Abstract
teashirt was initially identified as a gene required for the specification of the trunk segments in Drosophila embryogenesis and encodes a transcription factor with zinc finger motifs. We report here that targeted expression of teashirt in imaginal discs is sufficient to induce ectopic eye formation in non-eye tissues, a phenotype similar to that produced from targeted expression of eyeless, dachshund, and eyes absent. Furthermore, teashirt and eyeless induce the expression of each other, suggesting that teashirt is part of the gene network that functions to specify eye identity.
Although the Drosophila compound eye is anatomically distinct from the vertebrate eye, recent studies have revealed striking parallels in eye development between flies and vertebrates. Mutations in the Pax-6 gene in Drosophila [eyeless (ey)], mice (Small eye), and humans (Aniridia) all lead to defects in eye development (1–4), whereas ectopic expression of the Drosophila or murine Pax-6 gene can result in ectopic eye formation in other fly tissues (5). Additional genes, including eyes absent (eya), sine oculis (so), and dachshund (dac), have also been implicated in the early steps of eye development, because loss-of-function mutations in these genes lead to complete loss of eyes in Drosophila (6–8). Moreover, targeted expression of either eya or dac can result in ectopic eyes in other tissues, and so can potentiate the activity of eya and dac in such assays (9–12). More recent experiments have revealed aspects of the relationship between these genes and ey in Drosophila eye specification (5, 9–13). Although ey is required for the initial expression of eya, so, and dac in the eye primordium, the later genes are also involved in a positive feedback loop to activate the expression of ey. Therefore, ey does not function simply as a “master regulatory gene” to activate a linear pathway specifying the eye fate; rather, ey, eya, so, and dac form part of a regulatory network that together “locks in” the eye specification program. Apparent orthologs of eya, so, and dac have been isolated and are expressed in the developing eye primordium in vertebrates, suggesting that these genes are likely to be involved in a similar regulatory network in vertebrate eye development.
Although eya and dac can induce ectopic eyes, they are much less potent in doing so than ey, even when eya or dac is simultaneously expressed with so (5, 9–12), suggesting the existence of genes that can be induced by ey but not by eya (or eya plus so) and dac (or dac plus so). In addition, ey itself cannot induce ectopic eye formation in all fly tissues (5, 13). These observations suggest that additional genes are yet to be identified that in combination with ey, eya, so, and dac form a gene network that lies at the highest level of the eye specification hierarchy. A diagnostic feature of such genes is that ectopic expression of these genes either alone or in combination with other eye specification genes should induce ectopic eyes in noneye tissues.
We report here that teashirt (tsh) is part of this gene network that controls eye specification. tsh was initially identified as a homeotic gene required for specifying the trunk segments in Drosophila embryogenesis (14). Loss-of-function mutations in tsh leads to trunk-to-head transformation, whereas ectopic expression of tsh results in head-to-trunk transformation (14, 15). However, unlike genes in the Antennapedia (Antp) and bithorax complexes (HOM-C), which encode homeobox-containing proteins, tsh encodes a nuclear protein with zinc finger motifs (14). The function of tsh during imaginal disc development is less well understood. A potential role for tsh in eye development was suggested by the observation that flies transheterozygous for tsh and certain gain-of-function Antp mutations exhibit a reduced-eye phenotype that is not associated with either mutation alone (16). Such a phenotype was interpreted as an eye-to-cuticle transformation. Here we show that targeted expression of tsh is sufficient to induce ectopic eye development in the antennal disc. These ectopic eyes have ommatidia with properly differentiated photoreceptor neurons. In addition, we investigated the relationship between tsh and other eye specification genes. We show that tsh induces the expression of ey, so, and dac, whereas ectopic ey induces the expression of tsh. Moreover, ectopic eye formation induced by tsh depends on eya and so activity. Our results suggest that tsh is intimately linked to the other eye specification genes and that these genes function together to specify eye identity.
MATERIALS AND METHODS
Plasmid Construction, P Element Transformation, and Plasmid Rescue.
To construct DMREP, the P element transposon used in our overexpression screen, an EcoRI–XhoI fragment that contains the dpp disc enhancer and the hsp70 basal promoter was excised from pDMR (17) and blunt-ended with Klenow. This fragment was then cloned into the P element transformation vector PEG117 (18) that was treated with NotI and Klenow. Dysgenic males containing DMREP on the X chromosome and a source of transposase (Δ2–3) on the third chromosome were individually crossed to w1118 females. Insertions on the autosomes were identified as red-eyed males in the progeny and were screened for phenotypes in the eye, the wing, and the thorax.
