Abstract
The T-synthase is the key β3-galactosyltransferase essential for biosynthesis of core 1 O-glycans (Galβ1–3GalNAcα1-Ser/Thr) in animal cell glycoproteins. Here we describe the novel ability of an endoplasmic reticulum-localized molecular chaperone termed Cosmc to specifically interact with partly denatured T-synthase in vitro to cause partial restoration of activity. By contrast, a mutated form of Cosmc observed in patients with Tn syndrome has reduced chaperone function. The chaperone activity of Cosmc is specific, does not require ATP in vitro, and is effective toward T-synthase but not another β-galactosyltransferase. Cosmc represents the first ER chaperone identified to be required for folding of a glycosyltransferase.
Keywords: Chaperones/Protein Folding, Cosmc, Endoplasmic Reticulum, Galactosyltransferase, Molecular Chaperone, T-synthase
Introduction
Proteins correctly fold into unique functional three-dimensional structures within very crowded intracellular environments, including the cytoplasm and the oxidizing environment of the endoplasmic reticulum (ER)2 (1–3). Whereas in vitro studies have demonstrated that proteins can fold independently under physiological conditions based on the primary amino acid sequence of the polypeptide (4), protein folding in vivo typically involves assistance of other proteins termed molecular chaperones, which recognize and selectively bind nonnative structures and prevent aggregation (5–10). Chaperones are typically either general or specific in their client recognition. For example, relatively general and less-specific chaperones in client recognition include BiP/GRP78 and GRP94 along with co-chaperones such as Hsp40, and lectin chaperones such as calnexin/calreticulin and ERp75 (8, 11–13). Client-specific chaperones include Hsp47, which assists in collagen assembly (14) and Shr3p, an ER chaperone in yeast required for assembly and correct tertiary structures of amino acid permeases (15).
Previous studies indicate that a unique and specific molecular chaperone in the ER is Cosmc, which is required for the formation of active core 1 β3-galactosyltransferase (core 1 β3GalT, T-synthase) (16, 17), an essential enzyme required for core 1 O-glycan (Galβ1–3GalNAcα1-Ser/Thr) biosynthesis on animal glycoproteins (18, 19). Acquired mutations in Cosmc, which is encoded by the X-chromosome gene (Xq24) in humans, leads to loss of T-synthase activity and expression of the abnormal Tn (GalNAcα1-Ser/Thr) and Sialyl Tn (NeuAcα2–3GalNAcα1-Ser/Thr) antigens (17, 20, 21), which are also known as tumor-associated carbohydrate antigens (22–24). Disruption of the T-synthase in mice results in embryonic lethality primarily caused by defective angiogenesis and lymphangiogenesis (18, 19). In cultured cells lacking Cosmc, inactive T-synthase forms oligomeric aggregates in the ER and is eventually degraded by ubiquitin-dependent pathways in the cytosolic proteasome (16, 17). Cosmc appears to bind directly to T-synthase and to ATP (16), but the mechanism by which Cosmc participates in correct folding of the T-synthase, and the possibility that Cosmc is specific for folding of the T-synthase, have not been explored.
To aid in understanding this interesting chaperone system, here we describe an in vitro reconstitution approach to explore Cosmc interactions with the unfolded T-synthase. Understanding the molecular mechanisms of the regulation of T-synthase should lead to a greater appreciation of human diseases and disorders involving altered expression and activity of the T-synthase and potential new therapeutic strategies for Tn antigen-related diseases.
EXPERIMENTAL PROCEDURES
Materials
GalNAc-α-phenyl, UDP-Gal, GlcNAc-β-S-pNp, β4-GalT from bovine milk, and firefly luciferase were obtained from Sigma-Aldrich. GRP78 (BiP) protein (active) was purchased from Abcam. Luciferase assay substrate and the luciferase assay buffer were purchased from Promega. UDP-6-[3H]Gal (40–60 Ci/mmol) was obtained from American Radiolabeled Chemicals, Inc. Insect cells (Hi-5 and Sf-9) were obtained from American Type Culture Collection. Sep-Pak C18 Cartridges were obtained from Waters Corporation. Restriction enzymes were obtained from New England Biolabs, Inc. pVL 1393 vector and transfection kit were obtained from BD Biosciences. Ni-nitrilotriacetic acid Superflow beads were obtained from Qiagen. SDS-PAGE gels were obtained from Invitrogen. Centricon 10-kDa molecular mass cut-off membrane was obtained from Millipore.
Preparation of Expression Construct
Soluble N-terminal 6×His-tagged soluble Cosmc (6×His-sCosmc) was prepared as described (16). For constructing plasmids for encoding E152K mutated form of Cosmc, a fragment from the digestion of the plasmid encoding the soluble form of the 6×His-tagged Cosmc, as described (16), was replaced by the fragment obtained by the digestion using SacI/NheI for full-length E152K mCosmc as described (25). The construct was confirmed by sequencing and termed 6×His-msCosmc.
Expression and Purification of 6×His-sCosmc, 6×His-smCosmc, and Soluble N-terminal HPC4-tagged Core 1 β3-Gal-T (T- synthase)
Soluble T-synthase was made by co-expressing N-terminal HPC4 epitope-tagged soluble T-synthase (HPC4-sT-syn) as described (26) with wild-type full-length Cosmc as described (25) in Hi-5 cells using the Baculovirus system. After 96 h of post-infection in Hi-5 cells, the medium was collected, and the epitope-tagged protein was absorbed on HPC4 antibody-conjugated Ultralink resin, washed, and eluted with elution buffer containing 10 mm EDTA. Using Centricon 10-kDa cut-off membranes, protein was concentrated in 5 mm Tris-HCl buffer, 30 mm NaCl, pH 7.8. Both 6×His-sCosmc and 6×His-msCosmc were made as described (16). Reducing SDS-PAGE was carried out followed by Coomassie Blue staining to determine purity (27). Gels were imaged on FluorChem Camera (Alpha Innotech), and the density of the bands was quantified using software FluorChemTM V.5.0.2.4 (Alpha Innotech).
