Abstract
Reactive oxygen species are known to participate in the regulation of intracellular signaling pathways, including activation of NF-κB. Recent studies have indicated that increases in intracellular concentrations of hydrogen peroxide (H2O2) have anti-inflammatory effects in neutrophils, including inhibition of the degradation of IκBα after TLR4 engagement. In the present experiments, we found that culture of lipopolysaccharide-stimulated neutrophils and HEK 293 cells with H2O2 resulted in diminished ubiquitination of IκBα and decreased SCFβ-TrCP ubiquitin ligase activity. Exposure of neutrophils or HEK 293 cells to H2O2 was associated with reduced binding between phosphorylated IκBα and SCFβ-TrCP but no change in the composition of the SCFβ-TrCP complex. Lipopolysaccharide-induced SCFβ-TrCP ubiquitin ligase activity as well as binding of β-TrCP to phosphorylated IκBα was decreased in the lungs of acatalasemic mice and mice treated with the catalase inhibitor aminotriazole, situations in which intracellular concentrations of H2O2 are increased. Exposure to H2O2 resulted in oxidative modification of cysteine residues in β-TrCP. Cysteine 308 in Blade 1 of the β-TrCP β-propeller region was found to be required for maximal binding between β-TrCP and phosphorylated IκBα. These findings suggest that the anti-inflammatory effects of H2O2 may result from its ability to decrease ubiquitination as well as subsequent degradation of IκBα through inhibiting the association between IκBα and SCFβ-TrCP.
Keywords: Immunology/LPS, Methods/Site Directed Mutagenesis, Oxygen/Reactive, Proteases/Ubiquitination, Protein/Protein-Protein interactions, Protein/Protein-Protein Interactions, Hydrogen Peroxide, Inflammation
Introduction
Reactive oxygen species (ROS)4 are generated during normal physiologic processes and participate in the maintenance of cellular homeostasis (1). However, increased production of ROS accompanies pathophysiologic conditions, such as chronic obstructive pulmonary disease, sepsis, and ischemia-reperfusion injury, that are characterized by activation of neutrophils, macrophages, and other cell populations to release cytokines and other proinflammatory mediators, many of which are under the regulatory control of the transcription factor NF-κB (2–5). Although initial reports indicated that ROS, such as superoxide and hydrogen peroxide (H2O2), exerted proinflammatory effects through activation of NF-κB, more recent studies have shown that ROS are not only responsible for inducing inflammation but also can have potent anti-inflammatory properties. In particular, increases in intracellular concentrations of H2O2 have been demonstrated to diminish TLR4 (Toll-like receptor 4)-induced activation of NF-κB and production of proinflammatory cytokines in neutrophils, epithelial cells, and other cell populations (6–8).
NF-κB p50/p65 heterodimers are retained in the cytoplasm by binding to the inhibitory molecule IκBα (inhibitor of NF-κB) (9). However, in response to external stimuli, such as engagement of TLR4, IκBα is degraded by a three-step process involving phosphorylation by IKK kinases, polyubiquitination by the SCFβ-TrCP (Skp1-cullin-F-box/β-transducin repeat-containing protein) complex, and degradation of ubiquitinated IκBα by the 26 S proteasome, thereby exposing the nuclear localization sequence in NF-κB and permitting translocation of NF-κB to the nucleus (10–12). Previous studies from our laboratory showed that exposure of LPS-stimulated neutrophils to H2O2 was associated with diminished degradation of IκBα and decreased translocation of NF-κB to the nucleus, providing a potential mechanism for the anti-inflammatory properties of H2O2. Increased intracellular concentrations of H2O2 did not affect LPS-induced activation of IKK or phosphorylation of IκBα (13). Although H2O2 has been shown to diminish 26 S proteasome activity, at least in part through oxidative modification and S-glutathionylation of the Rpn2 regulatory particle (14), the extent of stabilization of IκBα in LPS-stimulated neutrophils cultured with H2O2 was greater than that produced by treatment with MG132, a specific inhibitor of the 26 S proteasome (6). Such results suggest that H2O2 may affect additional processes, such as the ubiquitination of IκBα, which are involved in targeting IκBα for degradation by the 26 S proteasome and which contribute to the activation of NF-κB (15–17).
SCF E3 ubiquitin ligases consist of three invariable core molecule components, Skp1, cullin-1 (Cul1), and Rbx1 (also known as Roc1 or Hrt1), associated with an F-box protein and ubiquitin-conjugating enzyme (E2) (18, 19). The individual F-box protein and E2 enzyme associated with the SCF ligase complex provide substrate specificity. For ubiquitination of IκBα, the specific F-box protein and E2 bound to SCF are β-TrCP and UbcH3/Cdc34, respectively (20–22). Association of phosphorylated IκBα with the β-TrCP component of SCFβ-TrCP permits proper positioning of IκBα for polyubiquitination by UbcH3/Cdc34 (19, 23, 24).
In the present experiments, we examined the ability of H2O2 to modulate IκBα ubiquitination and SCFβ-TrCP activity after TLR4 engagement. We found that exposure to H2O2 or inhibition of catalase, a situation associated with increased intracellular concentrations of H2O2 (6, 13), resulted in diminished association of SCFβ-TrCP with IκBα as well as decreased ubiquitination of IκBα.
EXPERIMENTAL PROCEDURES
Mice
Male C57BL/6, C3HeB/FeJ, or acatalasemic C3Ga.Cg-Cat B/J mice, 8–12 weeks of age, were purchased from Jackson Laboratory (Bar Harbor, ME). The mice were kept on a 12-h/12-h light/dark cycle with free access to food and water. All experiments were conducted in accordance with institutional review board-approved protocols (University of Alabama at Birmingham Institutional Animal Care and Use Committee).
Materials
Hydrogen peroxide, lipopolysaccharide (LPS; from Escherichia coli O4:B111), 3-amino-1,2,4-triazole (ATZ), and MG132 were purchased from Sigma. Anti-cullin-1, anti-β-TrCP, and anti-c-Myc antibodies were from Zymed Laboratories Inc. (South San Francisco, CA). Rabbit anti-IκBα, mouse anti-phospho-IκBα, and mouse anti-ubiquitin antibodies were from Cell Signaling Technology (Danvers, MA). Mouse anti-β-catenin antibodies were from BD Transduction Laboratories (San Jose, CA). Goat anti-mouse IgG (H + L)-horseradish peroxidase conjugate and goat anti-rabbit IgG (H + L)-horseradish peroxidase conjugate were from Bio-Rad, whereas goat anti-mouse κ-chain-horseradish peroxidase was from SouthernBiotech (Birmingham, AL).
Neutrophil Isolation and Culture
Bone marrow neutrophils were isolated as described previously (6, 25, 26). Neutrophil purity was consistently >97%, as determined by Wright-Giemsa-stained cytospin preparations. Neutrophils were cultured in RPMI 1640 medium containing 0.5% fetal bovine serum and treated as indicated in the figure legends. Neutrophil viability as determined by trypan blue staining was consistently >95%.
Cell Culture, Transfection, and Generation of Stable Cell Lines
Human embryonic kidney cells (HEK 293) cells were maintained in RPMI 1640 (Sigma) containing 10% fetal bovine serum (Atlanta Biologics), and penicillin/streptomycin solution (1:10; Sigma). 293-hTLR4/MD2-CD14 cells, an isolated HEK 293 clone stably transfected with hTLR4, MD2, and CD14 genes (catalog number 293-htlr4-md2-cd14, Invivogen), were maintained according to the manufacturer's instructions. In experimental procedures, all treatments were performed in serum-free media as described in the figure legends. Cells were transfected using Lipofectamine 2000TM reagent. Stable cell lines overexpressing β-TrCP were generated by transfecting 293-hTLR4/MD2-CD14 cells with β-TrCP-FLAG plasmid DNA using Lipofectamine 2000TM reagent followed by G418 (Sigma) selection.
Acute Lung Injury Model
Acute lung injury was induced by intratracheal administration of 1 mg/kg LPS in 50 μl of phosphate-buffered saline as described previously (8, 13, 27, 28). Briefly, mice were anesthetized with isoflurane and then suspended by their upper incisors on a 60° incline board. The tongue was then gently extended, and LPS solution was deposited into the pharynx (8, 25, 29). Mice were pretreated with saline or ATZ (500 mg/kg body weight dissolved in 0.9% saline) intraperitoneally, and 4 h later, LPS (1 mg/kg) was administered intratracheally. Lungs were harvested 24 h after LPS administration.
