Abstract
Na+/Ca2+ exchangers (NCX) constitute a major Ca2+ export system that facilitates the re-establishment of cytosolic Ca2+ levels in many tissues. Ca2+ interactions at its Ca2+ binding domains (CBD1 and CBD2) are essential for the allosteric regulation of Na+/Ca2+ exchange activity. The structure of the Ca2+-bound form of CBD1, the primary Ca2+ sensor from canine NCX1, but not the Ca2+-free form, has been reported, although the molecular mechanism of Ca2+ regulation remains unclear. Here, we report crystal structures for three distinct Ca2+ binding states of CBD1 from CALX, a Na+/Ca2+ exchanger found in Drosophila sensory neurons. The fully Ca2+-bound CALX-CBD1 structure shows that four Ca2+ atoms bind at identical Ca2+ binding sites as those found in NCX1 and that the partial Ca2+ occupancy and apoform structures exhibit progressive conformational transitions, indicating incremental regulation of CALX exchange by successive Ca2+ binding at CBD1. The structures also predict that the primary Ca2+ pair plays the main role in triggering functional conformational changes. Confirming this prediction, mutagenesis of Glu455, which coordinates the primary Ca2+ pair, produces dramatic reductions of the regulatory Ca2+ affinity for exchange current, whereas mutagenesis of Glu520, which coordinates the secondary Ca2+ pair, has much smaller effects. Furthermore, our structures indicate that Ca2+ binding only enhances the stability of the Ca2+ binding site of CBD1 near the hinge region while the overall structure of CBD1 remains largely unaffected, implying that the Ca2+ regulatory function of CBD1, and possibly that for the entire NCX family, is mediated through domain interactions between CBD1 and the adjacent CBD2 at this hinge.
Keywords: Calcium/Binding Proteins, Calcium/Transport, Cell/Neuron, Exchange/Sodium/Calcium, Membrane/Proteins, Methods/X-ray Crystallography, Protein/Structure, Transport/Calcium
Introduction
The Na+/Ca2+ exchanger (NCX)3 plays an important role in eukaryotic Ca2+ homeostasis. This transporter functions as a Ca2+ efflux mechanism across cell membranes and broadly participates in Ca2+-mediated cellular signaling. NCXs have been identified in numerous tissues and cell types from several different species. In cardiac muscle, NCX1.1 plays a critical role in transsarcolemmal Ca2+ efflux, an essential requirement for cardiac relaxation (1). In neuronal tissues, a variety of exchangers are intricately involved in the control of excitation-secretion signaling (2). Notably, all characterized mammalian Na+/Ca2+ exchangers exhibit a common Ca2+-dependent regulatory mechanism, whereby their activity requires the presence of low concentrations of Ca2+ on their intracellular surface, and their activity is augmented in parallel with elevated intracellular Ca2+ levels (3). This important regulatory property may permit the timely coupling of exchange function to alterations in intracellular Ca2+ concentrations to meet the continuous needs for overall Ca2+ balance.
The general similarities of exchange function and regulatory properties within the large NCX protein family are ascribed to their conserved structural arrangements: nine predicted transmembrane (TM) segments form the ion translocation pathway and a large loop of ∼500 amino acid residues splits TM helix-5 and -6 on the intracellular side of the molecule (4). Ca2+-dependent regulation is attributed exclusively to Ca2+ interactions on the intracellular loop (5). A pair of Ca2+ binding domains (CBD1 and -2), called CALX-β motifs, has been identified (6). Sequence analysis revealed that CBD1 has conserved Ca2+ binding sites throughout the NCX family, whereas greater sequence diversity and/or Ca2+ binding capabilities occurs in CBD2 (7, 27). Given that CBD1 exhibits a higher Ca2+ affinity than CBD2 (8), it has been suggested that CBD1 acts as the primary sensor in the pair of CBDs. Mutations of carboxylate residues at CBD1 result in a pronounced reduction of the affinity for functional Ca2+ regulation (9). The Ca2+-bound structures of CBD1 of NCX1 have recently been determined by NMR, and more recently by x-ray crystallography (8, 11). The crystal structures revealed that four Ca2+ ions bind at the end of a β-sandwich structure of CBD1. More recently, a detailed study by backbone NMR suggested that Ca2+ binding induces a selective conformational change of CBD1 limited to the residues in the binding site, whereas the core of the β-sandwich structure remains unaffected (12). These observations raise a fundamental question of how the primary sensor role of CBD1 is conducted to the TM segments to control exchange activity.
There is currently no structure available for the apoform of CBD1. Consequently, there is no related mechanistic information or insight into how Ca2+ binding induces the conformational change of CBD1 required for transduction of this signal. In the reported Ca2+-bound NCX1-CBD1 crystal structure, the Ca2+ binding site was saturated with four Ca2+ atoms (11). Whether these four Ca2+ access the binding site of CBD1 simultaneously or in a sequential way is unknown. Information of this type is critical toward understanding whether exchange function is simply switched on or off by Ca2+ or whether various degrees of exchange function are graded by different levels of Ca2+ occupancy.