Multiple Four eyes alleles were isolated in the screen. Some alleles are homozygous lethal, and others are homozygous viable. They all complement the lethality of tsh8, a null allele of tsh, indicating that they do not inactivate the tsh gene. To determine the location of the Four eyes insertions, DNA flanking the DMREP insertion was isolated from one Four eyes allele by plasmid rescue and sequenced. This analysis revealed that the P element is inserted within an intron in the 5′-untranslated region of the tsh gene, with the dpp enhancer/hsp70 promoter cassette reading in the same direction as the endogenous tsh gene. This intron is located between +270 and +271 of the published tsh cDNA sequence (14), and the predicted translation start site of tsh is located at +1009 of the tsh cDNA.
To confirm that the Four eyes mutations are caused by overexpression of tsh, a full-length tsh cDNA was cloned into pDMR (17) and introduced into w1118 flies by standard procedures.
Genetics.
All crosses were done at 25°C. so (8), eya (7), dac (6), and tsh (14) mutants used in this study have been described previously.
For the analysis of hypomorphic tsh mutants, tsh4/Df(2L)TW161 and tsh1/Df(2L)TW161 were used. For the analysis of tsh mutant clones, tsh8 (14) was used. X-ray induction of somatic clones was carried out according to standard procedures (19). A P[w+] inserts 39E-F was used to mark the wild-type chromosome. An isogenized w1118 stock was used as a wild-type control.
Histology and Microscopy.
Enhancer trap lines dacP, so7, and tsh1 were used to monitor the expression of dac, so, and tsh, respectively. ey-lacZ (kindly provided by Walter Gehring, University of Basel, Basel) and GMR-lacZ were used to monitor the expression of ey and glass, respectively. β-Galactosidase activity staining and immunohistochemical staining of imaginal discs were carried out as described (6).
RESULTS
Isolation of Four eyes, a Gain-of-Function Mutation of tsh.
To isolate genes that may play important roles in imaginal disc development, we carried out a gain-of-function genetic screen with P element-mediated gene overexpression. In such screens, a P element transposon that contains an enhancer/promoter cassette reading off one P element end is mobilized. An overexpression phenotype may result if the enhancer/promoter cassette contained within the transposon drives the expression of a nearby endogenous gene (20, 21). In our screen, we used the dpp disc enhancer coupled with the hsp70 basal promoter. The dpp disc enhancer was chosen because this element is known to drive gene expression in all imaginal discs (22), allowing for the examination of overexpression phenotypes in a variety of tissues. In addition, because this enhancer drives gene expression in only a subset of cells in each imaginal disc, the deleterious effects of gene overexpression on animal viability is minimized.
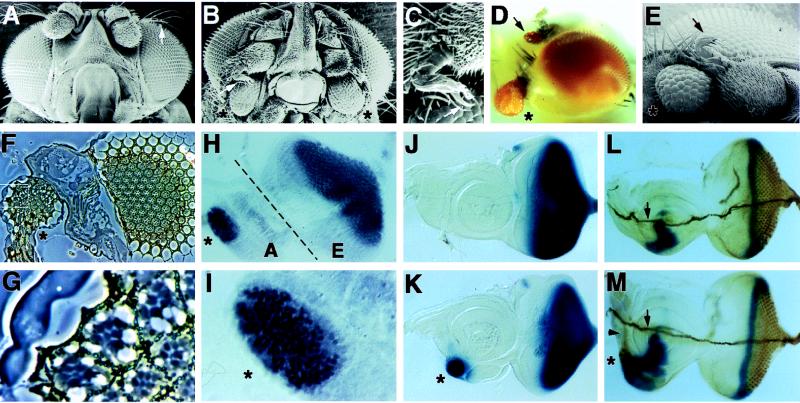
In the course of this genetic screen, we isolated five lines that have ectopic eyes in the anterior region of the head, just ventral to the antenna. These mutations were named Four eyes because often two ectopic eyes were seen in each fly (in Fig. 1 compare A and B). The position of ectopic eye formation on the head corresponds to the ventral region of the antennal disc where the dpp disc enhancer is known to be active, suggesting that the Four eyes mutations are caused by overexpression of gene(s) mediated by the dpp disc enhancer. The frequency of flies having at least one ectopic eye varied from 20% to 80% depending on the line. Ectopic eyes were also seen on the antenna (Fig. 1D), although they are relatively smaller and occurred at a lower frequency. Ectopic eyes were never observed in any other adult tissue. Another phenotype associated with the Four eyes mutations is the transformation of the arista of the antenna into a leg-like structure (Fig. 1 B and C).
