Abstract
Members of the 70-kDa heat shock family can control and manipulate a host of oncogenic client proteins. This role of Hsp70 in both the folding and degradation of these client proteins makes it a potential drug target for certain forms of cancer. The phenothiazine family of compounds, as well as the flavonoid myricetin, was recently shown to inhibit Hsp70-ATPase activity, whereas members of the dihydropyrimidine family stimulated ATPase function. Akt, a major survival kinase, was found to be under the regulation of Hsp70, and when the ATPase activity of Hsp70 was increased or decreased by these compounds, Akt levels were also increased or decreased. Also, increasing Hsp70 levels concurrent with inhibition of its ATPase function synergistically reduced Akt levels to a greater extent than either manipulation alone, providing new insights about client fate decisions. Akt reductions mediated by Hsp70 inhibitors were prevented when Hsp70 expression was silenced with small interfering RNA. Inhibiting Hsp70 ATPase function produced cytotoxic events only in breast cancer cell lines where Akt dysfunction was previously shown, suggesting therapeutic specificity depending on the Hsp70 client profile. Thus, increasing Hsp70 levels combined with inhibiting its ATPase function may serve to dramatically reduce Akt levels and facilitate cell death in certain types of cancer.
Keywords: Cancer/Breast, Chaperones/Protein Folding, Chaperones/Heat Shock, Protein/Degradation, Protein/Folding, Protein/Stability, Signal Transduction/Protein Kinases, Therapeutics
Introduction
Heat shock proteins are the primary regulators of protein folding and degradation in the cell. The ATPase activity of Hsp70 and Hsp90 variants serves as the hub whereby all other chaperones and co-chaperones are activated, either to facilitate recycling or for clearance of chaperone substrates or clients. Much of the effort surrounding chaperone research has focused on either altering the expression levels of these heat shock proteins by activating the heat shock transcription factor (HSF1) or by inhibiting the activity of Hsp90 (1, 2). Small molecules have been identified that can produce either or both of these outcomes (3, 4). However, despite its clear role in a number of diseases of aging, very little emphasis has been placed on the enzymatic ATPase activity of Hsp70. To address this deficiency, we recently identified several compounds that could either stimulate or inhibit Hsp70 ATPase function without affecting its levels (5). These compounds bind to Hsp70 allosterically, affecting the rate of ATP consumption, which is tied to opening and closing of the protein lid over the substrate binding domain. When ATP is converted to ADP, the lid closes, preventing release of the client. When ADP is exchanged for ATP, the lid opens, and the client is released. We demonstrated that Hsp70 inhibitors force this lid to stay open, whereas activators increase the rate of lid opening and closing (5). With these compounds defined, we are now able to compare the effects of increasing Hsp70 expression levels with modulating its ATPase function for distinct disease-related clients.
With this in mind, and based on previous findings that the prosurvival kinase, Akt, was a well established substrate of the chaperone network (6–9), we contrasted the impact of both Hsp70 expression and activity on Akt stability (10–12). Because kinases in general have traditionally been viewed as Hsp90 clients, most of the existing literature has focused on this relationship; however, more recent work suggests that not only is the heat shock-inducible form of Hsp70 (Hsp72) influencing cell survival and Akt levels, but the constitutive variant Hsc70 might also be playing a role (13). Interestingly, our investigations here using an siRNA2-screening assay initially suggested that these two extremely similar Hsp70 variants were having opposing activities on Akt protein levels. This unexpected result led us to further explore the relationship, and we have found that inducible Hsp70 is a critical mediator of Akt proteostasis. Then, using our newly described Hsp70 inhibitors, we discovered a second mechanistic paradox between Hsp70 levels and function. Lastly, we show that inhibiting Hsp70 ATPase function can rapidly and selectively kill cancer cells that are dependent on Akt for survival. Together, these results provide new mechanistic and therapeutic insights into the relationship between chaperones and Akt.
MATERIALS AND METHODS
Cell Lines
The Hs578T breast cancer cell line and the syngenic noncancer control Hs578Bst fibroblast line collected from the unaffected breast of the same patient (14) were obtained from ATCC. We also utilized MDA-MB-231, MDA-MB-468, MDA-MB-361, MCF7, and MDA-MB-453 cells (15). HeLa cells were also obtained from ATCC.
Antibodies, Plasmids, siRNAs, and Chemicals
Anti-Hsp70 and anti-Hsc70 antibodies were purchased from Stressgen (Ann Arbor, MI) and anti-Akt (total Akt) antibody was purchased from Cell Signaling Technology, and all were used at 1:1,000 dilutions. Anti-actin antibody was obtained from Sigma Aldrich and used at a 1:1,000 dillution. All antibodies were diluted in 7% nonfat dry milk in TBS solution. CA-Akt plasmid, a constitutively active, via myristoylation, form of mouse Akt-1, was provided by Ben Wolozin (Boston University). All siRNAs were acquired from Qiagen and were described previously and characterized at a 20 nm final concentration (5, 16). Methylene blue was obtained from Thermo Fisher Scientific. Azure C, SW02, 115–7C, and myricetin were described previously (5). KNK-437 was obtained from Sigma Aldrich.