In Vitro Reconstitution of Heat-denatured T-synthase and Heat-denatured β4-GalT
Recombinant HPC4-sT-syn and β4-GalT (∼0.25 μg in 32 μl) were denatured by heating over time at ∼54 °C or 62 °C, respectively in 10 mm HEPES buffer pH 7.8 containing 12 mm MgCl2 then cooled to room temperature. Renaturation was initiated by the addition of 6×His-sCosmc and ATP to a final concentration of ∼2.27 μm and 5 mm, respectively, in the reconstitution buffer except in Fig. 1, E and F, where the amount of 6×His-sCosmc and BiP were both at a final concentration of 1.8 μm. After preincubation at room temperature for 45 min, T-synthase activity was measured using the methods used previously except varying the concentration of UDP-Gal to 0.2 mm final concentration and without Triton-X100 (26). In Fig. 1, E and F preincubation was carried out at room temperature for 75 min, and T-synthase activity was measured. β4-GalT activity was determined using pNP-β-S-GlcNAc as the acceptor as described (28). Relative T-synthase or β4-GalT activities were calculated using 100% as the activity of the untreated enzymes.
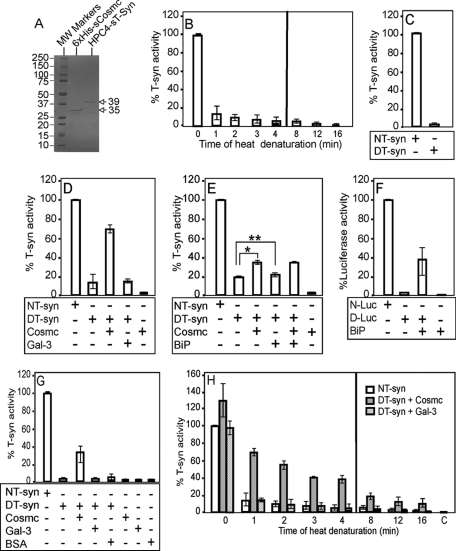
FIGURE 1.
Cosmc-dependent reconstitution of active T-synthase from denatured T-synthase. A, SDS-PAGE analysis of purified recombinant human 6×His-sCosmc and HPC4-sT-syn. The human N-terminal 6×His-tagged soluble Cosmc (6×His-sCosmc) and the N-terminal HPC4 epitope-tagged soluble T-synthase (HPC4-sT-syn) were expressed in Hi-5 cells and purified directly from the medium. Protein (2 μg each) was subjected to polyacrylamide gel electrophoresis and stained by Coomassie Blue, showing one major band in each lane (arrows). The lanes represent protein standards (lane 1), 6×His-sCosmc (lane 2), and HPC4-sT-syn (lane 3). B, HPC4-sT-syn was heat-denatured in reconstitution buffer over time and percent specific activities of native, and each preparation of denatured, HPC4-sT-syn were determined. C, purified soluble human HPC4-sT-syn was treated with GnHCl, and the percent specific activities of both treated (DT-syn) and untreated (NT-syn) T-synthase were determined. D, reconstitution of the heat-denatured HPC4-sT-syn, which was heated for 1min at 54 °C, was initiated by the addition of 6×His-sCosmc and the percent of restored T-synthase activity was determined, whereas galectin-3 (Gal-3), a control protein for specificity, did not support reconstitution. E, reconstitution of the heat-denatured HPC4-sT-syn was initiated by the addition of ER general chaperone Bip, which did not support the reconstitution of heat-denatured HPC4-sT-syn whereas (F) reconstitution of Bip appears to restore the activity of heat-denatured luciferase. N-Luc, native luciferase, D-Luc, denatured luciferase. G, reconstitution of GnHCl-denatured HPC4-sT-syn was initiated by the addition of recombinant 6×His-sCosmc, Gal-3, or BSA, as indicated, and percent T-synthase activity was determined. H, restoration of activity of denatured HPC4-sT-syn heated over time by addition of 6×His-sCosmc was measured. 6×His-sCosmc was added to denatured HPC4-sT-syn at each time point. A parallel reconstitution experiment of denatured HPC4-sT-syn was initiated by the addition of Gal-3, and percent T-synthase activity was determined. C, 6×His-sCosmc alone. A vertical line separates experiments performed at two different times (B and H). In B, D, and H, each assay was performed in duplicate, three replicate experiments were performed, and data represent the average of all experiments. In C and H, each assay was performed in duplicate and four replicate experiments were performed. In E and F, at least three replicate experiments were performed, and data represent the average of all experiments. Error bars, ± 1 S.D. from the average. * and ** represent p values p < 0.01 and p = 0.62, respectively.
Chemical Denaturation and Renaturation of T-synthase
HPC4-sT-syn (35 μg) was denatured in 1 ml of 6 m guanidinium hydrochloride (GnHCl), pH 7.2 for 90 min at room temperature. The sample was concentrated to ∼70 μl using Centricon 10,000 cutoff membranes. The sample was diluted 100 times in reconstitution buffer (10 mm HEPES, 150 mm NaCl, 12 mm MgCl2, pH 7.8) then 34-μl aliquots of the diluted sample were used for reconstitution reactions. Reconstitution was initiated by the addition of 6×His-sCosmc and ATP where the final concentration was 2.27 μm and 5 mm, respectively. Parallel control reconstitution experiments were initiated either by the addition of BSA or galectin-3 where the final concentration was 2.27 μm and 5 mm ATP, respectively. The reaction was incubated for 45 min at room temperature followed by assay for T-synthase activity.