Figure 1.
Targeted expression of tsh induces ectopic retinal development. (A and B) Scanning electron microscopy pictures of wild-type and Four eyes head morphology, respectively. Note the presence of two ectopic eyes (asterisks) in the Four eyes mutant. Also note that the arista (arrow in A), a thin branched structure on the antenna, was replaced with a leg-like structure in Four eyes (arrow in B; shown at higher magnification in C). Like normal legs, a claw (arrow in C) is present at the distal tip of this leg-like structure. (D) A light microscope image of a Four eyes fly. One ectopic eye is present at the anterior ventral surface of the head (asterisk), and another ectopic eye is present on a proximal segment of the antenna (arrow). (E) Scanning electron microscopy image of a transgenic fly expressing a full-length tsh cDNA in the pDMR vector. Note the presence of the ectopic eye (asterisk) and the arista-to-leg transformation (arrow). (F) A section through an ectopic eye (marked by an asterisk, to the left) and the normal eye (to the right) in a Four eyes mutant fly. A higher magnification of the outlined area in the ectopic eye is shown in G. Note the presence of cone cells, pigment granules, and rhabdomeres in the ectopic eye and that some ommatidia have normal numbers of photoreceptors. (H) A Four eyes eye (E)-antenna (A) disc stained with the Elav antibody. Note the presence of ectopic Elav staining in the antennal disc (asterisk), which normally does not express Elav. (I) A higher magnification of a portion of the antennal disc where ectopic Elav staining is observed. (J and K) glass expression in wild-type and Four eyes eye-antenna discs, respectively. Note the presence of ectopic glass expression (asterisk in K) in the antennal disc in Four eyes. Anterior is to the left and dorsal is up. (L and M) Wild-type and Four eyes eye-antenna discs, respectively, stained for dpp/lacZ (blue) and 22C10 (brown). Note that ectopic 22C10 staining can be seen both in the cell bodies of the ectopic photoreceptors (asterisk) and on their axon tracts (slightly out of focus, arrowhead in M). On a different focal plane (data not shown), we observed that the axons projected medially and then stopped; they did not fasciculate with the Bolwig’s nerve (arrows in L and M).
We mapped the P elements associated with three Four eyes lines to the 40A region of the second chromosome. Plasmid rescue and sequencing of the genomic DNA flanking the P element in one of these Four eyes alleles revealed that it was inserted into the 5′-untranslated region of the tsh gene, with the dpp enhancer/hsp70 promoter cassette reading into tsh. To confirm that the Four eyes mutations are caused by overexpression of tsh, we generated transgenic flies that expressed a full-length tsh cDNA under the direct control of the dpp enhancer/hsp70 promoter cassette with the pDMR vector (17). Consistent with tsh being responsible for the Four eyes mutations, such transgenic flies exhibited ectopic eyes in the head (Fig. 1E). Moreover, these transgenic flies showed an arista-to-leg transformation similar to that observed in the Four eyes mutants (Fig. 1E). The arista-to-leg transformation is also consistent with the known role of tsh in specifying trunk versus head identities (15). Taken together, these data demonstrate that the Four eyes mutations are gain-of-function alleles of tsh caused by its ectopic expression under the control of the dpp enhancer.
Normal Retinal Development in the Ectopic Eyes.
