Abstract
The main function of the corpus luteum (CL) is the production of progesterone. Adequate luteal progesterone is crucial for determining the physiological duration of the estrous cycle and for achieving a successful pregnancy. The CL is regulated not only by hypophyseal gonadotropin, but also by a number of cytokines that are locally produced. Tumor necrosis factor-α (TNF) and its specific receptors (TNFR) are present in the CL of many species. TNF plays multiple and likely important roles in CL function throughout the estrous cycle. TNF appears to have luteotropic and luteolytic roles in the CLs. In contrast, Fas ligand (Fas L), another member of TNF super family (TNF-SF), is primarily recognized for its apoptotic actions. Presumably, Fas L binds its cognate receptor (Fas) to induce structural luteolysis. This review is designed to focus on recent studies documenting the expression of TNF and Fas L, their receptors, and intracellular signaling mechanisms in the CL.
Introduction
The corpus luteum (CL) is a transient ovarian organ established by cells of the follicle following ovulation. The primary product of the CL, progesterone, is required for the establishment and maintenance of pregnancy [1]. The mammalian CL is composed of a heterogeneous mixture of cell types that consists of not only steroidogenic luteal cells but also non-steroidogenic cells, i.e., vascular endothelial cells, fibroblasts, and immune cells such as lymphocytes and macrophages [2,3]. There is increasing evidence that a number of factors locally produced by the non-steroidogenic cells modulate the CL function.
The function of CL varies from day to day after ovulation. When animals do not become pregnant, regression of the CL is essential for normal cyclicity as it allows the development of a new ovulatory follicle. Functional regression of CL, characterized by inhibition of progesterone production, is followed by structural regression. During luteolysis, cells of the CL undergo apoptosis, a process that has been described by morphological and biochemical parameters in many domestic species including the cow [4,5], pig [6] and sheep [7].
It is generally accepted that the immune system plays a central role in apoptosis of several tissues and cell types [8]. Furthermore, since the number of leukocytes in bovine CL (e.g., T lymphocytes, macrophages) increase at the time of luteolysis [9], it is assumed that leukocytes mediate apoptosis during CL regression. Leukocytes are known to produce a variety of cytokines, including tumor necrosis factor-α (TNF) and interferon-γ (IFN), which have been shown to affect luteal cell function in vitro [10].
TNF, a non-glycosylated protein with a molecular weight of 17 kDa, was first described as a tumoricidal factor produced by activated macrophages [11]. This protein possesses a wide repertoire of biological actions that include not only the regulation of proinflammatory responses but also the control of cell differentiation, tissue renewal and restructuring [12]. Extensive research during the last decade suggests that TNF physiologically plays multiple roles in ovarian function in a variety of species [13-15]. Furthermore, the release of TNF is associated with the expression of TNF alpha converting enzyme (TACE) in cells [16] and intracellular concentrations of TACE may be crucial for modulating TNF production and subsequent local actions. TNF belongs to TNF super family (TNF-SF), which consists of 18 members. The TNF-SF members have a conserved C-terminal domain coined the TNF homology domain. This trimeric domain, which is responsible for receptor binding, has 20–30% sequence identity between family members. The receptors for the TNF-SF ligands also constitute a TNF receptor super family (TNFR-SF). The disulfide bonds from "cysteine-rich domains" that are the hallmark of the TNFR-SF. The familiar as well as standardized names of these proteins together with their gene locations are listed in following address http://www.gene.ucl.ac.uk/nomenclature/genefamily/tnftop.html. Fas ligand (Fas L), another member of the TNF-SF primarily engages its membrane receptor (Fas) to induce apoptosis [17]. Fas L is expressed at high levels on activated T lymphocytes [18] and mediates apoptosis of target cells [17]. Recent studies suggest that Fas-mediated apoptosis plays an important role in structural regression of the CL in a variety of species [19-22]. It is also possible that Fas L works in conjunction with other cytokines (TNF and IFN) to regulate luteal regression [22,23]. This review will focus on studies documenting roles of TNF-SF members in the CL, with emphasis on TNF and its receptors and Fas L and its receptors in the structural CL regression, i.e., apoptosis following the functional CL regression. Furthermore, possible roles of TNF in the CL throughout the estrous cycle and in the gestation period will be discussed.
TNF and Fas L in luteal formation and development
Immunoreactive or bioactive TNF is present in follicles during follicular development in a variety of animals [24,25,32-34,38]. Likewise, the TNF receptor (TNFR) is also present on granulosa and theca cells [39,42,45]. For more detailed information on the expression of TNF and TNFR in the ovaries of several species, and the methods used for detecting TNF and TNFR see Table 1. From a functional standpoint, TNF has been shown to inhibit FSH-, insulin- or insulin-like growth factor-I (IGF)-induced estradiol-17β (estradiol) production in granulosa cells [52,53] and LH-stimulated androstenedione production in theca cells [53]. These observations led to the idea that TNF plays one or more physiological roles in regulating follicular cell function. One possible role of TNF in the follicles is to mediate the mechanism of ovulation. In cows, maximum levels of bioactive TNF are observed in the dominant follicles [24]. In addition, intrafollicular injection of TNF antibodies after administration of GnRH blocked ovulation in sheep [36]. Other studies focusing on the role of TNF in ovulation have been conducted in rats [54] and sheep [55].
Table 1.
Ovarian TNF and TNF receptors (TNFR) in various animals1)
| Species | Sources | Stages of the estrous cycle2) | Method of detection | Ref. | |||||
| F | I | II | III | R | P | ||||
| TNF | |||||||||
| Cow | Follicular fluid | + | Bioactivity | [24] | |||||
| Theca cells | + | IHC | [25] | ||||||
| Corpus luteum | + | + | MDS/RIA | [26] | |||||
| Corpus luteum | + | + | + | + | + | RT-PCR/ELISA | [27] | ||
| Corpus luteum | + | RT-PCR | [28] | ||||||
| Pig | Corpus luteum | + | + | + | + | + | RT-PCR | [29] | |
| Macrophages/CL | + | + | + | IHC | [30] | ||||
| EC/CL | + | + | + | IHC/Western blot | [31] | ||||
| Human | Follicular fluid | + | ELISA | [32] | |||||
| Granulosa cells | + | IHC | [33] | ||||||
| Theca cells | + | In situ hybridization | [34] | ||||||
| Corpus luteum | + | + | + | + | IHC | [35] | |||
| Sheep | EC/TC | + | IF-microscopy | [36] | |||||
| Corpus luteum | + | Bioactivity | [37] | ||||||
| Rat3) | Granulosa cells | + | IHC | [25] | |||||
| Mouse3) | GC/TC/CL | + | + | + | IHC | [38] | |||
| TNFR | (RI or RII) | ||||||||
| Cow | GC/TC | + | RRA/cells | [39] | |||||
| Corpus luteum (RI) | + | + | + | + | RT-PCR/RRA | [27] | |||
| Corpus luteum | + | RRA | [28] | ||||||
| EC/CL | + | RRA/cells | [40] | ||||||
| EC/LLC/SLC (RI) | + | + | + | + | RT-PCR | [41] | |||
| Pig | Granulosa cells | + | IHC | [42] | |||||
| LLC/SLC | + | RRA/cells | [43] | ||||||
| Corpus luteum | + | + | + | + | + | RRA | [44] | ||
| Rat3) | Ovarian cells (RI, RII) | + | RT-PCR/RRA | [45] | |||||
1)Abbreviations; TC: theca cells, GC: granulose cells, EC: endothelial cells, LLC: large luteal cells, SLC: small luteal cells, IHC: immunohistochemistry, RRA: radioreceptor assay. 2)Each stage of the estrous cycle was classified as follows; follicular (F), early-CL (I), mid-CL (II), late-CL (III), regressing CL (R), and pregnancy (P). 3)Each stage of the estrous cycle was classified as follows; estrus (I), metestrus (II), diestrus (III), proestrus (R), and pregnancy or pseudopregnancy (P).