Construction of Expression Plasmids and Recombinant Protein Expression
A full-length human β-TrCP cDNA was purchased from Open Biosystems and cloned into 3×FLAG-CMV10 (Sigma) for mammalian expression. Four FLAG-tagged point mutant constructs of β-TrCP-C308A (MB1), C348A (MB2), C471A (MB5), and C511A (MB6) were generated using PCR mutagenesis. An IKKβ cDNA containing N-terminal amino acids 1–420 was obtained from Open Biosystems and cloned into 3×FLAG-CMV10. Full-length human Roc1, Skp1, and UBCH3/Cdc34 (Open Biosystems) were cloned into pcDNA-Myc vector for mammalian expression as Myc-tagged proteins. A full-length cDNA for β-catenin was purchased from Open Biosystems. The IκBα construct in pET15b was kindly provided by Dr. Gourisankar Ghosh (University of California, San Diego, La Jolla, CA). IκBα and β-catenin were cloned into pGEX vector (GE Healthcare) for bacterial expression as N-terminal GST fusion proteins. GST-tagged recombinant proteins were purified using glutathione-Sepharose (GE Healthcare).
In Vitro Phosphorylation of IκBα and β-Catenin
Phosphorylation of IκBα or β-catenin was performed using 2 μg of GST-tagged substrate protein, 50 ng of IKKβ (Cell Signaling, Danvers, MA), or GSK3β (SignalChem, Richmond, Canada), in 50 μl of 1× kinase buffer (Cell Signaling) and 2 mm ATP for 1 h at room temperature. The phosphorylated products were stored at −80 °C until used.
In Vitro Ubiquitination Assay
Cultured cells and neutrophils were lysed, or lungs of mice were homogenized in lysis buffer consisting of 50 mm Tris, pH 8.0, 5 mm EDTA, 150 mm NaCl, 10 mm NaF, 2 mm Na3VO4, protease inhibitor mixture (1:100, v/v) (Sigma), and 0.5% Nonidet P-40. Protein concentrations were determined using Bradford's reagent (Bio-Rad). To immunoprecipitate SCFβ-TrCP, 1 mg of cell lysates or lung homogenates was incubated with anti-cullin-1 mouse monoclonal antibody in 1 ml of lysis buffer containing 5% glycerol for 2 h, followed by the addition of 30 μl of Protein A-Sepharose beads (Sigma) and incubation overnight at 4 °C with continuous stirring. The beads were then washed three times with lysis buffer and twice with ligase buffer (50 mm Tris, pH 7.5, 25 mm MgCl2, 2 mm Na3VO4, 10 mm NaF). Ubiquitination was performed with the washed beads resuspended in 30 μl of solution containing 100 ng of E1-GST (Boston Biochem, Cambridge, MA), 500 ng of His-tagged UbcH3 (Boston Biochem), 1 μg of ubiquitin-FLAG (BioMol, Plymouth Meeting, PA), Energy Regeneration Solution (Boston Biochem) (a mixture that contains MgCl2, ATP, and ATP-regenerating enzymes to recycle hydrolyzed ATP (i.e. AMP and ADP to ATP)), 100 μm ATP, and 200 ng of phosphorylated substrate for 1 h at 30 °C. The reaction was then stopped by adding SDS-PAGE loading buffer, followed by boiling the samples for 15 min, and resolution on 6% SDS-PAGE. Ubiquitination of substrates was detected by either anti-ubiquitin antibodies or antibodies to IκBα or β-catenin.
GST Pull-down of β-TrCP Using Phospho-GST-IκBα or Phospho-GST-β-Catenin
Phosphorylated GST-IκBα or phosphorylated GST-β-catenin (500 ng) was added to 1 mg of protein obtained from cell lysates or lung extracts in 1 ml of lysis buffer containing 5% glycerol. Glutathione-Sepharose beads were added, and the mixture was incubated at 4 °C with continuous stirring for 1 h. The beads were washed three times with lysis buffer, and IκBα- or β-catenin-bound β-TrCP was analyzed by immunoblotting with specific antibodies to FLAG or β-TrCP.
Imaging of DCF Fluorescence
Intracellular levels of ROS, including hydrogen peroxide, were measured using the redox-sensitive probe DCFH-DA (30) in conjunction with fluorescent microscopy (1, 6, 8, 31–33). Briefly, neutrophils, HEK 293 cells, or 293-hTLR4/MD2-CD14 cells (∼80% confluent) were incubated in a 4-well chambered coverglass (Nalge; Naperville, IL) with dichlorofluorescein diacetate (10 μm) for 60 min, followed by treatment with various concentrations of H2O2 or LPS at 37 °C. Images were acquired by single bidirectional scans of live cells using a Leica DMIRBE inverted epifluorescence/Nomarski microscope outfitted with Leica TCS NT laser confocal optics. The pinhole setting was 0.2 Airy units, and laser excitation was set for 5% to avoid dye photo-oxidation. The levels of fluorescence were averaged using SimplePCI software (Compix; Cranberry Township, PA). Images were processed using IPLab Spectrum and Adobe Photoshop (Adobe Systems) software.
Cytokine Enzyme-linked Immunosorbent Assay
Levels of tumor necrosis factor-α from neutrophils into culture media were determined using commercially available enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, MN), according to the manufacturer's instructions and as previously described (7, 8, 13, 34).
Measurement of Proteasome Activity
26 S proteasomal chymotrypsin-like and trypsin-like activity was measured in HEK 293 cells using the fluorogenic peptide substrate succinyl-Leu-Leu-Val-Tyr-7-amido-4-methylcoumarin or t-butoxycarbonyl-Leu-Arg-Arg-7-amido-4-methylcoumarin, respectively, as previously described (13, 35–38).
Labeling of β-TrCP Free Cysteine Thiols
The extent of free (unoxidized) cysteine residues within β-TrCP was determined using the biotinylated iodoacetyl ethylenediamine (BIAM)-labeling assay (14, 39–42). Briefly, cell lysates (0.5 mg/ml) obtained from HEK 293 cells that transiently expressed β-TrCP-FLAG were incubated with BIAM (100 μm) for 30 min at room temperature, and then excess BIAM was removed by passing the extracts through Bio-Gel P10. Next, BIAM-protein conjugates were precipitated with streptavidin-agarose for 1 h at 4 °C. Samples were washed four times with lysis buffer containing 0.05% SDS and then subjected to reducing SDS-PAGE and Western blot analysis with antibodies to β-TrCP or FLAG peptide. Cells were also directly incubated with BIAM (200 μm) for 20 min, and then cell lysates were passed through Bio-Gel P10 to remove excess BIAM. The amount of BIAM-β-TrCP adduct formation was determined using streptavidin-agarose pull-down followed by Western blot analysis with antibodies specific to β-TrCP or FLAG peptide.
Statistical Analyses
For each experiment, neutrophils were isolated and pooled from groups of mice (n = 3–4), and all conditions were studied at the same time. Data are presented as means ± S.D. for each experimental group. One-way analysis of variance, the Tukey-Kramer multiple comparison test (for multiple groups), or Student's t test (for comparisons between two groups) were used. p < 0.05 was considered significant.
RESULTS
Inhibitory Effects of Hydrogen Peroxide on TLR4-induced IκBα Degradation
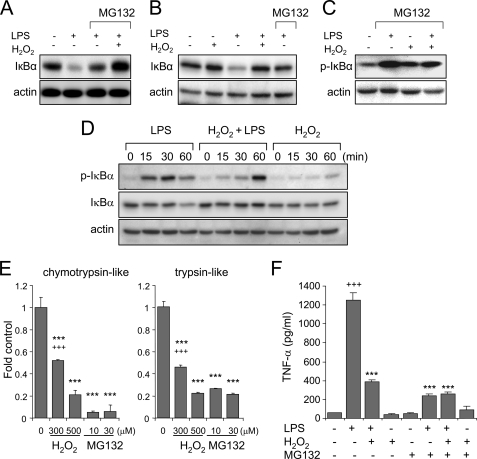
In recent studies, we demonstrated that increased intracellular levels of H2O2 were associated with diminished degradation of IκBα and reduced 26 S proteasomal activity in LPS-stimulated neutrophils (6, 8). As shown in Fig. 1, A–C, whereas exposure of LPS-stimulated neutrophils or 293-hTLR4/MD2-CD14 cells to H2O2 resulted in diminished degradation of IκBα, the extent of IκBα stabilization in H2O2-treated cells was greater than that found after blockade of 26 S proteasomal function with MG132 in the absence of H2O2. These results suggested that the mechanisms by which H2O2 prevents IκBα degradation may extend beyond proteasomal inhibition.
