Abstract
BACKGROUND:
Klebsiella oxytoca is a cause of antibiotic-associated hemorrhagic colitis. Few reports of the occurrence of K oxytoca within stool exist and there is no gold standard method for its isolation.
METHODS:
MacConkey agar was modified to culture K oxytoca. Ampicillin was added and adonitol was substituted for lactose. Rectal swabs from 200 patients being screened for vancomycin-resistant enterococci (VRE) and stool specimens from 429 patients who tested negative for Clostridium difficile cytotoxin were cultured. K oxytoca isolates were evaluated for cytotoxicity to HEp-2 cells. Available charts of K oxytoca-positive patients and a convenience sample of 93 K oxytoca-negative patients who underwent testing for C difficile cytotoxicity were reviewed retrospectively for documentation of bloody stool.
RESULTS:
K oxytoca was isolated from 14 of 200 patients (7.0%) being screened for VRE; only one of the 14 isolates (7.1%) was cytotoxic. The organism was isolated from 42 of 429 patients (9.8%) tested for C difficile cytotoxicity; 10 isolates (23.8%) were cytotoxic. Differences in isolation and cytotoxicity rates between groups were not statistically significant. Two of 13 (15.4%) K oxytoca-positive patients screened for VRE, three of 27 (11.1%) K oxytoca-positive patients tested for C difficile cytotoxicity, and 11 of 93 (11.8%) patients from the convenience sample had documented bloody stool.
CONCLUSIONS:
A medium that greatly facilitates isolation of K oxytoca was developed. Occurrence of K oxytoca colonization was similar in the two patient populations studied and isolation of cytotoxic K oxytoca was not usually associated with hematochezia. Current understanding of the occurrence and causality of antibiotic-associated hemorrhagic colitis is insufficient for clinical laboratories to begin culturing K oxytoca and testing for cytotoxicity.
Keywords: Adonitol, Antibiotic-associated hemorrhagic colitis (AAHC), Klebsiella oxytoca, MacConkey agar
Abstract
HISTORIQUE :
Le Klebsiella oxytoca est une cause de colite hémorragique secondaire aux antibiotiques. Peu de rapports portent sur la présence de K oxytoca dans les fécès, et il n’existe pas de méthode de référence pour l’isoler.
MÉTHODOLOGIE :
Les auteurs ont modifié l’agar de MacConkey pour cultiver le K oxytoca. Ils y ont ajouté de l’ampicilline et ont substitué l’adonitol au lactose. Ils ont mis en culture des écouvillons rectaux prélevés sur 200 patients dépistés pour des entérocoques résistants à la vancomycine (ERV) et effectué des coprocultures prélevées sur 429 patients négatifs à la cytotoxine du Clostridium difficile. Ils ont évalué la cytotoxicité aux cellules HEp-2 des isolats de K oxytoca. Ils ont procédé à l’examen rétrospectif des dossiers disponibles de patients positifs au K oxytoca et un échantillon de commodité de 93 patients négatifs au K oxytoca ayant subi un test de cytotoxicité au C difficile afin de documenter les selles sanguinolantes.
RÉSULTATS :
Les auteurs ont isolé le K oxytoca chez 14 des 200 patients (7,0 %) dépistés pour l’ERV. Un seul de ces 14 isolats (7,1 %) était cytotoxique. Ils ont isolé l’organisme chez 42 des 429 patients (9,8 %) ayant subi un test de cytotoxicité au C difficile. Dix isolats (23,8 %) étaient cytotoxiques. Les différences dans les taux d’isolation et de cytotoxicité entre les groupes n’étaient pas statistiquement significatives. Deux des 13 patients (15,4 %) positifs au K oxytoca dépistés pour l’ERV, trois des 27 patients (11,1 %) positifs au K oxytoca ayant subi un test de cytotoxicité au C difficile et 11 des 93 patients (11,8 %) de l’échantillon de commodité avaient des selles sanguinolantes.
