Abstract
Muscle contraction is tightly regulated by Ca2+ binding to the thin filament protein troponin. The mechanism of this regulation was investigated by detailed mapping of the dynamic properties of cardiac troponin using amide hydrogen exchange-mass spectrometry. Results were obtained in the presence of either saturation or non-saturation of the regulatory Ca2+ binding site in the NH2 domain of subunit TnC. Troponin was found to be highly dynamic, with 60% of amides exchanging H for D within seconds of exposure to D2O. In contrast, portions of the TnT-TnI coiled-coil exhibited high protection from exchange, despite 6 h in D2O. The data indicate that the most stable portion of the trimeric troponin complex is the coiled-coil. Regulatory site Ca2+ binding altered dynamic properties (i.e. H/D exchange protection) locally, near the binding site and in the TnI switch helix that attaches to the Ca2+-saturated TnC NH2 domain. More notably, Ca2+ also altered the dynamic properties of other parts of troponin: the TnI inhibitory peptide region that binds to actin, the TnT-TnI coiled-coil, and the TnC COOH domain that contains the regulatory Ca2+ sites in many invertebrate as opposed to vertebrate troponins. Mapping of these affected regions onto the troponin highly extended structure suggests that cardiac troponin switches between alternative sets of intramolecular interactions, similar to previous intermediate resolution x-ray data of skeletal muscle troponin.
Keywords: Calcium/Binding Proteins, Methods/Mass Spectrometry, Protein/Allosteric, Protein/Contractile, Regulation Allosteric, Signal Transduction/Calcium
Introduction
Striated muscle contraction is reversibly activated and tightly controlled by Ca2+ binding to the key regulatory protein of the contractile apparatus, troponin (Tn2, reviewed in Ref. 1, 2). In vertebrate troponins, this regulation involves a Ca2+-dependent attachment of subunit TnC NH2 domain to a ∼10-residue, amphipathic, “switch” helix of subunit TnI (3–5). The switch helix is within a 9-kDa portion of TnI that appears to interact with actin and tropomyosin so as to shut off muscle contraction (6, 7), specifically when Ca2+ (and the switch helix) dissociate from the TnC regulatory domain.
Ca2+-saturated cardiac and skeletal muscle troponin structures each have been solved by x-ray crystallography at high resolution (4, 5). Results agree with each other in many details, and the findings are important advances after many years when only much smaller portions of troponin had been solved at atomic resolution. On the other hand, the position of the TnC regulatory NH2 domain differs between these two structures. In addition, the connecting residues between this domain and the remainder of troponin are not seen in the cardiac data. The mechanistic implications of these differences are unclear. In the presence of Mg2+/EGTA, an atomic model of skeletal muscle troponin has been hypothesized from 9 Å x-ray data (5). High resolution data have not been reported for either isoform in the presence of low Ca2+.
Several other aspects of the regulatory mechanism remain unknown. The inhibitory effects of TnI on the thin filament are not understood at atomic resolution, and are functionally complex (8–12). Also, there is little understanding of the effects of regulatory Ca2+ dissociation on parts of troponin outside the TnC NH2 region. Finally, the regulatory action of many invertebrate troponins cannot be comprised by the mechanism stated above. These troponins have Ca2+ binding sites only within the COOH domain of TnC (13, 14), and none in the TnC NH2 domain. Thus, there are major gaps in the current understanding of how troponins control muscle contraction.
The present work represents a new approach to this subject: amide hydrogen exchange (15–19) of troponin. Solvent-exposed amide hydrogens in peptides rapidly exchange with solution H (or D in D2O), with rates of ∼10 s−1 (20). In contrast, exchange is blocked by hydrogen bonding, such as that of most backbone amide hydrogens in folded proteins. Exchange rates in proteins do not correspond to unfolding or refolding rates. Rather, they are very much slower. This is because, under usual conditions, local refolding rates greatly exceed the H/D exchange rate of the unfolded region. Exchange at the slowest exchanging amides tends to be governed, i.e. protected from the solvent-exposed rate, by global protein folding stability. Other amide hydrogens exhibit faster exchange, intermediate between those of solvent-exposed hydrogens and core hydrogens, reflecting wide variation in local flexibility or folding stability across different regions of an overall folded protein. The degree of H/D exchange protection is a measure of local folding stability within the context of a globally folded protein (15–19). By characterizing exchange rates at multiple sites, either by NMR or by mass spectrometry, the dynamic behavior of the protein can be mapped. Results loosely correlate with crystallographic B-factors, but reflect different protein properties and provide different information (21). Finally, by measuring the effects of physiological perturbations, such as Ca2+ binding to troponin, on H/D exchange rates, one may detail intramolecular signal transduction.
In the present study, mass spectrometry is used to map amide hydrogen exchange in the human cardiac troponin core domain (TnC-TnI-TnT-(183–288)). Data for two conditions are reported: in the presence of either full saturation or subsaturation of the regulatory Ca2+ binding site (site II), which is located in the TnC NH2 domain. Troponin is a highly extended protein, ∼20 nm in length (1), without a large globular region that might be highly protected from H/D exchange. Even the troponin “core domain,” which includes TnC, most of TnI, and the COOH terminus of TnT, lacks a globular truly core-like region (4). Correspondingly, in the current study much of troponin is found to undergo H/D exchange rapidly. Exchange is completed fully within seconds or a minute of exposure to D2O. The most prominent exception to this pattern is within the 46-residue TnT-TnI coiled-coil portion of troponin. Parts of the coiled-coil are protected from H/D exchange for hours and evidently comprise the most tightly folded portion of the ternary troponin complex.
Current results also show that Ca2+ saturation of site II affects H/D exchange rates across many parts of troponin. Effects are not confined to the TnC NH2 domain and the TnI switch helix. Rather, Ca2+ binding has long range allosteric effects. Based on these detailed results, we suggest that the intra-troponin switch regulating muscle contraction involves a conversion between two states of the troponin core domain. The states are stabilized by two different sets of intra- and intersubunit interactions, and involve the coiled-coil and TnC COOH domain as well as the region of troponin where Ca2+ binds. This proposal is discussed in terms of previous structural studies of cardiac and skeletal muscle troponins. It gives added weight to a hypothesized atomic structure for skeletal muscle troponin in the presence of low Ca2+ concentration, derived from 9 Å x-ray data (5).
