Abstract
Mammalian cloning by nuclear transfer from somatic cells has created new opportunities to generate animal models of genetic diseases in species other than mice. Although genetic mouse models play a critical role in basic and applied research for numerous diseases, often mouse models do not adequately reproduce the human disease phenotype. Cystic fibrosis (CF) is one such disease. Targeted ablation of the cystic fibrosis transmembrane conductance regulator (CFTR) gene in mice does not adequately replicate spontaneous bacterial infections observed in the human CF lung. Hence, several laboratories are pursuing alternative animal models of CF in larger species such as the pig, sheep, rabbits, and ferrets. Our laboratory has focused on developing the ferret as a CF animal model. Over the past few years, we have investigated several experimental parameters required for gene targeting and nuclear transfer (NT) cloning in the ferret using somatic cells. In this review, we will discuss our progress and the hurdles to NT cloning and gene-targeting that accompany efforts to generate animal models of genetic diseases in species such as the ferret.
Introduction
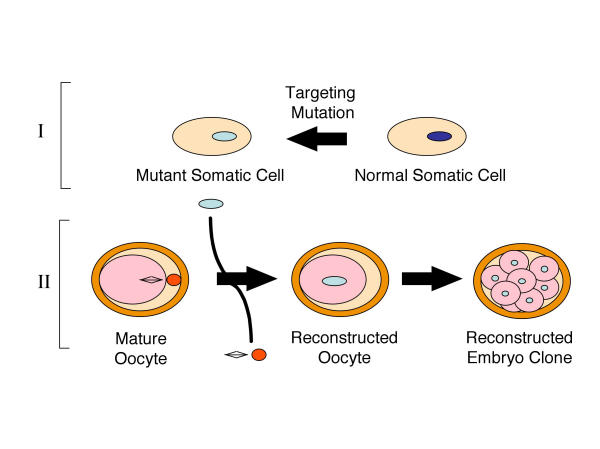
Until recently, the generation of gene-targeted animal models has primarily relied upon homologous recombination following direct introduction of transgenes into embryonic stem cells (ES cells). While this technique has been successful for animal modeling in the mouse, it has thus far proven significantly more difficult in larger species. To date, the most exciting and promising research in transgenesis involves the use of fetal and adult somatic cells to produce genetically identical animals through nuclear transplantation [1,2]. Successful production of cloned animals derived from somatic cells was first demonstrated in sheep [3,4] and has more recently been demonstrated in mice [5], cattle [6], goats [7], pigs [8], cats [9], rabbits, [10] and mules [11]. Transgenic calves [12], gene-targeted sheep [13], and α-1,3-galactosyltransferase knockout pigs [14,15] have also been obtained by nuclear transfer from somatic cells. These successes have made animal modeling using nuclear transfer in less-studied species, such as the ferret, more feasible. Since somatic cell nuclear donors can be easily maintained in vitro and readily targeted for gene mutations, somatic cell-based embryo cloning is undoubtedly the future method of choice for generating genetically modified larger animals. Two major steps are required to clone genetically defined animals (Figure 1). First, gene targeting must be achieved in a somatic cell type appropriate for nuclear cloning, and karyotypically normal clonal cell lines must be isolated. Second, the nucleus from the mutant somatic cell must be used to reprogram enucleated recipient oocytes. The reconstructed embryos must then be implanted in foster mothers to generate cloned genetically defined animals.
Figure 1.
General strategy for generating gene-targeted animal models using somatic cell nuclear cloning. Two major steps include: 1) Targeting gene mutations in appropriate somatic cell nuclear donor and 2) embryo reconstruction and cloning of animal.
CF is a recessive inherited genetic disease in the Caucasian population, with a frequency of about 1 in 3,000 newborns [16]. CF is caused by a defect in an epithelial chloride channel called the cystic fibrosis transmembrane conductance regulator (CFTR) [16]. CF patients suffer from recurrent bacterial infection in the lung, leading to bronchiectasis, compromised lung function, and ultimately death. Substantial efforts have been made to generate mouse models capable of reproducing the lung pathology seen in CF patients. However, due to differences in lung biology between mice and humans, CFTR-deficient and mutant mice do not develop spontaneous lung disease as seen in humans [17,18]. This lack of appropriate CF animal models has hindered progress in the development and testing of therapies for this disease.
The domestic ferret, Mustela Putorius Furos, has proven to be an excellent animal model for studying CFTR lung biology. Several aspects of ferret lung biology make this species an attractive model for CF lung disease. First, in contrast to mice, the ferret has marked similarities to humans in terms of lung physiology, airway morphology, and cell types [19-24]. Second, the expression of CFTR in the ferret airway epithelium and submucosal glands is identical to that in humans [25,26]. Third, amino acid identity between ferret and human nucleotide binding domain 1 (NBD1) of CFTR is a striking 97% [25], which is just as high as for non-human primates (96%, Macaca nemestrina) and is significantly higher than for rodents (80%, rat and mouse). Fourth, the ferret had been a useful model for viral and bacterial lung infections seen in humans [27-32]. Lastly, the ferret, with a gestation period of 42 days and 6 months to sexual maturity, has obvious advantages over larger species for animal modeling.