Cell Culture and Transfections
Hs578T and MCF-7cells were grown in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (FBS), 1% PenStrep (Invitrogen), and 0.01 mg/ml bovine insulin (Sigma Aldrich). Hs578Bst cells were grown in HybriCare medium (ATCC) containing 10% FBS, 1% PenStrep, and 30 ng/ml epidermal growth factor (Sigma Aldrich). MDA-MB-468, MDA-MB-361, and MDA-MB453 cells were grown in Dulbecco's modified Eagle's medium containing 10% FBS and 1% PenStrep. HeLa cells were grown in Opti-MEM media containing 10% FBS and 1% PenStrep. MDA-MB-231 and T47D cells were grown in RPMI 1640 media containing 10% FBS and 1% PenStrep. siRNA transfections were performed using SilentFect (Bio-Rad) and left to incubate for 48 h before harvest or treatment, with the exception of the KNK-437 experiment, where 100 μm of the reagent was added 24 h post-transfection. Plasmid transfections were performed using Lipofectamine 2000 (Invitrogen) using 1 μg plasmid per reaction; cells were left to incubate for 48 h before harvest or drug treatment.
Protein Collection, Quantitation, and Western Blotting
Supernatants of selected experiments were harvested using mammalian protein extraction reagent (Invitrogen) containing phosphatase inhibitor mixtures 1 and 2 (Sigma Aldrich) at 1:100, protease inhibitor cocktail set III at 1:100 (Calbiochem), and phenylmethylsulfonyl fluoride (Fisher BioReagents) at 1:100. Protein levels of the lysates were measured using the Pierce BCA kit. All proteins were standardized following BCA in BupH-modified phosphate-buffered saline (Thermo Fisher Scientific). Western blotting was performed by running SDS-PAGE, which was followed by transfer and probing with antibodies and visualization by ECL (SignaGen). Western blot quantitation was performed using Scion Image for Windows (Scion Corp.) version Alpha 4.0.3.2.
In-cell Western (ICW)
siRNA and Silentfect were mixed in serum-free medium at a ratio of 20 nm to 1 μl and distributed into indicated wells. Suspended HeLa cells were added to these mixtures and incubated for 72 h. Medium was removed from adherent cells and an in-cell Western assay was performed as described previously (17). Rabbit anti-Akt antibody (1:500 as determined from supplemental Fig. S1) and mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (1:1,000) from BioDesign were incubated overnight. Secondary antibodies labeled with near-infrared fluorophores were applied at 1:500 (anti-rabbit Alexa Fluor 680 from Molecular Probes and anti-mouse IR800CW from Rockland). Plates were imaged using the LiCor Odyssey system.
Lactate Dehydrogenase (LDH) Assay
Breast cancer cell lines were plated using designated media, according to ATCC. Once cells reached ∼95% confluency, methylene blue or vehicle treatments were applied in fresh serum-free Opti-MEM (Invitrogen) media. 6 h later, medium was collected and centrifuged to pellet dead cells. Supernatant was then added to a 96-well plate in triplicate along with reagents from Cytotox96 kit (Promega) following the supplied protocol.
RESULTS
Hsp70 and Hsc70 Reciprocally Affect Akt Levels
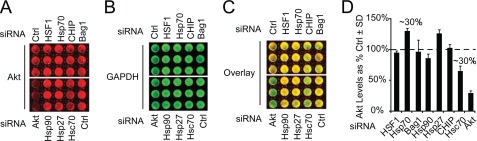
Previous work from our lab and others suggested that Akt was critically modulated by the chaperone network (10, 18, 19). To determine which chaperones and chaperone-related proteins were most effective at regulating Akt stability, we developed an ICW assay to screen chaperone siRNAs in a standard HeLa cell line, which was selected for its high transfection efficiency, high levels of endogenous Akt, and robust properties of adhesion and viability to withstand a semi-high throughput procedure (i.e. multiple washing steps, shaking, etc.; supplemental Fig. S1). Using this system, we found that siRNAs for Hsp70 or Hsc70 increased or decreased endogenous Akt levels by ∼30%, respectively. Use of Akt siRNA resulted in ∼75% knockdown of Akt. Both Hsp90 and CHIP siRNA also caused reductions in Akt levels, although to a lesser extent than Hsc70. Hsp27 siRNA also increased Akt levels. Neither Bag1 nor HSF1 siRNA had any effect on endogenous Akt levels. Akt levels were normalized to GAPDH, and increases or decreases are shown as a percentage of the nonsilencing control (Fig. 1, A–D). These findings, coupled with our hypothesis that the Hsp70 family of proteins may be involved in Akt stability, led us to further investigate their relationship.
FIGURE 1.
Small scale siRNA screen reveals opposing effects of Hsp70 and Hsc70 on Akt levels. HeLa cells were plated in 96-well plates at ∼40% confluency and transfected with indicated siRNAs for 72 h. siRNAs have been previously validated by Western blotting in this cell line. In-cell Western analysis for Akt (A, red) and GAPDH (B, green) showed an ∼30% increase in Akt levels when Hsp70 or Hsp27 was knocked down and an ∼30% decrease in Akt levels when Hsc70 was knocked down. Hsc70 siRNA decreased Akt levels most potently. An overlaid image of Akt and GAPDH is presented for contrast (C). Triplicate wells were quantified, and all values are shown as a percent of control siRNA (Ctrl) after GAPDH normalization ± S.D. (D).
