Abstract
CD4+ T cells can be instructed by nonantigen-specific signals to differentiate into functionally distinct lineages with mutually exclusive patterns of cytokine production. The molecular events that drive interferon-γ (IFNγ) production during Th1 development are well understood, but mechanisms that silence this cytokine during Th2 polarization are not clear. In this study, we find that the tbx21 gene encoding the Th1 master regulator T-bet is a direct target of the transcriptional repressor Ikaros. In Th2 cells, which do not express T-bet, strong Ikaros binding could be detected at the endogenous tbx21 promoter, whereas this gene was not occupied by Ikaros in T-bet-expressing Th1 cells. Inhibition of Ikaros DNA binding activity during Th2 polarization resulted in loss of Ikaros promoter occupancy, increased T-bet expression, and inappropriate T-bet-dependent production of IFNγ. Ikaros was also required for epigenetic imprinting of the ifnγ locus during Th2 polarization, and loss of Ikaros function in vivo led to an inappropriate Th1 response to the parasite Shistosoma mansoni. These studies demonstrate that Ikaros, a factor with an established role in lymphocyte development, also regulates the development of peripheral T helper responses.
Keywords: Chromatin/Epigenetics, Cytokines/Interferons, DNA/Methylation, Gene/Regulation, Immunology, Transcription/Regulation
Introduction
CD4+ helper T cells can differentiate into distinct subsets of effector cells that perform discrete functions during an immune response. Two well defined T cell differentiation pathways are T helper 1 (Th1),2 characterized by production of the cytokines IFNγ and interleukin (IL)-2, and T helper 2 (Th2), characterized by production of IL-4, IL-5, and IL-13. A combination of transcriptional activation and repression appears to polarize these modes of differentiation into mutually exclusive pathways. For instance, signaling through IFNγ and IL-12 receptors induces the activity of transcription factors like T-bet, Hlx, Stat4, Stat1, and Runx3, which drive ifnγ gene expression, whereas signaling through the IL-4 receptor induces Gata-3, c-Maf, and Stat6, which positively control transcription of the il4 gene (1–3). These mutually exclusive patterns of transcription factor expression are also reinforced by negative feedback, as Stat6 can actively repress expression of the tbx21 (T-bet) gene, and Stat4 down-regulates Gata-3. The mechanisms by which these signals lead to the repression of Gata-3 and T-bet expression are not known. Th1 and Th2 polarization is also associated with epigenetic changes in chromatin structure and DNA methylation at the ifnγ and il4 loci (3, 4). Although some of these changes are associated with binding of Stat6 and T-bet to the il4 and ifnγ loci, respectively, the molecular basis by which cytokine gene loci are remodeled epigenetically is not fully understood.
Ikaros is a zinc finger DNA binding protein that is required for lymphocyte development (5) and is known to interact with the NURD, CtBP, and Sin3 chromatin remodeling and DNA methylation complexes (6). An important role for this transcription factor has recently been defined in mature T cells, where it acts as a repressor of chromatin remodeling and transcription at the il2 gene during the induction of clonal anergy (7, 8). Because of its role in silencing cytokine gene expression during T cell tolerance, we hypothesized that Ikaros might mediate gene silencing in the context of T helper polarization. Using short hairpin RNA (shRNA)-mediated knockdown of Ikaros or a dominant-negative allele of Ikaros that allows lymphoid development but results in peripheral T cells with 90% reduced Ikaros DNA binding activity (7, 9), we show that Ikaros activity is not necessary for induction of IL-4 during Th2 differentiation, but that it is required to silence ifnγ gene expression in CD4+ T cells responding to Th2-promoting signals in vitro and in vivo. We show that Ikaros achieves this by binding to and repressing transcription of the tbx21 gene.
EXPERIMENTAL PROCEDURES
Mice, Antibodies, Plasmids, and Reagents
Wild-type C57BL/6 (B6), T-bet-deficient B6 (10) (The Jackson Laboratory), or B6 mice with one wild-type Ikaros allele and one allele deleted for the DNA binding domain (9) (ages 4–8 weeks) were used for all experiments. All procedures were approved by the Children's Hospital of Philadelphia/Stokes animal use and care committee. Monoclonal antibody against CD3 (2C11) and CD28 (37.51) were obtained from BioExpress, bioactive IL-12 was purchased from eBioscience, and IL-4 and monoclonal antibody against IL-4 (11B11), anti-IFNγ (XMG1) and IL-12 (17.8) were purchased from BD Biosciences. Antibody (Ab) against the C terminus (sc-9861) and N terminus (sc-13039) of Ikaros were purchased from Santa Cruz Biotechnology. MIGR1-based vectors encoding Ik1 and Ik7 have been described (11), MIGR1-dnT-bet was a gift from S. Reiner (University of Pennsylvania), and pSUPER-based vectors encoding control and Ikaros shRNAs (see supplemental Table 1) were purchased from OligoEngine.
In Vivo Th2 Immune Model
For in vivo induction of a Th2-polarized CD4+ T cell response, S. mansoni eggs were injected in the footpad (2,500 eggs) or intraperitoneally (5,000 eggs). Six days later, spleen and popliteal lymph node cells were isolated and restimulated in vitro with phorbol 12-myristate 13-acetate (PMA)/ionomycin or soluble egg antigen (SEA, 50 μg/ml). IL-4 and IFNγ secretion was measured at 6 h by flow cytometry or at 48 h by enzyme-linked immunosorbent assay (ELISA).
Cell Culture and Retroviral Transduction
CD8-depletion or CD4-positive selection from spleen and lymph node cells was achieved using Miltenyi columns. Peripheral lymphocytes from IkDN/+ mice were prescreened to ensure utilized mice were free of double-negative thymomas. These tumor cells do not express a functional T cell receptor and do not respond by proliferation or cytokine secretion in the cultures described below. CD4+ T helper polarization was achieved using either soluble or plate-bound anti-CD3/28 Abs with the addition of IL-12 (10 ng/ml) and anti-IL-4 Ab (10 μg/ml) for Th1, or IL-4 (40 ng/ml), anti-IFNγ (50 μg/ml), and anti-IL-12 (10 μg/ml) for Th2. In some experiments, naïve phenotype (CD62LhiCD44lo) CD4+ cells were enriched using magnetic beads (Miltenyi) before culturing. Post-sorted cells were >96% CD62Lhi and >88% CD44lo. All T cell cultures utilized standard RPMI 1640 with the addition of 10% fetal bovine serum, l-glutamine, 2-mercaptoethanol, and HEPES. Transduction of activated CD4+ T cells and Ikarosnull JE131 cells with MIGR- and pSUPER-based retroviral vectors was achieved as described previously (7, 12), achieving transduction efficiencies >85% (see supplemental Fig. 1).
