Abstract
Serotonin (5-HT) contributes to the prenatal development of the central nervous system, acting as a morphogen in the young embryo and later as a neurotransmitter. This biologically active agent influences both morphological and biochemical differentiation of raphe neurons, which give rise to the descending serotonergic paths that regulate the processing of acutely evoked nociceptive inputs. The involvement of 5-HT in the prenatal development of tonic nociceptive system has not been studied. In the present study we evaluated the effects of a single injection (400 mg/kg, 2 ml, i.p.) of the 5-HT synthesis inhibitor, para-chlorophenylalanine (pCPA), given to pregnant rats during the critical period fetal serotonin development. The functional integrity of the tonic nociceptive response was investigated in 25 day old rats using the classic formalin test. Morphological analysis of brain structures involved in formalin-induced pain and 5-HT levels in the heads of 12-day embryos were also evaluated. Embryonic levels of 5-HT were significantly lowered by the treatment. The juvenile rats from pCPA-treated females showed altered brain morphology and cell differentiation in the developing cortex, hippocampus, raphe nuclei, and substantia nigra. In the formalin test, there were significant decreases in the intensity and duration of the second phase of the formalin-induced response, characterizing persistent, tonic pain. The extent of impairments in the brain structures correlated positively with the level of decrease in the behavioral responses. The data demonstrate the involvement of 5-HT in the prenatal development of the tonic nociceptive system. The decreased tonic component of the behavioral response can be explained by lower activity of the descending excitatory serotonergic system originating in the raphe nuclei, resulting in decreased tonic pain processing organized at the level of the dorsal horn of the spinal cord.
Background
Serotonin (5-HT) plays an important role in regulating the development of the central nervous system [1-4]. Deficiency of 5-HT in early embryogenesis, when this biologically active substance influences cell and tissue development, causes disorders in neurogenesis [5,6] and abnormalities of development of brain and its transmitter systems. Consequences of these abnormalities persist into postnatal life and are accompanied by a number of functional disorders [6]. The involvement of 5-HT in the prenatal development of the processes that mediate tonic nociception remains to be understood. To understand the role of 5-HT in the development of pain processing, we decreased 5-HT content in fetal brain during the period when there is active development of nociceptive systems. In the present research, we focused our attention on investigation of the tonic component of nociceptive system in the juvenile (25-day-olds) offspring of rats born to females, who during a critical period of development of fetal serotonergic development were treated with an inhibitor of 5-HT synthesis, para-chlorophenylalanine (pCPA). We were particularly interested in the functional abnormalities of nociception in the behavioral response in the widely used formalin test [7] and in morphological characteristics of brain structures involved that response. The behavioral response to injection of formalin is presented by two phases. The first, short, acute phase reflects acute pain and the second, prolonged, tonic phase – tonic pain. Between the phases, there is an interphase, a period of inactivity, characterizing a process of inhibition in the central nervous system [10]. At 25 days of age rat pups the biphasic behavioral response in the formalin test has matured [8,9]. The formalin test, unlike other tests that are usually used for nociception estimation, favors studies of the feedback-type modulation effects and endogenous systems of pain regulation, including the descending serotonergic system.
Material and Methods
Rats
Experiments were performed in 25-day-old rat pups (30 females, 35 males) that were the offspring of 18 female Wistar rats. The guidelines published by the Committee for Research and Ethical Issues of the IASP on ethical standards for investigations of experimental pain in animals were followed [11]. Adult female and male rats obtained from the vivarium of I.P. Pavlov Institute of Physiology, were placed together in plastic cages (55 × 35 × 20 cm) for a night in our laboratory vivarium. Next morning vaginal smears were used to determine pregnancy. The presence of sperm was declared gestational day 0 (GD0). The day of birth of offspring was defined as postnatal day 0 (P0). Two days after birth each litter was culled to eight pups, four females and four males. Each litter was housed in a plastic cage and kept in the temperature-controlled laboratory vivarium (20–22°C) with a 12 h light/12 h dark cycle beginning at 07:00 h, and with full access to food and water.