FIGURE 1.
Effects of H2O2 and 26 S proteasomal inhibition on IκBα degradation and cytokine production in LPS-stimulated cells. A–C, neutrophils or 293-hTLR4/MD2-CD14 cells were cultured with H2O2 (0 or 300 μm) for 5 min and then with LPS (0 or 100 ng/ml) for an additional 60 min. In specified experiments, cells were preincubated with the 26 S proteasomal inhibitor MG132 (10 μm) for 1 h before the addition of H2O2 and LPS to the cultures. Representative Western blots show the levels of IκBα in neutrophils (A) as well as IκBα (B) and phospho-IκBα (C) in 293-hTLR4/MD2-CD14 cells. D, neutrophils were cultured with LPS or H2O2 or the combination of H2O2 and LPS for the indicated time period. Western blot analysis of phospho-Ser-32/36IκBα, total IκBα, and actin is shown. E, proteasomal chymotrypsin-like and trypsin-like activity was measured in neutrophils incubated with MG132 (0 or 10 μm) or H2O2 (0, 300, or 500 μm) for 60 min. Values are means ± S.D. (***, p < 0.001 compared with untreated cells; +++, p < 0.001 comparing treatment with H2O2 (300 μm) with MG132 (10 or 30 μm)). F, levels of tumor necrosis factor-α were measured in cell culture supernatants from neutrophils cultured with LPS, H2O2, or H2O2 and LPS for 5 h. In specified experiments, cells were preincubated with MG132 (0 or 10 μm) for 1 h before LPS or H2O2 treatment (means ± S.D.; n = 3; +++, p < 0.001 comparing LPS with untreated; ***, p < 0.001 comparing LPS treatment with cells incubated with either LPS and H2O2 or MG132).
Because phosphorylation of IκBα is required for its polyubiquitination and subsequent degradation by the 26 S proteasome, we hypothesized that a mechanism by which H2O2 might diminish TLR4-induced degradation of IκBα was through inhibiting IκBα phosphorylation. To examine this possibility, 293-hTLR4/MD2-CD14 cells were stimulated with LPS in the presence or absence of H2O2. As shown in Fig. 1C, H2O2 exposure appeared to have no effect on LPS-induced phosphorylation of IκBα. Although exposure to LPS or LPS and H2O2 both resulted in enhanced phosphorylation of IκBα, the amount of total IκBα decreased in LPS-treated cells but not in those treated with both LPS and H2O2 (Fig. 1D). Next, we examined the effects of H2O2 and MG132 on 26 S proteasomal activity. As shown in Fig. 1E, incubation of HEK 293 cells with MG132 resulted in inhibition of trypsin-like and chymotrypsin-like proteasomal activity; such inhibition of 26 S proteasomal function was more pronounced in cells treated with MG132 as compared with that found in cells treated with H2O2 alone (Fig. 1E). As shown in Fig. 1F, preincubation with H2O2 or MG-132 had inhibitory effects on the release of tumor necrosis factor-α from LPS-stimulated neutrophils.
The experiments shown in Fig. 1, A–D, indicate that although H2O2 appeared to be a less potent inhibitor of 26 S proteasomal activity than was MG132, exposure of LPS-treated cells to H2O2 appeared to stabilize IκBα levels to a greater extent than did MG132. These results suggest that in addition to its inhibitory actions on the 26 S proteasome, H2O2 also may affect upstream events that result in IκBα stabilization, such as through modulating IκBα ubiquitination.
Effects of H2O2 on IκBα Ubiquitination by SCFβ-TrCP
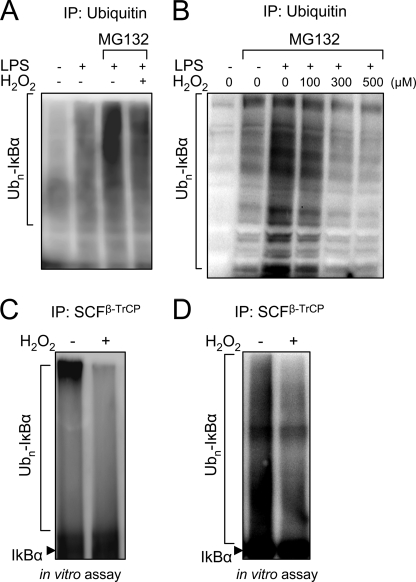
Ubiquitination of IκBα occurs after TLR4 engagement (43) and is required for the processing and degradation of IκBα by the 26 S proteasome. As shown in Fig. 2, A and B, exposure of neutrophils or 293-hTLR4/MD2-CD14 cells to LPS resulted in increased ubiquitination of IκBα, an effect that was enhanced by blockade of 26 S proteasomal activity following the addition of the proteasomal inhibitor MG132 to the cultures. In contrast, LPS-induced ubiquitination of IκBα was diminished in a concentration-dependent manner after exposure of cells to H2O2.
FIGURE 2.
Exposure to H2O2 diminishes SCFβ-TrCP-dependent ubiquitination of IκBα. A and B, levels of ubiquitinated IκBα (Ubn-IκBα) were determined in neutrophils (A) or in 293-hTLR4/MD2-CD14 cells that transiently expressed Myc-tagged IκBα, IKKβ-FLAG, or Ub-HA. In B, cells were treated with combinations of H2O2, LPS, and MG132 as indicated, and cell extracts were subjected to immunoprecipitation (IP) with anti-ubiquitin antibodies. Representative Western blots show the amounts of Ubn-IκBα conjugates detected with antibodies specific for phospho-IκBα. Two additional experiments provided similar results. C and D, neutrophils (C) or 293-hTLR4/MD2-CD14 cells stably expressing β-TrCP-FLAG (D) were cultured with H2O2 (0 or 300 μm) for 60 min, and then the SCFβ-TrCP complex was immunoprecipitated from cell extracts using anti-cullin-1 antibodies, followed by determination of SCFβ-TrCP-dependent ubiquitination of phosphorylated recombinant IκBα. Representative Western blots show levels of Ubn-IκBα as detected with anti-phospho-IκBα antibodies. A second experiment provided similar results.
The E3 ubiquitin ligase SCFβ-TrCP is responsible for the ubiquitination of IκBα (15, 44, 45). Therefore, given the diminished ubiquitination of IκBα in H2O2-exposed cells, it seemed possible that H2O2 might affect SCFβ-TrCP activity. As shown in Fig. 2, C and D, SCFβ-TrCP isolated from neutrophils or 293-hTLR4/MD2-CD14 cells cultured with H2O2 was less able to ubiquitinate IκBα than was SCFβ-TrCP purified from cells that had not been exposed to H2O2.
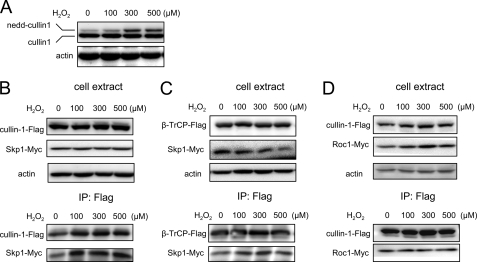
In initial experiments to determine the mechanism for the inhibitory actions of H2O2 on SCFβ-TrCP ubiquitin ligase activity, we examined the effect of H2O2 on cullin-1 neddylation, a process that has been shown to result in enhanced SCFβ-TrCP activity (45). Despite the reduction of SCFβ-TrCP activity in H2O2-exposed cells, incubation of HEK 293 cells with H2O2 resulted in increased cullin-1 neddylation (Fig. 3A). Such results indicate that the ability of H2O2 to diminish SCFβ-TrCP activity was not due to inhibition of cullin-1 neddylation.
FIGURE 3.