Hsc70 Modulates Akt Levels Indirectly via Hsp70
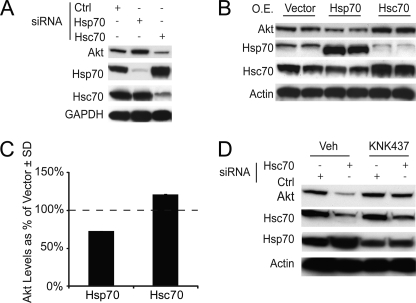
To confirm our ICW results, we analyzed lysates from HeLa cells transfected with either Hsc70 siRNA, Hsp70 siRNA, or a nonsilencing control by Western blotting. Akt levels confirmed the results from our ICW, showing increased levels of Akt in the presence of Hsp70 siRNA and decreased Akt levels in the presence of Hsc70 siRNA (Fig. 2A). Specific knockdown was confirmed for both siRNAs using Hsp and Hsc70 antibodies. Interestingly, this analysis revealed that Hsp70 levels were increased when Hsc70 was knocked down, whereas Hsc70 levels seemed unaffected by Hsp70 knockdown. To probe this further, we overexpressed Hsp or Hsc70 and explored not only the effects on Akt, but also their effects on each other. Indeed, Hsp70 overexpression decreased Akt levels, whereas Hsc70 increased Akt levels, reciprocal to the siRNA results (Fig. 2, B and C). Moreover, increasing the level of Hsc70 decreased Hsp70 levels, again opposing the results seen with siRNA. This suggested that the effects of Hsc70 on Akt were actually an indirect consequence of increasing Hsp70 expression. We considered two possible mechanisms for this; either Hsc70 was contributing to Hsp70 turnover, or it was regulating Hsp70 transcription. To test the latter, cells transfected with either Hsc70 siRNA or a nonsilencing control were treated with vehicle or KNK-437, a compound that inhibits heat shock transcription by blocking the binding of HSF1 to heat shock elements (HSE) in Hsp promoters (20–22). Western blot analysis showed that Akt clearance mediated by Hsc70 siRNA was blocked when Hsp70 transcription was inhibited, indicating that Hsp70, not Hsc70, was critically linked to Akt stability (Fig. 2D). Therefore, we endeavored to further explore how Hsp70 might be involved in fate decisions for Akt and what role, if any, this has in altering cell viability.
FIGURE 2.
Silencing and overexpression of Hsp70 and Hsc70 inversely affect Akt and each other; effect is blocked by heat shock transcription inhibition. HeLa cells were transfected with Hsp70 or Hsc70 siRNA or a nonsilencing control siRNA (Ctrl) for 48 h and then analyzed by Western blotting (20 μg protein/lane) (A). HeLa cells at ∼90% confluency were transfected with Hsp70 and Hsc70 expression vectors for 48 h and analyzed by Western blotting (40 μg protein/lane) (B). Akt levels resulting from the overexpression (O.E.) of Hsp70 and Hsc70 data were quantitated ± S.D. (C). HeLa cells, at ∼50% confluency, were transfected with siRNA for Hsc70 or a nonsilencing control; at the time of transfection, cells were treated with either vehicle (Veh) or 100 μm heat shock element inhibitor KNK-437 for 48 h; results were analyzed by Western blotting (20 μg protein/lane) (D).
Inhibition of Hsp70ATPase Function Reduces Akt Levels
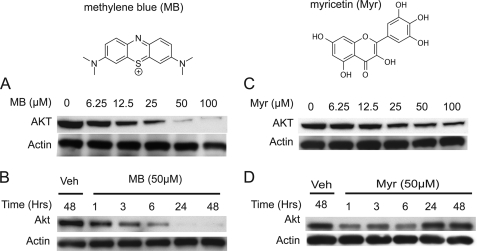
To determine whether Hsp70 levels and ATPase function could converge to regulate Akt stability, we analyzed Akt levels in HeLa cells following treatment with a group of recently identified Hsp70 ATPase chemical modulators (5). Interestingly, Hsp70 ATPase inhibitors (methylene blue (MB) and myricetin) caused robust decreases in Akt levels in a dose-dependent manner (Fig. 3A). These reductions were evident within 1 h (Fig. 3B). Reductions were more pronounced over a 48-h time course with MB; however, the flavonoid, myricetin, lost activity after 24 h (Fig. 3, C and D), likely due to the inherent instability of all flavonoid compounds (23). Because of the robust activity demonstrated by MB, further studies defining the mechanisms involved in Hsp70-mediated Akt clearance focused on this compound and its demethylated derivative, Azure C (AC).
FIGURE 3.
Hsp70 ATPase inhibitors show reductions in Akt levels in HeLa cells. Structure of MB and myricetin (Myr) pictured at top. A 6-h dose response was performed with MB (A) or myricetin (C) at indicated concentrations in HeLa cells. A time course of 50 μm MB (B) or myricetin (D) was also performed at indicated time points in HeLa cells. Shown is a 6-h myricetin dose response at indicated dosages in HeLa cells, treated at ∼90% confluency. All blots contained 20 μg protein/lane. Veh, vehicle.
Activators of Hsp70 ATPase Function Facilitate Akt Accumulation
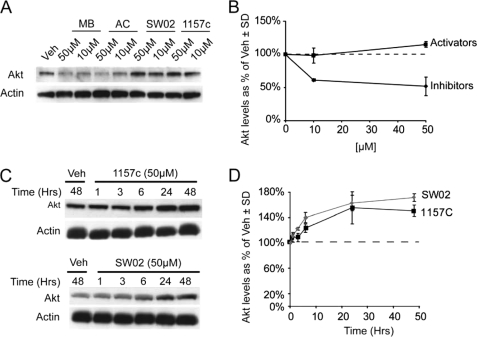
From our previous chemical screening platform (5, 24), we not only identified inhibitors of Hsp70 ATPase function but also identified activators. Therefore, we tested the effects of these dihydropyrimidine activators, SW02 and 1157C, on Akt levels in HeLa cells. These activators, along with MB and AC, were incubated with cells at two doses (10 and 50 μm) for 24 h. Activation of Hsp70 ATPase function with SW02 and 1157c increased Akt levels in a dose-dependent manner, relative to vehicle, whereas Hsp70 inhibition with MB and AC reduced Akt levels (Fig. 4, A and B). Time course analyses for 50 μm doses of 1157C and SW02 showed accumulation of Akt levels over time (Fig. 4, C and D). These results were somewhat unexpected because Hsp70 overexpression caused a similar outcome for Akt stability, as did Hsp70 enzymatic inhibition, whereas Hsp70 activation preserved Akt levels. One obvious explanation would be that the drugs are simply having off-target effects, which should not be overlooked, given that MB and myricetin can both regulate mitochondrial respiration. To address this issue, we investigated whether overexpression of Hsp70 combined with Hsp70 inhibition synergistically reduced Akt levels. A dose-response analysis was performed with MB and AC in cells transfected with Myc-tagged Hsp70 or empty vector. We found that although Hsp70 did decrease Akt levels, inhibition of Hsp70 ATPase activity coupled with its overexpression was more effective than either alone (supplemental Fig. S2); however, this still did not definitively show that MB and AC were working via Hsp70 to reduce Akt levels. There was still a possibility that both manipulations were simply facilitating Akt clearance through two distinct pathways.