Strategy and Progress in Cloning a CF Ferret
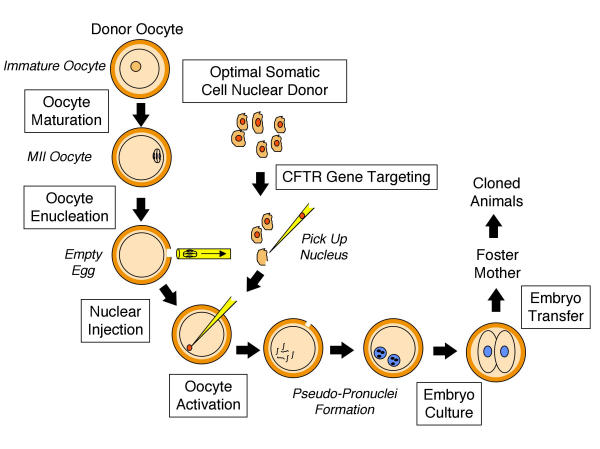
Unlike sheep and cattle, for which NT cloning procedures are well established, cloning in the ferret presents numerous challenges since experimental parameters for embryo manipulation have not been defined. Over the past few years, several of the critical steps required for NT cloning in the ferret have been established [33-35]. This review will focus on progress in the various steps highlighted in Figure 2. Furthermore, we will discuss approaches currently under investigation to facilitate efficient gene targeting in somatic cells, a process required to ultimately clone a CF ferret.
Figure 2.
The important steps involved in somatic cell nuclear cloning are shown schematically. Progress in each of the boxed methodologies will be discussed in detail in subsequent sections [33-35]. Italicized labels indicate descriptive markings, not methodological steps per se.
In Vitro Culture of Ferret Embryos and Successful Production of Offspring from Implanted Embryos
A critical first step toward genetic manipulation in a new species is defining the parameters required for embryo culture and adoptive transfer into pseudopregnant recipient females. Optimal superovulation in the ferret (19.3 ± 0.6 oocytes and/or embryos per female) was achieved by a combination of hormonal injections, including 100 IU eCG and 150 IU hCG at 72-hr intervals [33]. This ovulation rate is more than double that induced by mating. Mating with a male immediately following hCG injection did not significantly alter the ovulated number of oocytes and/or embryos, indicating that mating is not required for superovulation in ferrets. Of embryos harvested at the one-cell stage, 64.5% and 47.1% developed into blastocysts when cultured in vitro in CZB or TCM-199 plus 10% fetal bovine serum (FBS) media, respectively. In contrast, only 17.1% of embryos cultured in vitro in NCSU-23 developed to the blastocyst stage. Both freshly retrieved and in vitro cultured embryos from cinnamon coat–colored parents produced live young when transferred at the 8-cell stage into albino coat–colored, pseudopregnant recipients. The percentage of kits delivered relative to embryos transferred was 61% when freshly retrieved embryos were used and 32% when in vitro cultured embryos were used. The most important achievement of these studies was the successful birth of cinnamon coat–colored pups from an albino female. These results demonstrate successful embryo transfer in the ferret and open the door to animal modeling with this species following embryo manipulation [33].
Conditions for In Vitro Maturation and Parthenogenetic Activation of Ferret Oocytes
One of the most critical and difficult steps in successful cloning of any species is the reprogramming of oocytes to divide in the absence of fertilization. Optimization of this step, termed parthenogenetic activation, is very empirical, and it varies greatly between species. To increase the yield of oocytes for NT cloning, ovaries are often harvested and immature oocytes extracted for use. This method requires a step involving in vitro maturation (IVM), which must also be optimized prior to activation. The success of activation is closely linked to the quality of IVM oocytes. To this end, we have optimized conditions for in vitro maturation and parthenogenetic activation of ferret haploid oocytes.
Immature oocytes (cumulus-oocyte complexes) harvested from ovaries of superovulated ferrets have been evaluated for in vitro maturation conditions using several types of media [34]. The optimal media for maturation of ferret oocytes consisted of TCM-199 containing 10% FBS, 10 IU/ml of eCG, and 5 IU/ml of hCG. In this media, the maturation rate of ferret oocytes was 72% at 24 hrs of IVM. Optimization of oocyte activation was evaluated using both electrical and chemical stimuli individually or in combination. Treatment with cycloheximide (5 μg/ml, 5 min) and 6-dimethylaminopurine (6-DMAP, 2 mM/ml, 4 h) following electrical stimulation (an alternating current pulse of 3 V for 5 secs, followed by one direct current pulse of 180 V/mm for 30 μsec) resulted in 43% of the oocytes developing to the blastocyst stage. Such an activation rate represented a significant improvement over those obtainable under other tested conditions, including individual treatment with electrical pulses (10%), cycloheximide (3%), or 6-DMAP (5%). Blastocysts derived from in vitro activation appeared to be normal morphologically and were composed of an appropriate number of both inner cell mass (10.3 ± 1.1) and trophectoderm (60.8 ± 2.9) cells when they were examined using a technique [36] that differentially stains the inner cell mass (ICM) and trophectoderm (TE) layer [34].