Although TNF exerts pleiotropic responses in various targets tissues, the engagement of Fas with Fas L is primarily known for its role in apoptosis in various cell types. As shown in Table 2, Fas and Fas L are expressed in ovarian granulosa or theca cells in multiple species [46-48,51]. Activation of Fas/Fas L system is thought as a key event in follicular atresia [56]. Although it is a very interesting topic, we will limit our detailed review to the Fas/Fas L system in the process of follicular development in the ovary.
Table 2.
Ovarian Fas and Fas ligand (Fas L) in various animals1)
| Species | Sources | Stages of the estrous cycle2) | Method of detection | Ref. | |||||
| F | I | II | III | R | P | ||||
| Fas | |||||||||
| Cow | GC/TC | + | RT-PCR | [46] | |||||
| Corpus luteum | + | + | + | + | RT-PCR | [22] | |||
| Human | Follicular fluid | + | ELISA | [47] | |||||
| Corpus luteum | + | + | + | + | IHC | [21] | |||
| Rat3) | Granulosa cells | + | TUNEL/IHC | [48] | |||||
| CL/LLC | + | RT-PCR | [49] | ||||||
| Mouse3) | Corpus luteum | + | RT-PCR | [50] | |||||
| Fas L | |||||||||
| Cow | GC/TC | + | RT-PCR | [51] | |||||
| Rat3) | Granulosa cells | + | TUNEL/IHC | [48] | |||||
| CL/SLC | + | RT-PCR | [49] | ||||||
1)Abbreviations; TC: theca cells, GC: granulose cells, EC: endothelial cells, LLC: large luteal cells, SLC: small luteal cells, IHC: immunohistochemistry, RRA: radioreceptor assay. 2)Each stage of the estrous cycle was classified as follows; follicular (F), early-CL (I), mid-CL (II), late-CL (III), regressing CL (R), and pregnancy (P). 3)Each stage of the estrous cycle was classified as follows; estrus (I), metestrus (II), diestrus (III), proestrus (R), and pregnancy or pseudopregnancy (P).
TNF and TNFR are evident in the early stage CL in cows [27], pigs [29,44], and human [35] (Table 1). TNF is a potent stimulator of luteal prostaglandins (PGs) including PGF2α, PGE2 and PGI2 [15,27,57]. Luteal PGs are known to stimulate progesterone production from bovine CL in vitro, suggesting that they are luteotropic agents [58,59]. Macrophages [11,30] and endothelial cells [31] are sources of TNF, and these cells infiltrate into newly formed CL concomitant with vascular angiogenesis [60]. Thus, it could be assumed that TNF contributes to the production of PGs by early CL, and may partly promote the formation of CL. Additional studies in human luteinized granulosa cells support a luteotropic role of TNF [61]. This effect is not limited to steroidogenic cells since TNF is capable of stimulating PGE2 secretion by the bovine luteal endothelial cells [40]. Since both TNF [62] and PGE2 [63] are known to affect the proliferation of endothelial cells derived from other tissues, TNF and TNF-induced PGE2 may be autocrine and/or paracrine regulators of vascular angiogenesis. Taken together, these findings suggest that TNF has a role in stimulating the early phase of luteal development.
Fas is expressed in human [21] and bovine [22] CL throughout the luteal phase. Its presence throughout the luteal phase could suggest multiple roles including luteal development, maintenance, and regression. However, the physiological significance of Fas or its ligand in the developing CL, if any, is not yet known. Bovine luteal cells express Fas mRNA and Fas L can induce luteal cell death [22,64]. Similar results have been shown in bovine granulosa cells cultured in a medium containing fetal bovine serum [65]. Quirk et al. [66] demonstrated that bovine granulosa cells become sensitive to Fas-mediated apoptosis when the cells are cultured in serum-free media. However, Fas mRNA expression in bovine granulosa cells was not different in the two culture systems (with and without serum).
Growth factors, such as IGF, block Fas-mediated cell death in a serum-free medium [66]. IGF is produced locally within the bovine CL [67] and is a potent luteotropic factor in bovine luteal cells [68]. In addition, luteal concentrations of mRNA encoding IGF and the receptor for IGF increase during luteal development [69]. Thus, the ability of Fas L to induce apoptosis in luteal cells is reduced under conditions in which the cells are regulated by survival factors such as IGF. Furthermore, Fas mRNA expression in cultured rat luteal cells has been clearly suppressed by treatment with progesterone [70]. These data support the idea that progesterone and IGF act as important factors that can change the sensitivity of CL to Fas/Fas L system.
It is interesting to note that progesterone suppresses apoptotic cell death in rat and bovine luteal cells [70,71]. The suppressive effects of progesterone on Fas-mediated luteal cell death were not directly related to the amount of Fas expression in cows [72], but were related in the mouse model [70]. Furthermore, treatment with a progesterone antagonist (onapristone) in cultured luteal cells resulted in reduced cell viability without the presence of Fas L [72]. Therefore, it remains to be clarified how progesterone controls the apoptotic cell death in the CL (e.g., the stage-dependent changes of progesterone receptors, and the effects of progesterone on the intracellular signaling cascade for apoptosis in the CL).
TNF and Fas L in luteolysis
Functional luteolysis
TNF has an inhibitory effect on gonadotropin-stimulated steroid production in the steroidogenic luteal cells in rats [13], pigs [14], and cows [15]. Furthermore, LH receptor and steroidogenic acute regulatory protein mRNA are reduced by TNF in rat CL [73]. Secretion of TNF in bovine CL is higher in the late stage CL than in mid luteal phase CL [26,27]. In addition, specific binding sites for TNF are present in the bovine CL throughout the luteal phase of estrous cycle [27]. These studies suggest that TNF affects CL function as a luteolytic agent. However, how TNF switches from a luteotropic agent to a luteolytic agent in the estrous cycle is unknown. Recent investigations about the synergistic or co-operative actions between TNF and other substances may clarify these points.
It is well recognized that uterine derived PGF2α is the primary initiator of luteolysis in many species, especially in ruminants. PGF2α decreases progesterone production by cultured luteal cells stimulated by gonadotropins in pigs [10] and cows [14]. PGF2α also increases endothelin-1 (ET-1) production from endothelial cells in the CL, and it is presumed that elevated ET-1 augments PGF2α induced inhibition of progesterone synthesis [41]. Specific binding sites for TNF are present in endothelial cells derived from bovine CL [40,41] and ET-1 secretion by the cells is significantly stimulated by treatment with TNF [40]. Since it has been shown that high concentrations of ET-1 decreased progesterone production by bovine luteal cells [74], TNF-induced ET-1 secretion from the endothelial cells may act on luteal cells as an antisteroidogenic agent in the CL concomitant with endometrial PGF2α in cattle. Moreover, PGF2α-induced steroid reduction results in an activation of inflammatory cells followed by TNF and IFN secretion in the CL of cows [9], pigs [75], and rabbits [76]. TNF in combination with IFN reduces progesterone production and the number of viable cells, although TNF alone shows little effect, if any [15,77]. Although to date there is no known study demonstrating IFN is present in the CL at the protein level, IFN mRNA has been detected in bovine luteal tissue collected at the end of the luteal phase of a naturally regressing CL and after induced luteolysis [9]. Treatment with IFN by itself can decrease progesterone levels and induce apoptosis [77]. Realistically, however, it is likely that TNF works in conjunction with IFN or other substances (e.g., ET-1 etc.) to inhibit progesterone synthesis (i.e., functional regression).