CALX, a Na+/Ca2+ exchange protein, was first identified in Drosophila photoreceptor cells (6, 13, 24). CALX is responsible for extruding intracellular Ca2+ from these cells and plays an essential role in light-mediated signaling in Drosophila sensory neurons (14). CALX shares 49% amino acid identity with the prototypical canine Na+/Ca2+ exchanger, NCX1.1. Functionally, it shares many properties found in mammalian exchangers (13). However, CALX exhibits a completely opposite response to regulatory Ca2+ compared with all other characterized mammalian NCX homologs: the highest activity of CALX occurs in the complete absence of regulatory Ca2+ and its activity is progressively inhibited, rather than stimulated, by elevations in intracellular regulatory [Ca2+]. This negative Ca2+ regulation of CALX leads to a considerable loss of signal amplification in the light response of the Drosophila visual system that triggers the photoreceptor cell cascade (14). CALX also possesses a pair of CBD domains on its intracellular loop. The mechanism underlying the negative Ca2+ regulatory phenotype observed for CALX is still elusive and enigmatic based on the existing structural information for NCX1. Our recent crystal structure of CALX-CBD2 showed that this site is not a functional Ca2+ binding site, suggesting that CBD1 must be the critical site involved in Ca2+ regulation of CALX (7). Furthermore, structural predictions, together with previous mutagenesis studies (7, 15) have strongly suggested that CALX-CBD1 possesses a similar Ca2+ binding site as does NCX1. To gain further insight into the Ca2+-binding mechanisms of Na+/Ca2+ exchange proteins in atomic detail and to investigate the negative Ca2+ regulatory property of CALX, we have determined crystal structures of CBD1 from CALX1.1 in the presence and absence of Ca2+ and studied the properties of this regulatory mechanism by mutagenesis and electrophysiology.
EXPERIMENTAL PROCEDURES
Expression and Purification of CALX-CBD1 Domains
The gene fragments encoding the CBD1 (amino acids 442–554) from a full length cDNA of Drosophila CALX1.1 were cloned into the vector pET28a (Novagen) with restriction sites of NdeI/XhoI. In the generated plasmid, CBD1 has an N-terminal His-tag spaced by a thrombin cleavage site. Protein expression was performed in Escherichia coli BL21(DE3) cells in the autoinduction medium (16) overnight at 25 °C. The cell pellet was suspended in a lysis buffer containing 50 mm sodium phosphate, 500 mm NaCl, 20 mm imidazole, pH 8.0, and ruptured by a high pressure homogenizer (Avestin). The lysate supernatant was applied to a nickel-nitrilotriacetic acid resin column (GE Healthcare), and the CALX-CBD1 protein was eluted with 300 mm imidazole. The purified protein was dialyzed overnight against a Tris-buffered saline buffer, pH 7.4, and was incubated with thrombin protease (GE Healthcare) overnight at 4 °C to truncate the His-tag. The proteolytic reaction mixtures were reapplied to nickel-nitrilotriacetic acid resin and the pass-through containing untagged CALX-CBD1 proteins was concentrated by Centricon (Millipore) and further purified by size-exclusion chromatography using a Superdex 75 10/300 GL column (GE Healthcare). The protein concentration was determined with a Coomassie protein determination kit (Pierce).
Crystallization of CALX-CBD1
All crystallization experiments were performed using the sitting-drop vapor diffusion method at 18 °C. CALX-CBD1, premixed with 1 mm CaCl2 at a protein concentration of 10 mg/ml, was crystallized using the following conditions: 50 mm MES, pH 6.0, 20% polyethylene glycol 3350. To obtain the apoform crystals, CALX-CBD1 protein was incubated with 10 mm EDTA for 1 h and then dialyzed against Tris-buffered saline buffer, pH 7.4, prior to the crystallization experiment. The apoform crystals were obtained under different conditions using 100 mm Bis-Tris, pH 6.5, 200 mm NH4Ac, 10 mm MgCl2, 15% polyethylene glycol 10,000.
Data Collection
All crystals were flash-cooled to 100 K with 25% glycerol as the cryoprotectant. Diffraction data for the Ca2+ form crystal of CBD1 were collected at beamline X06SA of the Swiss Light Source (Villigen). A long wavelength (1.90 Å) was used for data collection with the intention of exploiting weak anomalous signals from Ca2+ atoms. The data collection for the apoform of CBD1 was carried out at the Advanced Light Sources beam line 4.2.2 (Berkeley, CA).
Data Processing and Structural Determination
Data processing, merging, and reduction were carried out with programs XDS and XSCALE (17). The CALX-CBD1 structures were solved using the molecular replacement method by the program PHASER (18) and using the CBD1 structure from NCX1 (Protein Data Bank code 2DPK) as a search model. Both structures were refined using the program Refmac (19). The model building was performed using COOT (20). Crystallographic data and the model refinement statistics are given in Table 1. The anomalous Fourier map in Fig. 1A was calculated using the program FFT (21) with phases from the final refined coordinates and observed anomalous difference in diffraction data. All figures were prepared using the program PyMOL (22).
TABLE 1.
Data processing and model refinement statistics for CALX-CBD1 structures
| CALX-CBD1 Ca2+-bound form | CALX-CBD1 apoform | |
|---|---|---|
| Data collection | ||
| Space group | P212121 | P212121 |
| Unit cell (Å) | 59.4, 73.7, 129.7 | 59.1, 76.5, 129.0 |
| Wavelength (Å) | 1.90 | 1.00 |
| Resolution range (Å)a | 20–2.25 (2.3–2.25) | 47.6–1.6 (1.70–1.60) |
| Observations (total/unique) | 196,637/26,203 | 553,318/146,906 |
| I/σ (I)a | 14.1 (3.1) | 15.6 (2.0) |
| Completeness (%)a | 94.4 (71.8) | 98.7 (95.8) |
| Rsym (%)a | 7.2 (54.4) | 10.7 (57.7) |
| Structure refinement | ||
| Rwork/Rfree | 20.8/26.5 | 19.2/22.1 |
| Number of atomsb | 3,596/16/120 | 3,482/6/461 |
| r.m.s.d.c bond length (Å) | 0.017 | 0.012 |
| r.m.s.d. bond angle | 1.76° | 1.63° |
| Ramachandran analysis (%) | ||
| Most favored | 91.2 | 91.5 |
| Additional favored | 8.8 | 8.2 |
| Generously allowed | 0 | 0.3 |
| B factors (average) | ||
| Monomer A/B/C/D | 47.7/45.3/46.1/62.3 | 20.4/18.7/21.2/23.2 |
| Ca2+ | 64.5 | 25.7 |
| Water | 39.1 | 32.1 |
a Values within parentheses refer to the high resolution shell.
b Non-hydrogen protein atoms/metal atoms/water molecules.
c r.m.s.d., root mean square deviation.