EXPERIMENTAL PROCEDURES
Protein Preparation, Protein Fragment Generation, and Protein Fragment Identification
Human cardiac troponin was prepared by reconstitution of bacterially expressed (22) TnI, TnC, and COOH-terminal TnT-(183–288) (expression plasmid obtained by PCR of full-length TnT cDNA.). After 1:1:1 mixture and serial dialysis to remove initial denaturant and to decrease 1 m NaCl to 0.1 m NaCl, the complex was isolated via ion exchange chromatography using a 6-ml Resource S column connected to an FPLC (AKTA, Amersham Biosciences), and stored at −80 °C.
To identify troponin fragments that could be examined for H/D exchange, 5 μg of troponin in 50 μl of 10 mm NaHPO4 (pH 7.0), 0.1 m NaCl, was mixed with 50 μl of 0.1 m NaHPO4 (pH 2.5), followed by the addition of 5 μg of porcine pepsin dissolved in 10 mm HCOONa (pH3.75). The digested sample (5 min 0 °C) was injected into a micropeptide trap connected to C18 HPLC column (5 cm × 1 mm, Vydac) attached to a Finnigan FT-ICRMS (Thermoelectron). Digested peptides were eluted at 90 μl/min for tandem mass spectrometry. Peptic fragments were identified using the search algorithm Bioworks 3.2 (Thermoelectron) and manual confirmation.
H/D Exchange of Troponin and Subsequent Isotope Analysis by HPLC-Electrospray Ionization FT-ICR MS
To initiate exchange, performed at 25 °C, 5 μg of troponin complex was transferred by Zeba Desalt Column (Pierce) into H2O buffer (10 mm NaHPO4 (pH 7.1), 0.1 m NaCl), and then diluted to 5.9 μm troponin by adding 9 parts (v/v) D2O buffer: 10 mm NaDPO4 (pD read 7.1), 0.1 m NaCl, 0 or 1 mm CaCl2. Exchange was quenched after different time intervals by adding an equal volume of iced 100 mm NaDPO4 (pD 2.5), 0.1 m NaCl, and quickly flash-freezing the sample in liquid nitrogen. Samples were stored at −80 °C.
For analysis, samples were quickly thawed and digested with 5 μg of pepsin (Sigma) on ice for 5 min followed by immediate injection into the micropeptide trap (Michrom Biosources, Inc) attached to C18 HPLC column connected to FT-MS. Peptides were eluted within 10–12 min using a gradient of 5–35% acetonitrile at a flow rate of 90 μl/min. Individual peptide envelopes were easily recognizable by correspondence to the possible post-exchange m/z of pre-H/D-identified peptides; 1/z Da increase per exchanged hydrogen. The centroid of each peptide was determined using the software package MagTran (23). To allow correction for backward D/H exchange during pepsin digest and HPLC-MS, fully deuterated control was prepared (and digested, and peptides assessed by MS) by incubation of troponin complex for 2 h at 40 °C in 2 m deuterated urea, 100 mm NaDPO4 (pD 2.5). Corrections for back exchange (D to H) during processing were determined for each peptide. The average back exchange was 23%.
Curve Fitting of Exchange Kinetic Data
Non-linear least squares curve fitting of peptide mass increases over time was performed with Scientist (Micromath). Peptides, subpeptides, and/or overlapping peptides were grouped for global curve fitting. This improved error estimates for some peptides, relative to the alternative approach of subtraction that is sometimes effective (24). (In this alternative, exchange in a subpeptide is subtracted from exchange in a larger, parent peptide, and the kinetics of the subtracted result are fit, with larger errors in some cases.) The globally fit groups of peptides were: TnC [25–56, 28–57, 36–56s, 36–57u] and [118–132s, 122– 130, 122–132]; TnI [27–53s, 29–49s, 29–53s], [54–66, 54–78], [62–85, 78–88s], [97–109, 97–116], [125–134, 125–152], [156–169, 162–169], and [191–197, 191–210]; TnT [224–242, 225–243, 230–243, 237–243] and [243–263, 244–250, 244–264, 249–263, 251–263], where peptide groups are enclosed in “[ ]”, and s or u indicates peptides only assessed under high or low Ca2+ conditions, respectively.
Primary Structure Assignments of H/D Exchange Protection
For peptides from most of troponin (TnT, TnI, and the TnC COOH-terminal domain), the sizes of the observed H/D exchange transitions matched the peptide lengths or length overlaps. This allowed straightforward assignment of the transitions to specific residues, as shown under “Results and Discussion.” In most of the remaining cases, a peptide (or non-overlapping subpeptide region) contained both a detectable transition and other hydrogens that exchanged before the first 5-s time point. Assignment was again straightforward for these peptides, because the observed transition, which indicated detectable protection from exchange, could be attributed to the subset of residues that were folded sufficiently to be identifiable by x-ray crystallography. The primary exception to this method of transition assignment was in the TnC NH2 domain, where there was no unambiguous basis for assignment of specific residue NHs to many of the kinetically identified transitions. For illustration of approximate mapping, the different rates were provisionally assigned to conform with previously published work, such as the troponin high resolution structural data and NMR studies of TnC.
The assigned exchange rates (kex) were converted to estimated protection factors (Kcl = kchem/kex), where kchem was the geometric average (range 1.8–8.2) of the unprotected H/D exchange rates expected (20) for each residue in the peptide. These protection factors can be considered to represent local folding stability, measured in the context of the globally folded troponin molecule. In the figures, protection factors are mapped on the troponin primary and tertiary structures. However, these maps are altered very little if direct exchange rate values are substituted and mapped instead.
Ca2+ Concentrations and Binding Saturation
Cardiac troponin sites III and IV bind Ca2+ with affinity ∼108 m−1, the regulatory site II binds Ca2+ with affinity ∼106 m−1, and site I is fully defunct (1, 25). To perform H/D exchange with sites II-IV saturated was straightforward: 1 mm CaCl2 was included. To specifically examine effects of site II Ca2+, we sought a condition in which site II was not saturated, but sites III and IV remained fully saturated. In principle, H/D exchange of some portions of troponin might be accelerated considerably by even minor subsaturation of sites III and IV. Therefore, to ensure that effects of lower Ca2+ concentrations were due to changes in site II saturation only, our lower Ca2+ condition remained in the μm range. Adding mm Mg2+ to an EGTA-chelated sample was an unused alternative, with the limitation that the Mg2+ affinity of sites III and IV is not high enough to assure saturation and also that Mg2+ is not Ca2+.