Scanning electron microscopy revealed that tsh-induced ectopic eyes contain nearly normal facets with interommatidial bristles (Fig. 1 B and E). Sections through such eyes indicate that the ectopic ommatidia consist of various cell types found in normal ommatidia, such as the cone cells, pigment cells, and photoreceptors with rhabdomeres (Fig. 1 F and G). To follow the development of the ectopic eyes, we analyzed several markers that are normally expressed in the developing retina. The nuclear protein Elav is normally expressed in all neurons, including the photoreceptors, and is not expressed in the antennal disc in third-instar larvae (23). In Four eyes mutants, ectopic Elav staining was observed in the antennal discs (Fig. 1 H and I). Glass, a gene that is only expressed in the visual system in Drosophila (ref. 24; Fig. 1J) was also induced in the antennal discs in the Four eyes mutants (Fig. 1K), suggesting that tsh not only induced neuronal differentiation but more specifically conferred retinal cell fate to these antennal disc cells. On the fate map of the antennal disc (25), the location of the ectopic retina development corresponds to the site where the ectopic eyes are formed on the adult head. As seen in normal photoreceptors, the ectopic photoreceptors elaborate axons that fasciculate with each other and project medially (Fig. 1 L and M). Taken together, these data indicate that tsh has the capacity to turn on a complete program of eye specification in the antennal disc.
tsh Plays a Redundant Role During Normal Eye Development.
Although the data presented so far indicate that tsh can induce eye cell fate in overexpression studies, they do not prove that tsh does play a role in specifying the eye identity during normal development. To address this issue, we first examined whether tsh is expressed at the right time and the right place to have a role in specifying the eye identity. Indeed, tsh mRNA is expressed in the eye disc, with the strongest expression anterior to the morphogenetic furrow (Fig. 2I). This pattern of expression is similar to that of ey (13), a gene that is known to play an essential role in specifying eye identity. Next we examined whether loss-of-function mutations of tsh affect eye development. We first examined several weak loss-of-function tsh alleles (14) and did not find any eye defects (see Materials and Methods for alleles used). Making mosaic clones of tsh null alleles with the FRT/FLP system was troublesome because tsh is located at 40A, exactly where the most proximal FRT insert on the left arm of the second chromosome is located (26). Therefore, we used x-ray-induced mitotic recombination to generate mutant clones of a null tsh allele, tsh8 (14). tsh mutant clones were recovered at a frequency similar to the wild-type control, and sections through the mutant clones revealed a normal ommatidial organization (Fig. 2J). These data suggest that tsh may play a redundant role during normal eye development, and the requirement for tsh may be masked by other factor(s) that play a role similar to tsh.
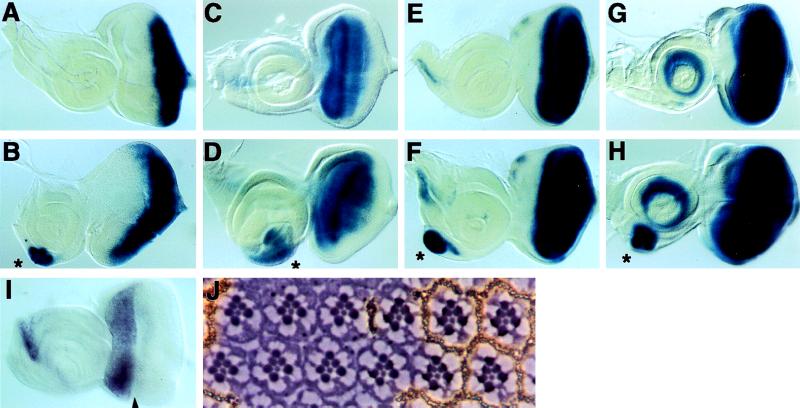
Figure 2.
Relationship among tsh, ey, so, and dac. (A and B) Expression of ey/lacZ in wild-type and Four eyes eye-antenna discs, respectively. Ectopic ey expression is observed at the ventral region of the antennal disc in Four eyes (marked with asterisk). (C and D) Expression of tsh in wild-type flies and flies of genotype UAS:ey × dpp GAL4, respectively. tsh expression was monitored with a P element enhancer trap tsh allele, tsh1. tsh is normally expressed only in the eye portion of the eye-antenna disc. Its expression is strongly induced in the ventral antennal region in flies bearing UAS:ey and dpp-GAL4 (marked with asterisk). (E and F) Expression of so in wild-type and Four eyes eye-antenna discs, respectively. so expression was monitored using a P element enhancer trap allele, so7. Ectopic so expression is observed at the ventral region of the antennal disc in Four eyes (marked with asterisk). (G and H) Expression of dac in wild-type and Four eyes eye-antenna discs, respectively. dac expression was monitored using a P element enhancer trap dac allele, dacP. Ectopic dac expression is observed at the ventral region of the antennal disc in Four eyes (marked with asterisk). (I) Expression pattern of tsh in the eye antenna disc as revealed by RNA in situ hybridization. Note that tsh is most strongly expressed anteriorly to the morphogenetic furrow (arrowhead). The tsh/lacZ expression in G and H extends more posteriorly, presumably because of the perdurance of β-galactosidase. (J) Normal ommatidial development in tsh mutant clones. Shown is a section through an adult eye containing a w− clone that is homozygous for a null tsh allele, tsh8. The mutant clone, which is located to the left, is recognizable by the lack of pigment granules. Note that the mutant ommatidia show normal number and organization of photoreceptors and accessory cells.