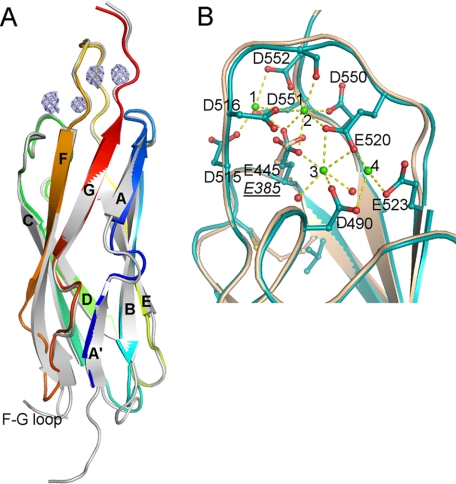
FIGURE 1.
Crystal structures of CALX-CBD1. A, structural alignment of the CBD1 from CALX and NCX1. CALX-CBD1 shown as rainbow ribbons was superimposed on the NCX1-CBD1 crystal structure shown in gray. The anomalous difference map is displayed in slate and contoured at 6.0σ to indicate the Ca2+ positions. The short F–G loop of CALX is highlighted, whereas that from NCX1 is invisible. B, structural alignment of the Ca2+ binding sites of CALX-CBD1 and NCX1-CBD1. The Ca2+-bound CALX-CBD1 structure in cyan is superimposed onto the NCX1-CBD1 structure (colored in wheat). Four Ca2+ and water molecules are represented as green and red spheres, respectively. The residues coordinating Ca2+ are shown as stick balls; Ca2+ interactions with residues are shown as yellow dashed lines. The label for the E385 residue in NCX1 is shown in underlined italics.
Circular Dichroism (CD) Spectroscopy
Prior to CD spectroscopic analysis, CALX-CBD1 protein used for crystallization was passed through a desalting column (GE Healthcare) equilibrated with a solution containing 200 mm NaF, pH 7.4, to remove any Cl−. The protein concentration was adjusted to 1 mg/ml before measurement. CD spectra of the CBD1 protein were collected at room temperature over a wavelength range from 190 to 260 nm with a Jasco J-720 spectrometer using a 0.02-cm cylindrical cell.
Mutational Analysis Using Giant Excised Patch Clamping
Mutations of CALX1.1 were introduced by a modified site-directed mutagenesis procedure (23). To exclude the possibility of random PCR errors on large cNDA of CALX (>3 kb), a minimal DNA fragment containing the sequencing-confirmed mutations was recloned back to the parent vector with appropriate restriction sites.
The effect of each mutation on Ca2+ regulation of CALX1.1 was measured by outward Na+-Ca2+ exchange current recordings using the giant, excised patch clamp technique, as described previously (24). Briefly, Xenopus laevis oocytes were injected with ∼23–35 ng of cRNA of wild-type or mutant CALX1.1 and maintained at 18 °C. Electrophysiological measurements were typically obtained from day 3 to 7 postinjection. Borosilicate glass pipettes were pulled and polished to a final, inner diameter of ∼20–30 μm, and coated with a Parafilm®:mineral oil mixture to enhance patch stability and reduce electrical noise. Oocytes were briefly (∼5–10 min) transferred to a solution containing: 100 mm KOH, 100 mm K-aspartate, 100 mm MES, 20 mm HEPES, 10 mm NH3SO3, 5.0 mm EGTA, 5.0 mm Mg(OH)2; pH 7.0, at room temperature (with MES) to allow sufficient shrinkage of the cells in order for their vitellin layers to be removed by dissection. Giga ohm seals were formed by gentle suction, and membrane patches were excised by progressive movements of the pipette tip. Rapid solution changes were introduced using computer-controlled, multi-channel perfusion devices. Axon Instruments® hardware and software were used for data acquisition. A holding potential of 0 mV was employed for all current measurements.
Pipette solutions contained 100 mm N-methyl-d-glucamine, 100 mm MES, 30 mm HEPES, 30 mm TEA-OH, 16 mm sulfamic acid, 8.0 mm CaCO3, 6.0 mm KOH, 0.25 mm ouabain, 0.1 mm flufenamic acid, 0.1 mm niflumic acid; pH 7.0, at room temperature (with MES). Currents were elicited by switching from Li+- to Na+-based perfusion solutions containing: 100 mm NaOH or LiOH, 100 mm l-aspartic acid, 20 mm CsOH, 20 mm HEPES, 20 mm TEA-OH, 10 mm EGTA, 3.00–21.84 mm NH3SO3, 0–9.91 mm CaCO3, 1.00–1.50 mm Mg(OH)2; pH 7.0, at 30 °C (with MES or LiOH). Stock solutions of CaCO3 and Mg(OH)2 were prepared in a ratio of 1:2 with sulfamic acid. Total Mg2+ and Ca2+ were adjusted to yield free concentrations of 1.00 mm and 0–30 μm, respectively, using MAXC software (25).