Chromatin Immunoprecipitation (ChIP) and DNA Methylation Analysis
ChIP analysis of in vivo Ikaros promoter occupancy was performed on purified CD4+ T cells as described previously (7), using primer sets for the tbx21 promoter (supplemental Table 1). Specific binding was calculated as the ratio of the specific anti-Ikaros ChIP signal over the background isotype Ab control ChIP (2(Ikaros ChIP Ct − input Ct)/2(control ChIP Ct − input Ct)). Bisulfite DNA methylation mapping of the ifnγ locus was performed as described previously (11). Full conversion was achieved as measured by 100% conversion of all cytosines not located 5′ to a guanine. All procedures utilized CD4+ T cells of >95% purity.
Measurement of Gene Expression
Transcription factor and cytokine mRNA levels in cultured cells or colonic tissue were quantified by qRT-PCR (Amplitaq Gold SYBR Master Mix, ABI) on a MyIQ real-time thermal cycler (Bio-Rad) using primers listed in supplemental Table 1. Ikaros expression in whole-cell extracts was assessed by immunoblotting using Ab specific for the N or C terminus. IL-2, IL-4, and IFNγ protein secretion was measured by ELISA (eBioscience) following stimulation with plate-bound anti-CD3 (1 μg/ml) and anti-CD28 (0.5 μg/ml) or by flow cytometry following a 5-h stimulation with PMA (30 ng/ml) and ionomycin (1 μm) in the presence of monensin (3 μm).
RESULTS
Ikaros DNA Binding Activity Is Required for Polarized Patterns of Cytokine Production by Helper T cells in Vitro
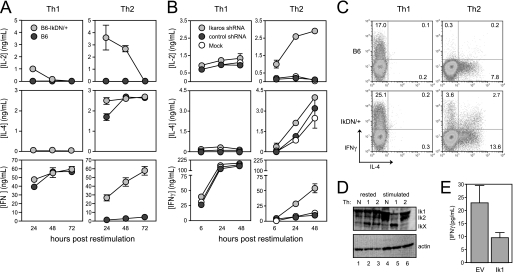
Recent studies have shown that Ikaros fulfills a previously unappreciated role in mature CD4+ T cells as a repressor of il2 gene expression during the induction of anergy (7, 8). We have also found that Ikaros actively represses the production of the Th1 cytokine IFNγ in anergic T cells,3 leading us to ask whether Ikaros plays a broader role in controlling “inappropriate” cytokine gene expression under other circumstances, such as during polarized T helper responses. To test this, we induced a loss of Ikaros function using two approaches. First, we utilized mice expressing one dominant-negative allele of Ikaros that allows lymphoid development but results in peripheral T cells with 90% reduced Ikaros DNA binding activity (7, 9). CD4+ T cells from wild-type or IkDN/+ mice as above were stimulated in vitro under neutral conditions, or in the presence of cytokines and antibodies that drive strong Th1 or Th2 differentiation. As observed previously (7), Ikaros-mutant CD4+ cells stimulated under neutral conditions produced more IL-2 than wild-type cells upon restimulation (data not shown). This effect of Ikaros on il2 gene expression was not limited to neutral cultures, as inhibition of Ikaros DNA binding activity by the dominant-negative mutant was able to augment IL-2 production by Th1 and especially Th2 cells as well (Fig. 1A, top panels). Second, we transduced wild-type CD4+ T cells with retroviral vector encoding a short hairpin RNA targeting the Ik1 transcript or a scrambled control shRNA and cultured these cells under Th1-or Th2-polarizing conditions (supplemental Fig. 1A). This approach resulted in a >80% decrease in Ikaros protein expression by both Th1 and Th2 cells compared with mock-transduced or control shRNA-transduced cells (supplemental Fig. 1B) and likewise led to a marked increase in IL-2 production particularly by transduced Th2 cells (Fig. 1B, top panels).
FIGURE 1.
Ikaros DNA binding activity is required for cytokine polarization during T helper development. A, CD8-depleted spleen cells from wild-type (dark gray circles) and IkDN/+ (light gray circles) mice were stimulated with soluble anti-CD3/28 Abs with the addition of IL-12 and anti-IL-4 Ab for Th1 or IL-4, anti-IFNγ, and anti-IL-12 for Th2. B, wild-type CD8-depleted spleen cells were stimulated under either Th1 or Th2 conditions (as in A above) and transduced with retroviral vector encoding Ik1 shRNA (light gray circles), control scrambled shRNA (dark gray circles), or mock-transduced (white circles). For both A and B, cultures were harvested, washed, and restimulated with plate-bound anti-CD3/28 Ab. Secretion of IL-2, IL-4, and IFNγ secretion was measured by ELISA. C, three days following restimulation, wild-type (top panels) and IkDN/+ (bottom panels) cultures were boosted by the addition of PMA and ionomycin in the presence of monensin for 5 h, and cells were stained for surface CD4 and intracellular IL-4 (x axis) and IFNγ (y axis). Plots are gated on CD4+ cells, and the percentage of CD4+ cells that are positive for one or both cytokines is shown. D, wild-type neutral (lanes 1 and 4), Th1 (lanes 2 and 5), and Th2 (lanes 3 and 6) cells were generated as in A and rested or restimulated with plate-bound anti-CD3/28 Ab for 18 h, and Ikaros expression was assessed by immunoblotting using an Ab against the N terminus. E, purified CD4+ T cells stimulated under neutral conditions were transduced with empty MIGR1 retroviral vector (EV) or MIGR1 encoding full-length Ikaros (Ik1). Cells were rested and restimulated for 24 h with plate-bound anti-CD3/28 Ab, and IFNγ secretion was measured by ELISA. The data shown are representative of three independent experiments. Error bars are S.E. for biological replicate cultures.