5-HT depletion
5-HT depletion was performed by injection of an aqueous suspension of the inhibitor of 5-HT synthesis, pCPA (400 mg/kg/2 ml, i.p.; ICN, U.S.A.), to the dams on day 9 of pregnancy. The day of injection was selected because the maximal effect of the drug (the greatest decrease of 5-HT concentration in the dam and in the embryo) is within 2–3 days after injection at this age. This developmental window is critical for development of the serotonergic system, since it is this time when dorsal raphe neurons start to differentiate [5,12]. The dose of pCPA was chosen because it had been reported that 5-HT concentration in rat's midbrain was decreased by 74% three days after comparable treatment [13]. To confirm the effect of maternal pCPA treatment on 5-HT concentration in heads of 12-day-old embryos, the females were anesthetized with ether, the fetuses were removed and females sacrificed. Embryos were placed into a petri dish with ice-cold phosphate buffered saline and the heads were cut off at the neck under control of bifocal microscope. The heads were placed into liquid nitrogen and kept at 70°C for no more than a week. Concentration of 5-HT was determined using high-performance liquid chromatography (HPLC) and was expressed as ng per mg of protein estimated by Lowry method [14]. The Biochemistry Laboratory of the Institute of Obstetrics and Gynecology (ST. Petersburg) performed these analyses.
The formalin test
At 25 days of age each rat pup was weighed and then placed into an experimental chamber (25 × 25 × 25 cm) with transparent walls for recording specific behaviors – flexing, shaking and licking – in response to formalin injection (2.5%, 10 μl into the plantar pad of the left hindpaw). We recorded the intensity of behaviors during each minute for 60 min, which is the duration of the response. We analyzed both the intensity (the number of flexes+shakes and licking duration in sec) and duration (in min) of the first and the second phase response and the duration of the interphase. These responses characterize pain sensitivity in the formalin test [7]. Control rat pups (7 females and 7 males) were injected with the saline of equivalent volume into the pad of the left hind paw.
Morphological analysis
Morphological analysis of the brain was carried out using routine histological techniques. The brain was fixed in the Bouin fixative, embedded in paraffin and frontal sections 7 μm thick were prepared. The sections were stained for Nissl substance.
Statistical analysis
Statistical analysis used the Wilcoxon rank pair test for comparison within each group, for dependent variables (along with Student's t-test for dependent variables) and the Mann-Whitney test for evaluation of different groups of animals, for independent variables. Non-parametric tests were used because scores were not normally distributed. For all tests P < 0.05 was considered as statistically significant.
Results
In the 25-day-old offspring of saline-treated female controls, the injection of formalin induced the biphasic behavioral response consisting of the first phase (1–4 min) and the second phase (12–24 min). The interphase between them lasted for 6–9 min during which the specific behaviors, flexing, shaking or licking, were not shown. Similar injection of saline to 25-day-old offspring from both pCPA- and saline-treated females did not produce any nociceptive behaviors.
The birth weights of pups from pCPA-treated females were somewhat lower than those of control pups, but during the postnatal development the weights of rat pups in both groups were equal (58.5 ± 0.9 g in 25-day-old rat pups from pCPA-treated females and 59.4 ± 1.1 g from saline-treated controls).
The treatment of rats with pCPA on day 9 of pregnancy led to significant changes in formalin-induced pain in their 25-day-old offspring and severe morphological alterations in brain structures involved in formalin-induced pain, compared to offspring from control females. Biochemical assays indicated that the levels of 5-HT content in heads of 12-day-old fetus from pCPA-treated females were 63,6% lower compared to those in control fetuses and were 0,05 ± 0,006 ng/mg (n = 5) and 0,137 ± 0,01 ng/mg (n = 6), respectively.