In contrast to ruminants, the CL of pigs, rodents or primates are known to secrete estradiol as well as progesterone. Intraluteal estradiol is a potent luteotropic factor, namely it stimulates progesterone secretion in the CL of pigs [78]. It has been demonstrated that PGF2α inhibited progesterone secretion by porcine luteal cells in vitro, whereas estradiol was stimulated [14]. Moreover, when the CL is pretreated with TNF, PGF2α reduces progesterone production more markedly than that in treatment with PGF2α alone [78]. Since TNF inhibits estradiol production of cultured luteal cells [29], TNF may reduce progesterone levels not only by a direct inhibition of progesterone secretion but also by inhibiting luteotropic-estradiol production in the CL of pigs. However, in human, it has been shown that estradiol is not luteotropic agent [79]. Thus, the mechanisms of TNF-mediated inhibition of progesterone secretion seem to be different among species.
Structural luteal regression
Structural regression of the CL must occur to allow new follicular development. The importance of interactions between Fas/Fas L system and other cytokines (i.e., TNF, IFN) in structural luteolysis has been demonstrated. Bovine luteal cells became sensitive to Fas L-induced cell death in the presence of IFN and IFN in combination with TNF [22]. IFN and TNF have been shown to stimulate Fas mRNA expression in a variety of ovarian cell types [22,50,65]. Moreover, the increased sensitivity to Fas L in bovine luteal cells was correlated with an increase of Fas mRNA expression induced by cytokines, suggesting that Fas L induces cell death of bovine luteal cells mediated via the Fas/Fas L system [22]. Furthermore, the expression of Fas L protein is elevated in the regressing postpartum rat CL [20]. Injections of an anti-Fas antigen antibody given to mice in the pro-estrous period made the CL disappear [19], suggesting that the Fas/Fas L system mediates apoptosis of CL cells in structural regression of the CL. In addition, administration of a Fas activating antibody to mice following a gonadotropin induced luteal phase resulted in increasing apoptosis [80].
As mentioned previously, the number of leukocytes increases at the time of luteolysis in the CL [9,75,76]. T-lymphocytes are known to abundantly express Fas L and to be a primary source of IFN, whereas macrophages are the main source of TNF [11,18]. Therefore, Fas L expressed on T-lymphocytes may transduce apoptotic signals to luteal cells in which Fas expression is induced by leukocyte-derived cytokines. Collectively, these reports suggest that the Fas/Fas L system may be involved in the physiological process of structural luteolysis in the CL. Interactions between TNF and IFN are also considered important in the structural regression of the CL. A decrease in progesterone levels and interruption of growth factor signaling in the CL may promote activation of inflammatory cells resulting in increased TNF and IFN production [75,76]. TNF induces a significant increase in the expression of major histocompatibility (MHC) class I glycoproteins in cultured bovine luteal cells [15]. It is hypothesized that these glycoproteins are recognized by cytotoxic T lymphocytes in order for the T lymphocytes to phagocytize luteal cells [15]. IFN also increases the expression of class II MHC [81]. In addition, IFN alone reduces the viability of bovine and murine luteal cells, and that TNF augments this effect [15,22,78]. Since IFN up-regulates TNFR in a variety of cell types [82], the luteal cells might become more sensitive to the action of TNF in the presence of IFN. These findings support the idea that TNF and IFN also play important roles in luteolysis, especially at time of structural regression by a mechanism not mediated via the Fas/Fas L system.
Intracellular signaling pathways of TNFR and Fas
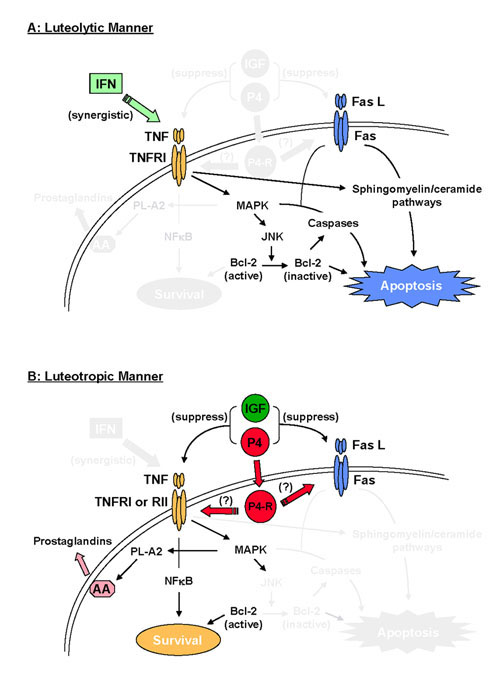
Possible actions of TNF and Fas L in the luteal cells are summarized in Figure 1. Two immunologically distinct TNFR of approximately 55 kDa (Type I; TNFRI) and 75 kDa (Type II; TNFRII) have now been identified [83]. TNFRI and TNFRII have different intracellular signaling pathways [84]. TNFRI contains an intracellular death domain, which is required for signaling pathways associated with apoptosis. In contrast, TNFRII can induce gene transcription for cell survival, growth, and differentiation. Our previous work demonstrated that TNFRI mRNA and the specific binding sites for TNF are present in bovine CL throughout the estrous cycle, and suggested that at least one of the receptors for TNF in bovine CL is the TNFRI [27]. The TNF/TNFRI complex activates phospholipase (PL)-A2 pathway [85], PL-C or protein kinase (PK)-C pathway [86], and PK-A pathway [87] in a variety of tissues. TNF stimulates PG secretion by cultured bovine luteal cells via its specific receptors [15,27]. A selective inhibitor of PL-A2 completely stopped the actions of TNF, whereas inhibitors of PL-C, PK-A and PK-C did not significantly inhibit TNF-induced PGF2α production [88,89]. These results suggest that TNF induces PL-A2 to produce PGF2α in the bovine steroidogenic luteal cells. In addition, a selective mitogen-activated protein kinase (MAPK) inhibitor was recently shown to suppress TNF-induced PGF2α secretion by cultured bovine luteal cells [88]. Since MAPK partly regulates cytoplasmic PL-A2 activation in a variety of cells [90], the MAPK cascade may couple with the PL-A2 pathway in bovine luteal cells. These findings suggest that TNF activates the MAPK kinase/MAPK signaling cascade and subsequently activates the PL-A2 pathway in bovine luteal cells to produce arachidonic acid and PG. Alternatively, TNF is known to activate cell survival pathways, involving activation of nuclear factor-κB (NFκB) [91]. NFκB has been shown to control transcription of cyclooxygenase (COX)-2 gene [92]. COX-2 is an inducible key rate-limiting enzyme for converting arachidonic acid to PG family [93]. Therefore, TNF-induced PG production may be also regulated by activation of NFκB and subsequent promotion of COX-2 expression.
Figure 1.
Possible actions of TNF and Fas L in the luteal cells. TNF and Fas show multiple actions on luteal cell function through complex intracellular pathways. Fig. 1A shows the possible luteolytic actions of TNF and Fas L in the luteal cells. Fig. 1B shows the possible luteotropic actions of TNF in the luteal cells. * Abbreviations; tumor necrosis factor-α (TNF), TNF receptor type I (TNFRI), TNF receptor type II (TNFRII), Fas antigen (Fas), Fas ligand (Fas L), interferon-γ (IFN), insulin-like growth factor-I (IGF), progesterone (P4), P4 receptor (P4-R), mitogen-activated protein kinase (MAPK), phospholipase-A2 (PL-A2), arachidonic acid (AA), nuclear factor-κB (NFκB), jun-n-terminal kinase (JNK).