Experiments using a Ca2+-sensitive fluorescence probe showed that site II was mostly but not completely empty under our low Ca2+ conditions. FuraFF (Invitrogen) spectra and Ca2+ affinities (measured with fluorescence titration data fit to simple binding isotherms) were characterized and used to examine solution Ca2+ concentrations. When troponin was transferred by spin column into the H2O buffer and then added in 1:10 part to the D2O buffer, the free Ca2+ rose by 4 μm, implying that 4 μm Ca2+ dissociated from 5.9 μm troponin (the final protein concentration). Accordingly, troponin site II was at minimum 2/3 dissociated and at most 1/3 bound to Ca2+ under these conditions, which replicated those used for H/D exchange measurements. This conclusion of substantial unsaturation of site II was confirmed by the observed differences in H/D exchange rates in the presence of low as opposed to high Ca2+. However, because site II was not completely empty under the low Ca2+ conditions examined, the true effects on exchange rates may be larger than those detected in the present report.
RESULTS
Kinetic Results
Extensive (5 s to 6 h) H/D exchange kinetic data were obtained for 43 peptides derived by digestion of troponin exposed to D2O in the presence of saturating, 1 mm Ca2+. In separate experiments, kinetic H/D exchange data were obtained from 37 of these same 43 peptides, plus one other, derived by digestion of troponin exposed to D2O containing 4 μm Ca2+. (This Ca2+ concentration was subsaturating for regulatory Ca2+ binding site II, under these experimental conditions.) The peptides are shown in Fig. 1. The high Ca2+ peptides provided data concerning 89% of the amide NH groups in the experimental troponin, and the lower Ca2+ peptides included 80% of the amide NH groups. The average peptide contained 15.5 exchangeable amide bonds, and overlapped in 60% of its length with one or more other peptides.
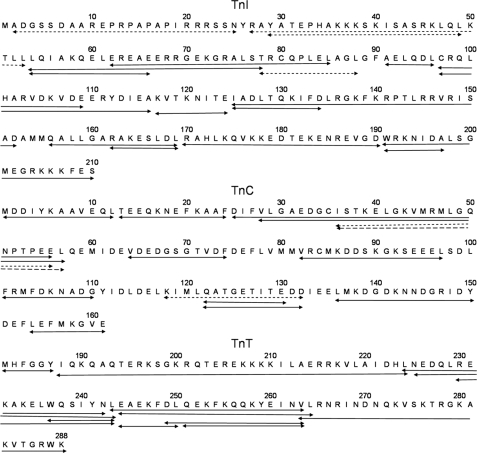
FIGURE 1.
Proteolytic troponin fragments used to characterize H/D exchange in native troponin. Fragments indicated by solid lines were characterized kinetically in the presence of both high and low Ca2+ concentrations. Short and long dashed lines indicate, respectively, peptides measured only in the presence of high or low Ca2+.
The exchange data (peptide mass increase versus duration of D2O exposure) spanned a wide time range. Results, therefore, could report transitions occurring over multiple timescales, from seconds to hours. This was in fact observed (Figs. 2 and 3, panels described in detail below). H/D exchange rates ranged across almost four orders of magnitude, from unprotected amide hydrogens that exchanged before the first, 5-s time point, to amide hydrogens that were unexchanged after 6 h in D2O. Often, there was more than one transition evident within an individual peptide. Note that all peptides reported in Figs. 2 and 3 begin at zero mass increase at zero time.
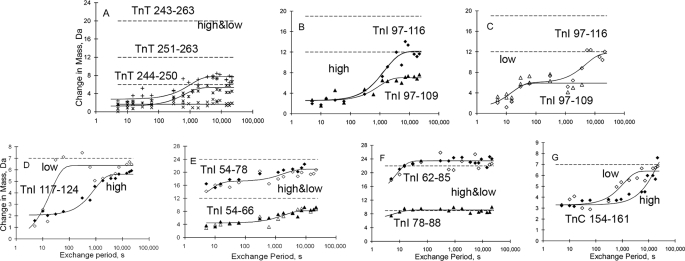
FIGURE 2.
Kinetics of H/D exchange distant from the regulatory Ca2+ binding site. A, TnT 244–250, 251–263, and 243–263; B and C, TnI 97–109 and 97–116; D, TnI 117–124; E, TnI 54–66 and 54–78; F, TnI 62–85 and 78–88; G, TnC 154–161. Dashed lines indicate the increase in mass corresponding to full exchange for the indicated peptides. Solid lines are non-linear least square fit curves. The words high and low refer to alternate Ca2+ concentration conditions. In panels A and D–G both high Ca2+ data (filled symbols), and low Ca2+ data (open symbols) are shown. In A, exchange was modest regardless of condition, and the high and low Ca2+ data are merged. Panels B and C contrast high (B) and low (C) Ca2+ data for the same two peptides.
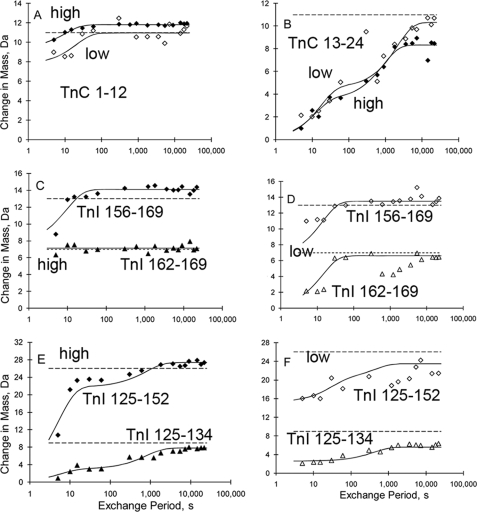
FIGURE 3.
Kinetics of H/D exchange within regions more proximal to the regulatory Ca2+ binding site. A, TnC 1–12; B, TnC 13–24; C (high Ca2+); and D (low Ca2+) - TnI 156–162 and 162–169; data; E, (high Ca2+) and F (low Ca2+) - TnI 125–134 and 125–152. High Ca2+, filled symbols. Low Ca2+, open symbols. Dashed and solid lines, and empty and filled symbols are as in Fig. 2.
The availability of so much data, and over such a broad time range, raised the possibility of achieving a near-comprehensive, local mapping of troponin dynamics. Accordingly, the kinetic analysis was conducted to provide assessments that were as full and as precise as possible. Curve fitting was employed to determine transition rate constants and transition magnitudes. Because of the wide time range examined, it was necessary when measuring rate constants to treat the data as the sum of exponentials, whenever the results so implied. Also, to optimize measurement of transitions, global curve fitting proved essential. Data from peptides and their subpeptides or overlapping peptides were combined and fit simultaneously. In the global fitting procedure, exchange rates of those NHs present in more than one peptide were required to be equal within all such peptides. Curve fitting was broadly successful for transition rate estimation, as shown in Figs. 2 and 3, and in Tables 1 and 2.
TABLE 1.