Luciferase Renaturation Assay
Approximately 10 nm commercial luciferase (firefly) in the reconstitution buffer (10 mm HEPES buffer containing 12 mm MgCl2 at pH 7.8) was denatured at 43 °C for 7 min and cooled to room temperature. Both ATP and BiP were added to a final concentration of 5 mm and 1.8 μm, respectively. Renaturation was carried out for ∼75 min, and the luciferase activity was immediately measured by the addition of 100 μl of assay reagents. The light produced was measured by a Top Count NXT Microplate Scintillation and Luminescence Counter. Luciferase activities were calculated using 100% as the activity of the untreated enzymes.
Time Dependence for Restoration of HPC4-sT-syn
Reconstitution of heat-denatured HPC4-sT-syn was carried out at 37 °C. For each reaction, 0.25 μg in 32 μl of recombinant HPC4-sT-syn was denatured by heat at ∼54 °C in 10 mm HEPES buffer containing 12 mm MgCl2 at pH 7.8 for 2 min and cooled to 37 °C. For reconstitution at 37 °C, 6×His-sCosmc, ATP, and other T-synthase reagents preincubated at 37 °C were added. Reconstitution of HPC4-sT-syn was carried out simultaneously for different time points, and the renaturation stopped at different times by diluting the reaction ∼15 times with cold water, following by assays for T-synthase activity.
RESULTS
Cosmc Promotes Renaturation of Denatured T-synthase in Vitro
To investigate whether the chaperone function of Cosmc can be measured in vitro, we expressed recombinant soluble 6×His-tagged Cosmc (6×His-sCosmc) and HPC4-tagged soluble T-synthase (HPC4-sT-syn), and purified each to apparent homogeneity (Fig. 1A), of apparent molecular mass of 35 and 39 kDa, respectively. Note that to generate the enzymatically active HPC4-sT-syn in Hi-5 insect cells, we co-expressed the HPC4-sT-syn with a construct encoding full-length, membrane-bound Cosmc, which insects lack. Because the full-length Cosmc is not secreted it does not contaminate preparations of the co-expressed HPC4-sT-syn. To generate the 6×His-sCosmc, we expressed a construct encoding that protein alone in Hi-5 cells. For these in vitro renaturation studies, we denatured HPC4-sT-syn either thermally or chemically. The denaturation status of HPC4-sT-syn was monitored by assaying T-synthase activity after being heated over time or being treated with 6 m guanidinium hydrochloride (GnHCl) for 90 min, as shown in Fig. 1, B and C, respectively. Treatments with either heat or GnHCl caused significant loss of enzyme activity. In exploring the ability of Cosmc to restore activity to denatured HPC4-sT-syn, the renaturation assay was initiated by the addition of purified 6×His-sCosmc into the denatured enzyme, which was followed by assaying enzyme activity. This involved measuring product formation with the donor UDP-[3H]Gal toward the acceptor GalNAcα1-O-phenyl (29). Incubation with 6×His-sCosmc caused significant restoration of activity of denatured HPC4-sT-syn either by heat treatment or GnHCl (Fig. 1, D and G, respectively). Whereas addition of 6×His-sCosmc induced significant restoration of heat-denatured HPC4-sT-syn, addition of a control protein, recombinant galectin-3, at equal amounts to 6×His-sCosmc had no effect on restoring the activity (Fig. 1D). Human galectin-3 was chosen as one of the controls, because like Cosmc it is also a non-glycosylated recombinant protein, and has a similar size (∼30 kDa) to Cosmc (30, 31). Importantly, addition of recombinant human BiP, a general ER chaperone, did not promote the renaturation of heat-denatured HPC4-sT-syn (Fig. 1E). Addition of BiP did not inhibit the reconstitution of heat-denatured HPC4-sT-syn by 6×His-sCosmc (Fig. 1E), indicating that BiP-containing buffer lacks inhibitory activity in the renaturation process. By contrast, addition of BiP to heat-denatured luciferase (Fig. 1F) caused partial restoration of luciferase activity, and BiP alone lacks contaminating luciferase activity (Fig. 1F). For HPC4-sT-syn denatured by GnHCl treatment, incubation with 6×His-sCosmc also caused restoration of activity, whereas addition of control proteins, BSA, or galectin-3, had little effect (Fig. 1G).
We examined Cosmc-dependent renaturation of heat-denatured HPC4-sT-syn over the time of heating. The results showed that the longer HPC4-sT-syn was heated, there was a reduction of the amount of restoration of enzyme activity by the same amount of 6×His-sCosmc (Fig. 1H). This reduction is likely caused by precipitation of denatured T-synthase with prolonged heating. 6×His-sCosmc alone had no contaminating T-synthase enzyme activity (Fig. 1, D, E, and G), but we observed that at time 0 before heating, the addition of 6×His-sCosmc to the recombinant HPC4-sT-syn led to elevated enzyme activity. This is likely to be due to the presence of some partly denatured HPC4-sT-syn in the starting preparation purified from insect cells. These results strongly support the conclusion that Cosmc functions as a chaperone for the denatured T-synthase, and it can act independently of other co-chaperones in this in vitro assay.