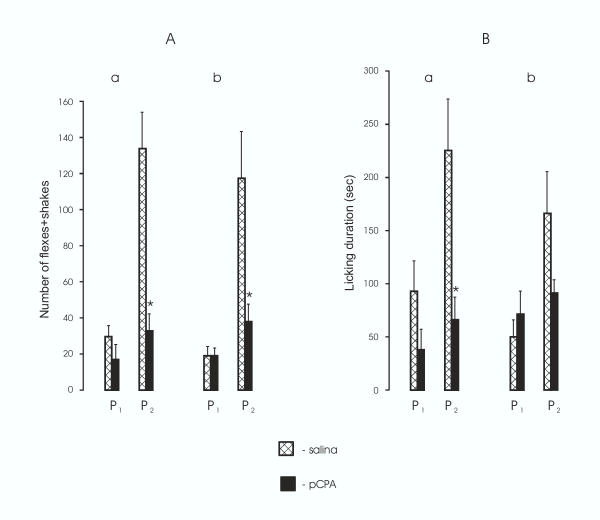
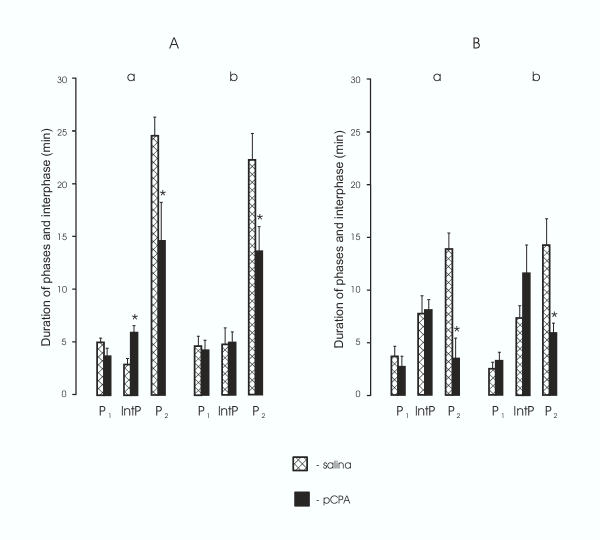
Of all prenatally pCPA-treated animals 12 of 14 female rat pups and 10 of 15 male rat pups showed reliable decreases in pain sensitivity during the second phase. The remainder of pups prenatally treated with pCPA (2 females and 5 males) failed to show any nociceptive specific behaviors in response to injection of formalin and therefore were not included in Figures 1 and 2 or the analyses. The reduction in nociception was evidenced by a significant decrease in number of flexes+shakes (p < 0.05; Fig. 1, Aa) and licking duration (p < 0.04; Fig. 1, Ba) in female pups and by a significant decrease (p < 0,02) of flexing+shaking behavior (Fig. 1, Ab) and a pronounced tendency for a decrease of licking duration (Fig. 1, Bb) in male pups, compared appropriate controls. Moreover, pCPA treatment of the pregnant rats compared to saline treatment produced in offspring a highly significant decrease in duration of the second phase of the response to formalin, both in female pups (flexing+shaking, p < 0.006, Fig. 2 Aa; licking p < 0.002, Fig. 2 Ba) and male pups (flexing+shaking, p < 0.02, Fig. 2, Ab; licking, p < 0.01, Fig. 2, Bb). Duration of the interphase increased (p < 0.02) in prenatally pCPA-treated female rat pups compared to prenatally saline-treated female pups (Fig. 2, Aa). No differences were found between female and male rat pups in the behavioral response in the formalin test.
Figure 1.
The effect of pCPA treatment of the pregnant rats on intensity of the biphasic behavioral response in the formalin test in 25-day-old offspring. A, The number of flexes+shakes; B, licking duration (sec); a, female, b, male rat pups. P1, P2, the first and second phases, respectively. The data are shown as the mean ± SEM. Asterisks indicate statistically significant differences between offspring of pCPA- and saline-treated (control) rats.
Figure 2.