Unlike IL-2, Ikaros did not appear to regulate the production of IL-4 to a significant degree. Th2 cells with reduced Ikaros function showed a slight increase in IL-4 production at early time points, but these cells accumulated as much IL-4 as wild-type Th2 cells over the entire culture period (Fig. 1, A and B, middle right panels). Also, neither dominant-negative Ikaros nor shRNA-mediated knockdown of Ikaros led to significant production of IL-4 by Th1 cells (Fig. 1, A and B, middle left panels).
Wild-type CD4+ T cells and CD4+ T cells with reduced Ikaros function secreted comparable amounts of IFNγ when primed under Th1-inducing conditions (Fig. 1, A and B, bottom left panels, and C, left panels). However, unlike wild-type Th2 cells, which effectively silenced ifnγ gene expression (Fig. 1A, bottom right panel, and C, top right panel), IkDN/+ Th2 cells produced as much IFNγ as polarized Th1 cells (Fig. 1A, bottom right panel). Similarly, Th2 cells in which Ikaros had been reduced via shRNA produced nearly 8-fold more IFNγ than Th2 cells expressing control shRNA (Fig. 1B, lower right panel). This correlated with a >10-fold increase in the frequency of IFNγ single producers in Ikaros-mutant cultures and also a >10-fold increase in the frequency of cells producing both IFNγ and IL-4 (Fig. 1C, right panels). Importantly, similar results were obtained from polarization cultures pre-enriched for naïve phenotype (CD44loCD62Lhi) CD4+ cells (supplemental Fig. 2), indicating that the IFNγ in Ikaros-mutant Th2 cultures is not derived from a subpopulation of pre-existing memory Th1 cells.
To gain further insight into Ikaros regulation of T helper polarization, we measured Ikaros expression in wild-type CD4+ T cells that were stimulated under Th1, Th2, or neutral conditions. Resting Th1, Th2, and neutral cells expressed roughly comparable levels of the Ik1 and Ik2 isoforms (Fig. 1D, lanes 1–3). However, upon restimulation (Fig. 1D, lanes 4–6), full-length Ikaros was specifically lost in Th1 cells, accompanied by the appearance of a smaller form of Ikaros that contains the N-terminal region (Fig. 1D, lane 5). This smaller isoform or degradation product lacks the C-terminal region of the protein, as an Ab against the C terminus of Ikaros did not detect this form (data not shown). These data suggest that down-regulation of Ikaros in Th1 cells facilitates high level production of IFNγ, whereas the maintenance of Ikaros expression in Th2 serves to repress ifnγ gene expression. Consistent with this, overexpression of full-length Ikaros in stimulated, neutral CD4+ T cells inhibited IFNγ production (Fig. 1E). Together, the data in Fig. 1 show that Ikaros is required to silence the ifnγ gene in Th2 cells and therefore exerts a strong influence on T helper polarization in vitro.
The tbx21 Gene Is a Direct Target of Repression by Ikaros in Th2 Cells
Our data above show that Ikaros is required for silencing of the ifnγ gene in Th2 cells. Ikaros could mediate this activity by direct binding and repression of the ifnγ locus or through regulation of other transcription factors that affect Th1 or Th2 differentiation. T-bet, a Th1-specific transcription factor encoded by the tbx21 gene, is necessary and sufficient for ifnγ gene expression in CD4+ T cells (13) and therefore represents a prime candidate for regulation by Ikaros.
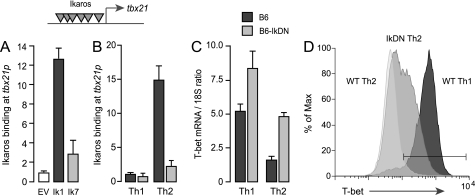
The primary sequence of the tbx21 promoter region contains at least 12 putative Ikaros binding elements, suggesting that this locus may be a direct target of Ikaros. Indeed, ChIP analysis showed that full-length Ikaros ectopically expressed in Ikarosnull JE131 cells binds strongly to the endogenous tbx21 promoter (Fig. 2A, dark gray bar). This promoter occupancy was dependent upon the DNA binding domain of Ikaros, as the Ik7 deletion mutant did not exhibit significant binding when expressed in JE131 cells (Fig. 2A, light gray bar). To determine whether the tbx21 gene is a target of native Ikaros in differentiating T helper cells, we isolated chromatin complexes containing Ikaros from wild-type or IkDN/+ CD4+ T cells early during primary Th1 versus Th2 polarization. These experiments showed strong binding of native Ikaros to the endogenous tbx21 promoter in wild-type Th2 cells but not in wild-type Th1 cells (Fig. 2B, dark gray bars) or in IkDN/+ Th2 cells (Fig. 2B, light gray bars). These results establish a strong negative correlation between Ikaros occupancy and T-bet expression in these T helper lineages and suggest that Ikaros may silence the tbx21 gene during Th2 differentiation.
FIGURE 2.
Ikaros is a direct transcriptional repressor of the endogenous tbx21 gene. A, Ikarosnull JE131 cells were transduced with empty MIGR1 retroviral vector or reconstituted with MIGR1 encoding FLAG-tagged Ikaros lacking the DNA binding domain (Ik7) or full-length, FLAG-tagged Ikaros (Ik1). Chromatin extracts were precipitated with Ab against the FLAG epitope, and precipitated DNA was probed for the tbx21 promoter by qRT-PCR. Ikaros binding was calculated as the ratio of the specific Ab ChIP signal over the background isotype Ab control ChIP signal. Gray inverted triangles indicate putative Ikaros binding elements within the tbx21 promoter. B, wild-type (dark gray bars) and Ik7DN/+ (light gray bars) CD4+ T cells were polarized under Th1 or Th2 conditions for 24 h, and chromatin extracts were precipitated with Ab against native Ikaros. Precipitated DNA was probed for the tbx21 promoter by qRT-PCR. C and D, wild-type (dark gray bars) and Ik7DN/+ (light gray bars) Th1 and Th2 cells were restimulated on plate-bound CD3/28 Ab for 3 days. RNA was isolated, and expression of T-bet mRNA was measured by qRT-PCR (C), and T-bet protein expression in the CD4+ subset was detected by flow cytometry using fluorochrome-conjugated Ab against T-bet (D). The white histogram depicts T-bet staining of wild-type Th2 cells; the light gray histogram depicts staining of IkDN Th2 cells; and the dark gray histogram depicts wild-type Th1 cells. T-bet staining in IkDN Th1 cells was comparable with wild-type Th1 cells (not shown). The gate represents the 98% confidence interval of the isotype control stain. The data depicted are representative of two separate experiments. Error bars are S.E. for biological replicate cultures.