H-D exchange rates in the presence of saturating Ca2+ concentration
| Subunit | Peptide or group of peptides | H-D exchange rate, s−1 | Log (protection factor) | Assigned exchange region |
|---|---|---|---|---|
| TnC | 1–12 | 0.10 ± 0.02 | 1.49 | 8–10 |
| 13–24 | 0.08 ± 0.03 | 1.83 | 14–16 | |
| “ | 0.0012 ± 0.0003 | 3.69 | 20–24 | |
| 25–57 | 0.004 | 2.65 | 26–28 | |
| ” | 0.0007 | 3.91 | 29–33 | |
| “ | 0.07 | 1.92 | 34–36 | |
| ” | 0.10 ± 0.03 | 1.68 | 37–42 | |
| “ | 0.00015 ± 0.00009 | 4.52 | 43–47 | |
| ” | 0.10 ± 0.03 | 1.68 | 48–57 | |
| 64–74 | 0.050 ± 0.017 | 1.97 | 64–65 | |
| 82–97 | 0.028 ± 0.015 | 2.39 | 95–97 | |
| 118–132a | 0.06 ± 0.02 | 1.79 | 119–122 | |
| “ | 0.038 ± 0.012 | 2.12 | 123–124 | |
| 154–161 | 0.000051 ± 0.000037 | 4.62 | 155–158 | |
| TnI | 27–53 | 0.0015 ± 0.0014 | 3.74 | 46–47 |
| ” | 0.073 ± 0.044 | 1.63 | 50–53 | |
| 54–78a | 0.0006 ± 0.0002 | 3.82 | 59–62 | |
| 62–88a | 0.14 ± 0.06 | 1.63 | 71–82 | |
| 91–96 | 0.00049 ± 0.00010 | 3.70 | 92–96 | |
| 97–116 | 0.0011 ± 0.0003 | 3.72 | 98–103 | |
| “ | 0.00043 ± 0.00021 | 3.80 | 112–116 | |
| 117–124 | 0.0011 ± 0.0002 | 3.54 | 118–124 | |
| 125–152 | 0.0013 ± 0.0003 | 3.54 | 126–131 | |
| ” | 0.16 ± 0.07 | 1.35 | 132–134 | |
| “ | 0.20 ± 0.04 | 1.43 | 135–152 | |
| 156–169 | 0.11 ± 0.02 | 1.67 | 157–162 | |
| TnT | 182–187 | 0.0018 ± 0.0006 | 3.87 | 183–184 |
| 188–224 | 0.024 ± 0.018 | 2.39 | 214–219 | |
| 224–243a | 0.0011 ± 0.0003 | 3.62 | 225–230 | |
| ” | 0.00015 ± 0.00004 | 4.37 | 231–233 | |
| “ | 0.00040 ± 0.00013 | 4.21 | 238–239 | |
| 243–264a | 0.0010 ± 0.0002 | 3.60 | 259–264 | |
| 263–288 | 0.23 ± 0.12 | 1.55 | 264–273 |
a Merged data, saturating and subsaturating Ca2+.
TABLE 2.
H-D exchange rates in the presence of subsaturating Ca2+ concentration
| Subunit | Peptide or group of peptides | H-D exchange rate, s−1 | Log (protection factor) | Assigned exchange region |
|---|---|---|---|---|
| TnC | 1–12 | 0.048 ± 0.031 | 1.79 | 8–10 |
| 13–24 | 0.060 ± 0.020 | 1.97 | 14–18 | |
| ” | 0.00048 ± 0.00013 | 4.07 | 19–24 | |
| 25–57 | 0.0006 | 3.43 | 26–28 | |
| “ | 0.028 ± 0.011 | 2.30 | 35–37 | |
| ” | 0.028 ± 0.011 | 2.25 | 38–42 | |
| “ | 0.0003 ± 0.0001 | 4.21 | 43–48 | |
| 64–74 | 0.067 ± 0.055 | 1.85 | 64–66 | |
| 118–132a | 0.06 ± 0.02 | 1.79 | 119–122 | |
| ” | 0.038 ± 0.012 | 2.12 | 123–124 | |
| 136–150 | 0.095 ± 0.040 | 1.85 | 137–140 | |
| 154–161 | 0.0009 ± 0.0003 | 3.37 | 155–157 | |
| TnI | 54–78a | 0.0006 ± 0.0002 | 3.82 | 59–62 |
| 62–88a | 0.14 ± 0.06 | 1.63 | 71–82 | |
| 91–96 | 0.00028 ± 0.00004 | 3.94 | 92–96 | |
| 97–116 | 0.066 ± 0.024 | 1.93 | 98–103 | |
| “ | 0.00025 ± 0.00016 | 4.04 | 111–116 | |
| 117–124 | 0.052 ± 0.014 | 1.89 | 118–124 | |
| 125–152 | 0.0024 ± 0.0012 | 3.18 | 128–131 | |
| ” | 0.034 ± 0.020 | 2.03 | 144–148 | |
| 156–169 | 0.07 ± 0.01 | 1.80 | 163–169 | |
| TnT | 182–187 | 0.06 ± 0.04 | 2.36 | 183–185 |
| 188–224 | 0.07 | 1.92 | 214–219 | |
| 224–243a | 0.0011 ± 0.0003 | 3.62 | 225–230 | |
| “ | 0.00015 ± 0.00004 | 4.37 | 231–233 | |
| ” | 0.0004 ± 0.0001 | 4.21 | 238–239 | |
| 243–264a | 0.0011 ± 0.0003 | 3.60 | 259–264 |
a Merged data saturating and subsaturating Ca2+.
Fast Hydrogen Exchange Occurs in Large Portions of Troponin
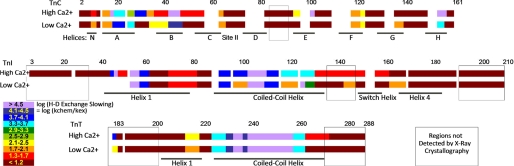
Before considering the detailed data across troponin, it useful to note the broad pattern of the results. Fig. 4 shows color-coded mapping of the degree of protection from H/D exchange. Results are delineated according to the troponin complex primary structure, and presented for both experimental conditions, high Ca2+ and low Ca2+. The largest single kinetic category was unprotected amide hydrogens that exchanged before the first, 5-s time point, with protection factor = log (kchem/kex) < 1.2. Fully 53% of the assessed amides (colored reddish-brown in the figure) exchanged before the first 5-s time point when troponin was placed in D2O containing low Ca2+. An almost equal fraction of amide hydrogens (47%) exchanged before 5 s in the presence of high Ca2+. Some of these fast exchanging hydrogens are in regions of troponin that are, as far as is known, predominantly disordered in the absence of binding partners actin and tropomyosin. However, most of these fast-exchanging hydrogens are in regions with structure identifiable by x-ray and/or NMR. Therefore, results suggest these regions are loosely folded rather than disordered. They appear to have too little protection from exchange to be detected in the present study. For example, amide hydrogens with < 20-fold protection would experience nearly complete hydrogen-deuterium exchange within 5 s. Summing the extent of these reddish brown-colored regions with the extent of the red-colored regions exhibiting fast but measurable exchange rates (1.3 < log(kchem/kex) < 1.7)), the H/D exchange rates of 66% of the troponin complex suggest loose or absent local folding in the presence of saturating Ca2+ concentration. Similarly, 58% of the examined portion of the protein has these properties (i.e. either red or reddish brown in the figure) in the presence of low Ca2+. The overall picture is of a highly dynamic molecule, regardless of whether site II is saturated with Ca2+.