Effects of H2O2 on SCFβ-TrCP complex composition. A, HEK 293 cells were treated with H2O2 (0, 100, 300, and 500 μm) for 1 h. Representative Western blot analysis with anti-cullin-1 antibody shows the levels of endogenous cullin-1 and neddylated cullin-1 (nedd-cullin 1). B–D, HEK 293 cells transiently expressing β-TrCP-FLAG and Skp1-Myc, cullin-1-FLAG and Skp1-Myc, or cullin-1-FLAG and Roc1-Myc were treated with H2O2 (0, 100, 300, and 500 μm) for 1 h. Western blots show the amounts of SCFβ-TrCP components before (cell extract) or after immunoprecipitation with anti-FLAG-agarose beads (IP: Flag). After immunoprecipitation, the amounts of β-TrCP-FLAG associated with Skp1-Myc (B) or of cullin-1-FLAG associated with Skp1-Myc (C) or of cullin-1-FLAG associated with Roc1-Myc (D) were determined by Western blot analysis with anti-Myc antibodies. A second experiment provided similar results.
Because the H2O2-induced decrease in SCFβ-TrCP ubiquitin ligase activity might be due to alterations in the composition of the SCFβ-TrCP complex, we determined binding of β-TrCP to Skp1, cullin-1 to Skp1, and cullin-1 to Roc1 in HEK 293 cells that were transfected with β-TrCP-FLAG and Skp1-Myc, cullin-1-FLAG and Skp1-Myc, or cullin-1-Flag and Roc 1-Myc and then exposed or not to increasing concentrations of H2O2. As shown in Fig. 3, B, C, and D, H2O2 did not appear to affect binding of Skp1 to cullin-1, β-TrCP to Skp1, or cullin-1 to Roc1. These data suggest that alterations in the composition of SCFβ-TrCP are not responsible for the ability of H2O2 to inhibit its ubiquitin ligase activity.
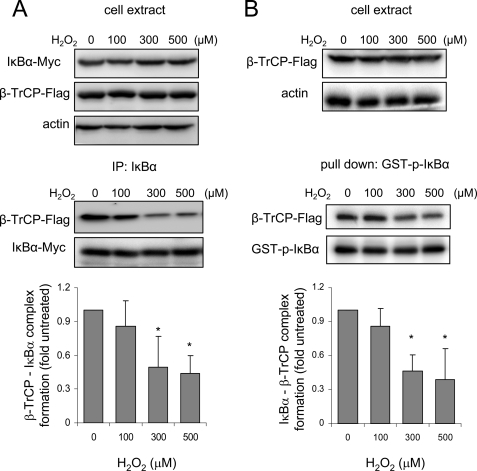
Because H2O2 does not affect SCFβ-TrCP complex formation, we next examined the effect of H2O2 on the binding affinity of SCFβ-TrCP to phosphorylated IκBα. Within the SCFβ-TrCP complex, the F-box protein β-TrCP is responsible for substrate acquisition, including binding to phosphorylated IκBα and β-catenin (18, 24). For these experiments, we transfected 293-hTLR4/MD2-CD14 cells with IκBα-Myc, β-TrCP-FLAG, and IKKβ-FLAG constructs and then treated the cells with increasing doses of H2O2 followed by immunoprecipitation of IκBα. As shown in Fig. 4A, incubation of the 293-hTLR4/MD2-CD14 cells with H2O2 diminished binding of IκBα to β-TrCP in LPS-stimulated cells. Similar results were found after cell extracts from 293-hTLR4/MD2-CD14 cells stably expressing β-TrCP-FLAG were incubated with phosphorylated IκBα tagged with GST (Fig. 4B).
FIGURE 4.
H2O2 diminishes SCFβ-TrCP-dependent binding to IκBα. A, 293-hTLR4/MD2-CD14 cells transiently expressing β-TrCP-FLAG, IκBα-Myc, and IKKβ-FLAG were treated with combinations of H2O2, LPS, and MG132 as noted in the figure. The amounts of IκBα associated with β-TrCP were determined after pull-down with anti-IκBα antibodies (IP: IκBα) with Western blot analysis using anti-FLAG antibodies. Representative Western blots show the level of IκBα, β-TrCP-FLAG, and actin before (cell extract) or after IκBα immunoprecipitation (IP: IκBα). B, 293-hTLR4/MD2-CD14 cells stably expressing β-TrCP-FLAG were treated with H2O2 (0, 100, 300, and 500 μm) for 1 h, and then the cell extracts were incubated with phosphorylated GST-IκBα, followed by pull-down with glutathione-Sepharose. Western blots show the amount of β-TrCP-FLAG bound to GST-IκBα (pull down) and amounts of GST-IκBα and β-TrCP-FLAG in the cell extracts before pull-down. The optical band density (means ± S.D.) obtained from three independent experiments (A and B) is provided (*, p < 0.05, comparing the amount of IκBα associated with β-TrCP in cells incubated with 300 or 500 μm H2O2 with that found in untreated cells).
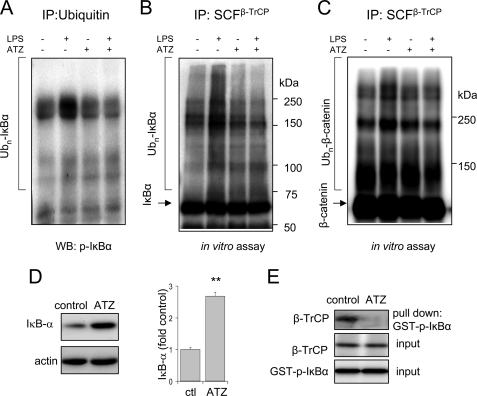
Enhanced Intracellular H2O2 Concentrations in Vivo Are Associated with Diminished SCFβ-TrCP Activity and Association with IκBα
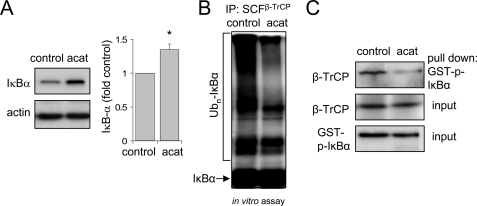
Intracellular concentrations of H2O2 are increased in acatalasemic mice and in mice treated with ATZ, a specific inhibitor of catalase (13). These conditions therefore permit exploration of the effects of increased intracellular H2O2 on SCFβ-TrCP activity under in vivo conditions. As shown in Figs. 5 and 6, SCFβ-TrCP isolated from the lungs of acatalasemic mice or mice treated with ATZ is less able to ubiquitinate IκBα and also demonstrates diminished binding to phosphorylated IκBα as compared with SCFβ-TrCP from control mice (Figs. 5B and 6D). In addition, intratracheal administration of LPS resulted in enhanced ubiquitination of IκBα in the lungs of control mice but not in the lungs of mice treated with ATZ before LPS exposure (Fig. 6A). Although SCFβ-TrCP isolated from the lungs of LPS-exposed mice showed increased ability to ubiquitinate either phosphorylated IκBα or phosphorylated β-catenin, there was no increase in ubiquitin ligase activity of SCFβ-TrCP that had been purified from mice treated with ATZ before LPS exposure (Fig. 6, B and C). These results are consistent with the ability of increased intracellular H2O2 levels to inhibit SCFβ-TrCP activity and decrease IκBα turnover even under basal conditions (Figs. 5A and 6D).
FIGURE 5.
SCFβ-TrCP activity is diminished in the lungs of acatalasemic mice. A and B, representative Western blots and mean optical band density show the levels of IκBα obtained from lung homogenates of control C3HeB/FeJ (control) or C3Ga.Cg-Cat B/J (acat) mice (means ± S.D., n = 3 per each group of mice; *, p < 0.05). C and D, SCFβ-TrCP complex was immunoprecipitated (IP) from lung homogenates of control or acatalasemic mice using anti-cullin-1 antibodies followed by measurement of IκBα ubiquitination in vitro. Representative Western blots using anti-phospho-IκBα antibodies show the amounts of Ubn-IκBα (C). In C, lung extracts from control and acatalasemic mice were incubated with recombinant phospho-IκBα-GST followed by pull-down with glutathione-Sepharose and Western blotting with anti-β-TrCP antibodies. The amounts of β-TrCP in the lung extracts as well as recombinant phospho-IκBα-GST added to lung extracts (input) are shown. Input indicates the levels of β-TrCP in lung homogenates obtained from control or acatalasemic mice. Similar results were obtained from three independent experiments.