All experiments were conducted at 30 °C. Origin® software was used for curve-fitting and statistical analyses. Pooled data are mean ± S.E. Student's t test or one-way analysis of variance and Tukey's post hoc test, were used for statistical determinations. p < 0.05 was considered significant.
RESULTS
Overview of CALX1.1-CBD1 Structure
The Ca2+-bound structure of CALX-CBD1 was determined at 2.25 Å resolution. The structure shows CALX-CBD1 has an immunoglobulin-like conformation formed by two anti-parallel β-sheets consisting of β-strands, A, B, G and C, D, E, F, respectively (Fig. 1A). CALX-CBD1 shares 60% sequence identity with that of canine NCX1-CBD1. Consequently, these two structures can be superimposed with a root mean square deviation of 0.84 Å for 113 aligned Cα atoms. The most significant difference between these two CBD1 structures occurs within their F-G loops. CALX-CBD1 has a rather short F–G loop with only 9 residues, whereas its counterpart from NCX1 consists of 28 residues and displays high flexibility, as indicated in the NMR structure (8).
Ca2+ Binding Site of CALX-CBD1
The Ca2+- bound CALX-CBD1 crystal diffraction data set was collected at 1.9 Å wavelength, allowing the assignment of the bound Ca2+ by examination of the anomalous signal (Fig. 1A). Four Ca2+ are clustered in the distal loops of the β-sandwich with ∼4 Å equal distance spacing between them. Nine carboxylate residues are involved in the coordination of Ca2+ (Fig. 1B). The side chains of these acidic residues are arranged in a zipper-like orientation, forming an extensive carboxylate cluster at the top of this β-sandwich. The E–F loop is the major component in the Ca2+ binding site with five of its residues involved in Ca2+ coordination. That is, the E–F loop crosses over the Ca2+ zipper line clamping Ca-1, -2, and -3 ions through coordination with carboxylate groups of Asp515, Asp516, and Glu520 together with the carbonyl oxygen atoms of Asp517 and Val518. Compared with Ca-1 or -2, which is penta- or hexacoordinated, Ca-4 is only tricoordinated with Asp490, Glu523, and Glu520. Consequently, Ca-4 displays the highest thermal B factor, suggesting it may be the most mobile species during these ligand interactions.
The Ca2+ binding sites of CALX-CBD1 are almost identical to those of NCX1 except for Glu455, which locates centrally on the basement of the binding site. Compared with the NCX1 structure, this residue rotates its carboxyl group by 90°, resulting in simultaneous coordination with three Ca2+ (Ca-1, Ca-2, and Ca-3) instead of one (Ca-2) shown in the NCX1-CBD1 structure (11). In addition, it appears that the disulfide bridge locking the A–B loop near Glu385 in the NCX1-CBD1 structure is absent in CALX-CBD1, as a valine replaces cysteine at position 453.
Conformational Change in the Apoform Structure
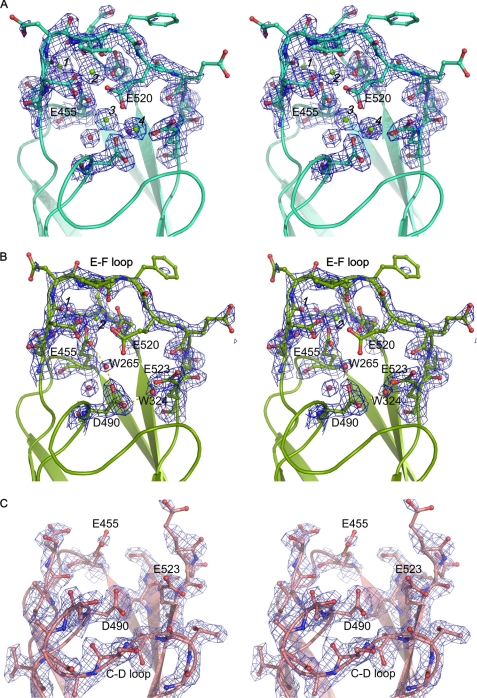
To gain additional structural information regarding Ca2+ binding to CBD1, the CBD1 protein was treated with 10 mm EDTA and dialyzed prior to crystallization; the apoform structure was determined at 1.6 Å resolution. The EDTA-treated sample gives the same crystal packing as that of the Ca2+-bound form; four monomers assemble in anti-parallel in an asymmetric unit. The overall structure of the apoform CBD1 shows no overall conformational changes compared with the Ca2+-bound form except within the Ca2+ binding sites. In the Ca2+-bound structure, Ca2+ binding sites of four monomers in an asymmetric unit were identically and fully occupied. In contrast, the same binding sites exhibit three distinct Ca2+ binding states in the apoform structure. Monomer B shows a full occupancy state as shown in the Ca2+-form structure, where four Ca2+ were found in the Ca2+ binding site and all residues involved in Ca2+ coordination are clearly visible (Fig. 2A).
FIGURE 2.
Stereo views of conformational changes in the Ca2+ binding sites of CALX-CBD1. 2Fo − Fc electron density maps contoured at 1.5σ level are shown in blue. Ca2+ and water molecules are represented as green and red spheres, respectively. The residues coordinating Ca2+ are shown as stick balls. Hydrogen bond interactions are shown as yellow dashed lines. A, B, and C represent three different conformations from the same asymmetric unit of the apoform CALX-CBD1 structure. A, monomer B (four Ca2+-bound); B, monomer A (two Ca2+-bound); and C, monomer C (no Ca2+-bound).