The effect of pCPA treatment of the pregnant rats on the duration of the phases and the interphase of the behavioral response in the formalin test in 25-day-old offspring. A, The duration (min) of the phases and the interphase in flexing+shaking behaviors. B, The duration (min) of the phases and the interphase in licking behavior. a, female, b, male rat pups. P1, P2, the first and second phase, respectively. IntP, the interphase. The data are shown as the mean ± SEM. Asterisks indicate statistically significant differences between offspring of pCPA- and saline-treated (control) rats.
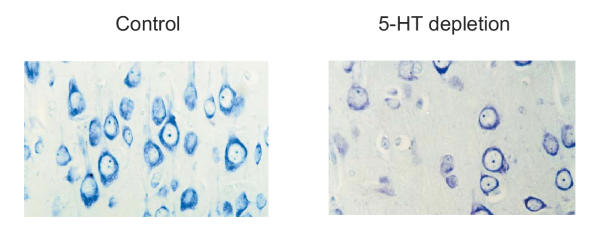
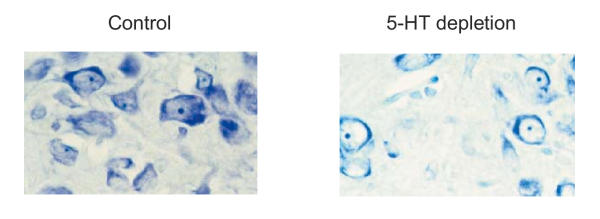
Figures 3 and 4 demonstrate the most typical morphological changes in the brain structures observed following prenatal pCPA treatment in rat pups that failed to show any behavioral response in the formalin test. Morphological analysis of the neocortex, hippocampus and raphe nuclei demonstrated that prenatally pCPA-exposed animals had a fully layered cortex but with reduced cortical thickness and fewer cells. The neurons of the neocortex had large round and very faintly stained nuclei with a thin rim of cytoplasm and little Nissl substance (Fig. 3). Hyperchromatosis-like loss of either single neurons or neuron clusters was found in all the layers of the cingular, occipital and parietal cortex and the substantia nigra. Chromatolysis was observed often in the upper cortical layers; shrunken cells were demonstrated in periamygdalar area and in the prepyriform cortex. Neurons in the CA4 zone of the hippocampus were inappropriately oriented and there were fewer cells in the raphe nuclei than in the control. Morphological characteristics of neurons in the raphe nuclei were similar to those found in neocortex (Fig. 4). In animals with decreased behavioral response the morphological changes were similar but less pronounced.
Figure 3.
Cells of neocortex (the 5th layer) in 25-day-old rat pup. Nissl staining. Magnification, X400; a – prenatally saline-treated (control), b – pCPA-treated rat pups, pCPA was injected at a dose of 400 mg/kg, 2 ml, intraperitoneally to the female rat on day 9 of pregnancy. Note the evident cell loss and morphological alterations of neurons, that have large round and very light nuclei with a thin rim of cytoplasm with little Nissl' substance in a prenatally pCPA-treated rat pup as compared with control.
Figure 4.
Cells of the dorsal raphe nuclei in 25-day-old rat pup. Nissl staining. Magnification, X400; a – a prenatally saline-treated (control), b – a pCPA-treated rat pup, pCPA was injected at a dose of 400 mg/kg, 2 ml, intraperitoneally to the female rat on day 9 of pregnancy. Note the evident cell loss and morphological alterations of neurons, that have large round and very light nuclei with a thin rim of cytoplasm with little Nissl' substance in a prenatally pCPA-treated rat pup as compared with control.
Discussion
The present study demonstrated that a single pCPA treatment of rats on day 9 of pregnancy, a critical day for the normal fetal development of serotonin, resulted in alterations of brain morphology and tonic nociception in 25-day-old offspring. Moreover, the study provides evidence for a significant decrease in the level of 5-HT content in heads of 12-day-old embryos from pCPA-treated compared to saline-treated females. These results are consistent with the data that maternal pCPA treatment significantly depleted 5-HT levels within embryonic 5-HT neurons [15].