FIGURE 6.
SCFβ-TrCP activity is diminished in the lungs of mice treated with aminotriazole. A–E, C57BL/6 mice were treated intraperitoneally with saline or the catalase inhibitor ATZ and then 4 h later were given LPS (1 mg/kg) intratracheally. Lung homogenates were obtained 2 h after LPS administration. In A, lung homogenates were subjected to immunoprecipitation (IP) with anti-Ub antibodies, and Ubn-IκBα conjugates were determined using Western blots (WB) developed with anti-phospho-IκBα antibodies. B and C, SCFβ-TrCP complexes were immunoprecipitated from lung homogenates of control or ATZ-treated mice with anti-cullin-1 antibodies followed by determination of the ability of the precipitated SCFβ-TrCP to ubiquitinate phospho-GST-IκBα or phospho-β-catenin in vitro. D, representative Western blots are shown as well as mean optical band density of the levels of IκBα obtained from lung homogenates of control and mice treated with ATZ (means ± S.D., n = 3 per each group of mice, **p < 0.01). E, lung homogenates were incubated with recombinant phospho-IκBα-GST and then precipitated with glutathione-Sepharose. The amounts of β-TrCP in the lung extracts as well as recombinant phospho-IκBα-GST added to lung extracts (input) are shown. Representative Western blots are shown. Two additional experiments provided similar results.
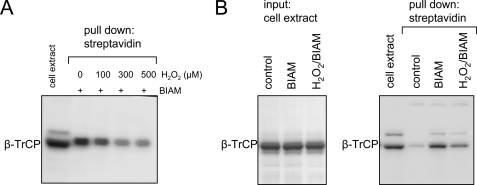
Cysteine Thiols of β-TrCP Undergo Oxidative Modification after Exposure to H2O2
Reactive oxygen species (and particularly H2O2) can affect intracellular signaling through posttranslational protein modification and particularly through oxidation of cysteine thiols (1, 42, 46, 47). To determine if cellular exposure to H2O2 produces modification of cysteines in β-TrCP, we labeled free, unmodified cysteine residues by the addition of BIAM to cells incubated with increasing concentrations of H2O2. As shown Fig. 7A, the number of unaltered cysteines in β-TrCP available for BIAM-cysteine adduct formation was decreased in a dose-dependent manner by cellular incubation with H2O2, a situation associated with increased intracellular concentrations of H2O2 and other reactive oxygen species (supplemental Fig. 1). Similarly, incubation of cell extracts with H2O2 resulted in diminished BIAM association with β-TrCP, consistent with H2O2-dependent oxidative modification of cysteine residues in β-TrCP (Fig. 7B).
FIGURE 7.
Exposure to H2O2 induces oxidation of β-TrCP cysteine thiols. A, cell extracts obtained from HEK 293 cells transiently expressing β-TrCP were incubated with H2O2 and BIAM. BIAM adduct formation in β-TrCP was determined by pull-down with streptavidin-agarose followed by Western blotting with β-TrCP antibody. B, HEK 293 cells transiently expressing β-TrCP were treated with H2O2 (300 μm) for 20 min followed by culture with BIAM (200 μm) for an additional 20 min. A representative Western blot shows the amount of total β-TrCP in the cell extract (left; input) and the BIAM-β-TrCP adduct formation obtained after precipitation with streptavidin-agarose (right; pull down). A second experiment provided similar results.
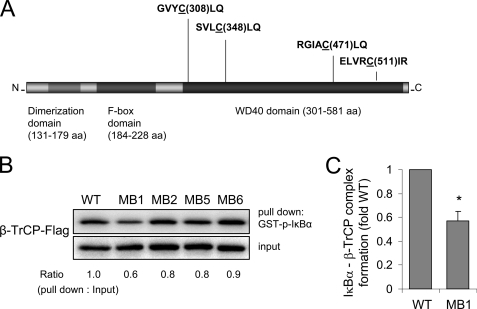
Cysteine 308 in the Blade 1 β-TrCP β-Propeller Region Is Required for Binding between β-TrCP and Phosphorylated IκBα
β-TrCP contains a total of 25 cysteine residues. The binding site of β-TrCP to phosphorylated substrates, including phosphorylated IκBα, has been localized to the top face of the WD40 domain of β-TrCP (19, 48–50). There are a total of 12 cysteines in the seven blades of the β-TrCP WD40 β-propeller region that are likely to interact with phosphorylated substrates, including phosphorylated IκBα; of these 12 cysteines, only four are likely to exist as thiolate anions as a result of being close to positively charged amino acids (i.e. Cys-Leu in Blade 1, Leu-Cys-Leu in Blade 2, Arg-Cys-Leu in Blade 5, and Arg-Cys-X-Arg in Blade 6) (Fig. 8A). These four cysteines in Blades 1, 2, 5, and 6 are therefore potentially more vulnerable to oxidation by H2O2 and were targets for site-directed mutagenesis in β-TrCP. In these experiments, Cys → Ala point mutations in β-TrCP were generated in the targeted cysteines in Blades 1, 2, 5, and 6, and then the FLAG-tagged mutants or wild type β-TrCP were expressed in HEK 293 cells.
FIGURE 8.
Cys-308 in β-TrCP is involved in the binding of phosphorylated IκBα. A, schematic diagram of the human β-TrCP gene showing the three primary domains: dimerization domain, F-box domain, and substrate binding WD40 domain (β-propeller region). Partial amino acid sequence of Blade 1, Blade 2, Blade 5, and Blade 6 is shown, and the cysteines targeted for mutagenesis are underlined. B, a representative Western blot (bottom) shows the concentrations of FLAG-tagged wild type β-TrCP or mutant β-TrCP with cysteine to alanine changes, specifically C308A (MB1), C348A (MB2), C478A (MB5), or C511A (MB6) transiently expressed in HEK 293 cells (input). Cell extracts were incubated with recombinant phospho-IκBα-GST and then subjected to glutathione-Sepharose precipitation (pull down). The amounts of β-TrCP associated with GST-phospho-IκBα were determined by Western blot analysis with anti-FLAG antibodies (top). Two additional experiments provided similar results. Mean optical density for IκBα associated with β-TrCP (wild type (WT)) or β-TrCP MB1 is shown in C (means ± S.D.; n = 3; *, p < 0.05). aa, amino acids; WT, wild type.
As shown in Fig. 8B, mutation of cysteine 308 in Blade 1 of β-TrCP to alanine reduces binding of phosphorylated IκBα with β-TrCP, whereas mutation of cysteine 348 in Blade 2, cysteine 471 in Blade 5, or cysteine 511 in Blade 6 in the WD40 domain of β-TrCP did not affect binding between β-TrCP and phosphorylated IκBα. These results demonstrate that cysteine 308 in Blade 1 of β-TrCP participates in binding between β-TrCP and phosphorylated IκBα. Of note, whereas exposure of cells to H2O2 increased intracellular oxidation of DCF-DA, an indicator of intracellular ROS formation, transient expression or β-TrCP or exposure to LPS had no effects on DCF-DA oxidation (supplemental Fig. 1).
DISCUSSION
Previous studies have shown that increased intracellular concentrations of H2O2 exert anti-inflammatory effects on TLR4 induced neutrophil activation (51, 52). Exposure of neutrophils to H2O2 stabilizes cytoplasmic concentrations of IκBα, both in resting cells and after TLR4 engagement, and also diminishes LPS-induced nuclear translocation of NF-κB (6–8, 13). Although initial experiments with HeLa cells suggested that exposure to H2O2 enhanced nuclear translocation of NF-κB, subsequent studies (51, 53–56) in other cell populations found the opposite effect, with H2O2 inhibiting NF-κB activation. Such disparate findings suggested that the role of H2O2 in affecting pathways relating to the activation of NF-κB is likely to be cell type-specific. Several potential mechanisms for the ability of H2O2 to inhibit degradation of IκBα and to diminish activation of NF-κB have been proposed (6, 13, 57–59). Phosphorylation of serine 32 and 36 in IκBα by the IKK complex is required to initiate the subsequent ubiquitination and degradation of IκBα in the 26 S proteasome (60–62). Although previous studies in C10 and aortic smooth muscle cells demonstrated that H2O2 treatment resulted in inhibition of IKKβ (52), we did not find any effects of H2O2 exposure on IKK activity in neutrophils (7, 51). Similarly, the present studies did not demonstrate any alterations in the phosphorylation of IκBα after incubation of 293-hTLR4/MD2-CD14 cells with H2O2.