FIGURE 4.
Hsp70 ATPase activators display effects opposite those of inhibitors. HeLa cells, at ∼90% confluency, were treated for 24 h with 10 and 50 μm of Hsp70 ATPase inhibitors (MB or AC) or Hsp70 activators (SW02 or 115–7C), and lysates were analyzed by Western blotting (A). Quantitation was performed using pixel density analysis and is shown as a percentage of vehicle (Veh)-treated cells ± S.D. (♦, inhibitor; ■, activator) (B). HeLa cells, at ∼90% confluency, were treated with 50 μm doses of either SW02 or 115-7C and analyzed by Western blotting (C). Quantitation was performed using pixel density analysis and is shown as a percentage of vehicle-treated cells ± S.D. (■, 1157c; ♦, SW02) (D). All blots contained 20 μg protein/lane.
Despite Pluripotency, Phenothiazines Primarily Affect Akt via Hsp70 Modulation
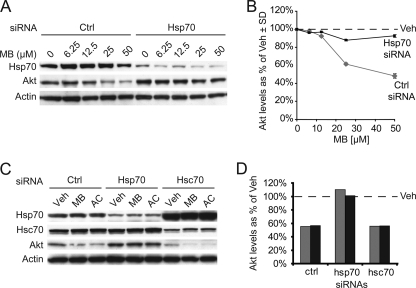
To definitively address whether MB and AC were regulating Akt levels by inhibiting Hsp70 ATPase function, we again turned to siRNA. We suppressed Hsp70 expression in HeLa cells with Hsp70 siRNA and performed a dose-response study with MB. Indeed, Hsp70 siRNA almost completely blocked MB-mediated decreases in Akt, relative to cells transfected with a nonsilencing control (Fig. 5, A and B). Subsequent investigation of this phenomenon with both MB and AC showed that Hsp70, not Hsc70, was necessary for Hsp70-inhibitor-mediated Akt reductions (Fig. 5, C and D). In fact, Hsc70 siRNA actually increased Hsp70 inhibitor efficacy, due to the increase in Hsp70 levels caused by Hsc70 knockdown. Thus, Hsp70 overexpression and Hsp70 ATPase inhibition can converge to reduce the prosurvival kinase Akt in cells. Although paradoxical, these results can actually be explained quite elegantly when considering the properties of Hsp70 itself and our recent data with these compounds and the microtubule-associated protein Tau (see Fig. 7 and “Discussion” for more detail regarding the model) (5). One final aspect of these studies was to test the potential therapeutic application of these inhibitors in cancer cells whose survival is dependent on high levels of Akt activity.
FIGURE 5.
Efficacy of Hsp70 ATPase inhibitors is dependent on levels of Hsp70. HeLa cells, at ∼50% confluency, were transfected with Hsp70 siRNA or a nonsilencing control (Ctrl) and were then treated with increasing doses of MB as indicated. Lysates were analyzed by Western blot (A), and Akt levels as a percent of vehicle (Veh) ± S.D. following pixel density analysis (■, Hsp70 siRNA; ♦, control siRNA) (B). HeLa cells, at ∼50% confluency, were transfected with nonsilencing siRNA (Ctrl), Hsp70 siRNA, or Hsc70 siRNA for 48 h, and then were treated with 50 μm MB, AC, or vehicle (C). Results were analyzed by Western blotting, and quantitation of Akt levels by pixel density analysis is shown as a percentage of corresponding vehicle treatments (gray bar, MB; black bar, AC) (D). All blots contained 20 μg protein/lane.
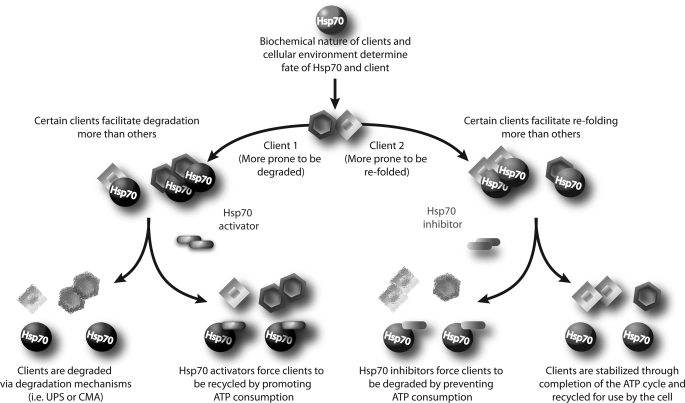
FIGURE 7.