To test this, we measured the expression of T-bet mRNA and protein in differentiating wild-type and mutant CD4+ T cells. Consistent with previous studies, we found high expression of T-bet mRNA (Fig. 2C, dark gray bars) and protein (Fig. 2D, dark gray histogram) in wild-type Th1 cells, whereas T-bet mRNA (Fig. 2C, dark gray bars) and protein (Fig. 2D, white histogram) was down-modulated in wild-type cells upon Th2 differentiation. However, inhibition of Ikaros DNA binding activity during Th2 differentiation resulted in the failure to down-regulate T-bet mRNA (Fig. 2C, light gray bars) and led to elevated T-bet mRNA in Th1 cells as well (Fig. 2C, light gray bars). Remarkably, nearly half of the Ikaros mutant CD4+ cells in Th2 cultures exhibited T-bet protein levels that were intermediate between wild-type Th2 cells and Th1 cells (Fig. 2D, light gray histogram).
These data show that Ikaros is a direct transcriptional repressor of the tbx21 gene in primary Th2 cells. Inhibition of Ikaros DNA binding activity also resulted in dysregulation of other transcription factors involved in T helper polarization, with both Th1 and Th2 cells exhibiting increased expression of the Th1-specific factors Eomes and Runx3 and reduced expression of the Th2-specific factors Gata-3 and c-Maf in the Th2 lineage (supplemental Fig. 3). Thus, in the absence of normal Ikaros function, Th2-instructive signals result in a transcriptional program dominated by positive regulators of the ifnγ gene, including the Th1 “master” regulator T-bet.
Ikaros Opposes IFNγ Production by Silencing T-bet Expression
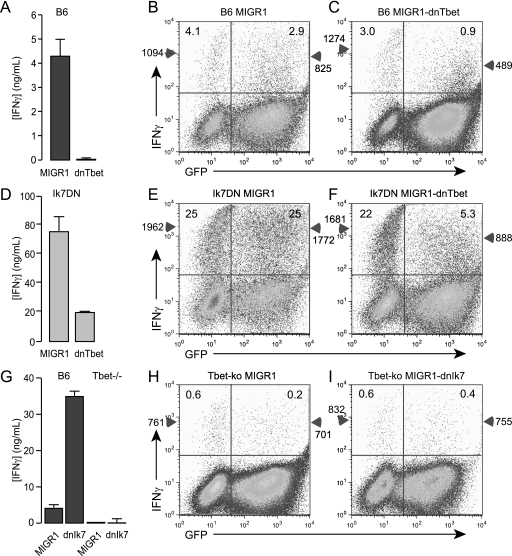
The results above show that the tbx21 is a direct target of Ikaros. However, we also observed weak but significant binding of native Ikaros to multiple regulatory regions of the ifnγ locus in both Th1 and Th2 cells.3 This raised the question to what extent inappropriate expression of the ifnγ gene in Ikaros-mutant Th2 cells may be a direct result of derepression of the ifnγ locus versus an indirect result of elevated T-bet expression. To determine whether T-bet is still required to transactivate the ifnγ gene in the absence of Ikaros repressive activity, we took two separate approaches. First, we transduced wild-type or IkDN/+ CD4+ T cells with a dominant-negative form of T-bet (14). Consistent with previous studies, dnT-bet strongly inhibited IFNγ production by wild-type CD4+ T cells (Fig. 3A). At the single cell level, this correlated with a 3-fold reduction in the frequency of IFNγ-positive cells specifically in the transduced (GFP+), but not the nontransduced (GFP−) cells (Fig. 3B and C, 2.9% versus 0.9%), and a 2-fold decrease in the mean fluorescence intensity (MFI) of IFNγ staining in the positive cells (Fig. 3, B and C, 825 versus 489). Inhibition of T-bet function in Ikaros mutant CD4+ T cells also resulted in a large decrease in IFNγ production (Fig. 3D). The significant residual IFNγ in the supernatants of IkDN/+ cultures is likely derived from the nontransduced cells, which produced IFNγ at a 6-fold increased frequency and 2-fold greater MFI compared with wild-type cells (Fig. 3, B and F, 25% versus 3–4% and 1,094 versus 1,962). Compared with the nontransduced cells or cells transduced with empty MIGR1, the GFP+ IkDN/+ cells were strongly affected by dnT-bet expression, exhibiting a 5-fold reduction in the frequency and a 2-fold decrease in the MFI of IFNγ producers (Fig. 3, E and F, 5% versus 25% and 888 versus 1,772). In a separate approach, wild-type or tbx21-deficient CD4+ T cells were transduced with empty MIGR1 or MIGR1-encoding dominant-negative Ikaros (Ik7). Although inhibition of Ikaros DNA binding activity in wild-type CD4+ T cells resulted in a strong increase in IFNγ production (Fig. 3G, B6), IkDN expression was unable to restore IFNγ production by tbx21-deficient cells, measured either in supernatants (Fig. 3G, T-bet−/−) or at the single cell level (Fig. 3, H and I). These experiments show that Ikaros mutant T cells are still dependent upon T-bet for the production of IFNγ. Although these data do not obviate a role for Ikaros in direct repression of the ifnγ gene, the studies clearly demonstrate that Ikaros silences IFNγ expression in Th2 cells in large part through repression of T-bet, a potent transactivator of the ifnγ gene.
FIGURE 3.