tsh and ey Can Turn on the Expression of Each Other.
Ectopic eye formation induced by tsh overexpression is remarkably similar to that resulting from the expression of eye specification genes, including ey, eya (or eya + so), and dac (or dac + so). Therefore, we examined the relationship between tsh and these eye specification genes. We first examined the relationship between tsh and ey, because during normal development ey activates the initial expression of the other eye specification genes and appears to play the most critical role in specifying the eye identity (13). We asked whether ey expression was induced during ectopic eye formation directed by the tsh gene. Normally, ey is expressed in the eye but not the antennal disc (Fig. 2A). In the Four eyes mutants, ectopic ey expression was detected in the ventral part of the antennal disc, where ectopic retinal development occurs (Fig. 2B). Although this result may be interpreted as tsh acting upstream of ey, we also observed that tsh expression is induced by ey (Fig. 2 C and D), suggesting that tsh may function both upstream and downstream of ey during retinal development.
tsh Acts Upstream of eya, so, and dac in Ectopic Eye Development.
We next examined the relationship between tsh and several other eye specification genes, including eya, so, and dac. We found that the expression of so (in Fig. 2, compare E and F) and dac (in Fig. 2, compare G and H) are induced in the antennal disc by the ectopic expression of tsh, suggesting that tsh may act upstream of these genes in eye development. We further examined the functional requirement of eya and so in tsh-induced ectopic eye formation. We found that the eye-specific eya1, eya2, and so1 mutations suppressed completely ectopic eye formation in Four eyes mutants (data not shown). These results suggest that tsh may function upstream of eya, so, and dac in ectopic eye development.
DISCUSSION
In this paper, we provide several lines of evidence suggesting that tsh is part of an interactive network of genes that function together at the highest level of the eye specification hierarchy. First, tsh is normally expressed in the eye imaginal disc, mostly anterior to the morphogenetic furrow. This pattern of expression is similar to that of ey (13), a gene that is known to play a critical role in eye specification. We noticed that unlike ey, whose expression in the eye primordium can be detected as early as in embryos, tsh is not expressed in the embryonic eye primordium (14). This feature of tsh expression is similar to that of eya, which is not expressed in the eye primordium until the second-instar larval stage (7). We suggest that like eya the initial expression of tsh in the eye proper is likely to be induced by ey at larval stages. Second, targeted expression of tsh is sufficient to induce ectopic retinal development in the antennal disc, a property shared by ey, eya, and dac, genes that are known to be part of the network that functions at the highest level of the eye specification hierarchy. Third, the reciprocal control of expression between ey and tsh is similar to that observed between ey and eya or between ey and dac, suggesting that the functions of all these genes are likely to be closely related.
Our studies lend further support to the view that eye development is executed by an interactive network of genes rather than a linear hierarchy dictated by ey. During normal development, ey is the first gene to be expressed in the eye primordium, and its expression leads to the initial expression of the other eye specification genes, including eya, so, dac, and tsh. However, positive feedback loops exist such that the later genes, when turned on by ey, may also positively regulate the expression of ey. It should be noted that ey is a much more potent inducer of ectopic eyes than any single gene in the later group, suggesting that none of these genes represents the sole direct transcriptional target of ey and that no single gene can recapitulate the full spectrum of ey activity. Previous studies suggested that eya and so may be direct targets of ey (9, 11, 13). Our studies suggest that tsh could also be a direct target of ey. It will be interesting to determine whether coexpression of eya, so, and tsh can recapitulate the full activity of ey. If so, these three genes may represent the only direct targets of ey.