Our laboratory and others have demonstrated that increased intracellular levels of H2O2 result in inhibition of 26 S proteasomal function in neutrophils or other cell populations (6, 63). If inhibition of the 26 S proteasome was the primary mechanism leading to stabilization of intracellular levels of IκBα in H2O2-treated cells, then one would also expect to find increased concentrations of polyubiquitinated IκBα after H2O2 exposure. However, as shown in the present experiments, incubation of LPS-stimulated neutrophils or 293-hTLR4/MD2-CD14 cells with H2O2 resulted in diminished ubiquitination of IκBα, consistent with inhibition of ubiquitin ligase activity. Our studies also found that exposure of neutrophils or HEK 293 cells to H2O2 resulted in inhibition of the activity of SCFβ-TrCP, the specific E3 ubiquitin ligase responsible for ubiquitination of IκBα, thereby providing a mechanism that may explain the ability of increased intracellular concentrations of H2O2 to prevent degradation of IκBα, activation of NF-κB, and expression of NF-κB proinflammatory genes, such as tumor necrosis factor-α, after TLR4 engagement (Fig. 9).
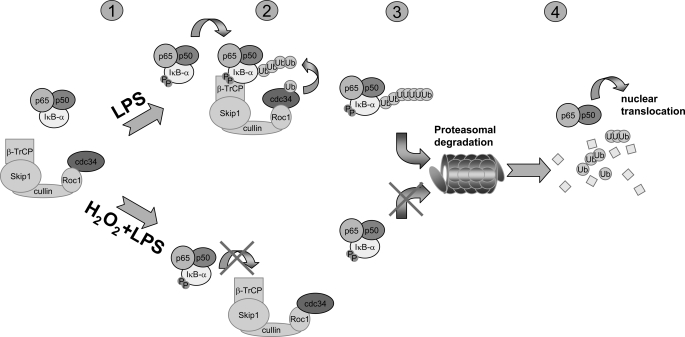
FIGURE 9.
Proposed mechanism for the ability of H2O2 to inhibit LPS-induced IκBα degradation and NF-κB activation. 1, in unstimulated cells, IκBα is bound to the p50/p65 NF-κB heterodimeric complex, thereby preventing nuclear translocation of NF-κB. 2, engagement of TLR4 by LPS leads to IKK-dependent phosphorylation of IκBα that then associates with and is ubiquitinated by the SCFβ-TrCP complex. Ubiquitinated IκBα is targeted for degradation by the 26 S proteasome (3), followed by nuclear translocation of NF-κB and enhanced transcription of NF-κB-dependent genes (4). Increases in the intracellular level of H2O2 suppress ubiquitination of phosphorylated IκBα through inhibiting association with β-TrCP and the SCFβ-TrCP complex (2) and also inhibits degradation of ubiquitinated IκBα by diminishing activity of the 26 S proteasome (3). Inhibition of IκBα ubiquitination and proteasomal degradation results in maintenance of cytoplasmic levels of IκBα and prevention of nuclear translocation of NF-κB.
Ubiquitination of target proteins is a multistep process. After activation by an E1 enzyme, ubiquitin is transferred to an active cystine of a ubiquitin-conjugating enzyme (E2). A ubiquitin ligase (E3) then transfers ubiquitin from the E2 ubiquitin-conjugating enzyme to the target protein either by forming an E3-ubiquitin thioester intermediate in the case of HECT E3 ubiquitin ligases or by facilitating the transfer of ubiquitin directly from the E2 to the substrate for RING finger E3 ubiquitin ligases (60, 61). SCFβ-TrCP, a RING finger E3, appears to be specific for ubiquitination of phosphorylated IκBα as well as for phosphorylated β-catenin (11, 24, 64, 65). SCFβ-TrCP includes several structural and functional components: β-TrCP, a F-box protein that binds to phosphorylated IκBα and β-catenin; the adapter protein SKP1, which binds to β-TrCP as well as the NH3-terminal region of cullin-1; and the RING finger protein Rbx1/Roc1/Hrt1, which binds to the COOH-terminal region of cullin-1 and also recruits the E2 enzyme UbcH3/Cdc34 that ubiquitinates phosphorylated IκBα and phosphorylated β-catenin (19, 48–50).
In the present experiments, we found that exposure of 293-hTLR4/MD2-CD14 cells to H2O2 was associated with diminished activity of SCFβ-TrCP. Because phosphorylated IκBα was used in ubiquitination assays containing purified E1 and E2, the decreased ubiquitination of IκBα found with SCFβ-TrCP immunoprecipitated from H2O2-treated cells must be due to decreased ubiquitin ligase activity of SCFβ-TrCP and not to diminished phosphorylation of the substrate, as might occur through oxidation-induced changes in IκBα or inhibition of IKK or to inactivation of the E1 or E2 enzymes through modification of cysteine or other amino acids by H2O2.
Neddylation of cullin-1 is involved in SCFβ-TrCP complex assembly and enhances its ubiquitin ligase activity (45, 66). A previous study showed that H2O2 can diminish SCFβ-TrCP ubiquitin ligase activity by inhibiting the neddylation of cullin-1 (67). Contrary to these previous findings, we found that cullin-1 neddylation is not affected in H2O2-treated cells although such conditions result in decreased activity of SCFβ-TrCP. Such results indicate that the inhibitory effects of H2O2 on SCFβ-TrCP activity are not due to modulation of cullin-1 neddylation.
A potential mechanism for the inhibitory effect of H2O2 on IκBα ubiquitination may be through alteration in SCFβ-TrCP complex formation. The results of the present experiments indicate that H2O2 exposure does not affect SCFβ-TrCP complex composition but does result in diminished binding between SCFβ-TrCP and phosphorylated IκBα. Because binding of phosphorylated IκBα to β-TrCP is required for ubiquitination of IκBα by the SCFβ-TrCP complex, the H2O2-induced inhibition of interaction between IκBα and SCFβ-TrCP provides a potential mechanism for the ability of H2O2 to inhibit IκBα ubiquitination. Because cysteine thiol modification is a common mechanism by which H2O2 modulates protein function (46, 68), it is possible that cysteines in β-TrCP that are critical for binding IκBα are oxidized in H2O2-exposed cells. In these studies, exposure of β-TrCP to H2O2 resulted in diminished BIAM adduct formation, consistent with oxidative modification of cysteine residues in β-TrCP.
Using site-directed mutagenesis of specific cysteines in the β-TrCP WD40 region that are likely to interact with phosphorylated IκBα, we found that Cys-308 in Blade 1 of the β-TrCP β-propeller region is required for optimal binding between phosphorylated IκBα and β-TrCP and is likely to be involved in the H2O2-induced reduction in binding between SCFβ-TrCP and phosphorylated IκBα. These results are consistent with the previously reported findings that alkylation of cysteine thiols of β-TrCP by N-ethylmaleimide significantly diminished ubiquitination of IκBα, whereas alkylation of cullin-1 and Roc1 with N-ethylmaleimide had no effect on IκBα ubiquitination (20).
Exposure of the lungs to LPS during Gram-negative pneumonia, during systemic Gram-negative infections, or through environmental factors produces acute inflammatory changes associated with neutrophil migration into the pulmonary parenchyma and airways, release of reactive oxygen species and proinflammatory cytokines, and the development of lung injury (69–71). Catalase facilitates the conversion of H2O2 to H2O and O2, and intracellular H2O2 concentrations are increased in neutrophils from acatalasemic mice or mice treated with aminotriazole, an inhibitor of catalase, reflecting the importance of catalase in regulating oxidant balance. There is diminished severity of LPS-induced pulmonary inflammation and lung injury in mice that are acatalasemic or treated with aminotriazole (13). The present experiments, showing diminished SCFβ-TrCP ubiquitin ligase activity in the lungs of aminotriazole-treated mice and of acatalasemic mice, provide a potential mechanism for the protective effects of catalase inhibition or its absence on LPS-associated lung injury. In particular, similar to the findings in H2O2-treated HEK 293 cells, there was decreased binding between phosphorylated IκBα and SCFβ-TrCP immunoprecipitated from the lungs of aminotriazole-treated and acatalasemic mice as well as diminished ability of SCFβ-TrCP to ubiquitinate phosphorylated IκBα or a second substrate, phosphorylated β-catenin. These results show that the inhibitory effects of H2O2 on SCFβ-TrCP ubiquitin ligase activity are not specific for IκBα but rather reflect a generic decrease of its ability to associate with and ubiquitinate its substrates.