Developmental Capacity of Ferret Embryos by Nuclear Transfer Using G0/G1-Stage Fetal Fibroblasts
With the ultimate goal of establishing experimental protocols necessary for cloning ferrets, we have begun to examine parameters for ferret cloning by nuclear transfer using G0/G1-stage donor fetal fibroblasts [35]. Cumulus-oocyte complexes were harvested from ovaries of superovulated ferrets and cultured in a maturation medium for 24 hrs. Following removal of cumulus cells, oocytes with normal morphology, uniform cytoplasm, and a first polar body were enucleated with a 25 μm (inside diameter, ID) glass pipette. Critical to successful embryo reconstruction by nuclear transfer is the complete removal of oocyte chromosomal DNA. Fluorescent DNA dye (such as Hoechst 33342) can be used to practice and evaluate enucleation efficiencies. However, these stains cannot be used during cloning since they affect the viability of the reconstructed embryo. Since the removal of too much of the oocyte cytoplasm also leads to poor cloning efficiencies, it is critical that the individual performing the work optimizes this step. The proportion of completely enucleated ferret oocytes was 80.8% ± 2.6 (n = 82) in the studies discussed below.
Following optimization of enucleation procedures, ferret fetal fibroblast cells (serum-starved for 14–16 hrs prior to NT) were injected directly into enucleated oocytes with a 10 μm (ID) PiezoDrill glass pipette. Reconstructed embryos were then activated by a combination of electrical pulses and chemical stimulations. Subsequently, the reconstructed and activated embryos were either cultured in vitro or transferred to pseudopregnant ferrets to evaluate their development capabilities in vitro and in vivo. Our results demonstrated that 56.3% of reconstructed embryos cleaved, while 26.0% and 17.6% developed to morula and blastocyst stages in vitro, respectively [35]. The blastocysts derived from NT embryos demonstrated normal morphology by differential staining and also contained cell numbers appropriate for normal blastocysts developed in vitro. In vivo developmental studies at 21 days post-transplantation demonstrated 8.8% of reconstructed embryos implanted into the uterine lining of recipients, while 3.3% formed fetuses (Figure 3). However, reconstructed embryos failed to develop to term (42 days). These results demonstrate that donor nuclei of G0/G1-stage fetal fibroblast cells can be reprogrammed to support the development of reconstructed ferret embryos in vitro and in vivo [35].
Figure 3.
Implantation and fetal development of NT-reconstructed ferret embryos. (A and B) Ovaries, oviducts and the uteri from two recipient albino ferrets were dissected at 21 days following transfer of ~25 NT-reconstructed embryos. The swollen regions of the uterus (denoted by arrows) are regions of implanted NT-reconstructed embryos. Recipient albino Jills were mated with vasectomized (sterile) male albino studs. Vasectomized studs were proven sterile by sperm count and mating. Albino oocytes were reconstructed with sable × cinnamon F1 fetal ferret fibroblasts. (C) Fetuses marked 1 and 2 were dissected from the swollen regions of the uterus in Panel B. (D) Normal 3-week ferret fetus developed from in vivo fertilization by normal mating. Embryo #1 appears to have normal 21-day development and is approximately 1.5 cm on the longest axis. Scale marker: Panels A and B, 1.5 cm; Panels C and D, 0.5 cm. Reproduced with permission from a published manuscript by Li et al [35].
One facet of cloning that has been highly variable between species is the narrow window of post-mating uterine development that is receptive for implantation of NT-reconstructed embryos. These differences are due to the developmental delay caused by NT reconstruction of embryos. To solve this problem, researchers have utilized asynchronous breeding schedules of the oocyte donors and the female recipients used for implantation of NT-reconstructed embryos [10]. Current efforts attempting to clone ferrets to live birth are optimizing asynchronous breeding schedules to improve survival in vivo.
Allele-Specific Targeting of Single Base-Pair Changes in Somatic Cells with Chimeric RNA/DNA Oligonucleotides
In addition to embryo manipulation procedures in the ferret, efficient strategies for introducing mutations into the ferret CFTR gene must be developed in order to generate appropriate somatic cell donors for NT cloning. Several criteria are important when considering the appropriate targeting strategy for somatic cells. First, the strategy cannot be dependent on expression of the target gene (ie., gene targeting using promoter-trapping) since the CFTR gene is not expressed in fibroblasts. Second, since low-passage somatic cells are preferred for NT cloning, the targeting strategy should be efficient and optimally not require selective pressure. Third, a highly sensitive and efficient screening method must be developed to isolate gene-targeted clonal cell lines at early passage. One advantage of NT cloning for generating genetically defined animal models is that theoretically, relatively few somatic cell donors are required to carry out the procedure. Hence, we have focused on developing methods based on nuclear injection of gene-targeting agents, followed by high-throughput screening of single-cell clones in 96-well plates for successful gene alterations. One suitable targeting agent we are currently evaluating utilizes chimeric RNA/DNA oligonucleotides (also called chimeraplasts).
To establish our ability to target the CFTR gene and rapidly screen large numbers of clonal cell lines, we began evaluating chimeraplasts in cell systems already established to target at high efficiencies. Chimeraplasts are self-folding RNA/DNA duplexes that create hairpin ends with flanking homology (10–30 bp) to the target base. Chimeraplasts have been reported to introduce single base-pair changes at the β-globin locus at efficiencies between 1 and 5% in Hela cells [37] and as high as 10% in other systems [38,39]. Since chimeraplasts are most efficient at introducing single base-pair alterations, we have evaluated the ability of chimeraplasts to target the G551D mutation to the CFTR gene that requires only a single-base alteration. With the ultimate goal of generating ferret fibroblast cell lines heterozygous for the G551D mutation, we first sought to develop targeting and screening methodologies in Hela cells. Since such targeting methods are non-selective, it is imperative that the targeting efficiency be ≥ 0.1% so that suitable somatic clones can be identified from a pool of fewer than 1000 cells.