T-bet is required for IFNγ production by Ikaros mutant CD4+ T helper cells. Purified CD4+ T cells from wild-type (A–C), IkDN/+ (d–F), or tbx2−/− (G–I) mice were stimulated with PMA, ionomycin, and IL-2 for 2 days, transduced with empty MIGR1, MIGR1-dnT-bet, or MIGR1-Ik7, and cultured for an additional 3 days in IL-2. Cells were washed and restimulated with plate-bound anti-CD3/28 Ab, and secretion of IFNγ was measured 24 h later by ELISA (A, D, and G). Restimulation cultures were also boosted by the addition of PMA (3 ng/ml) and ionomycin (1 μm) in the presence of monensin (3 μm) for 5 h, and cells were assessed for surface CD4, GFP (x axis), and IFNγ (y axis) (B, C, E, F, H, and I). Plots are gated on CD4+ cells, and numbers in each upper quadrant represent the % of GFP− or GFP+ CD4+ cells that are positive for IFNγ. Numbers on the left with the triangles on the y-axes indicate the MFI of IFNγ staining in the GFP− population, and numbers on the right with triangles indicate the MFI of IFNγ staining in the GFP+ population. The data shown are representative of two separate experiments. Error bars are S.E. for biological replicate cultures.
Ikaros DNA Binding Activity Is Required for DNA Hypermethylation at the ifnγ Locus in Th2 Cells
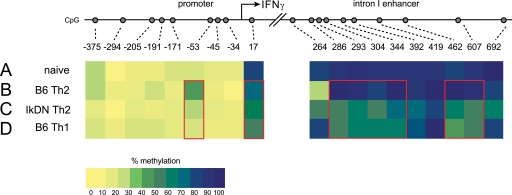
Mutually exclusive patterns of IFNγ and IL-4 production in polarized helper T cell subsets is reinforced through epigenetic modification of the genes encoding these cytokines (4, 15). To determine whether Ikaros is involved in epigenetic imprinting of the ifnγ locus, we assessed DNA methylation at the promoter and intronic enhancer in wild-type versus Ikaros mutant Th1 and Th2 cells by bisulfite conversion-sequencing analysis.
In naïve CD4+ T cells, the CpG dinucleotides within intronic enhancer were hypermethylated, exhibiting 90% methylation on average at each CpG site (Fig. 4A and Table 1). The promoter showed strong methylation near the transcriptional start site (80% at +17 bp), and 5–15% methylation at the more 5′ distal CpGs (Fig. 4A and Table 1). Wild-type CD4+ T cells stimulated under Th2-inducing conditions maintained DNA hypermethylation at the intronic enhancer, exhibiting equal or up to 20% increased methylation at the CpG dinucleotides between +286 and +607 bp (Fig. 4B and Table 1). Wild-type Th2 cells likewise showed a nearly 3-fold increase in methylation at the −53 CpG dinucleotide (Fig. 4B and Table 1). This site is located within a cis-element crucial for ifnγ promoter activity (16), and binding of the ATF2 and cAMP-responsive element-binding protein trans-activators to this element is opposed by methylation of this CpG in Th2 cells (17, 18). However, instead of exhibiting increased CpG methylation, Ikaros mutant CD4+ T cells stimulated under Th2-polarizing conditions exhibited dramatic DNA demethylation of the ifnγ locus. Consistent with the ability of these cells to produce high levels of IFNγ, IkDN/+ Th2 cells exhibited 30% less total methylation at the intronic enhancer compared with wild-type Th2 cells (62% versus 86%, Fig. 4C and Table 1), with certain CpG dinucleotides showing as much as a 2-fold decrease in methylation. Ikaros mutant Th2 cells also exhibited markedly less CpG methylation at the −53 bp ATF2/cAMP-responsive element-binding protein binding element compared with wild-type Th2 cells (25% versus 39%, Fig. 4C and Table 1). Indeed, Ikaros mutant CD4+ T cells subjected to a Th2-polarizing stimulus display a methylation pattern at the ifnγ promoter and intronic enhancer closely resembling that of IFNγ-producing Th1 cells (Fig. 4D and Table 1). These data demonstrate that Ikaros DNA binding activity is required for the appropriate epigenetic imprinting of the ifnγ locus during Th2 polarization.
FIGURE 4.
Ikaros DNA binding influences DNA methylation at the ifnγ locus. Genomic DNA was purified from wild-type CD4+ naïve (A), wild-type Th2 (B), IkDN/+ Th2 (C), and wild-type Th1 (D) cells and subjected to bisulfite-mediated C > T conversion. Nested primer sets were used to amplify the ifnγ promoter and intron 1 enhancer from converted DNA, and the amplicons were cloned and sequenced. The proportion of the sequenced alleles that were methylated at each CpG site is listed in Table 1 and is depicted in pseudocolor in A–D (blue, 100% methylated; yellow, 0% methylated). The data depicted are derived from 25–27 individual cloned and sequenced alleles. Red boxes highlight regions with differential methylation.
TABLE 1.
Bisulfite conversion sequence analysis of DNA methylation at CpG dinucleotides in the ifnγ promoter and intron 1 enhancer regions in wild-type versus Ikaros mutant T helper cells
| CpG site | Promoter |
Intron 1 enhancer |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −375a | −294 | −205 | −191 | −171 | −53 | −45 | −34 | +17 | total | +264 | +286 | +293 | +304 | +344 | +392 | +419 | +462 | +607 | +692 | Total | |
| Naïve Th0 | 25b | 0 | 5 | 5 | 15 | 15 | 10 | 10 | 79 | 18 | 81 | 89 | 96 | 92 | 89 | 92 | 92 | 81 | 89 | 100 | 89 |
| B6 Th1 | 12.5 | 4.2 | 16.7 | 16.7 | 8.3 | 16.7 | 4.2 | 4.2 | 54.5 | 14 | 80 | 50 | 60 | 60 | 60 | 75 | 100 | 40 | 50 | 80 | 66 |
| IkDN Th1 | 12.5 | 8.3 | 16.7 | 16.7 | 8.3 | 8.3 | 8.3 | 4.2 | 41.7 | 14 | 90 | 85 | 100 | 85 | 90 | 95 | 85 | 75 | 85 | 80 | 87 |
| B6 Th2 | 26 | 4.3 | 8.7 | 17.4 | 8.7 | 39 | 4.3 | 0 | 68 | 18 | 25 | 100 | 95 | 80 | 100 | 85 | 100 | 100 | 95 | 80 | 86 |
| IkDN Th2 | 10 | 0 | 10 | 5 | 10 | 25 | 10 | 5 | 65 | 14 | 55 | 55 | 60 | 50 | 70 | 70 | 80 | 60 | 50 | 65 | 62 |
a Base pairs relative to transcriptional start site.
b Proportion of alleles methylated at each site.