Model for targeting clients toward destructive or productive pathways via Hsp70. As distinct clients (i.e. Client 1 and Client 2) are bound by Hsp70, there are two distinct fates which can emerge based on the client. These fates can be classified as either profolding or prodegradation. The biochemical nature of the client bound to Hsp70 along with the state of the cellular environment are the key factors that guide the Hsp70-client complex toward a fate: pro-folding for those designated to be lead away from potentially nonproductive misfolding pathways; or prodegradation for those requiring turnover as indicated by internal mechanisms. However, the exact mechanisms contributing to either of these decisions are unknown. Increasing the levels of Hsp70 is more likely to decrease certain clients while preserving others. By chemically modulating the ATPase activity of Hsp70, we are able to dictate client fate. Hsp70 activators can promote the accumulation of clients that would normally be degraded by Hsp70. Hsp70 inhibitors can promote the degradation of clients that would normally be preserved. Thus, Hsp70 overexpression combined with Hsp70 inhibition would synergistically affect clients that typically prefer degradation. Conversely, for clients that are more prone to refolding, overexpression of Hsp70 would have minimal impact on the levels of these clients, but inhibition of Hsp70 ATPase function would cause reductions.
Phenothiazines Selectively Kill Breast Cancer Cells
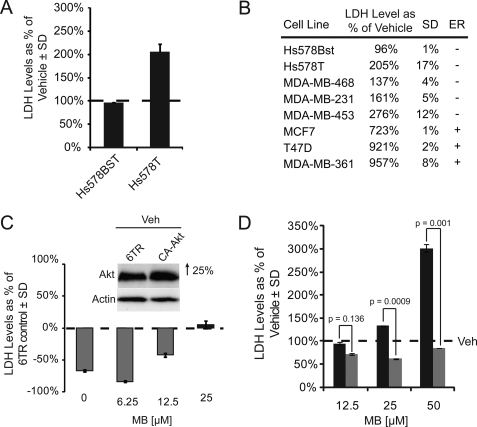
Although heat shock proteins have traditionally been deemed “protective” for cells, the results from our previous experiments indicated that cancer cell lines, in which Akt has been found to be critical for cell survival, may be uniquely sensitive to Hsp70 inhibition with MB. We analyzed the effects of Hsp70 inhibition on cell survival using seven breast cancer lines. We emphasized the Hs578T cell line because it has high levels of active Akt (25), and we previously showed effects with CHIP and Hsp90 inhibitors in this line (10). We compared the effects of MB on the Hs578T line with the Hs578Bst fibroblast line, which was isolated from the opposite breast of the same patient. The effects of MB on the Hs578T cells were dramatic; cells appeared morbid and nonadherent after only 1 h of a 50 μm MB treatment, with nearly complete cell death after 24 h. The Hs578Bst line, on the other hand, was completely unaffected by treatment. We turned to lactate dehydrogenase analysis to quantitatively show the cytotoxicity. Cells were again treated with a 50 μm concentration of MB for 6 h, and lactate dehydrogenase analysis showed double the amount of lactate dehydrogenase in spent medium compared with that of the Hs578Bst media (Fig. 6A). We then investigated the effects of Hsp70 inhibition on additional breast cancer cell lines with Akt dysfunction; MDA-MB-453, MDA-MB-361, MCF7, MDA-MB-468, MDA-MB-231, MCF-7, T47D, and MDA-MB-361. Indeed, increased cytotoxicity was observed in each of these cell lines to varying degrees, with the three estrogen receptor (+) cell lines (MCF-7, T47D, and MDA-MB-361) being the most affected (Fig. 6B). Because we did not observe MB mediated toxicity in other standard cell lines, such as HeLa and HEK293 (data not shown), we speculated that it was indeed Akt that was essential for survival in these particular breast cancer cells. To test this, we overexpressed constitutively active Akt (CA-Akt) in the Hs578T line, and performed a dose-response analysis with MB, again measuring lactate dehydrogenase levels. We found that at lower doses, Akt overexpression could abrogate cytotoxicity (Fig. 6C), despite the poor DNA transfection efficiency of this cell line (only ∼25% increase in expression). In fact, the higher 25 μm dose was able to override the low levels of CA-Akt. We then made a second attempt to rescue the cytotoxic effects of Hsp70 inhibition by pretreating Hs578T cells with the Hsp70 ATPase activator, SW02, 24 h prior to MB treatment. We found that activating Hsp70 function before treating HeLa cells with an inhibitor completely blocked the cytotoxicity previously seen at matching doses of MB (Fig. 6D). These data indicated that inhibition of Hsp70 by MB could reduce Akt levels and kill specific cancer types that require Akt for survival. Moreover, we were able to rescue these effects by overexpressing Akt or activating Hsp70 ATPase function.
FIGURE 6.
Hsp70 inhibitors produce Akt-dependent cytotoxicity in breast cancer cell lines. LDH assays were performed on media from Hs578Bst and Hs578T cell lines, both at ∼90% confluency, and treated with 50 μm MB or vehicle (Veh) for 6 h. Levels are shown as a percent of vehicle treated cells ± S.D. (A). LDH assays were performed on media from MDA-MB-468, MDA-MB-231, MDA-MB-453, MCF-7, T47D, and MDA-MB-361 cell lines, all at ∼90% confluency, treated with 50 μm MB or vehicle for 6 h. LDH levels are shown as a percent of vehicle treated cells ± S.D. and estrogen receptor (ER) expression (B). LDH assays were performed on media collected from Hs578T cells transiently transfected at ∼90% confluency with either a 6TR noncoding vector or a myristoylated Akt vector (CA-Akt) and treated with increasing dosages of MB or a vehicle control for 6 h. LDH levels are shown as a percent of cells overexpressing CA-Akt relative to treatment matched 6TR control transfected cells ± S.D. Inset verifies Akt overexpression by pixel density analysis of Western blot run on cells receiving vehicle treatment (C). LDH assays were performed on Hs578T cells, at ∼90% confluency, receiving a 24-h 50 μm dose of Hsp70 ATPase activator SW02 (gray bars) or vehicle (black bars) followed by a 6-h dose response treatment of Hsp70 ATPase inhibitor MB or vehicle. LDH levels are shown as a percent of vehicle ± S.D. (D). p values were determined by Student's t test. All blots contained 20 μg protein/lane.