The cross-regulatory relationship between tsh and ey, a homeobox-containing protein, is especially interesting in light of a similar relationship between tsh and HOM-C proteins in embryonic development. Previous studies of tsh in embryogenesis demonstrated that tsh functions as a transcription factor that may directly control the transcription of its downstream target genes. These studies further indicated that tsh function is closely linked to the HOM-C proteins and that tsh may function upstream, downstream, or at the same level as HOM-C proteins, depending on the developmental context. tsh plays at least two essential roles during embryogenesis. First, tsh is required for the subdivision of the midgut mesoderm. In this process, tsh simply acts downstream of HOM-C proteins such as ANTP and UBX (27, 28). Second, combinatorial action of TSH, ANTP, and bithorax complex proteins is required for the thoracic and abdominal (trunk) identities, through repression of the head homeotic gene labial (29). Because the initial expression of tsh is independent of Antp and bithorax complex genes and vice versa (29), tsh likely functions in parallel to Antp and bithorax complex genes. Clearly, in this process, tsh functions both in parallel with (Antp and bithorax complex) and upstream of (labial) HOM-C genes. The cross-regulatory relationship between tsh and homeobox-containing transcription factors in both eye development and embryonic development may reflect a similar molecular mechanism of tsh function in both processes.
Our studies also underscore the importance of gain-of-function genetics. Although ey, eya, so, and dac were identified as eye specification genes based on their loss-of-function phenotypes, tsh does not have a visible mutant eye phenotype under normal conditions. Its role in eye development could be revealed only under certain sensitized genetic backgrounds such as in the presence of Antp mutations, or as in our study, through overexpression. Indeed the majority of the genes in eukaryotes do not have easily assayable loss-of-function phenotypes (30). Therefore, gain-of-function genetics through gene overexpression is an important alternative approach to probe gene-regulatory networks and will be useful in elucidating many complex genetic pathways.
Acknowledgments
We thank Walter Gehring, Graeme Mardon, Matthew Scott, and Larry Zipursky for fly strains and reagents; Tom Serano for providing Fig. 2I; and Miro Pastrnak for help with plasmid rescue. Brian Avery and Tom Serano provided useful comments concerning the manuscript.
ABBREVIATIONS
- tsh, teashirt
ey, eyeless
- dac
dachshund
- eya
eyes absent
- Antp, Antennapedia.
References
- 1.Hill R E, Favor J, Hogan B L, Ton C C, Saunders G F, Hanson I M, Prosser J, Jordan T, Hastie N D, van Heyningen V. Nature (London) 1992;355:750. doi: 10.1038/355750a0. [DOI] [PubMed] [Google Scholar]
- 2.Glaser T, Jepeal L, Edwards J G, Young S R, Favor J, Maas R L. Nat Genet. 1994;7:463–471. doi: 10.1038/ng0894-463. [DOI] [PubMed] [Google Scholar]
- 3.Ton C C, Hirvonen H, Miwa H, Weil M M, Monaghan P, Jordan T, van Heyningen V, Hastie N D, Meijers-Heijboer H, Drechsler M, et al. Cell. 1991;67:1059–1074. doi: 10.1016/0092-8674(91)90284-6. [DOI] [PubMed] [Google Scholar]
- 4.Quiring R, Walldorf U, Kloter U, Gehring W J. Science. 1994;265:785–789. doi: 10.1126/science.7914031. [DOI] [PubMed] [Google Scholar]
- 5.Halder G, Callaerts P, Gehring W J. Science. 1995;267:1788–1792. doi: 10.1126/science.7892602. [DOI] [PubMed] [Google Scholar]