Initially, we based our chimeraplast design on the original reports describing this technology [38,39]. The non-coding strand in the oligonucleotide contains two 10–15-nt long, 2'-O-methyl RNA stretches, flanking a 5 bp DNA sequence, with the mutation to be generated in the central position. A 68-mer chimeric oligonucleotide, shown in Figure 4a, was synthesized and purified by IDT (Iowa City, IA) and transfected into Hela cells using standard Lipofectamine (Gibco)-mediated methods. Although direct nuclear injection was used in similar targeting strategies for primary ferret fetal fibroblasts, transfection efficiencies in Hela cells were high enough to work out the methodologies of screening. The efficiency of transfection, which was tested with a FITC-labeled oligonucleotide of similar length, was demonstrated to be >95% (data not shown). Specifically, 1.8 μg of chimeraplasts were transfected into 50% confluent 5 × 104 Hela cells in 200 μl serum-free DMEM medium/lipofectamine reagent at RT for 30 min in a 24-well plate. The cells were allowed to grow to confluence following the addition of 10% FBS. They were then trypsinized and serially diluted into 96-well plates for cloning. The cell concentration was determined before seeding so that only 50% of the wells would lead to the clonal outgrowth of a single cell. After the wells reached confluence, approximately 5000 cells were removed by scraping with a multi-channel pipette. The remaining cells were fed fresh media and allowed to grow back to confluence. Harvested cells from each clone were then pelleted and lysed directly in 60 μl of denaturing buffer containing 500 mM NaOH, 2.0 M NaCl, and 25 mM EDTA. After boiling for 5 min, 6 μl of 1 M Tris-Cl was added to cell lysates for neutralization. Nested PCR was then performed with 6 μl of the neutralized lysates as templates, using the following primers:
Figure 4.
Chimeraplast targeting of the G551D mutation to the human CFTR gene in Hela cells. (A) The 25 bp segment homologous to CFTR is bolded, with the mutated nucleotide (G → A) indicated by asterisks. This structure is based on the traditional design with a 5 bp DNA (capitalized and underlined) core region flanked by two 10-nt 2'-O-methyl RNA stretches (lowercased). Only one base-pair change exists in parentheses for generating such an oligo for ferret CFTR. (B, C) Cell lysates prepared with 44 targeted Hela cell clones after G551D chimeric oligonucleotide transfection were amplified by 2 rounds of PCR and analyzed by ASO hybridization against (B) wild-type CFTR and (C) G551D mutant oligonucleotide probes. Three of these cell clones (2b, 3c, and 6a) were highly positive for both the wild-type and the G551D genotype, as indicated by the asterisks. Positive plasmid cDNA controls for the wild-type (1e) and the G551D (1f) CFTR sequences were also run as standards. PCR blanks are shown in 1 g and 1 h. All other wells contain experimental Hela cell clone PCR material. Wells that showed no hybridization to either probe probably contained no DNA material, which was likely due to insufficient cells for efficient PCR.
1st round 5'-ACATTAGAAGGAAGATGTGCC-3'/5'-GTGCCTTTCAAATTCAGATTGAGC-3'
2nd round 5'-GGGCACAGATTCTGAGTAACC-3'/5'-AATGTGATTCTTAACCCACTAGCC-3'
One tenth of each PCR product was loaded onto a Nylon membrane using a slot-blotting apparatus and screened for mutations by allele-specific oligonucleotide (ASO) hybridization. The sequences for the wild-type and mutant ASO primers were as follows: 1) wild-type, GAGTGGAGGTCAACGAG, and 2) G551D mutant, GAGTGGAGATCAACGAG. Primers were end-labeled with [γ-32]ATP, and hybridization was performed overnight in a solution containing 3 M tetramethylammonium chloride (TMAC), 0.6% SDS, 1 mM EDTA, 10 mM Na3PO4 (pH 6.8), 5 × Denhardt, and 40 μg/ml yeast DNA. The blots were washed in 3 M TMAC, 1 mM EDTA, 10 mM Na3PO4 (pH 6.8), and 0.6% SDS twice at RT, followed by 2 more washes at 53°C. Membranes were then exposed to film. Since the G to A mutation leads to the loss of a Hindi restriction site and a gain of DpnI and DpnII sites, positive clones detected by ASO were confirmed by restriction digestion analysis following amplification of genomic DNA.
Since gene targeting efficiencies were expected to be fairly low, we initiated this line of research by developing methods for mutant screening that were amenable to analyzing 1000 cell clones. We settled on using a 96-well plate to screen crude cell lysates by PCR. These plates could also be replicated easily by simply scraping cells from one plate into another without the need for trypsinization. This approach will be of considerable benefit when applied to ferret fibroblasts since it requires minimal amplification of cell clones, and will thus likely increase cellular competence for NT experiments. A variety of lysis buffers were tested for efficient amplification of the target by nested PCR. The lysis buffer discussed above allowed successful PCR from as few as 1,000 cells. ASO screening of 44 single-cell clones generated from chimeraplast-transfected Hela cells resulted in 3 positive clones that strongly hybridized to the G551D mutant oligonucleotide (Figure 4b and 4c). Each of these targeted clones demonstrated the expected restriction site changes caused by a G → A conversion. In summary, we feel that this preliminary data has provided the means for rapid effective screening of targeted primary fibroblasts or any other cell type useful for NT cloning. The 7.6% targeting efficiency for the human CFTR gene found in this study also suggests that chimeraplasts may be a useful targeting strategy in the generation of mutant ferret somatic cells.