More recently, it has been shown that TNF-induced signaling in steroidogenic luteal cells resulted in an increase in phosphorylated p38MAPK and jun-n-terminal kinase (JNK) [94]. Elevations in JNK activity have been shown to induce phosphorylation and inactivation of Bcl-2 protein in the KYM-1 cell lines [95]. Generally, one of the effects of Bcl-2 is known to suppress apoptotic cell death, and intracellular levels of Bcl-2 are reduced by stimulation of TNF in human granulosa cells [96]. These observations suggest that TNF may augment Fas L or IFN to induce apoptosis through the inactivation of Bcl-2 protein following activation of JNK in the luteal cells. In addition, the engagement of TNF with TNFRI has been demonstrated to activate sphingomyelin-ceramide pathways, which results in apoptosis in some cell types [97]. The multiple actions of TNF are due to the activation of complex intracellular signaling pathways [98].
In contrast to TNFRI, information on the TNFRII in the ovary is limited [99]. Recently, TNFRII was demonstrated in the granulosa cells of porcine ovaries. Based on its presence, TNFRII was suggested to have a role in follicle atresia [99]. Whether or not TNFRII has any significance in the CL remains to be proven. In contrast to TNFR, numerous studies have provided evidence that Fas mediates apoptotic cell death through various intracellular signaling pathways. Both soluble Fas L and anti-Fas monoclonal antibody induced apoptosis in bovine luteal cells [64] and rat thecal/interstitial cells [100] via activations of sphingomyelin-ceramide pathways. Moreover, it is thought that the ratio of Bcl-2 to Bax expression is the critical determinant of cell fate, such that elevated Bcl-2 favors extended survival of cells, whereas increasing levels of Bax expression accelerate cell death [101,102]. Bcl-2 mRNA levels in the human CL during the menstrual cycle were highest in the midluteal phase and lowest in the regressing CL [103]. In contrast, bax mRNA levels were highest in the human regressing CL [103]. These findings suggest that apoptosis in the CL is regulated by a conserved pathway composed of these central apoptosis-related genes. Recent study demonstrated that the apoptosis-regulating factors Bcl-2, Bax, caspase-3 and NFκB are present in human CL throughout the estrous cycle [35]. The physiological importance of TNFRII and intracellular mechanisms of Fas-induced apoptosis in the CL are not completely understood. Further studies are needed to clarify these points.
Gestation period
The establishment of pregnancy is the result of a number of interactions between the conceptus and mother. When pregnancy is established, the CL is sustained and continuously produces progesterone to maintain the pregnancy. TNF and its receptor have been shown to be present in the gravid uterus, placenta, oviduct and embryo [104]. In the CL, it has been also demonstrated that high-affinity binding sites for TNF were present throughout the gestation period, and that the concentrations of TNFR changed during the gestational stages [28]. Furthermore, TNF mRNA has been detected in the bovine [28] and porcine [29] CL during the gestation period. These findings suggest that locally produced TNF plays one or more roles in the pregnant CL as an autocrine and/or paracrine mediator. Since the concentration of TNFR as well as the abundance of TNF mRNA were high in the CL of the late gestation period [28], it is possible that TNF locally produced in the CL contributes to luteal resorption during or following parturition.
Specific binding sites for TNF were also found in the bovine CL of the early and mid-gestational stages [28]. Furthermore, the affinity and the concentration of the receptor in the CL of pregnancy are comparable with those in the CL of the regressed stage [27]. Thus, TNF may also act on CL function in the early and mid-gestational stages. TNF stimulates PGE2 as well as PGF2α secretion by cultured bovine luteal cells in a dose-dependent fashion [27]. Both luteal PGE2 and PGF2α in the CL are known to be luteotropic agents, e.g., they stimulate progesterone production by bovine CL in vitro [58,59]. Therefore, it could be assumed that the luteal TNF contributes to the maintenance of pregnancy by stimulating the production of PGF2α and PGE2 by the CL of pregnancy, indirectly resulting in an increase of progesterone output from the CL of pregnancy. The CL of cows from day 200 of pregnancy secretes more PGE2 and PGF2α in vitro than those of Day 14 of the estrous cycle [105]. Furthermore, progesterone production in vitro by CL from day 200 of pregnancy was increased by PGE2 but not by LH [106]. These findings may support the above supposition that TNF indirectly contributes to produce progesterone by stimulation of luteal PGE2 in bovine CL during the gestation period.
The physiological significance of Fas/Fas-L system in the CL of pregnancy is still unknown. The only evidence for the presence of Fas in the CL of pregnancy has been demonstrated in mice [50]. Based on the characteristics of Fas/Fas L system, it is possible that Fas-mediated apoptosis contributes to the resorption of the pregnant CL. Further studies are needed to understand the physiological meanings of Fas/Fas L system in the pregnant CL.
Possible action of other TNF super family members on CL function
The TNF-SF is comprised of 18 members including TNF and Fas L, and the TNFR-SF is comprised of 29 members in human. Some members of the TNF-SF, such as EDA, lymphotoxins (α and β), CD40, RANKL and TRAIL are demonstrated their function in regulating immune systems in several species (reviewed in Ref. [107]). More recently, DR5-like death receptors, which can bind TRAIL, have been demonstrated in hen ovarian follicles [108]. Furthermore, TRAIL and its receptors have been detected in the follicles of pigs [109]. These findings suggest that TRAIL could regulate follicular development in mammals. However, very little effort has been made to recognize the physiological significance of these and other members of the TNF-SF or TNFR-SF in luteal function. Consequently, the roles of the other TNF-SF members in the CL will need to be delineated, so that we could promote a greater understanding of cytokine-induced regulation of CL function.
Conclusions
TNF plays multiple and important roles in CL function via its specific receptors throughout the estrous cycle. TNF seems to play a luteotropic role through the stimulation of luteal PGF2α and PGE2 in early luteal phase. On the other hand, luteolytic events, i.e., functional and structural luteolysis, are induced by this cytokine with other factors. IFN appears to be an important modulator of luteolysis, although the exact source and factors that contribute to IFN production in the CL are unknown. A Fas L, a member of TNF-SF, is recognized as an apoptotic agent. The apoptotic function is exerted by binding its specific receptor (Fas) to induce structural luteolysis. TNF and IFN appear to play big roles in completing the structural luteolysis mediated by Fas/Fas L system. Many factors, such as progesterone and IGF, are believed to inhibit Fas mediated apoptosis in luteolysis in vitro.
Acknowledgments
Acknowledgements
This research was supported by a Grant-in-Aid for Scientific Research (B) (No. 14360168) from the Japan Society for the Promotion of Science (JSPS). The authors thank Dr. Bo Rueda, Massachusetts General Hospital for his critical reading of the manuscript.
Contributor Information
Kiyoshi Okuda, Email: kokuda@cc.okayama-u.ac.jp.
Ryosuke Sakumoto, Email: sakumoto@affrc.go.jp.