CONCLUSIONS :
Les auteurs ont mis au point un médium qui facilitait grandement l’isolation du K oxytoca. L’occurrence de colonisation par le K oxytoca était similaire dans les deux populations de patients à l’étude. D’ordinaire, l’isolation du K oxytoca ne s’associait pas à l’hématochézie. Les connaissances actuelles au sujet de l’occurrence et de la causalité de la colite hémorragique secondaire aux antibiotiques sont insuffisantes pour que les laboratoires cliniques se mettent à cultiver le K oxytoca et à effectuer des tests de cytotoxicité.
Antibiotic-associated hemorrhagic colitis (AAHC) was first described in 1978 (1). The condition has been associated with beta-lactam, quinolone and pristinamycin therapy (2–4). AAHC usually resolves rapidly following discontinuation of the inciting antibiotic (4,5).
The accumulated evidence implicates Klebsiella oxytoca as a probable cause of AAHC (2,5–12). This hypothesis was recently confirmed when Koch’s postulates were fulfilled (4). The pathogenic mechanism by which K oxytoca causes hemorrhagic colitis is thought to involve a chromosomally encoded low-molecular-weight toxin that inhibits nucleic acid synthesis (13,14). The cytotoxin has been shown to induce mucosal hemorrhage in rabbit ileum and is toxic to HEp-2, Vero, CHO and HeLa cells (13,15). K oxytoca also produces a chromosomally encoded beta-lactamase that renders it resistant to aminopenicillins. Therapy with these antibiotics and others to which K oxytoca is resistant presumably contributes to its overgrowth and the development of AAHC.
The ability of penicillins to select for K oxytoca has been exploited by researchers to more easily culture the organism from stool (5). However, this technique still allows for the growth of a significant number of aerobic stool organisms in addition to K oxytoca. We formulated a modified version of MacConkey agar that facilitates the selection and differentiation of Klebsiella species from other fecal flora.
We used this modified MacConkey agar and a simple screening process to determine the background prevalence of both cytotoxic and noncytotoxic K oxytoca in patients at our institution undergoing screening for other pathogens both related and unrelated to antibiotic-associated diarrhea. Few studies have investigated the prevalence of K oxytoca in stool, yet such knowledge is necessary to interpret the significance of cultures performed in patients with suspected K oxytoca-associated hemorrhagic colitis and to determine if the spectrum of illness caused by K oxytoca extends beyond frankly hemorrhagic diarrhea.
METHODS
Patient selection
Two hundred rectal swabs submitted for vancomycin-resistant enterococci (VRE) screening and 429 Clostridium difficile cytotoxin-negative stool specimens were screened for K oxytoca. Subsequent specimens from previously screened patients were excluded. Specimens were submitted to the microbiology laboratory at the Queen Elizabeth II Health Sciences Centre in Halifax, Nova Scotia, between June and August, 2007. Approval to use clinical specimens for the present study and to review the clinical records was granted by the Capital Health Research Ethics Board.
Culture and screening for K oxytoca
MacConkey agar was modified from the common formulation (16) in two ways: the agar was supplemented with 30 mg/L of ampicillin, and 10 g/L of adonitol was substituted for lactose. Colonies that appeared pink or red, indicating adonitol fermentation, were subcultured onto blood agar and an indole spot test was performed. Positive colonies were further screened with a motility-indole-ornithine test, a triple sugar iron test and a phenylalanine deaminase test. These tests were performed as described in the Clinical Microbiology Procedures Handbook (17). Bacterial isolates that exhibited typical reactions were identified using the Vitek 1 automated instrument (bioMérieux Canada, Inc). Bacterial isolates were considered to be K oxytoca if identified as such by Vitek 1 with at least 90% confidence.
Cytotoxicity assays
C difficile cytotoxicity assays were performed using standard methods. Briefly, cell-free extract from each stool sample was introduced into two microtitre wells lined by human foreskin cell monolayers: one well with C difficile-neutralizing antitoxin (TechLab, USA) and the other without. Samples were considered to be C difficile cytotoxin-positive if cytopathic effects were noted by 48 h in wells without antitoxin and absent in wells containing antitoxin.