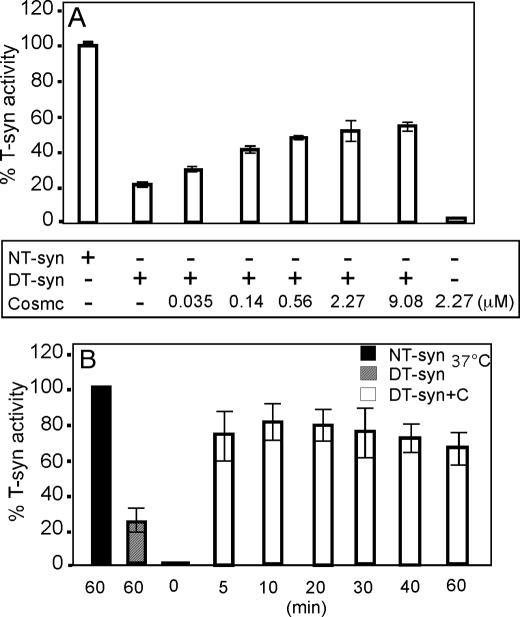
Cosmc Promotes Renaturation of Heat-denatured T-synthase in a Dose-dependent Manner
To further characterize the role of 6×His-sCosmc in the reconstitution of heat-denatured HPC4-sT-syn, we performed in vitro reconstitution experiments of heat-denatured HPC4-sT-syn (0.18 μm) with varying concentrations of 6×His-sCosmc. The ability of 6×His-sCosmc to aid in reconstitution of heat-denatured HPC4-sT-syn was concentration dependent (Fig. 2A). In this in vitro assay, significant restoration of HPC4-sT-syn activity occurred when the molar ratio of 6×His-sCosmc and HPC4-sT-syn was ∼1:1 (∼0.14 μm 6×His-sCosmc), as shown in Fig. 2A. We also measured the time dependence of restoration of activity of heat-denatured HPC4-sT-syn by 6×His-sCosmc. In this experiment, HPC4-sT-syn was denatured by treatment at ∼54 °C for 2 min, and then incubated at 37 °C with 6×His-sCosmc in the presence of all components of the HPC4-sT-syn activity assay for the indicated times. Therefore, the renaturation and measurement of HPC4-sT-syn activity was carried out simultaneously. Within the first 5 min, which was the minimal feasible time for conducting this type of experiment, we observed that maximum HPC4-sT-syn activity was regained (Fig. 2B). The results show that inactive HPC4-sT-syn regained activity within 5 min after the addition of 6×His-sCosmc.
FIGURE 2.
Cosmc restoration of activity of heat-denatured T-synthase is concentration dependent. A, purified HPC4-sT-syn (NT-syn) was heat-denatured in reconstitution buffer. Renaturation of the activity of the heat-denatured HPC4-sT-syn (DT-syn) was initiated by the addition of increasing concentrations of 6×His-sCosmc as indicated to DT-syn preparations, and percent T-synthase activity of each reaction was determined. B, time dependence of restoration of T-synthase activity from DT-syn by 6×His-sCosmc, in which reconstitution and assay of T-synthase was conducted at 37 °C, and the percent restored activity was determined at different time points as indicated. In A, each assay was performed in duplicate, two replicate experiments were performed, and data represent the average of all experiments. In B, each assay was performed in duplicate, three replicate experiments were performed, and data represent the average of all experiments. Error bars, ± 1 S.D. from the average.
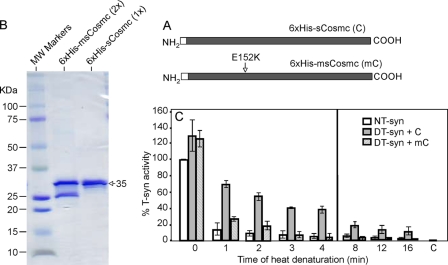
Mutated Form of Cosmc Does Not Restore the Activity of Heat-denatured T-synthase as Efficiently as Soluble Cosmc
Cosmc with Glu (E) to Lys (K) mutation at position 152 (E152K) was originally identified in a patient with Tn syndrome (25) (Fig. 3A). Co-expression of this mutated Cosmc with human HPC4-sT-syn in insect cells indicated that 6×His-msCosmc (E152K) has little ability to function as a chaperone in a cell expression system for forming active T-synthase (25). To investigate the characteristics of mutated Cosmc in vitro, we expressed and purified the tagged, soluble form of mutated Cosmc (6×His-msCosmc) in Hi-5 cells (Fig. 3B). 6×His-msCosmc (∼35kDa) is not as stable as wild-type 6×His-sCosmc and appears to be partly degraded (∼50%) to a smaller form (∼27 kDa). Therefore, the SDS-PAGE and Coomassie Blue-stained gel shown in Fig. 3B utilized twice as much 6×His-msCosmc (8 μg) compared with 6×His-sCosmc (4 μg). A scan of the gel shows that the amount of material at ∼35 kDa in size for both 6×His-msCosmc (lane 2) and 6×His-sCosmc (lane 3) are similar. Using this preparation of 6×His-msCosmc, we compared its ability to 6×His-sCosmc to assist renaturation of heat-denatured HPC4-sT-syn, and tested the effect over time. To compensate for the partial degradation observed for 6×His-msCosmc, we used twice the concentration of 6×His-msCosmc as compared with 6×His-sCosmc. Whereas 6×His-sCosmc was effective in assisting renaturation of heat-denatured HPC4-sT-syn, 6×His-msCosmc was weakly active and had no significant activity in restoring HPC4-sT-syn activity heated for 3 min or more (Fig. 3C). These results show that mutated Cosmc is not as effective as soluble wild-type Cosmc in this in vitro assay in assisting refolding activity of T-synthase.
FIGURE 3.
Mutated Cosmc has little effect on restoration of denatured T-synthase activity. A, depiction of the 6×His-sCosmc and 6×His-msCosmc constructs, where the latter has a point mutation E152K. B, SDS-PAGE analysis of purified recombinant soluble human 6×His-sCosmc and 6×His-msCosmc, as indicated. Lane 1, molecular weight standards; lane 2, 6×His-msCosmc (∼8 μg); lane 3, human N-terminal 6×His-sCosmc (∼4 μg). A densitometry scan of the protein bands in lanes 2 and 3 showed that the amount of protein corresponding to the apparent molecular mass of ∼35 kDa for both 6×His-smCosmc and 6×His-sCosmc were similar. C, renaturation of heat-denatured HPC4-sT-syn (DT-syn) was initiated by addition of 6×His-msCosmc and with recombinant 6×His-sCosmc, and percent T-synthase activity was determined. In C, each assay was performed in duplicate, three replicate experiments were performed, and data represent the average of all experiments. Error bars, ± 1 S.D. from the average. The vertical line separates experiments done at two different time points.