References
- Niswender GD, Nett TM. The corpus luteum and its control. In: Knobil E, Neil J, editor. The Physiology of Reproduction. New York, Raven Press; 1988. pp. 489–525. [Google Scholar]
- Farin CE, Moeller CL, Sawyer HR, Gamboni F, Niswender GD. Morphometric analysis of cell types in the ovine CL throughout the estrous cycle. Biol Reprod. 1986;35:1299–1308. doi: 10.1095/biolreprod35.5.1299. [DOI] [PubMed] [Google Scholar]
- Penny LA. Monocyte chemoattractant protein 1 in luteolysis. Rev Reprod. 2000;5:63–66. doi: 10.1530/revreprod/5.2.63. [DOI] [PubMed] [Google Scholar]
- Juengel JL, Garverick HA, Johnson AL, Youngquist RS, Smith MF. Apoptosis during luteal regression in cattle. Endocrinology. 1993;132:249–254. doi: 10.1210/en.132.1.249. [DOI] [PubMed] [Google Scholar]
- Rueda BR, Tilly KI, Botros IW, Jolly PD, Hansen TR, Hoyer PB, Tilly JL. Increased bax and interleukin-1-converting enzyme messenger ribonucleic acid levels coincide with apoptosis in the bovine corpus luteum during structural regression. Biol Reprod. 1997;56:186–193. doi: 10.1095/biolreprod56.1.186. [DOI] [PubMed] [Google Scholar]
- Bacci ML, Barazzoni AM, Forni M, Costerbosa GL. In situ detection of apoptosis in regressing corpus luteum of pregnant sow: evidence of an early presence of DNA fragmentation. Domest Anim Endocrinol. 1996;13:361–372. doi: 10.1016/0739-7240(96)00049-5. [DOI] [PubMed] [Google Scholar]
- Rueda BR, Wegner JA, Marion SL, Wahlen DD, Hoyer PB. Internucleosomal DNA fragmentation in ovine luteal tissue associated with luteolysis: in vivo and in vitro analyses. Biol Reprod. 1995;52:305–312. doi: 10.1095/biolreprod52.2.305. [DOI] [PubMed] [Google Scholar]
- Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- Penny LA, Armstrong D, Bramley TA, Webb R, Collins RA, Watson ED. Immune cells and cytokine production in the bovine corpus luteum throughout the oestrous cycle and after induced luteolysis. J Reprod Fertil. 1999;115:87–96. doi: 10.1530/jrf.0.1150087. [DOI] [PubMed] [Google Scholar]
- Pate JL. Involvement of immune cells in regulation of ovarian function. J Reprod Fertil Suppl. 1995;49:365–377. [PubMed] [Google Scholar]
- Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci USA. 1975;72:3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- Adashi EY, Resnick CE, Packman JN, Hurwitz A, Payne DW. Cytokine-mediated regulation of ovarian function: tumor necrosis factor α inhibits gonadotropin-supported progesterone accumulation by differentiating and luteinized murine granulosa cells. Am J Obstet Gynecol. 1990;162:889–899. doi: 10.1016/0002-9378(90)91289-o. [DOI] [PubMed] [Google Scholar]
- Pitzel L, Jarry H, Wuttke W. Effects and interactions of prostaglandin F2α, oxytocin, and cytokines on steroidogenesis of porcine luteal cells. Endocrinology. 1993;132:751–756. doi: 10.1210/en.132.2.751. [DOI] [PubMed] [Google Scholar]
- Benyo DF, Pate JL. Tumor necrosis factor-α alters bovine luteal cell synthetic capacity and viability. Endocrinology. 1992;130:854–860. doi: 10.1210/en.130.2.854. [DOI] [PubMed] [Google Scholar]
- Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumor-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- Sakamaki K, Yoshida H, Nishimura Y, Nishikawa S, Manabe N, Yonehara S. Involvement of Fas antigen in ovarian follicular atresia and luteolysis. Mol Reprod Dev. 1997;47:11–18. doi: 10.1002/(SICI)1098-2795(199705)47:1<11::AID-MRD2>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Roughton SA, Lareu RR, Bittles AH, Dharmarajan AM. Fas and Fas ligand messenger ribonucleic acid and protein expression in the rat corpus luteum during apoptosis-mediated luteolysis. Biol Reprod. 1999;60:797–804. doi: 10.1095/biolreprod60.4.797. [DOI] [PubMed] [Google Scholar]
- Kondo H, Maruo T, Peng X, Mochizuki M. Immunological evidence for the expression of the Fas antigen in the infant and adult human ovary during follicular regression and atresia. J Clin Endocrinol Metab. 1996;81:2702–2710. doi: 10.1210/jc.81.7.2702. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, Yokomizo Y, Okuda K. Fas/Fas ligand mediates luteal cell death in bovine corpus luteum. Biol Reprod. 2002;66:754–759. doi: 10.1095/biolreprod66.3.754. [DOI] [PubMed] [Google Scholar]
- Kuranaga E, Kanuka H, Bannai M, Suzuki M, Nishihara M, Takahashi M. Fas/Fas ligand system in prolactin-induced apoptosis in rat corpus luteum: possible role of luteal immune cells. Biochem Biophys Res Commun. 1999;60:167–173. doi: 10.1006/bbrc.1999.0858. [DOI] [PubMed] [Google Scholar]
- Zolti M, Meirom R, Shemesh M, Wollach D, Mashiach S, Shore L, Ben Rafael Z. Granulosa cells as a source and target organ for tumor necrosis factor-α. FEBS lett. 1990;261:253–255. doi: 10.1016/0014-5793(90)80565-Z. [DOI] [PubMed] [Google Scholar]
- Roby KF, Terranova PF. Localization of tumor necrosis factor (TNF) in the rat and bovine ovary using immunohistochemistry and cell blot: evidence for granulosa production. In: Hirshfield AN, editor. Growth Factors and the Ovary. New York, Plenum Publishing Corporation; 1989. pp. 273–278. [Google Scholar]
- Shaw DW, Britt JH. Concentrations of tumor necrosis factor α and progesterone within the bovine corpus luteum sampled by continuous-flow microdialysis during luteolysis in vivo. Biol Reprod. 1995;53:847–854. doi: 10.1095/biolreprod53.4.847. [DOI] [PubMed] [Google Scholar]
- Sakumoto R, Berisha B, Kawate N, Schams D, Okuda K. Tumor necrosis factor-α and its receptor in bovine corpus luteum throughout the estrous cycle. Biol Reprod. 2000;62:192–199. doi: 10.1095/biolreprod62.1.192. [DOI] [PubMed] [Google Scholar]
- Sakumoto R, Murakami S, Kishi H, Iga K, Okano A, Okuda K. Tumor necrosis factor-α and its receptor in the corpus luteum of pregnant cows. Mol Reprod Dev. 2000;55:406–411. doi: 10.1002/(SICI)1098-2795(200004)55:4<406::AID-MRD8>3.3.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Wuttke W, Pitzel L, Knoke I, Theiling K, Jarry H. Immune-endocrine interactions affecting luteal function in pigs. J Reprod Fertil Supple. 1997;52:19–29. [PubMed] [Google Scholar]
- Zhao Y, Burbach JA, Roby KF, Terranova PF, Brannian JD. Macrophages are the major source of tumor necrosis factor α in the porcine corpus luteum. Biol Reprod. 1998;59:1385–1391. doi: 10.1095/biolreprod59.6.1385. [DOI] [PubMed] [Google Scholar]
- Hehnke-Vagnoni KE, Clark CL, Taylor MJ, Ford SP. Presence and localization of tumor necrosis factor α in the corpus luteum of nonpregnant and pregnant pigs. Biol Reprod. 1995;53:1339–1344. doi: 10.1095/biolreprod53.6.1339. [DOI] [PubMed] [Google Scholar]