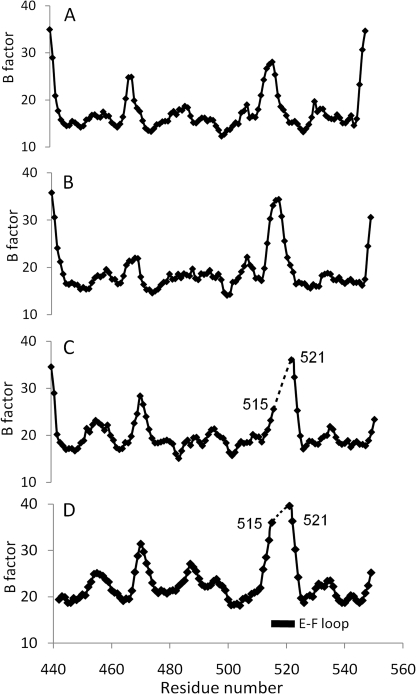
However, monomer A displays a partial Ca2+ binding state. Only Ca-1 and -2 (the primary Ca2+ pair) were found at similar positions as in monomer B (Fig. 2B). The absence of Ca-3 and Ca-4 (named as the secondary Ca2+ pair) does not result in any significant structural change compared with monomer A with root mean square deviation values of 0.28 Å for backbone atoms and 0.68 Å for all atoms. The conformational change occurs exclusively at Glu520, whose side chain is invisible in the electron density map. The backbone of the entire E–F loop is still clearly resolved and appears to be stabilized by the primary Ca2+ pair. However, the thermal B factors of the residues on the E–F loop are considerably increased compared with those in monomer B (Fig. 3). A water molecule at position 265 was found at Ca-3 position, which forms two hydrogen bonds with Glu455 and Asp490. No density can be observed in the Ca-4 position. The conformations of Asp490 and Glu523 remain unchanged; they are stabilized by a hydrogen bond network with a water molecule at position 324.
FIGURE 3.
Thermal B values versus residues in each monomer of an asymmetric unit of CALX-CBD1 structure. A, monomer B; B, monomer A; C, monomer C; and D, monomer D.
Strikingly, both monomers C and D represent the true apoform, and no Ca2+ can be found in corresponding regions (Fig. 2C). Nearly all residues involved in Ca2+ binding become invisible, particularly the entire E–F loop (Asp516–Glu522), which strongly suggests that the primary Ca2+ pair plays a critical role in stabilizing the entire Ca2+ binding region. The disorder of the Ca2+ binding region has no impact on the overall β-sheet structure of CBD1 and the neighboring C–D loop containing Asp490 is still clearly resolved. Both Ca2+-free forms superimpose well with similar root mean square deviation values of 0.44 Å for backbone atoms and 0.97 Å for all atoms. Notably, no Ca2+ binding region from these four monomers is involved in crystal packing, and all four monomers display comparable thermal B factors (Table 1 and Fig. 3).
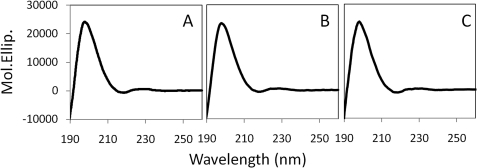
Our structural observations indicate that no gross conformational changes occur during Ca2+ binding. To exclude any possible effects of crystallization constraints, the Ca2+ extraction experiment was performed in solution with monitoring by CD spectroscopy. The result shows CBD1 protein exhibits a full β-strand conformation, as expected (Fig. 4A). No detectable change of protein secondary structure was observed by addition of either 2 mm Ca2+ (Fig. 4B) or 10 mm EDTA (Fig. 4C). Notably, 10 mm EDTA causes Ca2+ unbinding of CBD1 of NCX1 (8).
FIGURE 4.
CD spectra of CALX-CBD1 upon Ca2+ binding. A, CBD1 protein after the protein purification; B, the CBD1 protein with 2 mm Ca2+; and C, the CBD1 protein with 10 mm EDTA.
Mutational Analysis of Ca2+ Binding Site of CBD1
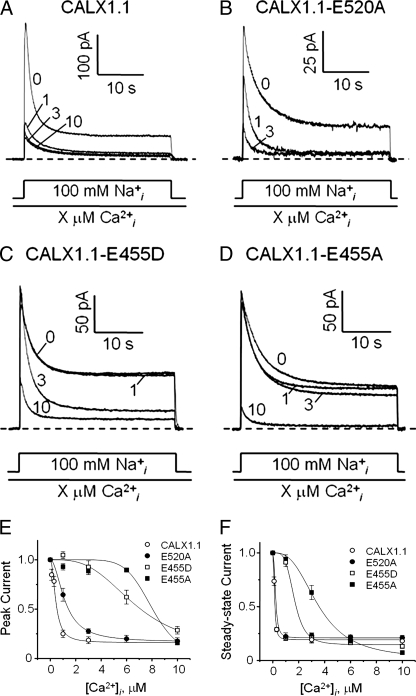
Two residues, Glu455 and Glu520, are involved in the coordination of three Ca2+ at the CBD1 site, although they differ in the specific Ca2+ ions which are involved (Glu455 with Ca-1, -2, and -3; Glu520 with Ca-2, -3, and -4). Our structural data suggest that Glu455, which coordinates the primary Ca2+ pair, plays a more important role in stabilizing the entire Ca2+ binding region than Glu520. To examine whether these two glutamate residues have unequal functions in the Ca2+-dependent regulatory mechanism, they were mutated into Asp and/or Ala. RNA from either the wild-type CALX1.1 or various mutant exchangers was injected into Xenopus laevis oocytes, and the outward currents were recorded to evaluate functional Ca2+ regulation. The overlapping traces in Fig. 5A show representative outward Na+-Ca2+ exchange currents for wild-type CALX1.1 at four different Ca2+ concentrations. In the absence of regulatory Ca2+, CALX is fully activated by the application of 100 mm Na+. However, both peak and steady-state currents become substantially suppressed by increasing the concentration of regulatory Ca2+. (Results obtained at 1, 3, and 10 μm are shown.) The corresponding IC50s for peak and steady-state currents are 0.4 ± 0.1 μm and 0.13 ± 0.002 μm, respectively.