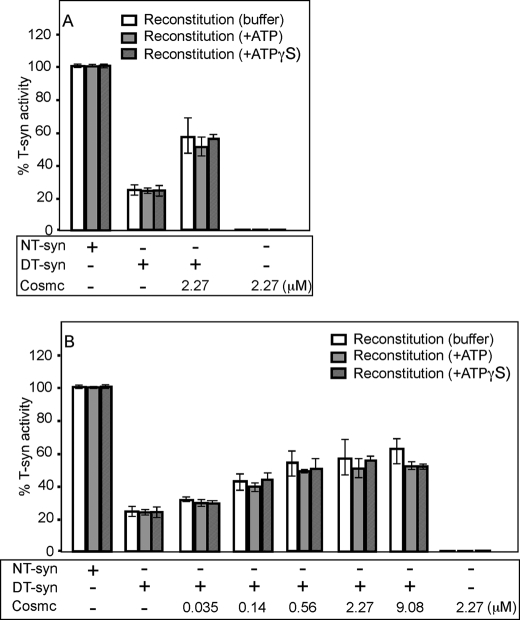
Cosmc Does Not Require ATP for Its in Vitro Renaturation of Heat-denatured T-synthase
Our previous study showed that Cosmc, but not the T-synthase, has ATP binding activity (16), which is consistent with a possible ATP-dependent chaperone function for the human Cosmc. It is interesting that in prior studies, the mouse homolog of Cosmc (NP_067525), which was studied before the function of Cosmc was identified, was also reported to have ATP binding activity (32). To explore whether ATP has any effect in the renaturation of heat-denatured T-synthase by Cosmc, we studied the effects of 6×His-sCosmc in vitro in the presence of ATP, the non-hydrolyzable analog ATPγS, and buffer without nucleotide. The 5 mm final concentration of nucleotide were chosen in this in vitro experiment because that amount was used in reconstitution of denatured luciferase by Hsp70 chaperone system (33). We first investigated the role of ATP where the 6×His-sCosmc concentration was ∼12-times higher than the concentration of HPC4-sT-syn. There was not a significant difference between the reconstitution of HPC4-sT-syn in the three different conditions (Fig. 4A). To further explore the potential effect of ATP, we varied the concentrations of 6×His-sCosmc in the presence of ATP and ATPγS as indicated. The data showed that neither ATP nor ATPγS had a significant difference in the restoration of activity of denatured HPC4-sT-syn (Fig. 4B). These results indicate that Cosmc does not require ATP for the folding of T-synthase in this in vitro assay.
FIGURE 4.
Cosmc can restore the activity of heat-denatured T-synthase independently of ATP in vitro. A, purified HPC4-sT-syn (NT-syn) was heat-denatured in reconstitution buffer. Reconstitution of denatured HPC4-sT-syn (DT-syn) activity was initiated by the addition of 6×His-sCosmc in reconstitution buffer with ATP, non-hydrolyzable ATP (ATPγS), or without nucleotide, where final concentration of nucleotides is 5 mm, and percent T-synthase activity was determined. B, reconstitution of heat-denatured HPC4-sT-syn by the addition of increasing concentration (μm) of 6×His-sCosmc in reconstitution buffer with ATP, ATPγS, or without nucleotide, where final concentration of nucleotides is 5 mm, and percent T-synthase activity was determined. Each assay was performed in duplicate, two replicate experiments were performed, and the data represent the average of all experiments. Error bars, ± 1 S.D. from the average.
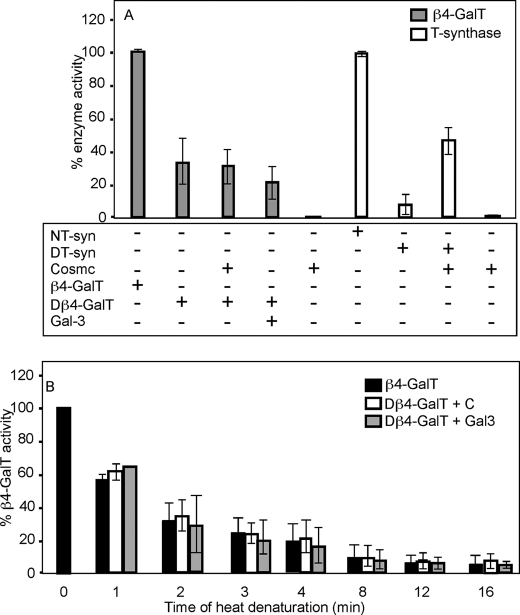
Cosmc Cannot Restore the Activity of Denatured β1–4-Galactosyltransferase (β4-GalT)
To further examine whether Cosmc is specific to the T-synthase, we investigated the nature of its specificity in vitro by conducting reconstitution experiments for another partially denatured enzyme in parallel to the experiments conducted with denatured HPC4-sT-syn. To this end, we selected the well-studied enzyme UDPGal:GlcNAc β1–4-galactosyltransferase (β4-GalT) (34–37). Like T-synthase, β4-GalT is also a Golgi-localized β-glycosyltransferase, uses the same donor substrate UDP-Gal, and is also an inverting β-galactosyltransferase. The in vitro reconstitution reaction was initiated by addition of either 6×His-sCosmc or galectin-3 to heat-denatured β4-GalT, and the activity of this enzyme was assayed. No reconstitution of denatured β4-GalT by either 6×His-sCosmc or galectin-3 was observed, whereas in parallel control experiments, 6×His-sCosmc was effective in restoring partial activity of heat-denatured HPC4-sT-syn (Fig. 5A). We further investigated the ability of Cosmc to function as a potential chaperone for β4-GalT, which was heated over time in the expectation that a diversity of intermediate folded forms might be present ranging from slightly unfolded to fully unfolded forms. The β4-GalT was heated over time at 62 °C, which resulted in time-dependent loss of its activity (Fig. 5B). However, addition of 6×His-sCosmc or galectin-3 had no significant effect on restoring activity of the β4-GalT. Taken together, these results show that Cosmc is specific for the T-synthase and is not able to restore activity of a related Golgi β-galactosyltransferase.