K oxytoca cytotoxicity assays were performed using previously described methods (4,5,18). HEp-2 monolayers were assessed for cell rounding and cell death at 24 h and 48 h. K oxytoca 06/19 O was used as a positive control and K oxytoca ATCC 13182 (Cedarlane Laboratories, Canada) was used as a negative control.
Chart review
Medical records for a subset of the patients colonized with K oxytoca were reviewed retrospectively and the incidence of diarrhea and bloody stool was noted. Data were available for 13 K oxytoca-positive patients who were screened for VRE and 27 K oxytoca-positive patients who underwent C difficile cytotoxicity testing. In addition, records of a convenience sample of 93 K oxytoca-negative patients who were also negative for C difficile cytotoxin were reviewed.
Statistical methods
Significance of association between variables was measured using χ2 analysis, except when an expected value was less than 10, in which case Fisher’s exact test was employed.
RESULTS
Prevalence of K oxytoca
K oxytoca was isolated from 14 of 200 patients (7.0%) tested for VRE and 42 of 429 patients (9.8%) screened for C difficile cytotoxicity (P>0.25), as shown in Table 1. One of the 14 isolates (7.1%) from patients screened for VRE and 10 of the 42 isolates (23.8%) from patients tested for C difficile cytotoxicity were cytotoxic to HEp-2 cells (P>0.25), as shown in Table 2.
TABLE 1.
Klebsiella oxytoca culture positivity among patients screened for vancomycin-resistant enterococci (VRE) or Clostridium difficile cytotoxicity
| Group | n | K oxytoca-positive, n (%) | 95% CI |
|---|---|---|---|
| VRE screened | 200 | 14 (7.0) | 4.1% to 11.5% |
| C difficile screened | 429 | 42 (9.8) | 7.3% to 13.0% |
TABLE 2.
Klebsiella oxytoca cytotoxicity to HEp-2 cells among isolates from patients screened for vancomycin-resistant enterococci (VRE) or Clostridium difficile cytotoxicity.
| Group | n | Cytotoxic to HEp-2 cells, n (%) | 95% CI |
|---|---|---|---|
| VRE screened | 14 | 1 (7.1) | <0.1% to 33.5% |
| C difficile screened | 42 | 10 (23.8) | 13.3% to 38.7% |
Selective and differential capability of MaConkey adonitol media and screening process
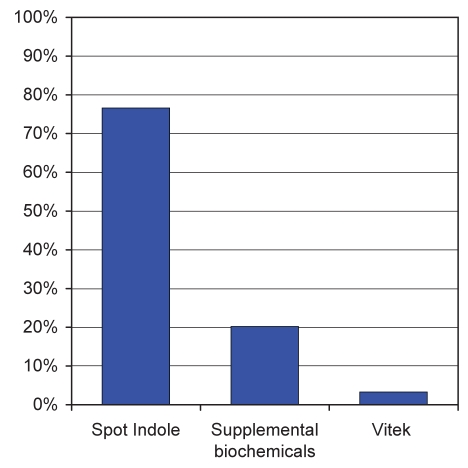
The substitution of adonitol for lactose within MacConkey agar allowed for the differentiation of K oxytoca from various other Gram-negative ampicillin-resistant organisms. A total of 222 of the 629 specimens (35.3%) examined grew one or more adonitol-negative colony morphotype, allowing ready differentiation from K oxytoca. One or more adonitol fermenting bacterial strain other than K oxytoca was isolated from 243 (38.6%) of the specimens screened; 187 (77.0%) of these specimens were excluded based on negative spot indole tests and a further 49 (20.2%) because of atypical biochemical reactions (Figure 1). Sixty-seven bacterial isolates from 66 clinical specimens were subjected to definitive examination by Vitek 1; only eight of these isolates were not K oxytoca. Other organisms isolated included Raoultella ornithinolytica, Escherichia hermannii, Escherichia coli and Enterobacter aerogenes.