Although studies with Hela cells have helped establish the screening protocols for selecting CFTR-targeted clonal cell lines, approaches for targeting primary fetal ferret fibroblasts have proven more difficult due to low transfection efficiencies. To this end, a single-cell injection approach was adopted in which targeting agents can be injected directly into the nucleus of primary ferret fibroblasts. Studies utilizing a mutant EGFP(Y66S) fluorescent recovery assay were established to help define the optimal conditions for gene targeting with chimeraplasts in ferret fetal fibroblasts [40]. Both episomal mutant EGFP plasmids and mouse transgenic fetal fibroblasts expressing the mutant EGFP gene were used as targets for chimeraplasts. Since the mutant EGFP protein is non-fluorescent, the simple index of fluorescent recovery could be used to assess the efficiency of gene targeting. Results from this study demonstrated that chimeraplasts could efficiently (~1–2%) target correction of mutant episomal DNA targets. However, they failed to correct an integrated mutant EGFP gene in fetal fibroblasts from transgenic mice. Although these results are disappointing, several advances in small oligonucleotide targeting may help to improve the efficiency of this approach. Recent reports have suggested that the polarity of the DNA segment in the targeting construct affects the efficiency of gene targeting by more than 1000-fold [41]. We have currently only tested for base alteration targeted to the antisense strand of the target gene (i.e., chimeraplast DNA segments encode the mutation in the sense strand). However, the alternative design, placing mutations in the anti-sense strand of the targeting DNA oligonucleotide, has proven significantly more effective [41]. Such adaptations to the described approach may be used to increase targeting efficiencies for integrated genes in primary fibroblasts.
Future Challenges in Mammalian Cloning of Genetic Disease Models
Mammalian cloning has been accomplished in several mammalian species by nuclear transfer of somatic cells. However, widespread use of this technology has been limited due to low efficiencies of cloning to live births. At present, cloning efficiency – as determined by the proportion of live offspring developed from all oocytes that receive donor cell nuclei – is no more than 3%, regardless of the developmental age of the donor cell or the type of cell used [42].
The low efficiency associated with cloning may be attributed to many factors that are not fully understood, such as the oocyte-donor cell interaction [3], the stage of the donor cell cycle [4,43-46], the type of donor cell used [47,48], and inappropriate or incomplete nuclear reprogramming following nuclear transfer [49,50]. In addition, technical skill greatly contributes to the cloning success rate. Even the slightest damage to the donor cell (cytoplasm and/or nucleus) may render the nuclei incapable of participating in normal embryo development.
Changes in DNA methylation patterns may also account for the low efficiency of present cloning approaches. DNA methylation is highly dynamic in cleavage-stage embryos of a number of mammalian species. Failure to properly recapitulate pre-implantation DNA methylation patterns in embryos derived by nuclear transfer may contribute to the low efficiency of nuclear transfer in producing live offspring [49]. It is natural to speculate that oocyte cytoplasm has 'special ingredients' that reprogram epigenetic imprinting from the somatic state to the zygotic state. Attempts to increase the efficiency of cloning by increasing the exposure time of donor nuclei to the oocyte's cytoplasm have met with some, though by no means dramatic success [45,51-53]. It was also somewhat surprising that using the so-called 'totipotent' ES cells for cloning was not as successful as expected [54,55]. However, more recent studies using out-bred F1 ES cell lines have demonstrated higher efficiencies in reconstructing oocytes capable of developing to live-born pups and increasing post-natal survival [56].
In conclusion, although mammalian cloning is still in its infancy, it is likely to change the face of animal modeling in the near future. As new methods for embryo manipulation and NT cloning merge with highly efficient gene targeting approaches, the ability to generate innovative larger animal models of genetic disease will significantly increase. Such efforts will greatly benefit the field of molecular medicine.
Acknowledgments
Acknowledgments
We gratefully acknowledge the editorial assistance of Leah Williams and NIDDK (DK47967/JFE), NHLBI (HL61234/MJW), and CFF research funding for the author's laboratory in the area of this review.
Contributor Information
Ziyi Li, Email: Ziyi-li@uiowa.edu.
John F Engelhardt, Email: John-engelhardt@uiowa.edu.