FIGURE 4.
Primary structure mapping of native state troponin H/D exchange. Results indicate the measured degree of exchange rate slowing relative to that estimated (20) for the unfolded state, and are color coded on a logarithmic scale. Both high Ca2+ and low Ca2+ findings are shown. Violet regions are the least dynamic, most protected from exchange (generally not exchanging after 6 h). Reddish-brown regions are the most dynamic, evidencing no protection (exchange completed by 5 s). This indicates either weak or absent folding within native troponin. In the TnC NH2 domain, the relative location of exchange transitions could not be determined within residues 14–24 and also within residues 26–57; the colors may be scrambled.
H/D Exchange Rates within Troponin Regions Distant from the Regulatory Ca2+ Binding Site
Fig. 4 shows that some regions of the primary structure contain hydrogens that are very highly protected from exchange. The violet-colored regions do not exchange in seconds. Very much to the contrary, they have protection from amide hydrogen exchange beyond the last 6-h time point. The largest structural region showing such protection is in the heart of the TnT-TnI coiled-coil, involving corresponding regions of both subunits. The direct kinetic data showing this is in Fig. 2A. This panel shows H/D exchange kinetic data for three nested TnT peptides. All exchange slowly, exemplifying the slow H/D exchange in much of the coiled-coil.
More generally, Fig. 2A can be used to illustrate how the kinetic exchange data were analyzed. Solid lines are best fit curves, providing transition sizes and rate constants used elsewhere in the present report. The three dashed lines in Fig. 2A, similar to all panels in Figs. 2 and 3, indicate the expected increases in the peptides masses if all of their amide hydrogens exchanged H for D. None of these peptide masses, together spanning residues 243–263, came close to approaching these maxima after 6 h of exposure to D2O. High and low Ca2+ results did not differ, and data are merged.
Similarly, TnI coiled-coil peptide 97–116 and subpeptide 97–109 did not fully exchange after 6 h, both in the presence of high Ca2+ (Fig. 2B) and in the presence of lower Ca2+ (Fig. 2C), reaching in each case only 50% maximal possible exchange (dashed lines). In contrast to many other regions of troponin that are highly dynamic according to the H/D exchange data, this region of the coiled-coil likely serves as a key component of the overall stability of the ternary troponin complex.
Close examination of Fig. 2C shows that an H/D exchange transition involving about six amides occurred during the first minute in both peptides, i.e. within amides 98–109 shared by the two peptides. H/D exchange rates for these and other hydrogens are listed in Tables 1 and 2. Remarkably, this Fig. 2C first minute transition was absent in the presence of mm Ca2+ (Fig. 2B). Similarly, much more exchange occurred in the first minute within TnI-(117–124) in the presence of lower Ca2+ than in the presence of higher Ca2+ (both curves shown in Fig. 2D). Thus two TnI peptides within the coiled-coil undergo significant allosteric effects detected by changes in H/D exchange rates, despite being located far from the Ca2+ binding site in the TnC NH2 domain and outside the portions of troponin generally implicated as part of regulatory Ca2+ signaling. The current findings suggest that regulatory signal transduction includes broad regions of troponin, beyond the TnC regulatory domain.
Similar effects were observed in the TnC COOH-terminal peptide 154–161 (Fig. 2G). Filled or open symbols indicate conditions of TnC site II saturation or unsaturation, in this as well as in the other panels. The TnC COOH domain includes high affinity Ca2+ sites III and IV, which were saturated under both conditions examined in the present study (1). Nevertheless, the figure shows a difference between the conditions. Specifically, 3 or 4 of the TnC-(154–161) amide hydrogens exchanged with a Ca2+ concentration-sensitive rate. They exchanged at ∼1,000 s when the Ca2+ concentration was μm and at ∼10,000 s when the Ca2+ was mm. (The remaining 3 or 4 hydrogens exchanged within the first 5 s.) This implies that the TnC H-helix, the end of which is within this peptide, is affected by Ca2+ binding to the TnC NH2 domain.
Of course, many of the mapped amides in troponin exhibited exchange rates that were indistinguishable, regardless of Ca2+. Fig. 2, E and F together show four distinct peptides from the long Helix 1 of TnI: 54–66 and 54–78 in 2E; 62–85 and 78–88 in 2F. Conditions of TnC site II saturation or unsaturation (filled versus open symbols) are indistinguishable. Combined evaluation of all four peptides allowed mapping (Fig. 4) of the locations of 4 timescales for H/D exchange: before 5 s (unprotected); within the first minute; at ∼1000 s (panel E only); and later than 6 h (panel E only). The overall pattern indicated no effect of Ca2+ and a dynamic helix, loosely folded except for high protection from exchange where the helix is in direct contact with the COOH domain of TnC (see below).
H/D Exchange Rates within Troponin Regions Proximal to the Regulatory Ca2+ Binding Site
One of the most diverse regions for exchange rates was the NH2 domain of TnC, which contains Ca2+ site II. Many of the amide hydrogens exchanged quickly (data exemplified by Fig. 3A). Others exchanged over a range of time scales, as shown by the variegated coloring of the TnC 2–57 region of Fig. 4, and as exemplified by Fig. 3B. Saturating Ca2+ resulted in fewer hydrogens that were completely unprotected from exchange in the TnC NH2 domain (Fig. 4). High Ca2+ appeared to increase the protection of some amide hydrogens and decrease the protection of others. Current results qualitatively agree (but are insufficient for quantitative comparison because of long peptides) with findings on isolated TnC or the TnC NH2 domain by x-ray crystallography (5, 26), NMR (25, 27), and H/D exchange (28), all of which indicate changes in this domain on Ca2+ binding.