Figure 1).
A total of 243 adonitol-positive specimens were excluded as possibly containing Klebsiella oxytoca by a sequential screening process that included the following tests in order: spot indole, growth in differential biochemicals (motility-indole-ornithine, triple sugar iron and phenylalanine deaminase media), and speciation by Vitek 1. The bars shown here represent the proportion of these 243 specimens that were excluded at each step of the screen
Chart review
Only two of 13 K oxytoca-positive patients (15.4%) from the group screened for VRE and three of 27 (11.1%) from the group screened for C difficile cytotoxicity, included in the chart review, had documentation of visibly bloody stool (Table 3). None of the five patients included with cytotoxic K oxytoca strains from the group tested for C difficile cytotoxicity had documented bloody stool. Blood was noted in the stool of 11 of 93 K oxytoca-negative control patients (11.8%) from the group tested for C difficile cytotoxicity. All 93 control patients and 27 K oxytoca-positive patients tested for C difficile cytotoxicity were presumed to have diarrhea as an indication for C difficile cytotoxicity testing. Only one patient in the K oxytoca-positive group screened for VRE had documented diarrhea.
TABLE 3.
Summary data for patients included in the chart review
| VRE screened | Clostridium difficile screened | Convenience sample | |
|---|---|---|---|
| Klebsiella oxytoca colonization status of group members | Positive | Positive | Negative |
| n | 13 | 27 | 93 |
| Bloody stool, n (%) | 2 (15.4) | 3 (11.1) | 11 (11.8) |
| Mean age, years | 65.8 | 68.8 | 64.3 |
| Male, n (%) | 7 (53.8) | 11 (40.7) | 40 (43.0) |
| Hospitalized at diarrhea onset, n (%) | – | 17 (63.0) | 42 (45.2) |
VRE Vancomycin-resistant enterococci
The three groups examined did not differ significantly with respect to age or sex, as shown in Table 3. Onset of diarrhea occurred in hospital for 17 K oxytoca-positive patients (63%) in the group screened for C difficile cytotoxicity and 42 K oxytoca-negative patients (45.2%) from the convenience sample (P>0.1).
DISCUSSION
Koch’s postulates were recently fulfilled to confirm that K oxytoca causes at least some cases of AAHC (4). We have used a modified form of MacConkey agar to elucidate the background prevalence of the organism by screening patients undergoing testing unrelated to AAHC (VRE screening). This is the first report of the prevalence of K oxytoca fecal colonization at a centre in North America. Previous studies in Austria and France have reported the prevalence of K oxytoca colonization in asymptomatic individuals as 1.6% and 9.0%, respectively (4,5). The latter study found 42.9% of K oxytoca isolates from asymptomatic individuals to be cytotoxic to HEp-2 cells (5). We isolated K oxytoca from 14 of 200 patients (7.0%) being screened for VRE; one isolate (7.1%) was cytotoxic to HEp-2 cells. These results are similar to those of a recent series that identified one cytotoxic and four noncytotoxic K oxytoca isolates in a cohort of 111 hospitalized patients with no associated antibiotic exposure or active diarrhea (19).
We also identified K oxytoca in the stool of 42 of 429 patients (9.8%) who had specimens submitted for C difficile cytotoxicity testing. We assume that the primary indication was clinical suspicion of antibiotic-associated diarrhea. Ten (23.8%) of these isolates were cytotoxic to HEp-2 cells. There was a trend toward more frequent isolation of cytotoxin-producing strains of K oxytoca in the group tested for C difficile-cytotoxicity relative to those screened for VRE, but it did not approach statistical significance (P>0.25). It is likely that most patients tested for C difficile cytotoxicity had recent antibiotic treatment, but this did not appear to increase the likelihood of K oxytoca-positive cultures in this population. Hospital records of prior antimicrobial use were incomplete and were not incorporated into this analysis.