- Wang LJ, Brännström M, Robertson SA, Norman RJ. Tumor necrosis factor α in the human ovary: presence in follicular fluid and effects on cell proliferation and prostaglandin production. Fertil Steril. 1992;58:934–940. doi: 10.1016/s0015-0282(16)55438-7. [DOI] [PubMed] [Google Scholar]
- Roby KF, Weed J, Lyles R, Terranova PF. Immunological evidence for a human ovarian tumor necrosis factor-alpha. J Clin Endocrinol Metab. 1990;71:1096–1102. doi: 10.1210/jcem-71-5-1096. [DOI] [PubMed] [Google Scholar]
- Maylor MS, Stamp GWH, Foulkes WD, Eccles D, Balkwill FR. Tumor necrosis factor and its receptors in human ovarian cancer. J Clin Invest. 1993;91:2194–2206. doi: 10.1172/JCI116446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaskivuo TE, Ottander U, Oduwole O, Isomaa V, Vihko P, Olofsson JI, Tapanainen JS. Role of apoptosis, apoptosis-related factors and 17β hydroxysteroid dehydrogenases in human corpus luteum regression. Mol Cell Endocrinol. 2002;194:191–200. doi: 10.1016/S0303-7207(02)00087-4. [DOI] [PubMed] [Google Scholar]
- Murdoch WJ, Colgin DC, Ellis JA. Role of tumor necrosis factor-α in the ovulatory mechanism of ewes. J Anim Sci. 1997;75:1601–1605. doi: 10.2527/1997.7561601x. [DOI] [PubMed] [Google Scholar]
- Ji I, Slaughter RG, Ellis JA, Ji TH, Murdoch WJ. Analyses of ovine corpora lutea for tumor necrosis factor mRNA and bioactivity during prostaglandin-induced luteolysis. Mol Cell Endocrinol. 1991;81:77–80. doi: 10.1016/0303-7207(91)90206-8. [DOI] [PubMed] [Google Scholar]
- Chen H, Marcinkiewicz JL, Sancho-Tello M, Hunt JS, Terranova PF. Tumor necrosis factor-α gene expression in mouse oocytes and follicular cells. Biol Reprod. 1993;48:707–714. doi: 10.1095/biolreprod48.4.707. [DOI] [PubMed] [Google Scholar]
- Spicer LJ. Receptors for insulin-like growth factor-I and tumor necrosis factor-α are hormonally regulated in bovine granulosa and thecal cells. Anim Reprod Sci. 2001;67:45–58. doi: 10.1016/S0378-4320(01)00114-2. [DOI] [PubMed] [Google Scholar]
- Okuda K, Sakumoto R, Uenoyama Y, Berisha B, Miyamoto A, Schams D. Tumor necrosis factor α receptors in microvascular endothelial cells from bovine corpus luteum. Biol Reprod. 1999;61:1017–1022. doi: 10.1095/biolreprod61.4.1017. [DOI] [PubMed] [Google Scholar]
- Friedman A, Weiss S, Levy N, Meidan R. Role of tumor necrosis factor α and its type I receptor in luteal regression: Induction of programmed cell death in bovine corpus luteum-derived endothelial cells. Biol Reprod. 2000;63:1905–1912. doi: 10.1095/biolreprod63.6.1905. [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J, Kreutzkamm C, Wehrenberg U, Rune GM. Role of tumor necrosis factor in preovulatory follicles of swine. Biol Reprod. 2001;65:928–935. doi: 10.1095/biolreprod65.3.928. [DOI] [PubMed] [Google Scholar]
- Richards RG, Almond GW. Identification and distribution of tumor necrosis factor α receptors in pig corpora lutea. Biol Reprod. 1994;51:1285–1291. doi: 10.1095/biolreprod51.6.1285. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Sakumoto R, Sakabe Y, Miyake M, Okano A, Okuda K. Tumour necrosis factor-α receptors are present in the corpus luteum throughout the oestrous cycle and during the early gestation period in pigs. Reprod Dom Anim. 2002;37:105–110. doi: 10.1046/j.1439-0531.2002.00324.x. [DOI] [PubMed] [Google Scholar]
- Balchak SK, Marcinkiewicz JL. Evidence for the presence of tumor necrosis factor alpha receptors during ovarian development in the rat. Biol Reprod. 1999;61:1506–1512. doi: 10.1095/biolreprod61.6.1506. [DOI] [PubMed] [Google Scholar]
- Porter DA, Vickers SL, Cowan RG, Huber SC, Quirk SM. Expression and function of Fas antigen vary in bovine granulosa and theca cells during ovarian follicular development and atresia. Biol Reprod. 2000;62:62–66. doi: 10.1095/biolreprod62.1.62. [DOI] [PubMed] [Google Scholar]
- Santos MJ, Anderson DJ, Racowsky C, Hill JA. Presence of Fas-Fas ligand system and bcl-2 gene products in cells and fluids from gonadotropin-stimulated human ovaries. Biol Reprod. 2000;63:1811–1816. doi: 10.1095/biolreprod63.6.1811. [DOI] [PubMed] [Google Scholar]
- Kim JM, Yoon YD, Tsang BK. Involvement of the Fas/Fas ligand system in p53-mediated granulosa cell apoptosis during follicular development and atresia. Endocrinology. 1999;140:2307–2317. doi: 10.1210/en.140.5.2307. [DOI] [PubMed] [Google Scholar]
- Kuranaga E, Kanuka H, Furuhata Y, Yonezawa T, Suzuki M, Nishihara M, Takahashi M. Requirement of the Fas ligand-expressing luteal immune cells for regression of corpus luteum. FEBS Lett. 2000;472:137–142. doi: 10.1016/S0014-5793(00)01426-5. [DOI] [PubMed] [Google Scholar]
- Quirk SM, Harman RM, Huber SC, Cowan RG. Responsiveness of mouse corpora luteal cells to Fas antigen (CD95)-mediated apoptosis. Biol Reprod. 2000;63:49–56. doi: 10.1095/biolreprod63.1.49. [DOI] [PubMed] [Google Scholar]
- Porter DA, Harman RM, Cowan RG, Quirk SM. Relationship of Fas ligand expression and atresia during bovine follicle development. Reproduction. 2001;121:561–566. doi: 10.1530/reprod/121.4.561. [DOI] [PubMed] [Google Scholar]
- Spicer L, Alpizar E. Effects of cytokines on FSH-induced estradiol production by bovine granulosa cells in vitro: dependence on size of follicle. Domest Anim Endocrinol. 1994;11:25–34. doi: 10.1016/0739-7240(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Spicer LJ. Tumor necrosis factor-α (TNF-α) inhibits steroidogenesis of bovine ovarian granulosa and thecal cells in vitro. Involvement of TNF-α receptors. Endocrine. 1998;8:109–115. doi: 10.1385/ENDO:8:2:109. [DOI] [PubMed] [Google Scholar]
- Brännström M, Bonello N, Wang LJ, Norman RJ. Effects of tumor necrosis factor α (TNFα) on ovulation in the rat ovary. Reprod Fertil Dev. 1995;7:67–73. doi: 10.1071/rd9950067. [DOI] [PubMed] [Google Scholar]
- Murdoch WJ. Proteolytic and cellular death mechanisms in ovulatory ovarian rupture. Biol Signals Recept. 2000;9:102–114. doi: 10.1159/000014629. [DOI] [PubMed] [Google Scholar]
- Asselin E, Xiao CW, Wang YF, Tsang BK. Mammalian follicular development and atresia: role of apoptosis. Biol Signals Recept. 2000;9:87–95. doi: 10.1159/000014627. [DOI] [PubMed] [Google Scholar]
- Schams D, Schmidt K, Schlegel W. Effects of growth factors on prostaglandin secretion of bovine luteal cells in vitro at different stages of the luteal phase. Biol Reprod. 1995;52:569. [Google Scholar]