Fig. 3C shows H/D exchange kinetics in the presence of mm Ca2+ for TnI peptide 156–169 and subpeptide 162–169. The longer of these two includes part of the TnI switch helix that binds to the TnC NH2 domain in a Ca2+-dependent manner. A transition over the first 10 s was observed only in the larger peptide (uppermost curve), defining the location of the transition as the switch helix. This is as expected: the switch helix is folded and has detectable protection from exchange under high Ca2+ conditions, when it is attached to TnC.
In contrast, data for the same two peptides (Fig. 3D) indicated no protection of the switch helix from H/D exchange in the presence of low Ca2+, i.e. unsaturated site II. The key within the panel to this inference is that by 5 s the larger peptide already incorporated at least 6 more deuteriums than did the smaller peptide. Thus, the additional six amide segment present in the larger peptide, a segment that includes the switch helix amides, underwent full exchange by 5 s. The data in Fig. 3D for TnI-(162–169) are of mediocre quality, but are of use when globally assessed with the higher quality TnI-(156–169) data. For example, because the longer peptide undergoes complete exchange by 60 s, the shorter peptide must do so as well.
Similarly, data for TnI peptide 125–152 and its subpeptide 125–134, shown in Fig. 3, E and F, allow assessment of exchange in another important region: TnI-(137–148). Early work on troponin (reviewed in Ref. 1) showed that the corresponding peptide from skeletal muscle troponin binds to the thin filament with inhibitory effect as an isolated peptide. This segment is included only in the larger of the two peptides shown in panels E and F. In the presence of mm Ca2+ (panel E), a large kinetic transition occurred at ∼5 s in the larger peptide, with magnitude such that the inhibitory segment must be included. This is consistent with uniform dynamics detected by EPR (29). A smaller transition occurred at ∼400 s, and is assignable to the end residues of the coiled-coil because it occurred in both peptides of the panel. (The shorter peptide comprises the end of the coiled-coil.)
Data from the same two TnI peptides indicated that release of Ca2+ from TnC site II, i.e. lower Ca2+ concentration conditions, altered exchange kinetics substantially (Fig. 3, F versus E). The longer peptide incorporated more deuterium within the first 5 s in the presence of lower Ca2+ than it did in the presence of higher Ca2+. However, this was not the only qualitative change for this longer peptide; there was a converse effect as well. With lower Ca2+ conditions, incorporation flattened by 5 s, so that by 15 s there was much more exchange into the longer peptide when the Ca2+ concentration was higher than when it was lower. These results imply large effects of Ca2+ binding to TnC site II on the dynamic properties of the TnI inhibitory region. Some residues exhibited faster exchange, and some exhibited slower exchange. The data at 30 s and beyond were relatively noisy for the larger peptide, so the global fit at later times was dominated primarily by the data for the shorter peptide. Neither peptide reached maximal H/D exchange by 6 h, another contrast from panel E.
DISCUSSION
Thin filament activation involves troponin movement, and troponin dynamics have proven a productive subject for understanding regulation (e.g., Ref. 12, 30)). The present report adds to this literature by employing amide hydrogen exchange, which provides particularly rich dynamic information. No exogenous probes need be attached, and insight is obtained for all of the protein backbone, including both mobile and immobile regions. Furthermore, when exchange rate constants can be determined as in the present report, a wealth of quantitative dynamic insight is gained.
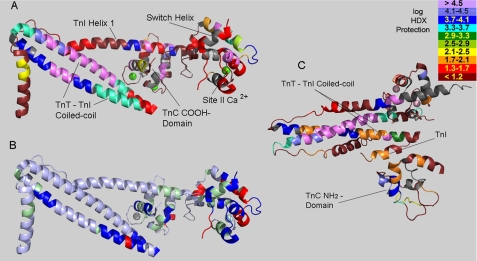
Fig. 5 illustrates concisely many of the findings in the present report. It shows the degree of protection from H/D exchange, as measured in the presence of mm Ca2+. The data are mapped onto the high resolution structure of the Ca2+-saturated cardiac troponin core domain, using a log scale color coding. Enormous variation was seen across this highly asymmetrical, extended protein. The most protected regions, in violet or dark blue, identify the components that are key to formation and overall stability of the ternary complex. These are the TnT-TnI coiled-coil, particularly the most highly protected portion in both subunits, and the attachment of TnI helix 1 to TnC. The H/D exchange results demonstrate these aspects both qualitatively and quantitatively. Furthermore, these findings can be seen in the figure to be in striking contrast to the overall dynamic aspects of troponin, most of which are shown in red or reddish brown, indicating weak local folding.
FIGURE 5.
Three-dimensional structure mapping of H/D exchange protection. A, high Ca2+ results from Fig. 4 are mapped onto the Ca2+-saturated structure of the cardiac troponin core domain. Gray indicates sections where no H/D exchange data were obtained. B, map of troponin regions with exchange rates altered by Ca2+ saturation of TnC site II. In high Ca2+ conditions relative to low Ca2+ conditions, blue indicates slower exchange, red indicates faster exchange, silver (light blue) indicates statistically insignificant alteration, and green indicates regions without data for comparison between high and low Ca2+. C, low Ca2+ results from Fig. 4 are mapped onto the proposed model of skeletal muscle troponin core domain under low Ca2+ conditions (Mg2+/EGTA) (5). The orientation in panel C is different from panels A and B, and only partial superposition is possible in any orientation. The indicated TnI segment is the inhibitory region, which is disordered so not shown in panels A and B.
Many features are notable from this mapping. The TnT-TnI coiled-coil was highly protected, but neither uniformly along its length, nor uniformly between TnT and TnI. The TnT was more protected, suggesting that when the coiled-coil strands transiently dissociate, the TnT strand is more stable. High protection began at the base of the coiled-coil, was most profound near the center, and greatly diminished near the COOH termini of both TnT and TnI components of the coiled-coil. In support of the validity of this mapping, there is an atomic resolution explanation for the most highly protected region that exists across both TnT and TnI strands: the region closely coincides with charged side chain networks that are important for troponin function, that extend across the coiled-coil, and that involve TnT residues E244 and K247 and TnI residues K106, D110, R79, and R103 (31).
The three-turn TnI switch helix in Fig. 5A is noteworthy. It overlies the TnC NH2 domain that contains regulatory site II, and is colored red to indicate weak but measurable protection from exchange (gray-colored portions were not measured.) H/D exchange within the TnI switch helix remained relatively fast, despite full saturation of site II via 1 mm Ca2+. Protection from exchange was a modest ∼50-fold. This suggests weak, energetically facile dissociation of the switch helix from Ca2+-saturated TnC, as may be required for regulatory switch function.