References
- Di Berardino MA. Animal cloning – the route to new genomics in agriculture and medicine. Differentiation. 2001;68:67–83. doi: 10.1046/j.1432-0436.2001.680201.x. [DOI] [PubMed] [Google Scholar]
- Renard JP, Zhou Q, LeBourhis D, Chavatte-Palmer P, Hue I, Heyman Y, Vignon X. Nuclear transfer technologies: between successes and doubts. Theriogenology. 2002;57:203–222. doi: 10.1016/S0093-691X(01)00667-7. [DOI] [PubMed] [Google Scholar]
- Campbell KH, McWhir J, Ritchie WA, Wilmut I. Sheep cloned by nuclear transfer from a cultured cell line. Nature. 1996;380:64–66. doi: 10.1038/380064a0. [DOI] [PubMed] [Google Scholar]
- Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- Wakayama T, Perry AC, Zuccotti M, Johnson KR, Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature. 1998;394:369–374. doi: 10.1038/28615. [DOI] [PubMed] [Google Scholar]
- Kato Y, Tani T, Sotomaru Y, Kurokawa K, Kato J, Doguchi H, Yasue H, Tsunoda Y. Eight calves cloned from somatic cells of a single adult. Science. 1998;282:2095–2098. doi: 10.1126/science.282.5396.2095. [DOI] [PubMed] [Google Scholar]
- Baguisi A, Behboodi E, Melican DT, Pollock JS, Destrempes MM, Cammuso C, Williams JL, Nims SD, Porter CA, Midura P, et al. Production of goats by somatic cell nuclear transfer. Nat Biotechnol. 1999;17:456–461. doi: 10.1038/8632. [DOI] [PubMed] [Google Scholar]
- Polejaeva IA, Chen SH, Vaught TD, Page RL, Mullins J, Ball S, Dai Y, Boone J, Walker S, Ayares DL, et al. Cloned pigs produced by nuclear transfer from adult somatic cells. Nature. 2000;407:86–90. doi: 10.1038/35024082. [DOI] [PubMed] [Google Scholar]
- Shin T, Kraemer D, Pryor J, Liu L, Rugila J, Howe L, Buck S, Murphy K, Lyons L, Westhusin M. A cat cloned by nuclear transplantation. Nature. 2002;415:859. doi: 10.1038/nature723. [DOI] [PubMed] [Google Scholar]
- Chesne P, Adenot PG, Viglietta C, Baratte M, Boulanger L, Renard JP. Cloned rabbits produced by nuclear transfer from adult somatic cells. Nat Biotechnol. 2002;20:366–369. doi: 10.1038/nbt0402-366. [DOI] [PubMed] [Google Scholar]
- Woods GL, White KL, Vanderwall DK, Li GP, Aston KI, Bunch TD, Meerdo LN, Pate BJ. A Mule Cloned from Fetal Cells by Nuclear Transfer. Science. 2003;29:29. doi: 10.1111/j.1728-4457.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- Cibelli JB, Stice SL, Golueke PJ, Kane JJ, Jerry J, Blackwell C, Ponce de Leon FA, Robl JM. Cloned transgenic calves produced from nonquiescent fetal fibroblasts. Science. 1998;280:1256–1258. doi: 10.1126/science.280.5367.1256. [DOI] [PubMed] [Google Scholar]
- McCreath KJ, Howcroft J, Campbell KH, Colman A, Schnieke AE, Kind AJ. Production of gene-targeted sheep by nuclear transfer from cultured somatic cells. Nature. 2000;405:1066–1069. doi: 10.1038/35016604. [DOI] [PubMed] [Google Scholar]
- Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, Im GS, Samuel M, Bonk A, Rieke A, Day BN, et al. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002;295:1089–1092. doi: 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- Dai Y, Vaught TD, Boone J, Chen SH, Phelps CJ, Ball S, Monahan JA, Jobst PM, McCreath KJ, Lamborn AE, et al. Targeted disruption of the alpha 1,3-galactosyltransferase gene in cloned pigs. Nat Biotechnol. 2002;20:251–255. doi: 10.1038/nbt0302-251. [DOI] [PubMed] [Google Scholar]
- Welsh MJ, Tsui L-C, Boat TF, Beaudet AL. Cystic fibrosis. In: Scriver CL, Beaudet AL, Sly WS, Valle D, editor. The Metabolic Basis of Inherited Disease. New York, McGraw-Hill; 1995. pp. 3799–3876. [Google Scholar]
- Davidson DJ, Rolfe M. Mouse models of cystic fibrosis. Trends Genet. 2001;17:S29–37. doi: 10.1016/S0168-9525(01)02452-0. [DOI] [PubMed] [Google Scholar]
- Grubb BR, Boucher RC. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol Rev. 1999;79:S193–214. doi: 10.1152/physrev.1999.79.1.S193. [DOI] [PubMed] [Google Scholar]
- Oldham MJ, Phalen RF, Huxtable RF. Growth of the ferret tracheobronchial tree. Lab Anim Sci. 1990;40:186–191. [PubMed] [Google Scholar]
- Leigh MW, Gambling TM, Carson JL, Collier AM, Wood RE, Boat TF. Postnatal development of tracheal surface epithelium and submucosal glands in the ferret. Exp Lung Res. 1986;10:153–169. doi: 10.3109/01902148609061490. [DOI] [PubMed] [Google Scholar]
- Plopper CG, Hill LH, Mariassy AT. Ultrastructure of the nonciliated bronchiolar epithelial (Clara) cell of mammalian lung. III. A study of man with comparison of 15 mammalian species. Exp Lung Res. 1980;1:171–180. doi: 10.3109/01902148009069646. [DOI] [PubMed] [Google Scholar]