FIGURE 5.
Representative outward Na+/Ca2+ exchange currents showing the regulatory Ca2+ dependence of the wild-type and various CALX1.1 mutant exchangers. 8 mm Ca2+ was present in the pipette, and the currents were activated by the addition of 100 mm Na+ to the cytoplasmic surface of the patch with regulatory Ca2+ absent or present at various concentrations. For wild-type (A), E455D (C) and E455A (D), exchange currents were recorded at four different Ca2+ concentrations (0, 1, 3, and 10 μm) as indicated. For E520A (C), only three different Ca2+ concentrations (0, 1, and 3 μm) were tested. Ca2+i dependence of pure outward currents for peak (E) and steady state (F) mediated by wild-type CALX1.1 and various mutants. Currents were normalized to those obtained at 0 Ca2+i within the same patch. Data points were averaged from four to seven individual patches. IC50 for peaks: 0.4 ± 0.1, 1.2 ± 0.01, 6.7 ± 1.3, and 8.1 ± 0.5 μm Ca2+ for CALX1.1, E520A, E455D, and E455A, respectively. IC50 for steady-state currents: 0.13 ± 0.002, 0.22 ± 0.06, 1.7 ± 0.1, and 3.5 ± 0.2 μm Ca2+ for CALX1.1, E520A, E455D, and E455A, respectively.
Even though Glu520 appears to have the equivalent Ca2+ coordinating capacity as Glu455 (i.e. three Ca2+), it exhibits far less importance in mediating the Ca2+ regulatory response. Mutations of these two residues illustrate their different functional roles. The Ca2+ dependence of peak and steady-state currents of these mutants are presented in Fig. 5, E and F. In the E520A mutation, there is only a slight reduction in the inhibitory potency of regulatory Ca2+ for peak currents, (IC50s = 1.2 ± 0.01 μm) and even less for steady-state currents (0.22 ± 0.06 μm) (Fig. 5B).
In sharp contrast, the Ca2+ response is much more sensitive to changes at residue Glu455. The E455A mutation results in a dramatic alteration of the Ca2+ regulatory response. Both peak and steady-state currents are not appreciably inhibited until the level of regulatory Ca2+ reaches 3 μm (Fig. 5D). The apparent Ca2+ affinity of E455A for regulation of peak currents is reduced by ∼20-fold to IC50 = 8.1 ± 0.5 μm. Steady-state currents show an even greater reduction in the inhibitory potency of regulatory Ca2+, which drops by 35-fold to 3.5 ± 0.2 μm. We also mutated Glu455 to an Asp. Similar to E455A, as shown in Fig. 5C, this conservative mutant shows a large shift in the affinity for functional Ca2+ regulation, which now appears at the level of 1 μm Ca2+. The inhibitory potency of regulatory Ca2+ is reduced by nearly ∼17-fold for both peak (6.7 ± 1.3 μm) and steady-state currents (1.7 ± 0.1 μm). These results clearly demonstrate that residue Glu455 is essential for Ca2+ binding and ultimately the transduction of the regulatory Ca2+ binding signal. The integrity of Glu455 is critical in maintaining normal exchanger regulation of CALX.
DISCUSSION
Ca2+ interactions occurring at CBD1 are essential for properly controlling sodium-calcium exchange activity and for the maintenance and reestablishment of resting Ca2+ levels in living cells. In this study, the Ca2+-bound structure of CALX-CBD1 precisely confirms the occupancy of four Ca2+ within this site by their anomalous signals. Our data shows that CALX has a similar overall structure and Ca2+ binding site of CBD1 as does the mammalian NCX1, irrespective of their opposite Ca2+ regulatory phenotypes. Therefore, Ca2+ binding within the CBD1 structures likely represents a general mechanism within the larger NCX family.
Despite the nearly identical sequence composition of the Ca2+ binding sites within CBD1 from either CALX or NCX1, both the Ca2+-bound and apoform CALX-CBD1 structures consistently demonstrate that Glu455 in the core of the Ca2+ binding site plays a significantly different role in Ca2+ coordination compared with Glu385 in the NCX1 structure. Possibly, this difference between CALX and NCX1 could be attributed to the lower resolution (2.5 Å) of the NCX1 structure (11). The oxygen atoms of the carboxylate group of Glu385 were not well defined, as seen in the electron density of the NCX1 structure. To date, no mutations of Glu385 in NCX1 have been reported. Given the remarkable impact of Glu455 mutations, we would predict that this Glu residue plays an important role in the Ca2+ regulatory mechanism of mammalian NCX1 and other exchanger proteins.
All characterized exchangers show concentration-dependent Ca2+ regulatory effects. For example, CALX, as seen in Fig. 5A, responds in a graded manner to the progressive administration of regulatory Ca2+. In contrast to NCX1-CBD2 (8, 10), CALX-CBD2 does not have Ca2+ binding capabilities (7). CBD1 is the only Ca2+ binding region within the entire Ca2+ regulatory domain that could be responsible for this progressive negative Ca2+ regulatory phenotype. Therefore, the graded Ca2+ regulation exhibited in Fig. 5A must represent regulatory Ca2+ binding at CBD1. However, with only Ca2+-bound structures, previously reported for CBD1 (8, 11), it is impossible to distinguish whether Ca2+ regulation at the level of a single exchanger protein constitutes an all-or-none versus a graded Ca2+ binding phenomenon.