The proportion of K oxytoca-positive patients for whom bloody stool was noted in the clinical record did not differ significantly between those screened for C difficile cytotoxicity relative to VRE. Nor did these rates differ significantly from the incidence of bloody stool within the K oxytoca-negative control population. None of the five patients with cytotoxic K oxytoca from the group screened for C difficile cytotoxicity whose charts were available had a notation of bloody stool in their record. Others have also observed that cytotoxic variants of K oxytoca are often isolated in the absence of disease (5). An alternative hypothesis is that the spectrum of diarrheal disease caused by cytotoxin-producing K oxytoca extends beyond AAHC; although the best evidence accumulated to date suggests otherwise, the possibility of such an association cannot be conclusively rejected (19). It is also possible that hematochezia was present in some of our patients but was not documented.
This is the first report of the use of a selective and differential growth medium incorporating adonitol for the isolation of Klebsiella species from stool. There is currently no gold standard growth medium used for this purpose. The most recent large-scale study in which K oxytoca was cultured from stool did not use growth medium with ampicillin or any other antibiotic (4). Because K oxytoca constitutively expresses a beta-lactamase and overgrowth of stool flora can decrease bacterial isolation rates, it is likely that the use of ampicillin here and by others (5) improves detection of K oxytoca within stool.
The differential property of our modified MacConkey agar also facilitated the isolation of K oxytoca from stool. Because K oxytoca (and other Klebsiella species) ferments adonitol and produces pink/red colonies on this modified MacConkey agar, the medium allows one to differentiate K oxytoca from organisms that do not ferment adonitol. This property allowed us to exclude bacterial colonies as possible K oxytoca isolates in 35.3% of the fecal specimens examined and decrease resource utilization. We have also demonstrated a simple screening process that allowed for the elimination of 97.2% of fecal specimens containing adonitol-positive isolates other than K oxytoca without the need for automated testing. The majority of such specimens (77%) were eliminated using simple spot indole tests.
We have determined the background prevalence of cytotoxic and noncytotoxic K oxytoca in stool and demonstrated a growth medium and screening process combination that allows for easy isolation of the organism. Such studies are necessary to facilitate the interpretation of positive K oxytoca cultures and cytotoxicity assays. Our data clearly show that both cytotoxic and noncytotoxic forms of K oxytoca are common within our institution. These results suggest that current understanding of the occurrence and causality of AAHC is insufficient for clinical laboratories to begin culturing K oxytoca and testing for cytotoxicity; positive and negative predictive values remain unknown.
REFERENCES
- 1.Toffler RB, Pingoud EG, Burrell MI. Acute colitis related to penicillin and penicillin derivatives. Lancet. 1978;312:707–9. doi: 10.1016/s0140-6736(78)92704-6. [DOI] [PubMed] [Google Scholar]
- 2.Benoit R, Dorval D, Loulergue J, et al. Post-antibiotic diarrheas: Role of Klebsiella oxytoca. Gastroenterol Clin Biol. 1992;16:860–4. [PubMed] [Google Scholar]