Ikaros Controls T Helper Polarization in Vivo
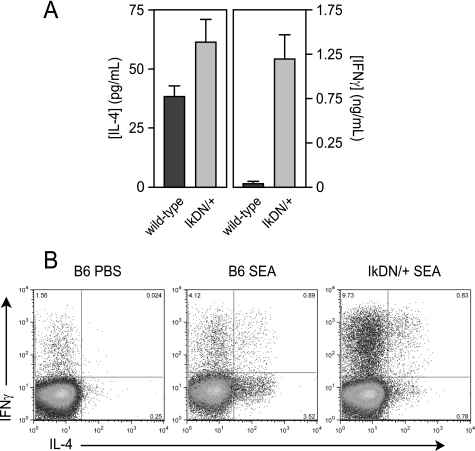
To determine whether Ikaros regulates the immune response to a strong, polarizing stimulus in vivo, we utilized the Th2-inducing pathogen S. mansoni. Eggs released during infection with this metazoan parasite result in the expansion of antigen-specific, IL-4-producing CD4+ T cells (19), and the acute stage of this Th2 response can be modeled by immunization with isolated S. mansoni eggs. Consistent with previous studies, restimulation of draining lymph node cells from wild-type egg-primed mice with a soluble shistosome egg extract (SEA) induced strong production of IL-4 (Fig. 5A, left panel, dark gray bar), but not IFNγ (Fig. 5A, right panel, dark gray bar). At the single cell level, SEA immunization of wild-type mice resulted in a >15-fold increase in CD4+ T cells able to produce IL-4 in response to PMA/ionomycin stimulation (Fig. 5B, left versus middle panels). Draining lymph node cells from SEA-immunized, Ikaros mutant mice exhibited a mild increase in IL-4 secretion upon restimulation with SEA in vitro (Fig. 5A, left panel, light gray bar), but secreted >25-fold more IFNγ than cells from immunized wild-type mice (Fig. 5A, right panel, light gray bar). This response correlated with a decreased frequency of IL-4-producing CD4+ T cells, in favor of an 8-fold increase in IFNγ-producing and IFNγ/IL-4-double producing cells (Fig. 5B, right panel). Thus, as in the in vitro polarization culture conditions, Ikaros is required for silencing of ifnγ gene expression by antigen-specific CD4+ T cells receiving Th2-polarizing signals in vivo.
FIGURE 5.
Ikaros is required for Th2 polarization in vivo. Wild-type (dark gray bars) and IkDN/+ (light gray bars) mice were immunized in the footpad with soluble S. mansoni egg antigen (SEA) or phosphate-buffered saline. Six days later, draining popliteal lymph node cells were restimulated in vitro with SEA (A) or PMA/ionomycin (B), and IL-4 and IFNγ secretion was measured by ELISA at 48 h (A) or by intracellular cytokine staining at 6 h (B). Contralateral popliteal lymph node from SEA-primed mice and popliteal lymph node from phosphate-buffered saline-injected mice failed to produce detectable levels of cytokine in response to SEA stimulation in vitro (not shown). The data shown are representative of two separate experiments. Error bars are S.E. for biological replicate mice.
DISCUSSION
Adaptive immunity is shaped by signals from the innate immune system and from pathogens. These signals promote discrete effector functions that are appropriate for immunity in certain situations but can lead to chronic inflammation, allergy, and immunopathology under other circumstances. The molecular events that turn on effector cytokine genes in these T helper subsets are relatively well characterized. T-bet in developing Th1 cells binds to the ifnγ locus and precipitates chromatin remodeling in concert with Runx1, Hlx, and Stat4 (20, 21), whereas Gata-3, c-Maf and Stat6 perform an analogous function at the il4-il5-il13 multilocus regulatory unit in Th2 cells (15). The mechanism by which the il4 gene is silenced in Th1 cells has been elucidated to a significant degree and involves cooperative binding of T-bet and Runx1 to the il4 silencer (21). However, less is known about how the ifnγ gene is silenced at a molecular level in Th2 cells. GATA-3 can indirectly oppose expression of the ifnγ gene (22–24) through down-regulation of IL-12 receptor/Stat4 signaling (23, 25), and Blimp-1 induced by IL-4 has been shown to repress the tbx21 gene (26). However, our studies show that these mechanisms are not sufficient to silence the ifnγ gene during Th2 differentiation but must work in concert with repressive mechanisms mediated by Ikaros.
Ikaros is a lymphocyte-specific zinc finger DNA binding protein required for the development of all lymphoid lineages (5, 27) and is a component of several co-repressor complexes, including NURD, Sin3, and CtBP (28–30). Ikaros regulates lymphocyte development by recruiting these complexes to genes involved in recombination and expression of B and T cell antigen receptors. Ikaros has also been shown to regulate cell cycle progression in mature T cells (31) and has recently been defined as a transcriptional repressor of the il2 gene in CD4+ T cells (7, 8). We now demonstrate that Ikaros functions in an analogous manner at the tbx21 locus to oppose T-bet and IFNγ expression by Th2 cells differentiated in vitro in the absence of IL-12 and IFNγ receptor signal transduction and in vivo in response to the strong Th2-polarizing parasite S. mansoni.