In this study, we determined the apoform structure of CBD1 as well as that of an intermediate with two, rather than four, Ca2+ bound. The apoform structure clearly indicates that the primary Ca2+ pair is critical for the stabilization of the entire Ca2+ binding site of CBD1 whereas the effect of the secondary Ca2+ pair is quite modest. This is consistent with our functional analysis, where mutations that altered Glu455 (coordinating the primary Ca2+ pair) strongly disrupted Ca2+ regulation, whereas mutations of Glu520 (coordinating the secondary Ca2+ pair) only resulted in subtle reductions of Ca2+ affinity. These two Ca2+ pairs also interact with other carboxylate groups (Fig. 1B). Given the modest effect of the other carboxylate residues in the Ca2+ binding site of CBD1 suggested by a previous mutagenesis study (15), the affinity of E520A mutant (0.13 μm) or E455A (3.5 μm) should approximate the affinities for the primary Ca2+ pair or the secondary Ca2+ pair, respectively. Calcium concentrations of 0.13–3.5 μm would correspond to the reactive concentration range of regulatory Ca2+ for CBD1. In the dynamic Ca2+ environment of living cells, the primary Ca2+ pair with its higher affinity is expected to access CBD1 initially to establish the conformational transitions. These observations clearly elucidate that the four Ca2+ access the binding site of CBD1 in a sequential manner, rather than by simultaneous occupation.
The mechanism through which occupancy of the CBDs by Ca2+ is ultimately transduced to the transport machinery within the TM segments remains unknown. A plausible theory would be that substantial conformational changes occur upon Ca2+ binding, which are subsequently transmitted to the transport machinery. In fact, a fluorescent resonance energy transfer study with NCX1 indicated that Ca2+ binding elicits a conformational change of CBD1 (26). The apoform structure shows that CBD1 maintains the integrity of its β-sandwich conformation regardless of Ca2+ binding, whereas conformational changes caused by Ca2+ interactions are limited to the residues in the Ca2+ binding region as examined by CD spectroscopy and x-ray crystallography, arguing that a change of quaternary structure of CBD1 is not a requirement for the Ca2+ regulatory mechanism. Occupancy by the primary Ca2+ pair only affects the stabilization of the Ca2+ binding site, especially within the E–F loop, as has also been shown for NCX1-CBD1 by NMR (12). Considering the fact that the E–F loop locates to the CBD1 domain surface, we propose that the Ca2+ binding signal is transduced from the E–F loop by communication to adjacent domain(s).
For all NCX proteins, the C terminus of CBD1 must connect with the N terminus of CBD2 in a very compact manner, providing the strong possibility that the Ca2+ binding site, particularly the E–F loop of CBD1, interacts with the N-terminal region of CBD2. Our mutational analysis shows that carboxylate residues in the Ca2+ binding sites of CBD1 serve unequal roles in the Ca2+ regulatory mechanism (Fig. 5). Notably, the essential residue Glu455 locates very close to the link or hinge region, further supporting the possibility that the signal of Ca2+ binding is transduced through this link via domain interactions between CBD1 and CBD2. This possibility is also strongly supported by our previous mutational analysis, where a proline mutation of Gly555 completely eliminated the Ca2+ regulatory properties of CALX (15). This proline mutation occurs at the joint between CBD1 and CBD2 and presumably disrupts CBD domain interactions.
Overall, our results support a mechanistic hypothesis that Ca2+ binding/unbinding at CBD1 is transduced through the interdomain interactions between the Ca2+ binding sites of CBD1 and CBD2 and that the E–F loop of CBD1 acts as a hinge for this domain interaction. As a consequence, Ca2+ binding at CBD1 stabilizes the E–F loop and in turn changes the orientation between these two rigid domains to influence the motion, ion access, or functionality of the transmembrane segments. Given that similar effects are also reported for the analogous mutation, G503P, in NCX1 (9), this general Ca2+ regulatory mechanism may also be applicable to the larger Na+/Ca2+ exchanger protein family.
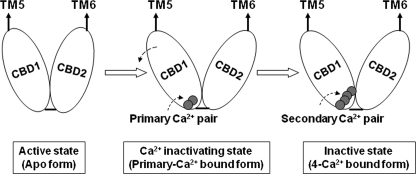
Based on our structural and functional analysis, we hypothesize that the Ca2+ regulatory mechanism occurring via CBD1 for CALX is performed in two sequential steps, as follows (Scheme 1): 1) CALX remains fully active when CBD1 exists in its apoform; 2) the primary pair of Ca2+ ions accesses the binding site to stabilize the E–F loop and alters the domain orientation angle between CBD1 and CBD2, generating a conformational change of the TM segments to initiate transporter inactivation; 3) as the Ca2+ concentration increases, the secondary Ca2+ pair binds to CBD1, stabilization of the E–F loop is further enhanced, and the TM transport machinery becomes more completely inactivated by stabilization of this inactive state. Notably, such notions are becoming increasingly testable based on existing structure-function studies.
SCHEME 1.
Hypothetical mechanism of Ca2+ regulation for the CALX Na+/Ca2+ exchanger.