DISCUSSION
Here, we have demonstrated for the first time that inhibition of Hsp70 ATPase activity and Hsp70 overexpression can each facilitate reductions in Akt stability. The ability of Hsp70 ATPase inhibitors to promote Akt clearance was dependent on the levels of Hsp70 in the cell. We also demonstrated that activating Hsp70 ATPase function increases Akt levels. Moreover, using a newly developed ICW assay for analysis of siRNAs targeting important chaperones, we found that Hsc70 and Hsp70 could differentially regulate Akt levels. Hsp27 siRNA also appeared to increase Akt levels. In subsequent analyses, we demonstrated that Akt reductions caused by Hsc70 siRNA were a direct result of a compensatory increase in Hsp70 levels via heat shock factor activation. We also demonstrated that certain breast cancer cell types with high levels of active Akt may be susceptible to Hsp70 inhibition. Ultimately, these studies suggest that Hsp70 is intimately involved with Akt stability, and modulating its levels and activity may be a relevant therapeutic strategy for the treatment of breast cancer.
The most interesting aspect of this study is the initially confounding result that Hsp70 overexpression and inhibition of its activity could lead to the same outcome for Akt. In fact, based on our preliminary data with Hsp70 overexpression, we initially speculated that Hsp70 activators would decrease Akt levels, whereas inhibitors would preserve it. However, these results, coupled with our findings with the Tau protein, suggest a mechanism that may change the way chaperone biology is currently viewed. The mechanisms that facilitate the decision of the Hsp70 complex to allow degradation or refolding a protein are not known. It is only known that the rate of hydrolysis and turnover of ATP can push the complex in either direction; more ATP consumption leads to more refolding; less ATP consumption leads to more degradation. Our data here, when placed in the context of our recent findings with the Tau protein, suggest an intriguing possibility: The nature of a specific client along with the state of the cellular environment can be destructive or productive. Some clients, like Tau, may mostly be targeted for refolding. Therefore, Tau levels are not dramatically reduced when Hsp70 levels are increased because this only produces more profolding Hsp70-Tau complexes that are not degraded; however, when we inhibit the ATPase activity of Hsp70, this forces the release of Tau from Hsp70, leading to its degradation (5). Conversely, because Hsp70 overexpression did reduce Akt levels, we would speculate that Akt is typically targeted for degradation. Then, when we inhibit Hsp70 ATPase function, the remaining pool of Hsp70-Akt complexes that was targeted for refolding is forced toward degradation, leading to further decreases in Akt levels. This principle is depicted graphically in Fig. 7. Therefore, what initially seemed paradoxical could actually shed new light on an area that is essential for our complete understanding of the chaperone process; how Hsp70 and Hsp90 “decide” when to promote degradation or refolding by accessory co-chaperones.
In addition to this interesting mechanistic insight, there are logical comparisons with this work that could be drawn to Hsp90 inhibitors (26). 17-Allyl-amino-17-demethoxygelanamycin, a specific Hsp90 ATPase inhibitor, has shown efficacy for reducing cell survival proteins such as Akt (27), propelling it to clinical trials as a potential therapy for various cancers (28–30). However, there seem to be several important distinctions between Hsp70 and Hsp90 inhibition. For example, because Hsp90 normally functions to bind HSF1 and prevent its translocation to the nucleus (3), Hsp90 inhibition induces a stress response, whereas Hsp70 inhibition does not. In fact, despite uncertainty about efficacy, this feature alone has prompted the use of 17-allyl-amino-17-demethoxygelanamycin in breast cancer trials simply as a heat shock inducer rather than an Hsp90 inhibitor (31–34). In addition to this major functional difference between Hsp70 and Hsp90, these proteins also possess unique structures, binding partners, and ATPase turnover kinetics. Despite these critical differences, the line separating these two proteins is often blurred when discussing their functional differences. Perhaps now, with these tools at our disposal, we can begin to further define the precise differences between Hsp70 and Hsp90 function in a way that was not previously possible.
This notion of functional redundancy is even more common when discussing Hsp70 and Hsc70, which often are used interchangeably in the literature. These proteins are extremely similar structurally, which suggests they also have similar functions. However, although these proteins may function similarly at the biochemical level, as we and others have shown, there are interesting differences between these two proteins with regard to the role of Hsc70 in regulating Hsp70 expression. For example, silencing of Hsp70 increased levels of Her2 in SKBr3 cells, whereas Hsc70 silencing decreased these levels in the same cell line (35). Moreover, our data here shows that Hsc70 is involved in Hsp70 expression, but not vice versa, suggesting that Hsc70 is a possibly more dominant form. Perhaps Hsp70 levels are partially regulated based on the existing levels of Hsc70 (13, 35). It is Hsp70, however, that appears to have the greater impact on Akt, as seen by the increases in Akt levels when Hsp70 is silenced and the decreases in Akt when Hsp70 is induced or overexpressed. The activity of Hsc70 on Akt regulation appears to only exist via induction of Hsp70 expression. It is important to note as well that the chemicals used in these studies are not selective for Hsc70 or Hsp70; they do inhibit both enzymes (24). Despite this, Hsp70 levels are not affected by these inhibitors (Fig. 5), suggesting that the induction in Hsp70 caused by Hsc70 siRNA is due to reduced levels of Hsc70 and not activity. Thus, Hsc70 and Hsp70 levels may be equally important for regulating Akt stability, but Hsp70 seems more likely to have a direct association with this important kinase.