How does Ikaros regulate expression of the ifnγ gene? Our results indicate a major mechanism is through regulation of T-bet, the primary transactivator of the ifnγ gene. Loss of Ikaros DNA binding in the absence of T-bet was not sufficient to allow production of IFNγ, even in cells stimulated under conditions permissive for Th1 differentiation. Also, forced expression of T-bet in Ikaros-sufficient Th2 cells could still activate the ifnγ gene (13). Ikaros mutant Th2 cultures exhibited in an increase in cells single-positive for IFNγ and in cells that had committed to IL-4 production but now also express IFNγ, a phenotype similar to Th2 cells with forced expression of T-bet (13). Loss of Ikaros function resulted in a ∼10–100-fold increase in T-bet expression and IFNγ production in Th2 cells (i.e. in the absence of IL-12- and IFNγ-mediated signaling). This indicates that relief of Ikaros-mediated repression is an important step in the Th1 differentiation program. However, these levels were still 5–10-fold below that observed in Th1 cells, especially at early time points post-restimulation. Therefore, the IL-12 and IFNγ receptors also contribute Ikaros-independent signals (e.g. Stat1 and Stat4) that promote the high level of tbx21 and ifnγ gene expression exhibited by Th1 cells. Conversely, removal of Ikaros repressive activity in Th2 cells (Fig. 1) or anergic T cells (7) was sufficient to allow full transcription of the il2 gene, which does not require T helper lineage-specific transcription factors for its expression.
How is the repressive activity of Ikaros differentially regulated in Th1 versus Th2 cells? Our results show that Th1 and Th2 cells generated by one round of polarization express similar levels of Ikaros protein, yet Th1 cells are able to produce T-bet and IFNγ, whereas Th2 cells are not. The differential expression of these genes is associated with direct occupancy of Ikaros at the tbx21 locus in wild-type Th2 cells, but not in Th1 cells. This indicates that signaling pathways coupled to the receptors for IL-12 and/or IFNγ are able to regulate Ikaros activity at a post-transcriptional level early during T helper polarization. A previous study showed that Ikaros can be phosphorylated by casein kinase 2 in vitro, and this post-translational modification reduces in vitro DNA binding activity (32). It is possible that IFNγ receptor or IL-12 receptor-coupled kinases may inactivate Ikaros in an analogous manner, leading to derepression of the tbx21 and ifnγ genes in Th1 cells. Alternatively, lineage-specific transcription factors induced by these same pathways could compete with Ikaros for binding to the regulatory regions of the tbx21 and ifnγ loci. Further study will be needed to establish exactly how lineage- and locus-specific regulation of Ikaros activity is achieved at a molecular level.
Our studies also show that Ikaros is necessary for progressive epigenetic imprinting of the ifnγ locus that occurs during Th2 development, as Ikaros mutant Th2 cells fail to induce DNA methylation in the promoter and enhancer regions and instead demethylate the locus-like IFNγ-producing Th1 cells. Although T-bet can induce chromatin remodeling at the ifnγ locus at early stages of Th1 differentiation, DNA demethylation occurs later and has been reported to be T-bet-independent (14). This could be due to direct targeting of DNA methyltransferase activity to the ifnγ locus by Ikaros in Th2 cells. Interestingly, our results show that the program of demethylation in Th1 cells is accompanied by the down-regulation of full-length Ikaros during the secondary phase of stimulation, suggesting a potential link between these phenomena. The mechanism by which Ikaros is down-regulated late during Th1 development is unclear, and additional studies will be needed to further dissect the relative roles of Ikaros versus T-bet in imprinting the Th1 versus the Th2 lineage decision.
In contrast to its role in regulating IFNγ production, we saw no effect of reducing Ikaros function on the production of IL-4 by Th2 or Th1 cells either in vitro or in vivo. This is consistent with a primarily repressive role for Ikaros in T helper development and is in contrast with a recent report that CD4+ T cells from Ikarosnull mice exhibit defective IL-4 production in response to a Th2-inducing stimulus (33). It is possible that the small amount of Ikaros activity remaining in the dominant-negative or shRNA-expressing cells in our studies is sufficient to allow positive regulation of the il4 gene, but not enough to mediate repressive effects at other genes such as il2 and tbx21. Alternatively, nonphysiologic development of CD4+ T cells in the complete absence of Ikaros may result in artifactual behavior of the il4 locus. For instance, a large proportion of the CD4+ T cells present in 3-week-old Ikarosnull mice in our colony have an activated phenotype and can rapidly produce IFNγ upon restimulation in vitro.3 The study by Quiron et al. (33) did not pre-enrich for naive CD4+ cells; therefore, the lack of IL-4 production in their system could be due to the presence of pre-existing effector/memory Th1 cells in the Ikarosnull cultures.
CD4+ T helper cells exhibit remarkable potential to be shaped into distinct lineages of effector cells with divergent function in response to unique sets of extracellular signals. We find that regulated binding of Ikaros to the gene encoding T-bet under Th2-promoting conditions represents a biochemical switch that silences “off lineage” expression of the ifnγ gene, allowing the mutually exclusive patterns of gene expression characteristic of T helper polarization. These studies provide important new insights into the molecular mechanisms by which T helper plasticity is restricted during an immune response.
Supplementary Material
Acknowledgments
We thank Drs. D. Artis, C. Zaph, W. Pear, and C. Hunter for technical and conceptual advice and Dr. S. Reiner for the gift of dnT-bet.
This work was supported, in whole or in part, by National Institutes of Health Grants AI070807 (to A. W.), AI059881 (to A. W.), AI032573 (to E. P.), and AI053825 (to E. P.) and by the Biesecker Pediatric Liver Center at The Children's Hospital of Philadelphia.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1 and Figs. 1–3.
R. M. Thomas and A. D. Wells, unpublished observations.
- Th1
- T helper 1
- IFNγ
- interferon-γ
- IL
- interleukin
- IFN
- interferon
- shRNA
- short hairpin RNA
- PMA
- phorbol 12-myristate 13-acetate
- SEA
- soluble egg antigen
- ELISA
- enzyme-linked immunosorbent assay
- Ab
- antibody
- ChIP
- chromatin immunoprecipitation
- qRT-PCR
- quantitative real-time PCR
- MFI
- mean fluorescence intensity
- GFP
- green fluorescent protein
- dn
- dominant-negative.