The graded nature of Ca2+ regulatory effects are even more pronounced for NCX1 than for CALX. In NCX1, Na+/Ca2+ exchange currents are triggered by submicromolar Ca2+ levels and are amplified almost continuously by higher Ca2+ levels exceeding 10 μm, presumably in response to the much larger changes in intracellular Ca2+ levels that occur in cardiac myocytes. Our structures provides solid evidence that stepwise regulation of exchange is accomplished through different Ca2+ binding states in the CBD1 domain through its communication between the two CBDs. Very recently, a comparably functional study with NCX1 CBD1 mutants supports further application of our hypothetic model (28). Additional understanding of this interplay will need to be addressed by future crystallographic studies of the entire intracellular loop in conjunction with mutagenesis studies.
Acknowledgments
We thank Magnus Hook for assistance with CD analyses, Joachim Diez at Swiss Light Source for help with data collection, and John L. Spudich for valuable discussion on the manuscript.
This work supported by grants from the American Heart Association (0830353N to L. Z.) and the Canadian Institutes of Health Research (to L. V. H.).
The atomic coordinates and structure factors (codes 3EAD and 3E9T) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
- NCX
- Na+/Ca2+ exchanger
- CBD1
- Ca2+ binding domain 1
- TM
- transmembrane
- MES
- 4-morpholineethanesulfonic acid.
REFERENCES
- 1.Bers D. M. (1991) Excitation-Contraction Coupling and Cardiac Contractile Force, pp. 71–92, Kluwer Academic Publications, London [Google Scholar]
- 2.Blaustein M. P., Fontana G., Rogowski R. S. (1996) Ann. N.Y. Acad. Sci. 779, 300–317 [DOI] [PubMed] [Google Scholar]
- 3.Linck B., Qiu Z., He Z., Tong Q., Hilgemann D. W., Philipson K. D. (1998) Am. J. Physiol. 274, C415–C423 [DOI] [PubMed] [Google Scholar]
- 4.Nicoll D. A., Ottolia M., Philipson K. D. (2002) Ann. N.Y. Acad. Sci. 976, 11–18 [DOI] [PubMed] [Google Scholar]
- 5.Matsuoka S., Nicoll D. A., Reilly R. F., Hilgemann D. W., Philipson K. D. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 3870–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwarz E. M., Benzer S. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 10249–10254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu M., Wang M., Nix J., Hryshko L. V., Zheng L. (2009) J. Mol. Biol. 387, 104–112 [DOI] [PubMed] [Google Scholar]
- 8.Hilge M., Aelen J., Vuister G. W. (2006) Mol. Cell 22, 15–25 [DOI] [PubMed] [Google Scholar]
- 9.Matsuoka S., Nicoll D. A., Hryshko L. V., Levitsky D. O., Weiss J. N., Philipson K. D. (1995) J. Gen. Physiol. 105, 403–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Besserer G. M., Ottolia M., Nicoll D. A., Chaptal V., Cascio D., Philipson K. D., Abramson J. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 18467–18472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicoll D. A., Sawaya M. R., Kwon S., Cascio D., Philipson K. D., Abramson J. (2006) J. Biol. Chem. 281, 21577–21581 [DOI] [PubMed] [Google Scholar]
- 12.Johnson E., Bruschweiler-Li L., Showalter S. A., Vuister G. W., Zhang F., Brüschweiler R. (2008) J. Mol. Biol. 377, 945–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hryshko L. V., Matsuoka S., Nicoll D. A., Weiss J. N., Schwarz E. M., Benzer S., Philipson K. D. (1996) J. Gen. Physiol. 108, 67–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang T., Xu H., Oberwinkler J., Gu Y., Hardie R. C., Montell C. (2005) Neuron. 45, 367–378 [DOI] [PubMed] [Google Scholar]
- 15.Dyck C., Maxwell K., Buchko J., Trac M., Omelchenko A., Hnatowich M., Hryshko L. V. (1998) J. Biol. Chem. 273, 12981–12987 [DOI] [PubMed] [Google Scholar]
- 16.Studier F. W. (2005) Protein Expr. Purif. 41, 207–234 [DOI] [PubMed] [Google Scholar]
- 17.Kabsch W. (1993) J. Appl. Cryst. 26, 795–800 [Google Scholar]
- 18.McCoy A. J., Grosse-Kunstleve R. W., Adams P. D., Winn M. D., Storoni L. C., Read R. J. (2007) Phaser crystallographic software. J. Appl. Cryst. 40, 658–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murshudov G. N., Vagin A. A., Dodson E. J. (1997) Acta Cryst. D53, 240–255 [DOI] [PubMed] [Google Scholar]
- 20.Emsley P., Cowtan K. (2004) Acta Cryst. D60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 21.Ten Eyck L. F. (1973) Acta Cryst. A29, 183–191 [Google Scholar]
- 22.DeLano W. L. (2002) The PyMOL Molecular Graphics System. DeLano Scientific LLC, Palo Alto, CA [Google Scholar]
- 23.Zheng L., Baumann U., Reymond J. L. (2004) Nucleic Acids Res. 32, e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omelchenko A., Dyck C., Hnatowich M., Buchko J., Nicoll D. A., Philipson K. D., Hryshko L. V. (1998) J. Gen. Physiol. 111, 691–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bers D. M., Patton C. W., Nuccitelli R. (1994) Methods Cell Biol. 40, 3–29 [DOI] [PubMed] [Google Scholar]
- 26.Ottolia M., Philipson K. D., John S. (2004) Biophys. J. 87, 899–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hilge M., Aelen J., Foarce A., Perrakis A., Vuister G. W. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 14333–14338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ottolia M., Nicoll D. A., Philipson K. D. (2009) J. Biol. Chem. 284, 32735–32741 [DOI] [PMC free article] [PubMed] [Google Scholar]