FIGURE 5.
Cosmc is unable to restore activity to denatured β4-GalT. A, β4-GalT was heat-denatured and reconstitution of denatured β4-GalT (Dβ4-GalT) was initiated by the addition of recombinant 6×His-sCosmc or Gal-3, and percent β4-GalT activity was determined. In parallel, reconstitution of the heat-denatured HPC4-sT-syn (DT-syn) was initiated by the addition of recombinant 6×His-sCosmc, and percent T-synthase activity was determined. B, β4-GalT was heat-denatured over time and reconstitution of denatured β4-GalT was initiated by the addition of recombinant 6×His-sCosmc (C) or Gal-3, and percent β4-GalT activity was determined. Each assay was performed in duplicate, two replicate experiments were performed, and data represent the average of all experiments. Error bars, ± 1 S.D. from the average.
DISCUSSION
Cosmc is the first known ER molecular chaperone required for expression of a glycosyltransferase that functions in the Golgi apparatus, but the mechanism of its action has not been clear. Recently, we reported that ER-localized Cosmc can be co-immunoprecipitated with T-synthase (16), and we hypothesized that Cosmc might play a direct role in the biosynthesis of the active form of T-synthase by interacting with newly synthesized nonnative T-synthase. The studies described here provide novel information as to how this ER chaperone functions and show that Cosmc directly interacts with partly unfolded and inactive T-synthase to partially restore enzyme activity. We showed previously that expression of active T-synthase in cells requires ER-localized Cosmc (16, 17), and the lack of Cosmc function results in inactive T-synthase aggregates (16).
The biochemical studies and the in vitro functional assays using purified 6×His-sCosmc show that Cosmc can restore activity from inactive forms of T-synthase following a brief period (<5 min) of incubation, that the addition of ATP had no effect in this assay format, and that restoration in vitro occurs independently of other chaperones/co-chaperones. Importantly, addition of the general ER chaperone BiP did not restore the activity of heat-denatured T-synthase, whereas BiP does support renaturation of luciferase. It has been shown previously that recombinant Hsc70 can support in vitro refolding of heat-denatured luciferase (38). Furthermore, 6×His-msCosmc E152K cannot restore the denatured HPC4-sT-syn in vitro as efficiently as 6×His-sCosmc and 6×His-sCosmc does not effect restoration of activity for another well-characterized β-galactosyltransferase (β4-GalT). Overall, our results provide experimental evidence in vitro that Cosmc is a specific chaperone for folding and maturation of the T-synthase. Moreover, these studies have a broad impact in understanding the expression of Tn and sialyl-Tn (22–24) which is associated with mutations in Cosmc and consequent deficiency of active T-synthase in human diseases, such as Tn syndrome (25) and human tumors (20, 21).
The approach taken here is modeled after studies on other chaperones showing their ability to restore the activity of thermally or chemically denatured substrate. Typically, the ability of a chaperone to restore activity to denatured substrates can result in restoration of from 1–2% to nearly 80% of initial activity. For example, the small heat shock protein α/β crystallin causes ∼14% restoration of a client protein citrate synthase and addition of ATP enhances that to 25% (39). Treatment of the Rubisco enzyme with 6 m GnHCl results in considerable loss of secondary structure, and addition of chaperone Cpn60 (GroEL) with its co-chaperone Cpn10 (GroES) can rescue its enzymatic activity up to ∼80% of initial (40). Additionally, chaperones such as Hsp70 or its prokaryotic homolog DnaK/DnaJ and GroEL-GroES, can reactivate both thermally and chemically denatured substrates (40–42). We observed that 6×His-sCosmc can cause up to ∼75% restoration of heat-denatured HPC4-sT-syn activity depending on how long the protein has been heat-treated (Fig. 1, D and H), and that it could restore ∼30% of activity to HPC4-sT-syn denatured by GnHCl treatment (Fig. 1G). Thus, Cosmc restoration of activity to denatured T-synthase is relatively similar to that seen for other chaperones with other clients in vitro, and probably reflects the heterogeneity of misfolded forms of the enzyme generated by these denaturing conditions.
Whereas Cosmc can restore the activity of denatured T-synthase activity independently of other co-chaperones in vitro, it is possible that co-chaperones play an important role in vivo within the crowded environment of the ER regulating the kinetics of T-synthase refolding in vivo. We previously reported that inactive oligomeric T-synthase accumulating in cells in the absence of Cosmc is associated with GRP78 (16). Thus, whereas other chaperones may associate with T-synthase, their interactions appear to be non-productive in helping to form active enzyme, but may be important in ER stress responses or eliminating/removing the inactive and oligomeric T-synthase from the ER lumen. Future studies are required to examine the chaperone folding complex and assess whether co-chaperones may function with Cosmc. Some known chaperones require co-chaperones and others do not. For example, chaperones like Hsp60, Hsp70, and Hsp90 require co-chaperones for their functional cycle (5), whereas small heat shock proteins Hsp18.1, Hsp17.7, Hsp27, and Hsp25 do not require co-chaperones and can function independently as molecular chaperones (43, 44).
The E152K mutated form of Cosmc found in Tn syndrome patients appears to lack any significant activity in vivo, because there is no detectable activity of T-synthase in the patient (25, 45). However, the mutated Cosmc is expressed. Therefore, we tested whether this mutated Cosmc can restore the activity of denatured T-synthase using our in vitro reconstitution experiment. Clearly, whereas the 6×His-msCosmc could not restore the activity of heat-denatured HPC4-sT-syn as efficiently as 6×His-msCosmc (Fig. 3C), there was a small but detectable level of restored activity. It is possible, but unlikely, that 6×His-msCosmc (E152K) generated recombinantly in insect cells might differ structurally or in other respects from the protein expressed in vivo, or that the 6×His tag might contribute in some indirect way to this partial functionality of the mutated Cosmc. The mutated protein expressed in vivo might not be folded correctly, localize differently, interact with different interacting partner/s, or the steady state level of the protein could be lower, resulting in its no detectable function in vivo.