- Duan D, Sehgal A, Yao J, Engelhardt JF. Lef1 transcription factor expression defines airway progenitor cell targets for in utero gene therapy of submucosal gland in cystic fibrosis. Am J Respir Cell Mol Biol. 1998;18:750–758. doi: 10.1165/ajrcmb.18.6.2987. [DOI] [PubMed] [Google Scholar]
- Kishioka C, Okamoto K, Kim J, Rubin BK. Regulation of secretion from mucous and serous cells in the excised ferret trachea. Respir Physiol. 2001;126:163–171. doi: 10.1016/S0034-5687(01)00214-6. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang Y, Amberson A, Engelhardt JF. New models of the tracheal airway define the glandular contribution to airway surface fluid and electrolyte composition. Am J Respir Cell Mol Biol. 2001;24:195–202. doi: 10.1165/ajrcmb.24.2.3918. [DOI] [PubMed] [Google Scholar]
- Sehgal A, Presente A, Engelhardt JF. Developmental expression patterns of CFTR in ferret tracheal surface airway and submucosal gland epithelia. Am J Respir Cell Mol Biol. 1996;15:122–131. doi: 10.1165/ajrcmb.15.1.8679216. [DOI] [PubMed] [Google Scholar]
- Engelhardt JF, Yankaskas JR, Ernst SA, Yang Y, Marino CR, Boucher RC, Cohn JA, Wilson JM. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat Genet. 1992;2:240–248. doi: 10.1038/ng1192-240. [DOI] [PubMed] [Google Scholar]
- Fenton RJ, Morley PJ, Owens IJ, Gower D, Parry S, Crossman L, Wong T. Chemoprophylaxis of influenza A virus infections, with single doses of zanamivir, demonstrates that zanamivir is cleared slowly from the respiratory tract. Antimicrob Agents Chemother. 1999;43:2642–2647. doi: 10.1128/aac.43.11.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh MW, Connor RJ, Kelm S, Baum LG, Paulson JC. Receptor specificity of influenza virus influences severity of illness in ferrets. Vaccine. 1995;13:1468–1473. doi: 10.1016/0264-410X(95)00004-K. [DOI] [PubMed] [Google Scholar]
- Collie MH, Rushton DI, Sweet C, Smith H. Studies of influenza virus infection in newborn ferrets. J Med Microbiol. 1980;13:561–571. doi: 10.1099/00222615-13-4-561. [DOI] [PubMed] [Google Scholar]
- Jakeman KJ, Rushton DI, Smith H, Sweet C. Exacerbation of bacterial toxicity to infant ferrets by influenza virus: possible role in sudden infant death syndrome [published erratum appears in J Infect Dis 1991 Jul;164(l):232] J Infect Dis. 1991;163:35–40. doi: 10.1093/infdis/163.1.35. [DOI] [PubMed] [Google Scholar]
- Husseini RH, Collie MH, Rushton DI, Sweet C, Smith H. The role of naturally-acquired bacterial infection in influenza-related death in neonatal ferrets. Br J Exp Pathol. 1983;64:559–569. [PMC free article] [PubMed] [Google Scholar]
- Kishioka C, Okamoto K, Hassett DJ, de Mello D, Rubin BK. Pseudomonas aeruginosa alginate is a potent secretagogue in the isolated ferret trachea. Pediatr Pulmonol. 1999;27:174–179. doi: 10.1002/(SICI)1099-0496(199903)27:3<174::AID-PPUL4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Li ZY, Jiang QS, Zhang YL, Liu XM, Engelhardt JF. Successful production of offspring after superovulation and in vitro culture of embryos from domestic ferrets (Mustela putorius furos) Reproduction. 2001;122:611–618. doi: 10.1530/reprod/122.4.611. [DOI] [PubMed] [Google Scholar]
- Li Z, Jiang Q, Rezaei Sabet M, Zhang Y, Ritchie TC, Engelhardt JF. Conditions for in vitro maturation and artificial activation of ferret oocytes. Biol Reprod. 2002;66:1380–1386. doi: 10.1095/biolreprod66.5.1380. [DOI] [PubMed] [Google Scholar]
- Li Z, Rezaei Sabet M, Zhou Q, Liu X, Ding W, Zhang Y, Renard JP, Engelhardt JF. Developmental capacity of ferret embryos by nuclear transfer using g0/g1-phase fetal fibroblasts. Biol Reprod. 2003;68:2297–2303. doi: 10.1095/biolreprod.102.012369. [DOI] [PubMed] [Google Scholar]
- Kidder JD, Giles JR, Foote RH, Richmond ME, Salerno M. Allocation of inner cell mass and trophectoderm cells to the preimplantation blastocyst of the domestic ferret, Mustela putorius furo. J Exp Zool. 1999;283:202–209. doi: 10.1002/(SICI)1097-010X(19990201)283:2<202::AID-JEZ11>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Santana E, Peritz AE, Iyer S, Uitto J, Yoon K. Different frequency of gene targeting events by the RNA-DNA oligonucleotide among epithelial cells. J Invest Dermatol. 1998;111:1172–1177. doi: 10.1046/j.1523-1747.1998.00403.x. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Cole-Strauss A, Yoon K, Gryn J, Kmiec EB. Targeted gene conversion in a mammalian CD34+-enriched cell population using a chimeric RNA/DNA oligonucleotide. J Mol Med. 1997;75:829–835. doi: 10.1007/s001090050172. [DOI] [PubMed] [Google Scholar]