Similarly, the data indicate that the TnI “inhibitory” region (boxed residues including TnI 140 in Fig. 4) is folded sufficiently under Ca2+-saturating conditions to be ∼30-fold protected from H/D exchange (i.e. the data show that kchem/kex ≈ 30). The inhibitory region was not identified in the high resolution x-ray structure of Ca2+-saturated cardiac troponin (4), but was ordered and identified in the high resolution structure of Ca2+-saturated skeletal muscle troponin (5). Our cardiac troponin data indicate that, in solution, the Ca2+-saturated cardiac isoform involves a folded rather than disordered state for the TnI inhibitory region, corresponding to the findings for skeletal muscle troponin. Thus, it is part of the intra-troponin Ca2+ switch, in addition to any role it may have in effecting inhibition of muscle contraction.
In contrast to the coiled-coil, TnT helix 1 (at far left of Fig. 5A) and most of the TnI helix 1 were highly dynamic. The exception to this within TnI helix 1 is high protection from exchange where it comes in contact with the globular, COOH domain of TnC. This suggests a tight interaction important for troponin complex formation.
The effects of Ca2+ on troponin dynamics are illustrated in Fig. 5B. Blue regions were more protected (inferred to be less flexible and more tightly folded) when Ca2+ was saturating, and red regions were less protected when Ca2+ was saturating. Regions with statistically insignificant alteration in exchange kinetics are colored silver (with a light blue tint). For green regions the effects of Ca2+ could not be assessed. Ca2+ increased the protection from exchange for some regions and decreased protection for others, with more of the former. The figure illustrates clearly that changes were not confined to the TnC NH2 domain and overlying TnI switch helix. Rather, allosteric effects were evident in the TnC COOH domain, in the TnI strand of the coiled-coil and at the COOH terminus of both strands of the coiled-coil. Actin and tropomyosin, of course are not present in these experiments, and cannot explain these effects. These Ca2+-mediated effects on many parts of this extended molecule raise two questions: how are the effects transmitted, and what relation do these effects have to the mechanism of thin filament regulatory function?
The answers to these questions, ultimately, should reference troponin and thin filament structures, which are known in significant part. In particular, the current results should be considered in relation to the proposed atomic structure of skeletal muscle troponin in low Ca2+, hypothesized from 9 Å x-ray data obtained in the presence of Mg2+/EGTA (5). In Fig. 5C, the low Ca2+ cardiac troponin H/D exchange data are mapped onto the homologous residues of this skeletal muscle troponin model. The orientation is different from that shown in Fig. 5, A and B. Also, regardless of orientation, the TnC NH2 domain has a different position within this structure. Importantly, just beyond the far end of the coiled-coil, the inhibitory region of TnI folds back sharply in this model to form tight contacts not observed in high Ca2+ conditions. This includes contacts with the TnC NH2 domain (bottom) and the end of the TnI strand of the coiled-coil.
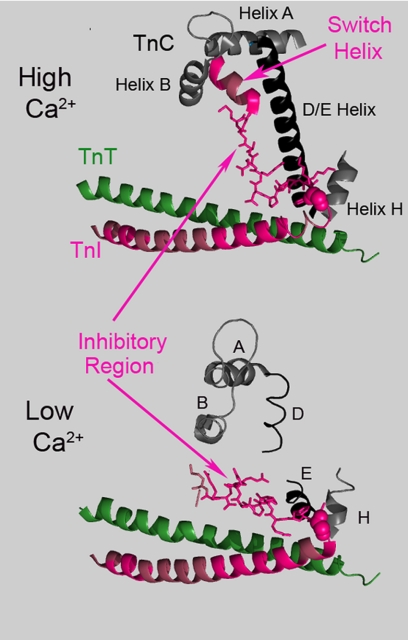
To explain the current H/D exchange findings, we propose that a similar conformation occurs in cardiac troponin, as part of an intra-troponin switch (Fig. 6). This conformation alters the H/D exchange properties of the inhibitory region and the end of the TnI strand of the coiled-coil, as well as the exchange properties of both the corresponding portion of the TnT strand, and the TnC COOH domain to which the TnT attaches. In this way, we suggest that the effect of Ca2+ on troponin is to promote a switch involving several components. In one conformational state the TnI switch helix attaches to the TnC regulatory domain, and the inhibitory region attaches to the D/E helix. In the second conformation, the inhibitory region of TnI attaches to the end of the coiled-coil. For vertebrate troponins, Ca2+ binding to the TnC NH2 domain influences this switching. We speculate that for many invertebrate troponins, switching instead is affected by Ca2+ binding to the TnC COOH domain, which interacts with the end of the coiled-coil.
FIGURE 6.
Illustration of the proposed effects of Ca2+ on troponin. Top, selected, indicated regions from the high resolution Ca2+-saturated skeletal muscle troponin structure. Bottom, same regions are shown within the proposed structure of Mg2+/EGTA skeletal muscle troponin, which was derived from intermediate resolution data in the same study (5). Cardiac TnI regions with Ca2+-sensitive H/D exchange rates are shown in hot pink, including the inhibitory region (stick representation), the switch helix, and much of the coiled-coil. A glycine at the coiled-coil terminus is shown in spherical representation, to indicate where the Ca2+-sensitive changes in the TnI inhibitory region commences in the proposed model. In high Ca2+, the cardiac TnI inhibitory region exhibits H/D exchange protection, as would be predicted from this skeletal muscle troponin structure, but not from the cardiac troponin x-ray structure (Fig. 5). Several changes occur when Ca2+ dissociates from the TnC NH2 domain. The TnI switch helix dissociates and becomes disordered. The inhibitory region turns to tightly interact with the end of the coiled coil and changes its position greatly. Interactions with TnC helix H are altered. The stabilizing effects of the inhibitory region on the D/E helix are lost.
Weaker binding between the two coiled-coil strands, when the Ca2+ concentration is low, is suggested by the faster H/D exchange properties extending through most of the TnI strand of the coiled coil in the presence of subsaturating Ca2+. This mechanism may explain how site II Ca2+ binding can have such a remote effect on H/D exchange. Furthermore, this may have functional significance (32). Increased dynamics of the TnI portion of the coiled-coil supports the possibility that it interacts with actin differently, depending upon Ca2+ conditions. At present there is little structural data regarding the interaction of the TnT-TnI coiled-coil with other thin filament proteins (33).