A comparative qualitative analysis of the state of neurons in brain structures (the cortical areas, hippocampus, raphe nuclei, substantia nigra) of rat pups from pCPA-treated females, revealed essential disorders in neuronal development and differentiation. These findings are noteworthy because of the lack of data on mechanisms of formalin-induced pain other than for spinal cord and brain stem.
It is important to note that the morphological damage described in our study in offspring from pCPA-treated females was accompanied by decreased nociceptive sensitivity during the second phase of behavioral response: the significant decrease in flexing/ shaking and licking behaviors organized at the spinal and supraspinal levels, respectively. Some rat pups from pCPA-treated females showed a total suppression of the behavioral responses. The formalin test gives an opportunity to assess quantitatively nociceptive sensitivity [7]. The second phase of biphasic behavioral response in the formalin test characterizes tonic pain and is determined by both central and peripheral sensitization, induced by the first phase and by afferent signaling from the inflammation focus [16,17]. The differences in the effects of prenatal 5-HT depletion on the indices of the first and second phases are attributable to the different neurophysiologic and neurochemical mechanisms mediating tonic and acute nociception [18,19]. Furthermore, in contrast to acute pain, which serves a protective function, tonic pain is associated with inflammation and is under prolonged control of the hypothalamic-pituitary-adrenal system, which is influenced by 5-HT [20]. Prenatal 5-HT depletion alters 5-HT-hormonal interactions, which in turn might alter tonic pain. The increased interphase duration in the female rat pups suggests increased inhibition in the central nervous system and is consistent with data showing that descending 5-HT raphe inputs slow the maturation of inhibitory systems in the spinal cord [21].
Our observations demonstrated that rat pups with total suppression of the behavioral response displayed more pronounced morphological impairments in cortical structures, hippocampus, raphe nuclei, substantia nigra than compared to those with decreased response. These structures are thought to be involved in the biphasic response to formalin [22,23] and receive projections from the serotonergic centers – the dorsal and medial raphe nuclei [24]. The individual differences in the behavioral responses and concomitant morphological alterations in brain structures in rat pups even from the same litter may be accounted for by the fact that ovulation and fertilization of eggs in females do not occur simultaneously. For this reason, the embryos located in the uterus at the moment of injection are at different stages of embryonic development [25]. Moreover, individual differences in pharmacokinetics may have also played a role.
The current studies are consistent with altered development of descending serotonergic pathways and 5-HT3 receptors [27]. The 5-HT3 receptors have been located both in brain and on primary sensory fibers in the dorsal horn [28]. There is a conclusive proof that 5-HT released from the descending bulbospinal neurons exerts a dual influence on spinal nociceptive transmission: facilitation through the 5-HT3 receptor and inhibition through the 5-HT1A receptor [29]. Furthermore, Green and Dickenson [26] reported that the descending serotonergic system is enhanced during formalin-induced inflammation, acting via excitatory 5-HT3 receptors in the dorsal horn of the spinal cord, thus, facilitating the response of nociceptive spinal neurons to peripheral irritant. Antagonists of 5-HT3 receptors, blocking the actions of 5-HT in the spinal cord, are antinociceptive [7]. The present data suggest that abnormal development of the dorsal raphe nuclei and other brain structures under study may impair the function of the descending excitatory serotonergic system. As a result, the nociceptive signals entering the supraspinal structures are inhibited, with a consequent decrease in the behavioral indices of the second phase in formalin-induced pain. The above-mentioned facts and our findings are consistent with the hypothesis that the activity of 5-HT3 receptors in rat pups after prenatal 5-HT depletion is strongly suppressed.
In summary, our study shows that a single pCPA treatment of the pregnant rat during the critical period of early development of the serotonergic system in the fetus leads to the decrease in the level of 5-HT content in fetus brains, and morphological alterations in developing cortical areas, hippocampus, raphe nuclei and substantia nigra, involved in formalin-induced pain. These impairments are accompanied by a severe loss of pain sensitivity during the second phase of the behavioral response in 25-day-old offspring. The data obtained demonstrate the involvement of 5-HT in the prenatal development of the tonic nociceptive system assessed in the formalin test in juvenile rats.