We previously reported that Cosmc binds to ATP-Sepharose, and can be cross-linked to [α-32P]azido-ATP (16). The ability of Cosmc to bind ATP is consistent with prior reports showing that the mouse homolog of Cosmc also appears to have ATP binding activity (32). The functional cycles of many chaperones for assisting protein folding are enhanced by their interaction with nucleotides such as ATP/ADP (5, 46). Our results indicate that ATP is not required in vitro for the renaturation of heat-denatured HPC4-sT-syn (Fig. 5A). Consistent with our data, several other ATP-independent chaperones have been identified, including Hsp47 (47), small Hsp (48), calnexin (49), SecB (50), and α-crystallin (51). Thus, the role, if any, of ATP in Cosmc function remains to be determined. It is possible that ATP binding by Cosmc may be important in a cycle of binding and release of T-synthase in vivo, through interactions with other proteins or perhaps co-chaperones.
The studies described here show that inactive and partly misfolded T-synthase can be partly restored to active form by incubation with 6×His-sCosmc and suggest that Cosmc activity is specific for T-synthase and not active toward another inverting β-galactosyltransferase. In other studies, we have generated mice deficient for Cosmc and found that embryonic cells from such null animals completely lack T-synthase activity but have apparently other normal protein glycosylation except for loss of core 1 O-glycans.3 The experimental results here are consistent with such observations and imply the apparently specific requirement of Cosmc for forming active T-synthase. Experiments are underway using mutants of Cosmc and T-synthase to define the molecular sites responsible for Cosmc/T-synthase intermolecular interactions.
Acknowledgments
We thank Dr. Sean Stowell for providing recombinant Galectin-3, Anthony Luyai for suggestions and critique of this manuscript, and Dr. Jamie Heimburg-Molinaro for help in preparing and editing the manuscript. We thank Sandy Cummings for technical support.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 GM068559 (to R. D. C.).
Y. Wang, T. Ju, and R. D. Cummings, unpublished observations.
- ER
- endoplasmic reticulum
- BSA
- bovine serum albumin
- β4-GalT
- β1–4-galactosyltransferase
- Cosmc
- core 1 β3-GalT-specific molecular chaperone
- Gal-3
- galectin-3
- GalNAc
- N-acetylgalactosamine
- GlcNAc
- N-acetylglucosamine
- GnHCl
- guanidinium hydrochloride
- HPC
- human protein C
- ATPγS
- adenosine 5′-O-(thiotriphosphate).
REFERENCES
- 1.Helenius A., Marquardt T., Braakman I. (1992) Trends Cell Biol. 2, 227–231 [DOI] [PubMed] [Google Scholar]
- 2.Hebert D. N., Molinari M. (2007) Physiol. Rev. 87, 1377–1408 [DOI] [PubMed] [Google Scholar]
- 3.Ellgaard L., Helenius A. (2003) Nat. Rev. Mol. Cell Biol. 4, 181–191 [DOI] [PubMed] [Google Scholar]
- 4.Anfinsen C. B. (1973) Science 181, 223–230 [DOI] [PubMed] [Google Scholar]
- 5.Fink A. L. (1999) Physiol. Rev. 79, 425–449 [DOI] [PubMed] [Google Scholar]
- 6.Ellis R. J. (1990) Semin. Cell Biol. 1, 1–9 [PubMed] [Google Scholar]
- 7.Hoshino M., Kawata Y., Goto Y. (1996) J. Mol. Biol. 262, 575–587 [DOI] [PubMed] [Google Scholar]
- 8.Schmid D., Baici A., Gehring H., Christen P. (1994) Science 263, 971–973 [DOI] [PubMed] [Google Scholar]
- 9.Blond-Elguindi S., Cwirla S. E., Dower W. J., Lipshutz R. J., Sprang S. R., Sambrook J. F., Gething M. J. (1993) Cell 75, 717–728 [DOI] [PubMed] [Google Scholar]
- 10.Buck T. M., Wright C. M., Brodsky J. L. (2007) Semin Cell Dev. Biol. 18, 751–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarty J. S., Buchberger A., Reinstein J., Bukau B. (1995) J. Mol. Biol. 249, 126–137 [DOI] [PubMed] [Google Scholar]
- 12.Xu Z., Horwich A. L., Sigler P. B. (1997) Nature 388, 741–750 [DOI] [PubMed] [Google Scholar]
- 13.Ranson N. A., Farr G. W., Roseman A. M., Gowen B., Fenton W. A., Horwich A. L., Saibil H. R. (2001) Cell 107, 869–879 [DOI] [PubMed] [Google Scholar]
- 14.Nakai A., Satoh M., Hirayoshi K., Nagata K. (1992) J. Cell Biol. 117, 903–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kota J., Ljungdahl P. O. (2005) J. Cell Biol. 168, 79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ju T., Aryal R. P., Stowell C. J., Cummings R. D. (2008) J. Cell Biol. 182, 531–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ju T., Cummings R. D. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 16613–16618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu J., Gerhardt H., McDaniel J. M., Xia B., Liu X., Ivanciu L., Ny A., Hermans K., Silasi-Mansat R., McGee S., Nye E., Ju T., Ramirez M. I., Carmeliet P., Cummings R. D., Lupu F., Xia L. (2008) J. Clin. Invest. 118, 3725–3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia L., Ju T., Westmuckett A., An G., Ivanciu L., McDaniel J. M., Lupu F., Cummings R. D., McEver R. P. (2004) J. Cell Biol. 164, 451–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ju T., Lanneau G. S., Gautam T., Wang Y., Xia B., Stowell S. R., Willard M. T., Wang W., Xia J. Y., Zuna R. E., Laszik Z., Benbrook D. M., Hanigan M. H., Cummings R. D. (2008) Cancer Res. 68, 1636–1646 [DOI] [PubMed] [Google Scholar]