The present findings, showing that H2O2 inhibits SCFβ-TrCP ubiquitin ligase activity through diminishing association with its substrates, including phosphorylated IκBα, provide a novel mechanism for the anti-inflammatory actions of H2O2 (Fig. 9) Although our results indicate that H2O2 can diminish association between phosphorylated IκBα and β-TrCP, it is also possible that H2O2 affects additional functions of the SCFβ-TrCP complex, such as coordination of Cdc34-dependent transfer of ubiquitin to IκBα. Although oxidation of cysteine thiols by H2O2 has been shown to participate in regulating the activity of kinases, such as IKK and Akt, as well as transcriptional factors, such as the p50 subunit of NF-κB, that are involved in inflammatory processes, there were no previous data demonstrating that H2O2 or other ROS could affect ubiquitination, an important regulatory pathway in physiologic and pathophysiologic processes. Although our experiments focused on interactions between H2O2, IκBα, and SCFβ-TrCP, it is possible that H2O2 may also participate in affecting the function of other ubiquitin ligases, providing an expanded role for ROS as participants in modulating cellular regulation and activation.
Supplementary Material
Acknowledgment
We thank Dr. Jack Lancaster, Jr. for helpful advice.
This work was supported, in whole or in part, by National Institutes of Health Grants HL62221, HL76206, and GM87748 (to E. A.). This work was also supported by grants from the Société Française d'Anesthésie et de Réanimation and the University Hospital of Amiens (France) (to E. L.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- ROS
- reactive oxygen species
- NF-κB
- nuclear factor-κB
- IKK
- IκB kinase
- LPS
- lipopolysaccharide
- ATZ
- 3-amino-1,2,4-triazole
- BIAM
- biotinylated iodoacetyl ethylenediamine
- GST
- glutathione S-transferase.
REFERENCES
- 1.Dröge W. (2002) Physiol. Rev. 82, 47–95 [DOI] [PubMed] [Google Scholar]
- 2.Cave A. C., Brewer A. C., Narayanapanicker A., Ray R., Grieve D. J., Walker S., Shah A. M. (2006) Antioxid. Redox Signal. 8, 691–728 [DOI] [PubMed] [Google Scholar]
- 3.Rahman I., Adcock I. M. (2006) Eur. Respir. J. 28, 219–242 [DOI] [PubMed] [Google Scholar]
- 4.Blackwell T. S., Blackwell T. R., Holden E. P., Christman B. W., Christman J. W. (1996) J. Immunol. 157, 1630–1637 [PubMed] [Google Scholar]
- 5.Arcaroli J. J., Hokanson J. E., Abraham E., Geraci M., Murphy J. R., Bowler R. P., Dinarello C. A., Silveira L., Sankoff J., Heyland D., Wischmeyer P., Crapo J. D. (2009) Am. J. Respir. Crit. Care Med. 179, 105–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zmijewski J. W., Zhao X., Xu Z., Abraham E. (2007) Am. J. Physiol. Cell Physiol. 293, C255–C266 [DOI] [PubMed] [Google Scholar]
- 7.Strassheim D., Asehnoune K., Park J. S., Kim J. Y., He Q., Richter D., Mitra S., Arcaroli J., Kuhn K., Abraham E. (2004) Am. J. Physiol. Cell Physiol. 286, C683–C692 [DOI] [PubMed] [Google Scholar]
- 8.Zmijewski J. W., Lorne E., Zhao X., Tsuruta Y., Sha Y., Liu G., Siegal G. P., Abraham E. (2008) Am J. Respir. Crit. Care Med. 178, 168–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baeuerle P. A., Baltimore D. (1988) Science 242, 540–546 [DOI] [PubMed] [Google Scholar]
- 10.Hayden M. S., Ghosh S. (2004) Genes Dev. 18, 2195–2224 [DOI] [PubMed] [Google Scholar]
- 11.Yaron A., Hatzubai A., Davis M., Lavon I., Amit S., Manning A. M., Andersen J. S., Mann M., Mercurio F., Ben-Neriah Y. (1998) Nature 396, 590–594 [DOI] [PubMed] [Google Scholar]
- 12.Ghosh S., Hayden M. S. (2008) Nat. Rev. Immunol. 8, 837–848 [DOI] [PubMed] [Google Scholar]
- 13.Zmijewski J. W., Lorne E., Zhao X., Tsuruta Y., Sha Y., Liu G., Abraham E. (2009) Am. J. Respir. Crit. Care Med. 179, 694–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zmijewski J. W., Banerjee S., Abraham E. (2009) J. Biol. Chem. 284, 22213–22221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vuillard L., Nicholson J., Hay R. T. (1999) FEBS Lett. 455, 311–314 [DOI] [PubMed] [Google Scholar]
- 16.Suzuki H., Chiba T., Kobayashi M., Takeuchi M., Furuichi K., Tanaka K. (1999) Biochem. Biophys. Res. Commun. 256, 121–126 [DOI] [PubMed] [Google Scholar]
- 17.Yaron A., Gonen H., Alkalay I., Hatzubai A., Jung S., Beyth S., Mercurio F., Manning A. M., Ciechanover A., Ben-Neriah Y. (1997) EMBO J. 16, 6486–6494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu G., Xu G., Schulman B. A., Jeffrey P. D., Harper J. W., Pavletich N. P. (2003) Mol. Cell 11, 1445–1456 [DOI] [PubMed] [Google Scholar]
- 19.Zheng N., Schulman B. A., Song L., Miller J. J., Jeffrey P. D., Wang P., Chu C., Koepp D. M., Elledge S. J., Pagano M., Conaway R. C., Conaway J. W., Harper J. W., Pavletich N. P. (2002) Nature 416, 703–709 [DOI] [PubMed] [Google Scholar]
- 20.Strack P., Caligiuri M., Pelletier M., Boisclair M., Theodoras A., Beer-Romero P., Glass S., Parsons T., Copeland R. A., Auger K. R., Benfield P., Brizuela L., Rolfe M. (2000) Oncogene 19, 3529–3536 [DOI] [PubMed] [Google Scholar]
- 21.Suzuki H., Chiba T., Suzuki T., Fujita T., Ikenoue T., Omata M., Furuichi K., Shikama H., Tanaka K. (2000) J. Biol. Chem. 275, 2877–2884 [DOI] [PubMed] [Google Scholar]
- 22.Hattori K., Hatakeyama S., Shirane M., Matsumoto M., Nakayama K. (1999) J. Biol. Chem. 274, 29641–29647 [DOI] [PubMed] [Google Scholar]
- 23.Petroski M. D., Deshaies R. J. (2005) Cell 123, 1107–1120 [DOI] [PubMed] [Google Scholar]
- 24.Winston J. T., Strack P., Beer-Romero P., Chu C. Y., Elledge S. J., Harper J. W. (1999) Genes Dev. 13, 270–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuruta Y., Park Y. J., Siegal G. P., Liu G., Abraham E. (2007) J. Immunol. 179, 7079–7086 [DOI] [PubMed] [Google Scholar]
- 26.Lorne E., Zmijewski J. W., Zhao X., Liu G., Tsuruta Y., Park Y. J., Dupont H., Abraham E. (2008) Am. J. Physiol. Cell Physiol. 294, C985–C993 [DOI] [PubMed] [Google Scholar]
- 27.Zhao X., Zmijewski J. W., Lorne E., Liu G., Park Y. J., Tsuruta Y., Abraham E. (2008) Am. J. Physiol. Lung Cell Mol. Physiol. 295, L497–L504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foster W. M., Walters D. M., Longphre M., Macri K., Miller L. M. (2001) J. Appl. Physiol. 90, 1111–1117 [DOI] [PubMed] [Google Scholar]