- Cole-Strauss A, Yoon K, Xiang Y, Byrne BC, Rice MC, Gryn J, Holloman WK, Kmiec EB. Correction of the mutation responsible for sickle cell anemia by an RNA-DNA oligonucleotide. Science. 1996;273:1386–1389. doi: 10.1126/science.273.5280.1386. [DOI] [PubMed] [Google Scholar]
- Tran ND, Liu X, Yan Z, Abbote D, Jiang Q, Kmiec EB, Sigmund CD, Engelhardt JF. Efficiency of chimeraplast gene targeting by direct nuclear injection using a GFP recovery assay. Mol Ther. 2003;7:248–253. doi: 10.1016/S1525-0016(02)00039-4. [DOI] [PubMed] [Google Scholar]
- Igoucheva O, Alexeev V, Yoon K. Targeted gene correction by small single-stranded oligonucleotides in mammalian cells. Gene Ther. 2001;8:391–399. doi: 10.1038/sj.gt.3301414. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R. Cloning: experience from the mouse and other animals. Mol Cell Endocrinol. 2002;187:241–248. doi: 10.1016/S0303-7207(01)00697-9. [DOI] [PubMed] [Google Scholar]
- Campbell KH, Loi P, Otaegui PJ, Wilmut I. Cell cycle co-ordination in embryo cloning by nuclear transfer. Rev Reprod. 1996;1:40–46. doi: 10.1530/revreprod/1.1.40. [DOI] [PubMed] [Google Scholar]
- Tani T, Kato Y, Tsunoda Y. Direct exposure of chromosomes to nonactivated ovum cytoplasm is effective for bovine somatic cell nucleus reprogramming. Biol Reprod. 2001;64:324–330. doi: 10.1095/biolreprod64.1.324. [DOI] [PubMed] [Google Scholar]
- Ono Y, Shimozawa N, Ito M, Kono T. Cloned mice from fetal fibroblast cells arrested at metaphase by a serial nuclear transfer. Biol Reprod. 2001;64:44–50. doi: 10.1095/biolreprod64.1.44. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Jouneau A, Brochard V, Adenot P, Renard JP. Developmental potential of mouse embryos reconstructed from metaphase embryonic stem cell nuclei. Biol Reprod. 2001;65:412–419. doi: 10.1093/biolreprod/65.2.412. [DOI] [PubMed] [Google Scholar]
- Kato Y, Tani T, Tsunoda Y. Cloning of calves from various somatic cell types of male and female adult, newborn and fetal cows. J Reprod Fertil. 2000;120:231–237. doi: 10.1530/reprod/120.2.231. [DOI] [PubMed] [Google Scholar]
- Solter D. Mammalian cloning: advances and limitations. Nat Rev Genet. 2000;1:199–207. doi: 10.1038/35042066. [DOI] [PubMed] [Google Scholar]
- Fairburn HR, Young LE, Hendrich BD. Epigenetic reprogramming: how now, cloned cow? Curr Biol. 2002;12:R68–70. doi: 10.1016/S0960-9822(01)00677-7. [DOI] [PubMed] [Google Scholar]
- Han YM, Kang YK, Koo DB, Lee KK. Nuclear reprogramming of cloned embryos produced in vitro. Theriogenology. 2003;59:33–44. doi: 10.1016/S0093-691X(02)01271-2. [DOI] [PubMed] [Google Scholar]
- Tsunoda Y, Kato Y. Full-term development after transfer of nuclei from 4-cell and compacted morula stage embryos to enucleated oocytes in the mouse. J Exp Zool. 1997;278:250–254. doi: 10.1002/(SICI)1097-010X(19970701)278:4<250::AID-JEZ6>3.3.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Kato Y, Yabuuchi A, Motosugi N, Kato J, Tsunoda Y. Developmental potential of mouse follicular epithelial cells and cumulus cells after nuclear transfer. Biol Reprod. 1999;61:1110–1114. doi: 10.1095/biolreprod61.4.1110. [DOI] [PubMed] [Google Scholar]
- Wells DN, Misica PM, Tervit HR. Production of cloned calves following nuclear transfer with cultured adult mural granulosa cells. Biol Reprod. 1999;60:996–1005. doi: 10.1095/biolreprod60.4.996. [DOI] [PubMed] [Google Scholar]
- Wakayama T, Rodriguez I, Perry AC, Yanagimachi R, Mombaerts P. Mice cloned from embryonic stem cells. Proc Natl Acad Sci U S A. 1999;96:14984–14989. doi: 10.1073/pnas.96.26.14984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rideout WM, 3rd, Wakayama T, Wutz A, Eggan K, Jackson-Grusby L, Dausman J, Yanagimachi R, Jaenisch R. Generation of mice from wild-type and targeted ES cells by nuclear cloning. Nat Genet. 2000;24:109–110. doi: 10.1038/72753. [DOI] [PubMed] [Google Scholar]
- Eggan K, Akutsu H, Loring J, Jackson-Grusby L, Klemm M, Rideout WM, 3rd, Yanagimachi R, Jaenisch R. Hybrid vigor, fetal overgrowth, and viability of mice derived by nuclear cloning and tetraploid embryo complementation. Proc Natl Acad Sci U S A. 2001;98:6209–6214. doi: 10.1073/pnas.101118898. [DOI] [PMC free article] [PubMed] [Google Scholar]