Authors' contributions
IP carried out pCPA treatment, the behavioral experiments in the formalin test, participated in the sequence alignment and drafted and translated the manuscript.
LI carried out the morphological analysis
ViAn carried out pCPA treatment, participated in the alignment, performed the statistical analysis and preparation of illustrations.
VlAl participated in the design of the study, drafted manuscript.
The Biochemistry Laboratory of the Institute of Obstetrics and Gynecology (ST. Petersburg) performed analyses of concentration of 5-HT in heads of rat fetuses.
All authors conceived of the study, read and approved the final manuscript.
Acknowledgments
Acknowledgments
This study was supported by the Russian Foundation for Basic Research (project N 02-04-48338) and by the Grant of the Leading Scientific School of Russia (ÍSH-116312003.4). We would like to thank Professor G.A. Barr for his editorial assistance.
Contributor Information
Irina P Butkevich, Email: but@kolt.infran.ru.
Ludmila I Khozhai, Email: otellin@infran.ru.
Victor A Mikhailenko, Email: mik@kolt.infran.ru.
Vladimir A Otellin, Email: otellin@infran.ru.
References
- Lauder JM. Otnogeny of the serotonergic system in the rat: serotonin as a developmental signal. Ann N Y Acad Sci. 1990;600:297–313. doi: 10.1111/j.1749-6632.1990.tb16891.x. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Druse M, Walker P, Lauder JM. Serotonin as a developmental signal. Behav Brain Res. 1996;73:19–29. doi: 10.1016/0166-4328(96)00071-X. [DOI] [PubMed] [Google Scholar]
- Verney C, Lebrand C, Gaspar P. Changing distribution of monoaminergic markers in the developing human cerebral cortex with special emphasis on the serotonin transporter. Anat Rec. 2002;267:87–93. doi: 10.1002/ar.10089. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Krebs H. Effects of p-chlorophenylalanine on time of neuronal origin during embryogenesis in the rat. Brain Res. 1976;107:638–644. doi: 10.1016/0006-8993(76)90153-0. [DOI] [PubMed] [Google Scholar]
- Otellin VA, Khozhai LI. Role of 5-HT in the prenatal development of brain and its pathology in mammalia. In: SPMA. St. Petersburg, editor. In Theoretical and applied aspects of embrional histogenesis. 2002. pp. 25–32. [Google Scholar]
- Dubisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- Barr GA. Maturation of the biphasic behavioral and heart rate response in the formalin test. Pharmacol Biochem Behav. 1998;60:329–335. doi: 10.1016/S0091-3057(97)00602-3. [DOI] [PubMed] [Google Scholar]
- Butkevich IP, Vershinina EA. Prenatal stress alters time characteristics and intensity of formalin-induced responses in juvenile rats. Brain Res. 2001;915:88–93. doi: 10.1016/S0006-8993(01)02819-0. [DOI] [PubMed] [Google Scholar]
- Henry JL, Yashpal K, Pitcher GM, Coderre TJ. Physiological evidence that the "interphase" in the formalin test is due to active inhibition. Pain. 1999;82:57–63. doi: 10.1016/S0304-3959(99)00033-0. [DOI] [PubMed] [Google Scholar]
- Committee for Research and ethical issue of the IASP Ethical standards for investigations of experimental pain in animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Towle AC, Patrick K, Henderson P, Krebs H. Decreased serotonin content of embryonic raphe neurons following maternal administration p-chlorophenylalanine: a quantitative immunocytochemical stydy. Dev Brain Res. 1985;20:107–114. doi: 10.1016/0165-3806(85)90092-6. [DOI] [PubMed] [Google Scholar]