- 21.Schietinger A., Philip M., Yoshida B. A., Azadi P., Liu H., Meredith S. C., Schreiber H. (2006) Science 314, 304–308 [DOI] [PubMed] [Google Scholar]
- 22.Brockhausen I. (2006) EMBO Rep. 7, 599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Itzkowitz S. H., Bloom E. J., Kokal W. A., Modin G., Hakomori S., Kim Y. S. (1990) Cancer 66, 1960–1966 [DOI] [PubMed] [Google Scholar]
- 24.Springer G. F. (1997) J. Mol. Med. 75, 594–602 [DOI] [PubMed] [Google Scholar]
- 25.Ju T., Cummings R. D. (2005) Nature 437, 1252. [DOI] [PubMed] [Google Scholar]
- 26.Ju T., Brewer K., D'Souza A., Cummings R. D., Canfield W. M. (2002) J. Biol. Chem. 277, 178–186 [DOI] [PubMed] [Google Scholar]
- 27.Laemmli U. K. (1970) Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 28.van Die I., van Tetering A., Schiphorst W. E., Sato T., Furukawa K., van den Eijnden D. H. (1999) FEBS Lett. 450, 52–56 [DOI] [PubMed] [Google Scholar]
- 29.Ju T., Cummings R. D., Canfield W. M. (2002) J. Biol. Chem. 277, 169–177 [DOI] [PubMed] [Google Scholar]
- 30.Barondes S. H., Castronovo V., Cooper D. N., Cummings R. D., Drickamer K., Feizi T., Gitt M. A., Hirabayashi J., Hughes C., Kasai K., Leffler H., Liu F. T., Lotan R., Mercurio A. M., Monsigny M., Pillai S., Poirer F., Raz A., Rigby P. I., Rini J. M., Wang J. L. (1994) Cell 76, 597–598 [DOI] [PubMed] [Google Scholar]
- 31.Stowell S. R., Qian Y., Karmakar S., Koyama N. S., Dias-Baruffi M., Leffler H., McEver R. P., Cummings R. D. (2008) J. Immunol. 180, 3091–3102 [DOI] [PubMed] [Google Scholar]
- 32.Inoue S., Sano H., Ohta M. (2000) Biochem. Biophys. Res. Commun. 268, 553–561 [DOI] [PubMed] [Google Scholar]
- 33.Laufen T., Mayer M. P., Beisel C., Klostermeier D., Mogk A., Reinstein J., Bukau B. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 5452–5457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Narimatsu H., Sinha S., Brew K., Okayama H., Qasba P. K. (1986) Proc. Natl. Acad. Sci. U.S.A. 83, 4720–4724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qasba P. K., Ramakrishnan B., Boeggeman E. (2008) Curr. Drug Targets 9, 292–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaper N. L., Shaper J. H., Meuth J. L., Fox J. L., Chang H., Kirsch I. R., Hollis G. F. (1986) Proc. Natl. Acad. Sci. U.S.A. 83, 1573–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hill R. L., Brew K. (1975) Adv. Enzymol Relat. Areas Mol. Biol. 43, 411–490 [DOI] [PubMed] [Google Scholar]
- 38.Terada K., Kanazawa M., Bukau B., Mori M. (1997) J. Cell Biol. 139, 1089–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muchowski P. J., Clark J. I. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 1004–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goloubinoff P., Christeller J. T., Gatenby A. A., Lorimer G. H. (1989) Nature 342, 884–889 [DOI] [PubMed] [Google Scholar]
- 41.Ziemienowicz A., Skowyra D., Zeilstra-Ryalls J., Fayet O., Georgopoulos C., Zylicz M. (1993) J. Biol. Chem. 268, 25425–25431 [PubMed] [Google Scholar]
- 42.Hafizur R. M., Yano M., Gotoh T., Mori M., Terada K. (2004) J Biochem. 135, 193–200 [DOI] [PubMed] [Google Scholar]
- 43.Rogalla T., Ehrnsperger M., Preville X., Kotlyarov A., Lutsch G., Ducasse C., Paul C., Wieske M., Arrigo A. P., Buchner J., Gaestel M. (1999) J. Biol. Chem. 274, 18947–18956 [DOI] [PubMed] [Google Scholar]
- 44.Lee G. J., Pokala N., Vierling E. (1995) J. Biol. Chem. 270, 10432–10438 [DOI] [PubMed] [Google Scholar]
- 45.Crew V. K., Singleton B. K., Green C., Parsons S. F., Daniels G., Anstee D. J. (2008) Br. J. Haematol. 142, 657–667 [DOI] [PubMed] [Google Scholar]
- 46.Sullivan W., Stensgard B., Caucutt G., Bartha B., McMahon N., Alnemri E. S., Litwack G., Toft D. (1997) J. Biol. Chem. 272, 8007–8012 [DOI] [PubMed] [Google Scholar]
- 47.Saga S., Nagata K., Chen W. T., Yamada K. M. (1987) J. Cell Biol. 105, 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jakob U., Gaestel M., Engel K., Buchner J. (1993) J. Biol. Chem. 268, 1517–1520 [PubMed] [Google Scholar]
- 49.Helenius A. (1994) Mol. Biol. Cell 5, 253–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Randall L. L., Hardy S. J. (1995) Trends. Biochem. Sci. 20, 65–69 [DOI] [PubMed] [Google Scholar]
- 51.Marini I., Moschini R., Del Corso A., Mura U. (2005) Cell Mol. Life Sci. 62, 599–605 [DOI] [PMC free article] [PubMed] [Google Scholar]