- 6.Mardon G, Solomon N, Rubin G M. Development (Cambridge, UK) 1994;120:3473–3486. doi: 10.1242/dev.120.12.3473. [DOI] [PubMed] [Google Scholar]
- 7.Bonini N M, Leiserson W M, Benzer S. Cell. 1993;72:379–395. doi: 10.1016/0092-8674(93)90115-7. [DOI] [PubMed] [Google Scholar]
- 8.Cheyette B N R, Green P J, Martin K, Garren H, Hartenstein V, Zipursky S L. Neuron. 1994;12:977–996. doi: 10.1016/0896-6273(94)90308-5. [DOI] [PubMed] [Google Scholar]
- 9.Bonini N M, Bui Q T, Gray-Board G L, Warrick J M. Development (Cambridge, UK) 1997;124:4819–4826. doi: 10.1242/dev.124.23.4819. [DOI] [PubMed] [Google Scholar]
- 10.Chen R, Amoui M, Zhang Z, Mardon G. Cell. 1997;91:893–903. doi: 10.1016/s0092-8674(00)80481-x. [DOI] [PubMed] [Google Scholar]
- 11.Pignoni F, Hu B, Zavitz K H, Xiao J, Garrity P A, Zipursky S L. Cell. 1997;91:881–891. doi: 10.1016/s0092-8674(00)80480-8. [DOI] [PubMed] [Google Scholar]
- 12.Shen W, Mardon G. Development (Cambridge, UK) 1997;124:45–52. doi: 10.1242/dev.124.1.45. [DOI] [PubMed] [Google Scholar]
- 13.Halder G, Callaerts P, Flister S, Walldorf U, Kloter U, Gehring W J. Development (Cambridge, UK) 1998;125:2181–2191. doi: 10.1242/dev.125.12.2181. [DOI] [PubMed] [Google Scholar]
- 14.Fasano L, Ràder L, Coré N, Alexandre E, Vola C, Jacq B, Kerridge S. Cell. 1991;64:63–79. doi: 10.1016/0092-8674(91)90209-h. [DOI] [PubMed] [Google Scholar]
- 15.de Zulueta P, Alexandre E, Jacq B, Kerridge S. Development (Cambridge, UK) 1994;120:2287–2296. doi: 10.1242/dev.120.8.2287. [DOI] [PubMed] [Google Scholar]
- 16.Bhojwani J, Shashidhara L S, Sinha P. Dev Genes Evol. 1997;207:137–146. doi: 10.1007/s004270050101. [DOI] [PubMed] [Google Scholar]
- 17.Pan D, Rubin G M. Cell. 1997;90:271–280. doi: 10.1016/s0092-8674(00)80335-9. [DOI] [PubMed] [Google Scholar]
- 18.Giniger E, Wells W, Jan L Y, Jan Y N. Roux’s Arch Dev Biol. 1993;202:112–122. doi: 10.1007/BF00636536. [DOI] [PubMed] [Google Scholar]
- 19.Ashburner M. Drosophila: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 20.Hay B A, Maile R, Rubin G M. Proc Natl Acad Sci USA. 1997;94:5195–5200. doi: 10.1073/pnas.94.10.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rorth P. Proc Natl Acad Sci USA. 1996;93:12418–12422. doi: 10.1073/pnas.93.22.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Staehling-Hampton K, Jackson P D, Clark M J, Brand A H, Hoffmann F M. Cell Growth Differ. 1994;5:585–593. [PubMed] [Google Scholar]
- 23.Robinow S, White K. J Neurobiol. 1991;22:443–461. doi: 10.1002/neu.480220503. [DOI] [PubMed] [Google Scholar]
- 24.Moses K, Rubin G M. Genes Dev. 1991;5:583–593. doi: 10.1101/gad.5.4.583. [DOI] [PubMed] [Google Scholar]
- 25.Cohen S M. The Development of Drosophila melanogaster. Plainview, NY: Cold Spring Harbor Lab. Press; 1993. pp. 747–841. [Google Scholar]
- 26.Xu T, Rubin G M. Development (Cambridge, UK) 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- 27.Mathies L D, Kerridge S, Scott M P. Development (Cambridge, UK) 1994;120:2799–2809. doi: 10.1242/dev.120.10.2799. [DOI] [PubMed] [Google Scholar]
- 28.McCormick A, Core N, Kerridge S, Scott M P. Development (Cambridge, UK) 1995;121:2799–2812. doi: 10.1242/dev.121.9.2799. [DOI] [PubMed] [Google Scholar]
- 29.Roder L, Vola C, Kerridge S. Development (Cambridge, UK) 1992;115:1017–1033. doi: 10.1242/dev.115.4.1017. [DOI] [PubMed] [Google Scholar]
- 30.Miklos G L, Rubin G M. Cell. 1996;86:521–529. doi: 10.1016/s0092-8674(00)80126-9. [DOI] [PubMed] [Google Scholar]