- 29.Brass D. M., Hollingsworth J. W., McElvania-Tekippe E., Garantziotis S., Hossain I., Schwartz D. A. (2007) Am. J. Physiol. Lung Cell Mol. Physiol. 293, L77–L83 [DOI] [PubMed] [Google Scholar]
- 30.Wrona M., Patel K., Wardman P. (2005) Free Radic. Biol. Med. 38, 262–270 [DOI] [PubMed] [Google Scholar]
- 31.Watanabe N., Zmijewski J. W., Takabe W., Umezu-Goto M., Le Goffe C., Sekine A., Landar A., Watanabe A., Aoki J., Arai H., Kodama T., Murphy M. P., Kalyanaraman R., Darley-Usmar V. M., Noguchi N. (2006) Am. J. Pathol. 168, 1737–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zmijewski J. W., Moellering D. R., Le Goffe C., Landar A., Ramachandran A., Darley-Usmar V. M. (2005) Am. J. Physiol. Heart Circ. Physiol. 289, H852–H861 [DOI] [PubMed] [Google Scholar]
- 33.Zmijewski J. W., Lorne E., Banerjee S., Abraham E. (2009) Am. J. Physiol. Lung Cell Mol. Physiol. 296, L624–L634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yum H. K., Arcaroli J., Kupfner J., Shenkar R., Penninger J. M., Sasaki T., Yang K. Y., Park J. S., Abraham E. (2001) J. Immunol. 167, 6601–6608 [DOI] [PubMed] [Google Scholar]
- 35.Bence N. F., Sampat R. M., Kopito R. R. (2001) Science 292, 1552–1555 [DOI] [PubMed] [Google Scholar]
- 36.Ding Q., Lewis J. J., Strum K. M., Dimayuga E., Bruce-Keller A. J., Dunn J. C., Keller J. N. (2002) J. Biol. Chem. 277, 13935–13942 [DOI] [PubMed] [Google Scholar]
- 37.Nam S., Smith D. M., Dou Q. P. (2001) J. Biol. Chem. 276, 13322–13330 [DOI] [PubMed] [Google Scholar]
- 38.Pajonk F., Riess K., Sommer A., McBride W. H. (2002) Free Radic. Biol. Med. 32, 536–543 [DOI] [PubMed] [Google Scholar]
- 39.Kim J. R., Yoon H. W., Kwon K. S., Lee S. R., Rhee S. G. (2000) Anal. Biochem. 283, 214–221 [DOI] [PubMed] [Google Scholar]
- 40.Choi K. S., Park S. Y., Baek S. H., Dey-Rao R., Park Y. M., Zhang H., Ip C., Park E. M., Kim Y. H., Park J. H. (2006) Prep. Biochem. Biotechnol. 36, 65–79 [DOI] [PubMed] [Google Scholar]
- 41.Cross J. V., Templeton D. J. (2006) Antioxid. Redox Signal. 8, 1819–1827 [DOI] [PubMed] [Google Scholar]
- 42.Ying J., Clavreul N., Sethuraman M., Adachi T., Cohen R. A. (2007) Free Radic. Biol. Med. 43, 1099–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taggart C. C., Greene C. M., McElvaney N. G., O'Neill S. (2002) J. Biol. Chem. 277, 33648–33653 [DOI] [PubMed] [Google Scholar]
- 44.Suzuki H., Chiba T., Kobayashi M., Takeuchi M., Suzuki T., Ichiyama A., Ikenoue T., Omata M., Furuichi K., Tanaka K. (1999) Biochem. Biophys. Res. Commun. 256, 127–132 [DOI] [PubMed] [Google Scholar]
- 45.Read M. A., Brownell J. E., Gladysheva T. B., Hottelet M., Parent L. A., Coggins M. B., Pierce J. W., Podust V. N., Luo R. S., Chau V., Palombella V. J. (2000) Mol. Cell. Biol. 20, 2326–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rhee S. G. (2006) Science 312, 1882–1883 [DOI] [PubMed] [Google Scholar]
- 47.Ward N. E., Stewart J. R., Ioannides C. G., O'Brian C. A. (2000) Biochemistry 39, 10319–10329 [DOI] [PubMed] [Google Scholar]
- 48.Bai C., Sen P., Hofmann K., Ma L., Goebl M., Harper J. W., Elledge S. J. (1996) Cell 86, 263–274 [DOI] [PubMed] [Google Scholar]
- 49.Deshaies R. J. (1999) Annu. Rev. Cell Dev. Biol. 15, 435–467 [DOI] [PubMed] [Google Scholar]
- 50.Skowyra D., Craig K. L., Tyers M., Elledge S. J., Harper J. W. (1997) Cell 91, 209–219 [DOI] [PubMed] [Google Scholar]
- 51.Torrie L. J., MacKenzie C. J., Paul A., Plevin R. (2001) Br. J. Pharmacol. 134, 393–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pantano C., Shrivastava P., McElhinney B., Janssen-Heininger Y. (2003) J. Biol. Chem. 278, 44091–44096 [DOI] [PubMed] [Google Scholar]
- 53.Matthews J. R., Wakasugi N., Virelizier J. L., Yodoi J., Hay R. T. (1992) Nucleic Acids Res. 20, 3821–3830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nishi T., Shimizu N., Hiramoto M., Sato I., Yamaguchi Y., Hasegawa M., Aizawa S., Tanaka H., Kataoka K., Watanabe H., Handa H. (2002) J. Biol. Chem. 277, 44548–44556 [DOI] [PubMed] [Google Scholar]
- 55.Jaspers I., Zhang W., Fraser A., Samet J. M., Reed W. (2001) Am. J. Respir. Cell Mol. Biol. 24, 769–777 [DOI] [PubMed] [Google Scholar]
- 56.Korn S. H., Wouters E. F., Vos N., Janssen-Heininger Y. M. (2001) J. Biol. Chem. 276, 35693–35700 [DOI] [PubMed] [Google Scholar]
- 57.Reynaert N. L., van der Vliet A., Guala A. S., McGovern T., Hristova M., Pantano C., Heintz N. H., Heim J., Ho Y. S., Matthews D. E., Wouters E. F., Janssen-Heininger Y. M. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 13086–13091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kanayama A., Inoue J., Sugita-Konishi Y., Shimizu M., Miyamoto Y. (2002) J. Biol. Chem. 277, 24049–24056 [DOI] [PubMed] [Google Scholar]
- 59.Kil I. S., Kim S. Y., Park J. W. (2008) Biochem. Biophys. Res. Commun. 373, 169–173 [DOI] [PubMed] [Google Scholar]
- 60.Ciechanover A. (1998) EMBO J. 17, 7151–7160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hochstrasser M. (1996) Annu. Rev. Genet. 30, 405–439 [DOI] [PubMed] [Google Scholar]
- 62.Chen Z. J., Parent L., Maniatis T. (1996) Cell 84, 853–862 [DOI] [PubMed] [Google Scholar]
- 63.Kotamraju S., Tampo Y., Keszler A., Chitambar C. R., Joseph J., Haas A. L., Kalyanaraman B. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 10653–10658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakayama K., Hatakeyama S., Maruyama S., Kikuchi A., Onoé K., Good R. A., Nakayama K. I. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 8752–8757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maniatis T. (1999) Genes Dev. 13, 505–510 [DOI] [PubMed] [Google Scholar]
- 66.Saha A., Deshaies R. J. (2008) Mol. Cell 32, 21–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kumar A., Wu H., Collier-Hyams L. S., Hansen J. M., Li T., Yamoah K., Pan Z. Q., Jones D. P., Neish A. S. (2007) EMBO J. 26, 4457–4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Landar A., Oh J. Y., Giles N. M., Isom A., Kirk M., Barnes S., Darley-Usmar V. M. (2006) Free Radic. Biol. Med. 40, 459–468 [DOI] [PubMed] [Google Scholar]
- 69.Craig A., Mai J., Cai S., Jeyaseelan S. (2009) Infect. Immun. 77, 568–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abraham E. (2003) Crit. Care Med. 31, Suppl. 4, S195–S199 [DOI] [PubMed] [Google Scholar]
- 71.Rubenfeld G. D., Herridge M. S. (2007) Chest 131, 554–562 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.