REFERENCES
- 1.Murphy K. M., Ouyang W., Farrar J. D., Yang J., Ranganath S., Asnagli H., Afkarian M., Murphy T. L. (2000) Annu. Rev. Immunol. 18, 451–494 [DOI] [PubMed] [Google Scholar]
- 2.Afkarian M., Sedy J. R., Yang J., Jacobson N. G., Cereb N., Yang S. Y., Murphy T. L., Murphy K. M. (2002) Nat. Immunol. 3, 549–557 [DOI] [PubMed] [Google Scholar]
- 3.Ansel K. M., Lee D. U., Rao A. (2003) Nat. Immunol. 4, 616–623 [DOI] [PubMed] [Google Scholar]
- 4.Lee G. R., Kim S. T., Spilianakis C. G., Fields P. E., Flavell R. A. (2006) Immunity 24, 369–379 [DOI] [PubMed] [Google Scholar]
- 5.Georgopoulos K., Bigby M., Wang J. H., Molnar A., Wu P., Winandy S., Sharpe A. (1994) Cell 79, 143–156 [DOI] [PubMed] [Google Scholar]
- 6.Georgopoulos K. (2002) Nat. Rev. Immunol. 2, 162–174 [DOI] [PubMed] [Google Scholar]
- 7.Thomas R. M., Chunder N., Chen C., Umetsu S. E., Winandy S., Wells A. D. (2007) J. Immunol. 179, 7305–7315 [DOI] [PubMed] [Google Scholar]
- 8.Bandyopadhyay S., Duré M., Paroder M., Soto-Nieves N., Puga I., Macián F. (2007) Blood 109, 2878–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winandy S., Wu P., Georgopoulos K. (1995) Cell 83, 289–299 [DOI] [PubMed] [Google Scholar]
- 10.Szabo S. J., Sullivan B. M., Stemmann C., Satoskar A. R., Sleckman B. P., Glimcher L. H. (2002) Science 295, 338–342 [DOI] [PubMed] [Google Scholar]
- 11.Northrop J. K., Thomas R. M., Wells A. D., Shen H. (2006) J. Immunol. 177, 1062–1069 [DOI] [PubMed] [Google Scholar]
- 12.Chen C., Rowell E. A., Thomas R. M., Hancock W. W., Wells A. D. (2006) J. Biol. Chem. 281, 36828–36834 [DOI] [PubMed] [Google Scholar]
- 13.Szabo S. J., Kim S. T., Costa G. L., Zhang X., Fathman C. G., Glimcher L. H. (2000) Cell 100, 655–669 [DOI] [PubMed] [Google Scholar]
- 14.Mullen A. C., Hutchins A. S., High F. A., Lee H. W., Sykes K. J., Chodosh L. A., Reiner S. L. (2002) Nat. Immunol. 3, 652–658 [DOI] [PubMed] [Google Scholar]
- 15.Ansel K. M., Djuretic I., Tanasa B., Rao A. (2006) Annu. Rev. Immunol. 24, 607–656 [DOI] [PubMed] [Google Scholar]
- 16.Penix L. A., Sweetser M. T., Weaver W. M., Hoeffler J. P., Kerppola T. K., Wilson C. B. (1996) J. Biol. Chem. 271, 31964–31972 [DOI] [PubMed] [Google Scholar]
- 17.Jones B., Chen J. (2006) EMBO J. 25, 2443–2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winders B. R., Schwartz R. H., Bruniquel D. (2004) J. Immunol. 173, 7377–7384 [DOI] [PubMed] [Google Scholar]
- 19.Pearce E. J., Krawczyk C. M., Sun J., J J. T., McKee A. S., Cervi L. (2004) Immunol. Rev. 201, 117–126 [DOI] [PubMed] [Google Scholar]
- 20.Mullen A. C., High F. A., Hutchins A. S., Lee H. W., Villarino A. V., Livingston D. M., Kung A. L., Cereb N., Yao T. P., Yang S. Y., Reiner S. L. (2001) Science 292, 1907–1910 [DOI] [PubMed] [Google Scholar]
- 21.Djuretic I. M., Levanon D., Negreanu V., Groner Y., Rao A., Ansel K. M. (2007) Nat Immunol 8, 145–153 [DOI] [PubMed] [Google Scholar]
- 22.Penix L., Weaver W. M., Pang Y., Young H. A., Wilson C. B. (1993) J. Exp. Med. 178, 1483–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ouyang W., Ranganath S. H., Weindel K., Bhattacharya D., Murphy T. L., Sha W. C., Murphy K. M. (1998) Immunity 9, 745–755 [DOI] [PubMed] [Google Scholar]
- 24.Lee H. J., Takemoto N., Kurata H., Kamogawa Y., Miyatake S., O'Garra A., Arai N. (2000) J. Exp. Med. 192, 105–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaminuma O., Kitamura F., Kitamura N., Miyagishi M., Taira K., Yamamoto K., Miura O., Miyatake S. (2004) FEBS Lett. 570, 63–68 [DOI] [PubMed] [Google Scholar]
- 26.Cimmino L., Martins G. A., Liao J., Magnusdottir E., Grunig G., Perez R. K., Calame K. L. (2008) J. Immunol. 181, 2338–2347 [DOI] [PubMed] [Google Scholar]
- 27.Georgopoulos K., Moore D. D., Derfler B. (1992) Science 258, 808–812 [DOI] [PubMed] [Google Scholar]
- 28.Kim J., Sif S., Jones B., Jackson A., Koipally J., Heller E., Winandy S., Viel A., Sawyer A., Ikeda T., Kingston R., Georgopoulos K. (1999) Immunity 10, 345–355 [DOI] [PubMed] [Google Scholar]
- 29.29 Koipally J., Georgopoulos K. (2000) J. Biol. Chem. 275, 19594–19602 [DOI] [PubMed] [Google Scholar]
- 30.Koipally J., Renold A., Kim J., Georgopoulos K. (1999) EMBO J. 18, 3090–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avitahl N., Winandy S., Friedrich C., Jones B., Ge Y., Georgopoulos K. (1999) Immunity 10, 333–343 [DOI] [PubMed] [Google Scholar]
- 32.Gómez-del Arco P., Maki K., Georgopoulos K. (2004) Mol. Cell. Biol. 24, 2797–2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quirion M. R., Gregory G. D., Umetsu S. E., Winandy S., Brown M. A. (2009) J. Immunol. 182, 741–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.