- 3.Koga H, Aoyagi K, Yoshimura R, Kimura Y, Iida M, Fujishima M. Can quinolones cause hemorrhagic colitis of late onset? Report of three cases. Dis Colon Rectum. 1999;42:1502–4. doi: 10.1007/BF02235056. [DOI] [PubMed] [Google Scholar]
- 4.Hogenauer C, Langner C, Beubler E, et al. Klebsiella oxytoca as a causative organism of antibiotic-associated hemorrhagic colitis. N Engl J Med. 2006;355:2418–26. doi: 10.1056/NEJMoa054765. [DOI] [PubMed] [Google Scholar]
- 5.Beaugerie L, Metz M, Barbut F, et al. Klebsiella oxytoca as an agent of antibiotic-associated hemorrhagic colitis. Clin Gastroenterol Hepatol. 2003;1:370–6. doi: 10.1053/s1542-3565(03)00183-6. [DOI] [PubMed] [Google Scholar]
- 6.David X, Pierrugues R, Michel H. Acute hemorrhagic colitis related to oral administration of cephalosporin. Gastroenterol Clin Biol. 1991;15:659. [PubMed] [Google Scholar]
- 7.Blanchi A, Pariente A. Acute hemorrhagic colitis after ingestion of amoxicillin. Gastroenterol Clin Biol. 1992;16:1012–4. [PubMed] [Google Scholar]
- 8.Gineston JL, Watine J, Bruna T, Lamblin G, Dubourdieu B. Acute hemorrhagic colitis caused by pristinamycin: Two cases with association of Klebsiella oxytoca and Clostridium difficile. Gastroenterol Clin Biol. 1993;17:773–5. [PubMed] [Google Scholar]
- 9.Fort E, Sevestre C, Cahiez M, Treppoz M, Dorval ED. Acute hemorrhagic colitis after oral ingestion of synergistin. Gastroenterol Clin Biol. 1993;17:231–2. [PubMed] [Google Scholar]
- 10.Grando V, Bellaiche G, Le Pennec MP, et al. Ulcerative hemorrhagic proctitis caused by Klebsiella oxytoca after treatment with an amoxicillin-clavulanic acid combination. Gastroenterol Clin Biol. 1994;18:536–7. [PubMed] [Google Scholar]
- 11.Bellaiche G, Le Pennec MP, Choudat L, Ley G, Slama JL. Value of rectosigmoidoscopy with bacteriological culture of colonic biopsies in the diagnosis of post-antibiotic hemorrhagic colitis related to Klebsiella oxytoca. Gastroenterol Clin Biol. 1997;21:764–7. [PubMed] [Google Scholar]
- 12.Chida T, Nakaya R, Tsuji M, et al. Intestinal microflora of patients with antibiotic-associated hemorrhagic colitis associated with Klebsiella oxytoca and Clostridium difficile enterotoxin. Kansenshogaku Zasshi. 1986;60:608–15. doi: 10.11150/kansenshogakuzasshi1970.60.608. [DOI] [PubMed] [Google Scholar]
- 13.Minami J, Okabe A, Shiode J, Hayashi H. Production of a unique cytotoxin by Klebsiella oxytoca. Microb Pathog. 1989;7:203–11. doi: 10.1016/0882-4010(89)90056-9. [DOI] [PubMed] [Google Scholar]
- 14.Minami J, Saito S, Yoshida T, Uemura T, Okabe A. Biological activities and chemical composition of a cytotoxin of Klebsiella oxytoca. J Gen Microbiol. 1992;138:1921–7. doi: 10.1099/00221287-138-9-1921. [DOI] [PubMed] [Google Scholar]
- 15.Minami J, Katayama S, Matsushita O, Sakamoto H, Okabe A. Enterotoxic activity of Klebsiella oxytoca cytotoxin in rabbit intestinal loops. Infect Immun. 1994;62:172–7. doi: 10.1128/iai.62.1.172-177.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atlas RM. Handbook of Microbiological Media. 3rd edn. CRC Press; 2004. [Google Scholar]
- 17.Isenberg HD. Clinical Microbiology Procedures Handbook. 2nd edn. ASM Press; 2004. [Google Scholar]
- 18.Higaki M, Chida T, Takano H, Nakaya R. Cytotoxic component(s) of Klebsiella oxytoca on HEp-2 cells. Microbiol Immunol. 1990;34:147–51. doi: 10.1111/j.1348-0421.1990.tb00999.x. [DOI] [PubMed] [Google Scholar]
- 19.Zollner-Schwetz I, Högenauer C, Joainig M, et al. Role of Klebsiella oxytoca in antibiotic-associated diarrhea. Clin Infect Dis. 2008;47:e74–8. doi: 10.1086/592074. [DOI] [PubMed] [Google Scholar]