- Miller GP, Cox RH, Snodgrass JWR, Maickel RP. Comparative effects of p-chlorophenylalanine, p-chloroamphetamine and p-chloro-N-methylamphetamine on rat brain norepinephrine, serotonin and 5-hydroxyindole-3 acetic acid. Biochemical Pharmacology. 1970;19:435–442. doi: 10.1016/0006-2952(70)90199-1. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Lauder JM. Hormonal and humoral influences on brain development. Psychoneuroendocrinology. 1983;8:121–155. doi: 10.1016/0306-4530(83)90053-7. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Katz J, Vaccarino AL, Melzack R. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain. 1993;52:259–285. doi: 10.1016/0304-3959(93)90161-H. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Sullivan AF. Peripheral origins and central modulation of subcutaneous formalin-induced activity of rat dorsal horn neurons. Neurosci Lett. 1987;83:207–211. doi: 10.1016/0304-3940(87)90242-4. [DOI] [PubMed] [Google Scholar]
- Dennis SG, Melzack R. Comparison of phasic and tonic pain in animals. In: Bonica JJ, editor. In Advances in Pain Research Therapy. Vol. 3. New York: Raven Press; 1979. pp. 747–761. [Google Scholar]
- Basbaum AI. Distinct neurochemical features of acute and persistent pain. Proc Natl Acad Sci USA. 1999;96:7739–7743. doi: 10.1073/pnas.96.14.7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouloff F. Physiopharmacological interactions between stress hormones and central serotonergic systems. Brain Res Rev. 1993;18:1–32. doi: 10.1016/0165-0173(93)90005-K. [DOI] [PubMed] [Google Scholar]
- Branchereau P, Chapton J, Meyrand P. Descending 5-hydroxytryptamine raphe inputs repress the expression of serotonergic neurons and slow the maturation of inhibitory systems in mouse embryonic spinal cord. J Neurosci. 2002;22:2598–2606. doi: 10.1523/JNEUROSCI.22-07-02598.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JE, Melzack R. Blocking NMDA receptors in the hippocampal dentate gyrus with AP5 produces analgesia in the formalin pain test. Exp Neurol. 2001;172:92–99. doi: 10.1006/exnr.2001.7777. [DOI] [PubMed] [Google Scholar]
- Morrow TJ, Paulson PE, Danneman PJ, Casey KL. Regional changes in forebrain activation during the early and late phase of formalin nociception: analysis using cerebral blood flow in the rat. Pain. 1998;75:355–365. doi: 10.1016/S0304-3959(98)00016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978;179:641–659. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Krishna M, Generoso WH. Timing of sperm penetration, pronuclear formation, pronuclear DNA synthesis and first cleavage in naturallyy ovulated mouse eggs. J Exper Zoology. 1977;202:245–267. doi: 10.1002/jez.1402020214. [DOI] [PubMed] [Google Scholar]
- Green GM, Scarth J, Dickenson A. An excitatory role for 5-HT in spinal inflammatory nociceptive transmission; state-dependent actions via dorsal horn 5-HT3 receptors in the anaesthetized rat. Pain. 2000;89:81–88. doi: 10.1016/S0304-3959(00)00346-8. [DOI] [PubMed] [Google Scholar]
- Bell J, III, Zhang X, Whitaker-Azmitia PM. 5-HT3 receptor-active drugs alter development of spinal serotonergic innervation: lack of effect of other serotonergic agents. Brain Res. 1992;571:293–297. doi: 10.1016/0006-8993(92)90667-X. [DOI] [PubMed] [Google Scholar]
- Kilpatrick GJ, Jones BJ, Tyers MB. Identification and distribution of 5-HT3 receptors in the brain using radioligand binding. Nature. 1987;330:746–748. doi: 10.1038/330746a0. [DOI] [PubMed] [Google Scholar]
- Oyama T, Ueda M, Kuraishi Y, Akaike A. Dual effect of serotonin on formalin-induced nociception in the rat spinal cord. Neurosci Res. 1996;25:129–135. doi: 10.1016/0168-0102(96)01034-6. [DOI] [PubMed] [Google Scholar]