In summary, the troponin role in the regulation of muscle contraction consists of a Ca2+-triggered change in quaternary structure, a switching between alternative sets of intra-troponin interactions. The current results are consistent with models of regulation in which COOH-terminal portions of TnI interact with actin and tropomyosin to inhibit muscle contraction under low Ca2+, relaxing conditions. The present work implies that intra-troponin interactions, evident in the H/D exchange data, and involving the end of the TnT-TnI coiled-coil, stabilize a troponin conformation that positions the TnI COOH terminus where it can affect actin and the position of tropomyosin on actin (7, 34), regulating muscle contraction.
Acknowledgments
We thank Drs. Richard van Breemen, Yan Wang, and Andrew Robertson for assistance in the early stages of this work. Contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
This work was supported, in whole or in part, by National Institutes of Health Grants HL063774 and HL038834. This work was also supported by the CBC/UIC Proteomics and Informatics Facility, established by a grant from The Searle Funds at the Chicago Community Trust to the Chicago Biomedical Consortium.
- Tn
- troponin
- MS
- mass spectrometry
- HPLC
- high performance liquid chromatography.
REFERENCES
- 1.Tobacman L. S. (1996) Annu. Rev. Physiol. 58, 447–481 [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi T., Solaro R. J. (2005) Annu. Rev. Physiol. 67, 39–67 [DOI] [PubMed] [Google Scholar]
- 3.Li M. X., Spyracopoulos L., Sykes B. D. (1999) Biochemistry 38, 8289–8298 [DOI] [PubMed] [Google Scholar]
- 4.Takeda S., Yamashita A., Maeda K., Maéda Y. (2003) Nature 424, 35–41 [DOI] [PubMed] [Google Scholar]
- 5.Vinogradova M. V., Stone D. B., Malanina G. G., Karatzaferi C., Cooke R., Mendelson R. A., Fletterick R. J. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 5038–5043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Syska H., Wilkinson J. M., Grand R. J. A., Perry S. V. (1976) Biochem. J. 153, 375–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galińska-Rakoczy A., Engel P., Xu C., Jung H., Craig R., Tobacman L. S., Lehman W. (2008) J. Mol. Biol. 379, 929–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tobacman L. S., Butters C. A. (2000) J. Biol. Chem. 275, 27587–27593 [DOI] [PubMed] [Google Scholar]
- 9.Heeley D. H., Belknap B., White H. D. (2006) J. Biol. Chem. 281, 668–676 [DOI] [PubMed] [Google Scholar]
- 10.Smith D. A., Maytum R., Geeves M. A. (2003) Biophys. J. 84, 3155–3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gafurov B., Chalovich J. M. (2007) FEBS J. 274, 2287–2299 [DOI] [PubMed] [Google Scholar]
- 12.Xing J., Chinnaraj M., Zhang Z., Cheung H. C., Dong W. J. (2008) Biochemistry 47, 13383–13393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doi T., Satoh A., Tanaka H., Inoue A., Yumoto F., Tanokura M., Ohtsuki I., Nishita K., Ojima T. (2005) Arch. Biochem. Biophys. 436, 83–90 [DOI] [PubMed] [Google Scholar]
- 14.Qiu F., Lakey A., Agianian B., Hutchings A., Butcher G. W., Labeit S., Leonard K., Bullard B. (2003) Biochem. J. 371, 811–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Englander S. W. (2000) Annu. Rev. Biophys. Biomol. Struct. 29, 213–238 [DOI] [PubMed] [Google Scholar]
- 16.Smith D. L., Deng Y., Zhang Z. (1997) J. Mass Spectrom. 32, 135–146 [DOI] [PubMed] [Google Scholar]
- 17.Arrington C. B., Robertson A. D. (2000) Methods Enzymol. 323, 104–124 [DOI] [PubMed] [Google Scholar]
- 18.Kim K. S., Fuchs J. A., Woodward C. K. (1993) Biochemistry 32, 9600–9608 [DOI] [PubMed] [Google Scholar]
- 19.Huyghues-Despointes B. M., Scholtz J. M., Pace C. N. (1999) Nat. Struct. Biol. 6, 910–912 [DOI] [PubMed] [Google Scholar]
- 20.Bai Y., Milne J. S., Mayne L., Englander S. W. (1993) Proteins 17, 75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Z., Post C. B., Smith D. L. (1996) Biochemistry 35, 779–791 [DOI] [PubMed] [Google Scholar]
- 22.Hinkle A., Tobacman L. S. (2003) J. Biol. Chem. 278, 506–513 [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z., Marshall A. G. (1998) J. Am. Soc. Mass Spectrom. 9, 225–233 [DOI] [PubMed] [Google Scholar]
- 24.Anand G. S., Hughes C. A., Jones J. M., Taylor S. S., Komives E. A. (2002) J. Mol. Biol. 323, 377–386 [DOI] [PubMed] [Google Scholar]
- 25.Li M. X., Gagné S. M., Spyracopoulos L., Kloks C. P., Audette G., Chandra M., Solaro R. J., Smillie L. B., Sykes B. D. (1997) Biochemistry 36, 12519–12525 [DOI] [PubMed] [Google Scholar]
- 26.Houdusse A., Love M. L., Dominguez R., Grabarek Z., Cohen C. (1997) Structure 5, 1695–1711 [DOI] [PubMed] [Google Scholar]
- 27.Spyracopoulos L., Gagné S. M., Li M. X., Sykes B. D. (1998) Biochemistry 37, 18032–18044 [DOI] [PubMed] [Google Scholar]
- 28.Wang F., Li W., Emmett M. R., Marshall A. G., Corson D., Sykes B. D. (1999) J. Am. Soc. Mass Spectrom. 10, 703–710 [DOI] [PubMed] [Google Scholar]
- 29.Brown L. J., Sale K. L., Hills R., Rouviere C., Song L., Zhang X., Fajer P. G. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 12765–12770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biesiadecki B. J., Kobayashi T., Walker J. S., John, Solaro R., de Tombe P. P. (2007) Circ. Res. 100, 1486–1493 [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto F., Maeda K., Chatake T., Maéda Y., Fujiwara S. (2009) Biochem. Biophys. Res. Commun. 382, 205–209 [DOI] [PubMed] [Google Scholar]
- 32.Luque I., Leavitt S. A., Freire E. (2002) Annu. Rev. Biophys. Biomol. Struct. 31, 235–256 [DOI] [PubMed] [Google Scholar]
- 33.Sun Y. B., Brandmeier B., Irving M. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 17771–17776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poole K. J., Lorenz M., Evans G., Rosenbaum G., Pirani A., Craig R., Tobacman L. S., Lehman W., Holmes K. C. (2006) J. Struct. Biol. 155, 273–284 [DOI] [PubMed] [Google Scholar]