt rules. Neuron 47: 155–166, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran VS. The Perception of Illusory Contours Springer: Berlin, 1987 [Google Scholar]
- Ramsden BM, Chou PH, Roe AW. Real and illusory contour processing in area V1 of the primate: a cortical balancing act. Cereb Cortex 11: 648–665, 2001 [DOI] [PubMed] [Google Scholar]
- Reynolds RI. Perception of an illusory contour as a function of processing time. Perception 10: 107–115, 1981 [DOI] [PubMed] [Google Scholar]
- Ringach DL, Shapley R. Spatial and temporal properties of illusory contours and amodal boundary completion. Vision Res 36: 3037–3050, 1996 [DOI] [PubMed] [Google Scholar]
- Ritzl A, Marshall JC, Weiss PH, Zafiris O, Shah NJ, Zilles K, Fink GR. Functional anatomy and differential time courses of neural processing for explicit, inferred, and illusory contours. an event-related fMRI study. Neuroimage 19: 1567–1577, 2003 [DOI] [PubMed] [Google Scholar]
- Roe AW. The Primate Visual System New York: CRC, 2003 [Google Scholar]
- Rosenberg A, Husson T, Mallik AK, Issa NP. Frequency-doubling in the early visual system underlies sensitivity to second-order stimuli. Vision Sciences Society Meeting, Naples, FL, May 2008, 281 [Google Scholar]
- Salvano-Pardieu W, Wink B, Taliercio A, Manktelow K, Meigen T. Can subthreshold summation be observed with the Ehrenstein illusion? Perception 35: 965–981, 2006 [DOI] [PubMed] [Google Scholar]
- Sceniak MP, Ringach DL, Hawken MJ, Shapley R. Contrast's effect on spatial summation by macaque V1 neurons. Nat Neurosci 2: 733–739, 1999 [DOI] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Hanes DP, Thompson KG, Leutgeb S, Schall JD, Leventhal AG. Signal timing across the macaque visual system. J Neurophysiol 79: 3272–3278, 1998 [DOI] [PubMed] [Google Scholar]
- Seghier M, Dojat M, Delon-Martin C, Rubin C, Warnking J, Segebarth C, Bullier J. Moving illusory contours activate primary visual cortex: an fMRI study. Cereb Cortex 10: 663–670, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth BR, Sharma J, Rao SC, Sur M. Orientation maps of subjective contours in visual cortex. Science 274: 2110–2115, 1996 [DOI] [PubMed] [Google Scholar]
- Song Y, Baker CL. Neural mechanisms mediating responses to abutting gratings: luminance edges vs. illusory contours. Vis Neurosci 23: 181–199, 2006 [DOI] [PubMed] [Google Scholar]
- Sugita Y. Grouping of image fragments in primary visual cortex. Nature 401: 269–272, 1999 [DOI] [PubMed] [Google Scholar]
- Vogels R, Orban GA. Illusory contour discrimination. Vision Res 27: 453–467, 1987 [DOI] [PubMed] [Google Scholar]
- von der Heydt R, Peterhans E. Mechanism of contour perception in monkey visual cortex. I. Lines of pattern discontinuity. J Neurosci 9: 1731–1748, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Nanez JE, Sr, Moreno MA. Depth release of illusory contour shape in the Ehrenstein grid. Vision Res 35: 2845–2851, 1995 [DOI] [PubMed] [Google Scholar]
- Wehrhahn C, Dresp B. Detection facilitation by collinear stimuli in humans: dependence on strength and sign of contrast. Vision Res 38: 423–428, 1998 [DOI] [PubMed] [Google Scholar]
- Westheimer G, Li W. Classifying illusory contours by means of orientation discrimination. J Neurophysiol 75: 523–528, 1996 [DOI] [PubMed] [Google Scholar]
- Westheimer G, Li W. Classifying illusory contours: edges defined by “pacman” and monocular tokens. J Neurophysiol 77: 731–736, 1997 [DOI] [PubMed] [Google Scholar]
- Westheimer G, Wehrhahn C. Real and virtual borders in the Poggendorf illusion. Perception 26: 12, 1997 [DOI] [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: I. Fitting, sampling and goodness-of-fit. Percept Psychophys 63: 1293–1313, 2001a [DOI] [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: II. Bootstrap-based confidence intervals and sampling. Percept Psychophys 63: 1314–1329, 2001b [DOI] [PubMed] [Google Scholar]
- Williams CB, Hess RF. Relationship between facilitation at threshold and suprathreshold contour integration. J Opt Soc Am A Opt Image Sci Vis 15: 2046–2051, 1998 [DOI] [PubMed] [Google Scholar]
- Zhan CA, Baker CL. Boundary cue invariance in cortical orientation maps. Cereb Cortex 16: 896–906, 2006 [DOI] [PubMed] [Google Scholar]