Another consideration from these studies is that both Akt and Hsp70 have demonstrated prosurvival functions in certain cell types. Recent evidence suggests that Hsp70 and Hsc70 play a role in preventing apoptosis, a process in which Akt is also intimately associated (13). Hsp70 can bind to the apoptosis precursor DIABLO (Smac) and the presence of a functional Hsp70 ATPase domain can prevent cell death (36). This suggests that an induction of Hsp70, by any means, would potentially be working against apoptosis. Therefore, at first glance, it is somewhat counterintuitive that Hsp70 overexpression and inhibition would both lead to reductions in Akt levels because Akt is known to promote cell survival; this function would likely antagonize any anti-apoptotic function of Hsp70. However, our results seem to point to a mechanism whereby the oncogenic client repertoire involved in specific types of cancer may be predictive of Hsp70 inhibitor efficacy. In other words, in tumor cells that are dependent on Akt activation for transformation and survival (similar to that found in the Hs578T line and the MDA-MB lines), Hsp70 overexpression combined with Hsp70 inhibition may be a very effective therapeutic strategy, simply because this would eliminate more Akt; however, either strategy may be entirely ineffective, or even detrimental, in other types of cancer. This aspect is an important consideration when attempting to manipulate oncogenic client proteins through modifications of the chaperone network. Given the low toxicity profile of MB in humans, this strategy could quickly move to the clinic for certain cancers based on client composition. Knowing whether chaperone clients are contributing to the initiation of oncogenic processes could be predictive of clinical success with chaperone-based therapies.
Finally, it is important to consider that MB and myricetin both are pluripotent compounds, affecting mitochondrial function, as well as a number of other processes in the cell (37–39). Therefore we cannot rule out that these “non-Hsp70” effects are contributing to cell death; however, our ability to rescue cell death by activating Hsp70 ATPase function suggests that Hsp70 inhibition is a primary activity of MB and related phenothiazine scaffolds, which is likely to prime certain cancer types for death. Although a therapeutic strategy involving Hsp70 for cancer requires additional validation, as well as identification of more specific compounds, our data support the idea that promoting Hsp70 levels combined with inhibiting its activity is potentially an effective strategy for reducing aberrant Akt levels in certain cancer types.
Supplementary Material
This work was supported by National Institutes of Health Grant R00AG031291 from NIA.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
- siRNA
- small interfering RNA
- FBS
- fetal bovine serum
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- LDH
- lactate dehydrogenase
- ICW
- in-cell Western
- MB
- methylene blue
- AC
- Azure C.
REFERENCES
- 1.Zou J., Guo Y., Guettouche T., Smith D. F., Voellmy R. (1998) Cell 94, 471–480 [DOI] [PubMed] [Google Scholar]
- 2.Grenert J. P., Sullivan W. P., Fadden P., Haystead T. A., Clark J., Mimnaugh E., Krutzsch H., Ochel H. J., Schulte T. W., Sausville E., Neckers L. M., Toft D. O. (1997) J. Biol. Chem. 272, 23843–23850 [DOI] [PubMed] [Google Scholar]
- 3.Bagatell R., Paine-Murrieta G. D., Taylor C. W., Pulcini E. J., Akinaga S., Benjamin I. J., Whitesell L. (2000) Clin. Cancer Res. 6, 3312–3318 [PubMed] [Google Scholar]
- 4.Sittler A., Lurz R., Lueder G., Priller J., Lehrach H., Hayer-Hartl M. K., Hartl F. U., Wanker E. E. (2001) Hum. Mol. Genet. 10, 1307–1315 [DOI] [PubMed] [Google Scholar]
- 5.Jinwal U. K., Miyata Y., Koren J., 3rd, Jones J. R., Trotter J. H., Chang L., O'Leary J., Morgan D., Lee D. C., Shults C. L., Rousaki A., Weeber E. J., Zuiderweg E. R., Gestwicki J. E., Dickey C. A. (2009) J. Neurosci. 29, 12079–12088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qian S. B., McDonough H., Boellmann F., Cyr D. M., Patterson C. (2006) Nature 440, 551–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickey C. A., Yue M., Lin W. L., Dickson D. W., Dunmore J. H., Lee W. C., Zehr C., West G., Cao S., Clark A. M., Caldwell G. A., Caldwell K. A., Eckman C., Patterson C., Hutton M., Petrucelli L. (2006) J. Neurosci. 26, 6985–6996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballinger C. A., Connell P., Wu Y., Hu Z., Thompson L. J., Yin L. Y., Patterson C. (1999) Mol. Cell. Biol. 19, 4535–4545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cyr D. M., Höhfeld J., Patterson C. (2002) Trends Biochem. Sci. 27, 368–375 [DOI] [PubMed] [Google Scholar]