- Miyamoto A, Lützow vH, Schams D. Acute action of prostaglandin F2α, E2, and I2 in microdialyzed bovine corpus luteum in vitro. Biol Reprod. 1993;49:423–430. doi: 10.1095/biolreprod49.2.423. [DOI] [PubMed] [Google Scholar]
- Okuda K, Uenoyama Y, Lee KW, Sakumoto R, Skarzynski DJ. Progesterone stimulation by prostaglandin F2α involves protein kinase C pathway in cultured bovine luteal cells. J Reprod Dev. 1998;44:79–84. doi: 10.1262/jrd.44.79. [DOI] [Google Scholar]
- Reynolds LP, Killilea SD, Grazul-Bilska AT, Redmer DA. Mitogenic factors of corpora lutea. Prog Growth Factor Res. 1994;5:159–175. doi: 10.1016/0955-2235(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Yan Z, Hunter V, Weed J, Hutchison S, Lyles RT, Terranova PF. Tumor necrosis factor-α alters steroidogenesis and stimulates proliferation of human ovarian granulosa cells in vitro. Fertil Steril. 1993;53:332–338. doi: 10.1016/s0015-0282(16)55676-3. [DOI] [PubMed] [Google Scholar]
- Leibovich SJ, Polverini PJ, Shepard HM, Shively V, Nuseir N. Macrophage-induced angiogenesis is mediated by tumor necrosis factor-α. Nature. 1987;329:630–632. doi: 10.1038/329630a0. [DOI] [PubMed] [Google Scholar]
- Form DM, Auerbach R. PGE2 and angiogenesis. Proc Soc Exp Biol Med. 1983;172:214–218. doi: 10.3181/00379727-172-41548. [DOI] [PubMed] [Google Scholar]
- Pru JK, Hendry IR, Davis JS, Rueda BR. Soluble Fas ligand activates the sphingomyelin pathway and induces apoptosis in luteal steroidogenic cells independently of stress-activated p38MAPK. Endocrinology. 2002;143:4350–4357. doi: 10.1210/en.2002-220229. [DOI] [PubMed] [Google Scholar]
- Vickers SL, Cowan RG, Harman RM, Poter DA, Quirk SM. Expression and activity of the Fas antigen in bovine ovarian follicle cells. Biol Reprod. 2000;62:54–61. doi: 10.1095/biolreprod62.1.54. [DOI] [PubMed] [Google Scholar]
- Quirk SM, Harman RM, Cowan RG. Regulation of Fas antigen (Fas, CD95)-mediated apoptosis of bovine granulosa cells by serum and growth factors. Biol Reprod. 2000;63:1278–1284. doi: 10.1095/biolreprod63.5.1278. [DOI] [PubMed] [Google Scholar]
- Amselgruber W, Sinowatz F, Schams D, Skottner A. Immunohistochemical aspects of insulin-like growth factors I and II in the bovine corpus luteum. J Reprod Fertil. 1994;101:445–451. doi: 10.1530/jrf.0.1010445. [DOI] [PubMed] [Google Scholar]
- Mamluk R, Creber Y, Meidan R. Hormonal regulation of messenger ribonucleic acid expression for steroidogenic factor-1, steroidogenic acute regulatory protein, and cytochrome p450 side-chain cleavage in bovine luteal cells. Biol Reprod. 1999;60:628–634. doi: 10.1095/biolreprod60.3.628. [DOI] [PubMed] [Google Scholar]
- Einspanier R, Miyamoto A, Schams D, Muller M, Brem G. Tissue concentration, mRNA expression and stimulation of IGF-I in luteal tissue during the oestrous cycle and pregnancy of cows. J Reprod Fertil. 1990;90:439–445. doi: 10.1530/jrf.0.0900439. [DOI] [PubMed] [Google Scholar]
- Kuranaga E, Kanuka H, Hirabayashi K, Suzuki M, Nishihara M, Takahashi M. Progesterone is a cell death suppressor that downregulates Fas expression in rat corpus luteum. FEBS Lett. 2000;466:279–282. doi: 10.1016/S0014-5793(00)01090-5. [DOI] [PubMed] [Google Scholar]
- Rueda BR, Hendry IR, Hendry III WJ, Stormshak F, Slayden OD, Davis JS. Decreased progesterone levels and progesterone receptor antagonists promote apoptotic cell death in bovine luteal cells. Biol Reprod. 2000;62:269–276. doi: 10.1095/biolreprod62.2.269. [DOI] [PubMed] [Google Scholar]
- Okuda K, Taniguchi H. Progesterone is a cell death suppressor in Fas-mediated apoptosis pathway in bovine corpus luteum. Biol Reprod. 2002;66:463. doi: 10.1095/biolreprod66.3.754. [DOI] [PubMed] [Google Scholar]
- Chen Y, Feng Q, Liu Y. Expression of the steroidogenic acute regulatory protein and luteinizing hormone receptor and their regulation by tumor necrosis factor-α in rat corpora lutea. Biol Reprod. 1999;60:419–427. doi: 10.1095/biolreprod60.2.419. [DOI] [PubMed] [Google Scholar]
- Girsh E, Milvae RA, Wang W, Meidan R. Effect of endothelin-1 on bovine luteal cell function: role in prostaglandin F2α induced anti-steroidogenic action. Endocrinology. 1996;137:1306–1312. doi: 10.1210/en.137.4.1306. [DOI] [PubMed] [Google Scholar]
- Hehnke KE, Christenson LK, Ford SP, Taylor M. Macrophage infiltration into the porcine corpus luteum during prostaglandin F2α-induced luteolysis. Biol Reprod. 1994;50:10–15. doi: 10.1095/biolreprod50.1.10. [DOI] [PubMed] [Google Scholar]
- Naftalin DM, Bove SE, Keyes PL, Townson DH. Estrogen withdrawal induces macrophage invasion in the rabbit corpus luteum. Biol Reprod. 1997;56:1175–1180. doi: 10.1095/biolreprod56.5.1175. [DOI] [PubMed] [Google Scholar]
- Jo T, Tomiyama T, Ohashi K, Saji F, Tanizawa O, Ozaki M, Yamamoto R, Yamamoto T, Nishizawa Y, Terada N. Apoptosis of cultured mouse luteal cells induced by tumor necrosis factor-alpha and interferon-gamma. Anat Rec. 1995;241:70–76. doi: 10.1002/ar.1092410110. [DOI] [PubMed] [Google Scholar]
- Wuttke W, Spiess S, Knoke I, Pitzel L, Leonhardt S, Jarry H. Synergistic effects of prostaglandin F2α and tumor necrosis factor to induce luteolysis in the pig. Biol Reprod. 1998;58:1310–1315. doi: 10.1095/biolreprod58.5.1310. [DOI] [PubMed] [Google Scholar]
- Endo T, Henmi H, Goto T, Kitajima Y, Kiya T, Nishikawa A, Manase K, Yamamoto H, Kudo R. Effects of estradiol and an aromatase inhibitor on progesterone production in human cultured luteal cells. Gynecol Endocrinol. 1998;12:29–34. doi: 10.3109/09513599809024967. [DOI] [PubMed] [Google Scholar]
- Carambula SF, Pru JK, Lynch MP, Matikainen T, Goncalves PBD, Flavell RA, Tilly JL, Rueda BR. Prostaglandin F2alpha- and FAS-activating antibody-induced regression of the corpus luteum involves caspase-8 and is defective in caspase-3 deficient mice. Reprod Biol Endocrinol. 2003;1:15. doi: 10.1186/1477-7827-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild DL, Pate JL. Interferon-γ induction of major histocompatibility complex antigens on cultured bovine luteal cells. Biol Reprod. 1989;40:453–457. doi: 10.1095/biolreprod40.3.453. [DOI] [PubMed] [Google Scholar]
- Bebo BF, Linthicum DS. Expression of mRNA for 55-kDa and 75-kDa tumor necrosis factor (TNF) receptors in mouse cerebrovascular endothelium: effects of interleukin-1β, interferon-γ and TNFα on cultured cells. J Neuroimmunol. 1995;62:161–167. doi: 10.1016/0165-5728(95)00113-5. [DOI] [PubMed] [Google Scholar]