- 10.Dickey C. A., Koren J., Zhang Y. J., Xu Y. F., Jinwal U. K., Birnbaum M. J., Monks B., Sun M., Cheng J. Q., Patterson C., Bailey R. M., Dunmore J., Soresh S., Leon C., Morgan D., Petrucelli L. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 3622–3627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai Q., Zhang C., Wu Y., McDonough H., Whaley R. A., Godfrey V., Li H. H., Madamanchi N., Xu W., Neckers L., Cyr D., Patterson C. (2003) EMBO J. 22, 5446–5458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Min J. N., Whaley R. A., Sharpless N. E., Lockyer P., Portbury A. L., Patterson C. (2008) Mol. Cell. Biol. 28, 4018–4025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powers M. V., Clarke P. A., Workman P. (2008) Cancer Cell 14, 250–262 [DOI] [PubMed] [Google Scholar]
- 14.Hackett A. J., Smith H. S., Springer E. L., Owens R. B., Nelson-Rees W. A., Riggs J. L., Gardner M. B. (1977) J. Natl. Cancer Inst. 58, 1795–1806 [DOI] [PubMed] [Google Scholar]
- 15.Yang L., Dan H. C., Sun M., Liu Q., Sun X. M., Feldman R. I., Hamilton A. D., Polokoff M., Nicosia S. V., Herlyn M., Sebti S. M., Cheng J. Q. (2004) Cancer Res. 64, 4394–4399 [DOI] [PubMed] [Google Scholar]
- 16.Dickey C. A., Kamal A., Lundgren K., Klosak N., Bailey R. M., Dunmore J., Ash P., Shoraka S., Zlatkovic J., Eckman C. B., Patterson C., Dickson D. W., Nahman N. S., Jr., Hutton M., Burrows F., Petrucelli L. (2007) J. Clin. Invest. 117, 648–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickey C. A., Eriksen J., Kamal A., Burrows F., Kasibhatla S., Eckman C. B., Hutton M., Petrucelli L. (2005) Curr. Alzheimer Res. 2, 231–238 [DOI] [PubMed] [Google Scholar]
- 18.Beliakoff J., Whitesell L. (2004) Anticancer Drugs 15, 651–662 [DOI] [PubMed] [Google Scholar]
- 19.Gao T., Newton A. C. (2002) J. Biol. Chem. 277, 31585–31592 [DOI] [PubMed] [Google Scholar]
- 20.Ohnishi K., Takahashi A., Yokota S., Ohnishi T. (2004) Int. J. Radiat. Biol. 80, 607–614 [DOI] [PubMed] [Google Scholar]
- 21.Voyer J., Heikkila J. J. (2008) Comp. Biochem. Physiol. A Mol. Integr. Physiol. 151, 253–261 [DOI] [PubMed] [Google Scholar]
- 22.Yokota S., Kitahara M., Nagata K. (2000) Cancer Res. 60, 2942–2948 [PubMed] [Google Scholar]
- 23.Boulton D. W., Walle U. K., Walle T. (1999) J. Pharm. Pharmacol. 51, 353–359 [DOI] [PubMed] [Google Scholar]
- 24.Chang L., Bertelsen E. B., Wisén S., Larsen E. M., Zuiderweg E. R., Gestwicki J. E. (2008) Anal. Biochem. 372, 167–176 [DOI] [PubMed] [Google Scholar]
- 25.Zhang M., Fang X., Liu H., Guo R., Wu X., Li B., Zhu F., Ling Y., Griffith B. N., Wang S., Yang D. (2007) Cancer Lett. 252, 244–258 [DOI] [PubMed] [Google Scholar]
- 26.Workman P. (2004) Cancer Lett. 206, 149–157 [DOI] [PubMed] [Google Scholar]
- 27.Pelicano H., Carew J. S., McQueen T. J., Andreeff M., Plunkett W., Keating M. J., Huang P. (2006) Leukemia 20, 610–619 [DOI] [PubMed] [Google Scholar]
- 28.Modi S., Stopeck A. T., Gordon M. S., Mendelson D., Solit D. B., Bagatell R., Ma W., Wheler J., Rosen N., Norton L., Cropp G. F., Johnson R. G., Hannah A. L., Hudis C. A. (2007) J. Clin. Oncol. 25, 5410–5417 [DOI] [PubMed] [Google Scholar]
- 29.Sharp S., Workman P. (2006) Adv. Cancer Res. 95, 323–348 [DOI] [PubMed] [Google Scholar]
- 30.Yano M., Naito Z., Tanaka S., Asano G. (1996) Jpn. J. Cancer Res. 87, 908–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Münster P. N., Srethapakdi M., Moasser M. M., Rosen N. (2001) Cancer Res. 61, 2945–2952 [PubMed] [Google Scholar]
- 32.Ciocca D. R., Clark G. M., Tandon A. K., Fuqua S. A., Welch W. J., McGuire W. L. (1993) J. Natl. Cancer Inst. 85, 570–574 [DOI] [PubMed] [Google Scholar]
- 33.Gabai V. L., Yaglom J. A., Waldman T., Sherman M. Y. (2009) Mol. Cell. Biol. 29, 559–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vargas-Roig L. M., Fanelli M. A., López L. A., Gago F. E., Tello O., Aznar J. C., Ciocca D. R. (1997) Cancer Detect. Prev. 21, 441–451 [PubMed] [Google Scholar]
- 35.Håvik B., Bramham C. R. (2007) Oncol. Rep. 17, 1501–1510 [PubMed] [Google Scholar]
- 36.Jiang B., Wang K., Liang P., Xiao W., Wang H., Xiao X. (2009) FEBS J. 276, 2615–2624 [DOI] [PubMed] [Google Scholar]
- 37.Kelner M. J., Bagnell R., Hale B., Alexander N. M. (1988) Basic Life Sci. 49, 895–898 [DOI] [PubMed] [Google Scholar]
- 38.Salaris S. C., Babbs C. F., Voorhees W. D., 3rd. (1991) Biochem. Pharmacol. 42, 499–506 [DOI] [PubMed] [Google Scholar]
- 39.Visarius T. M., Stucki J. W., Lauterburg B. H. (1997) FEBS Lett. 412, 157–160 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.