- Tartaglia LA, Goeddel DV. Two TNF receptors. Immunol Today. 1992;13:151–153. doi: 10.1016/0167-5699(92)90116-O. [DOI] [PubMed] [Google Scholar]
- Beutler B, van Huffel C. Unraveling function in the TNF ligand and receptor families. Science. 1994;264:667–668. doi: 10.1126/science.8171316. [DOI] [PubMed] [Google Scholar]
- Clark MA, Chen MJ, Crooke ST, Bomalaski JS. Tumour necrosis factor (cachectin) induces phospholipase A2-activating protein in endothelial cells. Biochem J. 1988;250:125–132. doi: 10.1042/bj2500125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachow R, Terranova P. Involvement of protein kinase C and protein tyrosine kinase pathways in tumor necrosis factor-α induced clustering of ovarian theca-interstitial cells. Mol Cell Endocrinol. 1993;97:37–49. doi: 10.1016/0303-7207(93)90209-3. [DOI] [PubMed] [Google Scholar]
- Zachow R, Tash J, Terranova P. Tumor necrosis factor-α attenuation of luteinizing hormone-stimulated androstenedione production by ovarian theca-interstitial cells: Inhibition at loci within the adenosine 3', 5'-monophosphate-dependent signaling pathway. Endocrinology. 1993;133:2269–2276. doi: 10.1210/en.133.5.2269. [DOI] [PubMed] [Google Scholar]
- Sakumoto R, Murakami S, Okuda K. Tumor necrosis factor-α stimulates prostaglandin F2α secretion by bovine luteal cells via activation of mitogen-activated protein kinase and phospholipase A2 pathways. Mol Reprod Dev. 2000;56:387–391. doi: 10.1002/1098-2795(200007)56:3<387::AID-MRD9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Townson DH, Pate JL. Mechanism of action of TNF-α-stimulated prostaglandin production in cultured bovine luteal cells. Prostaglandins. 1996;52:361–373. doi: 10.1016/S0090-6980(96)00104-9. [DOI] [PubMed] [Google Scholar]
- Lin LL, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- Xiao CW, Asselin E, Tsang BK. Nuclear factor κB-mediated induction of flice-like inhibitory protein prevents tumor necrosis factor α-induced apoptosis in rat granulosa cells. Biol Reprod. 2002;67:436–441. doi: 10.1095/biolreprod67.2.436. [DOI] [PubMed] [Google Scholar]
- Jobin C, Morteau O, Han DS, Balfour Sartor R. Specific NF-κB blockade selectively inhibits tumour necrosis factor-α-induced COX-2 but not constitutive COX-1 gene expression in HT-29 cells. Immunology. 1998;95:537–543. doi: 10.1046/j.1365-2567.1998.00646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita I. Distinct functions of COX-1 and COX-2. Prostaglandins Other Lipid Mediat. 2002;68–69:165–175. doi: 10.1016/S0090-6980(02)00029-1. [DOI] [PubMed] [Google Scholar]
- Rueda BR, Hendry IR, Ndjountche L, Sutter J, Davis JS. Stress-induced mitogen-activated protein kinase signaling in the corpus luteum. Mol Cell Endocrinol. 2000;164:59–67. doi: 10.1016/S0303-7207(00)00235-5. [DOI] [PubMed] [Google Scholar]
- Roulston A, Reinhard C, Amiri P, Williams LT. Early activation of c-Jun N-terminal kinase and p38 kinase regulate cell survival in response to tumor necrosis factor α. J Biol Chem. 1998;273:10232–10239. doi: 10.1074/jbc.273.17.10232. [DOI] [PubMed] [Google Scholar]
- Sasson R, Winder N, Kees S, Amsterdam A. Induction of apoptosis in granulosa cells by TNFα and its attenuation by glucocorticoids involve modulation of Bcl-2. Biochem Biophys Res Commun. 2002;294:51–59. doi: 10.1016/S0006-291X(02)00431-X. [DOI] [PubMed] [Google Scholar]
- Peraldi P, Hotamisligil GS, Buurman WA, White MF, Spiegelman BM. Tumor necrosis factor (TNF)-alpha inhibits insulin signaling through stimulation of the p55 TNF receptor and activation of sphingomyelinase. J Biol Chem. 1996;271:13018–13022. doi: 10.1074/jbc.271.22.13018. [DOI] [PubMed] [Google Scholar]
- Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Manabe N, Inoue N, Matsui T, Miyamoto H. Changes in the expression of tumor necrosis factor (TNF) α, TNFα receptor (TNFR) 2, and TNFR-associated factor 2 in granulosa cells during atresia in pig ovaries. Biol Reprod. 2003;68:530–535. doi: 10.1095/biolreprod.102.004820. [DOI] [PubMed] [Google Scholar]
- Foghi A, Ravandi A, Teerds KJ, Van Der Donk H, Kuksis A, Dorrington J. Fas-induced apoptosis in rat thecal/interstitial cells signals through sphingomyelin-ceramide pathway. Endocrinology. 1998;139:2041–2047. doi: 10.1210/en.139.4.2041. [DOI] [PubMed] [Google Scholar]
- Oltivai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Williams GT, Smith CA. Molecular regulation of apoptosis: genetic controls on cell death. Cell. 1993;74:777–779. doi: 10.1016/0092-8674(93)90457-2. [DOI] [PubMed] [Google Scholar]
- Sugino N, Suzuki T, Kashida S, Karube A, Takiguchi S, Kato H. Expression of Bcl-2 and Bax in the human corpus luteum during the menstrual cycle and in early pregnancy: regulation by human chorionic gonadotropin. J Clin Endocrinol Metab. 2000;85:4379–4386. doi: 10.1210/jc.85.11.4379. [DOI] [PubMed] [Google Scholar]
- Terranova PF, Hunter VJ, Roby KF, Hunt JS. Tumor necrosis factor-alpha in the female reproductive tract. Proc Soc Exp Biol Med. 1995;209:325–342. doi: 10.3181/00379727-209-43905b. [DOI] [PubMed] [Google Scholar]
- Weems YS, Lammoglia MA, Vera-Avila HR, Randel RD, Sasser RG, Weems CW. Effects of luteinizing hormone (LH), PGE2, 8-Epi-PGE1, 8-Epi-PGF2 alpha, trichosanthin and pregnancy specific protein B (PSPB) on secretion of prostaglandin (PG) E (PGE) or F2 alpha (PGF2 alpha) in vitro by corpora lutea (CL) from nonpregnant and pregnant cows. Prostaglandins other Lipid Mediat. 1998;55:359–376. doi: 10.1016/S0090-6980(98)00030-6. [DOI] [PubMed] [Google Scholar]
- Weems YS, Lammoglia MA, Vera-Avila HR, Randel RD, King C, Sasser RG, Weems CW. Effect of luteinizing hormone (LH), PGE2, 8-Epi-PGE1, 8-Epi-PGE2, trichosanthin, and pregnancy specific protein B (PSPB) on secretion of progesterone in vitro by corpora lutea (CL) from nonpregnant and pregnant cows. Prostaglandins other Lipid Mediat. 1998;55:27–42. doi: 10.1016/S0090-6980(98)00003-3. [DOI] [PubMed] [Google Scholar]
- Locksley RM, Killeen N, Lenardo ML. The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Bridgham JT, Johnson AL. Avian TVB (DR5-like) death receptor expression in hen ovarian follicles. Biochem Biophys Res Commun. 2002;291:226–232. doi: 10.1006/bbrc.2002.6429. [DOI] [PubMed] [Google Scholar]
- Wada S, Manabe N, Nakayama M, Inoue N, Matsui T, Miyamoto H. TRAIL-decoy receptor 1 plays inhibitory role in apoptosis of granulosa cells from pig ovarian follicles. J Vet Med Sci. 2002;64:435–439. doi: 10.1292/jvms.64.435. [DOI] [PubMed] [Google Scholar]