Abstract
Zinc-α2-glycoprotein (ZAG, also listed as AZGP1 in the MGI Database), a lipid-mobilising factor, has recently been suggested as a potential candidate in the modulation of body weight. We investigated the effect of increased adiposity on ZAG expression in adipose tissue and the liver and on plasma levels in obese (ob/ob) mice compared with lean siblings. The study also examined the effect of the pro-inflammatory cytokine tumour necrosis factor-α (TNFα) on ZAG expression in adipocytes. Zag mRNA levels were significantly reduced in subcutaneous (fourfold) and epididymal (eightfold) fat of ob/ob mice. Consistently, ZAG protein content was decreased in both fat depots of ob/ob mice. In the liver of obese animals, steatosis was accompanied by the fall of both Zag mRNA (twofold) and ZAG protein content (2·5-fold). Plasma ZAG levels were also decreased in obese mice. In addition, Zag mRNA was reduced in epididymal (fivefold) and retroperitoneal (fivefold) adipose tissue of obese (fa/fa) Zucker rats. In contrast to Zag expression, Tnfα mRNA levels were elevated in adipose tissue (twofold) and the liver (2·5-fold) of ob/ob mice. Treatment with TNFα reduced Zag gene expression in differentiated adipocytes, and this inhibition was chronic, occurring at 24 and 48 h following TNFα treatment. It is concluded that ZAG synthesis in adipose tissue and the liver is downregulated, as are its circulating levels, in ob/ob mice. The reduced ZAG production may advance the susceptibility to lipid accumulation in these tissues in obesity, and this could be at least in part attributable to the inhibitory effect of TNFα.
Introduction
Obesity is now a major health problem as it predisposes to insulin resistance, type 2 diabetes and cardiovascular malfunction. Although the pathogenesis of obesity and its associated co-morbidities are multifactorial, growing evidence suggests that altered production of adipose-derived protein factors (adipokines), such as leptin, tumour necrosis factor-α (TNFα), adiponectin and chemerin, plays an important role. Zinc-α2-glycoprotein (ZAG, also called AZGP1) is a secreted, soluble protein that has been found in plasma and other body fluids in humans (Burgi & Schmid 1961, Tada et al. 1991). Crystal structure analysis has demonstrated that ZAG belongs to the major histocompatibility complex (MHC) class I family (Sanchez et al. 1999). Unlike other MHC molecules, ZAG is not membrane bound, and its functions seem to differ from that of MHC class I molecules (Sanchez et al. 1999, McDermott et al. 2006). Although the biological roles of ZAG have not been fully characterised, the protein has been shown to be identical to a lipid-mobilising factor (LMF), purified from the urine of patients with cancer cachexia (Todorov et al. 1998). Treatment with purified ZAG causes a selective reduction in body fat in both obese (ob/ob) and normal mice (Hirai et al. 1998, Bing et al. 2002). In vitro, ZAG stimulates lipolysis in isolated murine and human adipocytes, and this appears to be attributable to its signalling through β3-adrenoceptors in rodents (Hirai et al. 1998). Further, there is evidence that ZAG directly promotes lipid utilisation, possibly by the upregulation of mitochondrial uncoupling proteins (Bing et al. 2002).
ZAG has been identified in various organs, including the liver, breast, lung and prostate (Tada et al. 1991). Recent work from our group has shown that ZAG is also expressed in mouse, rat and human adipose tissue (Bing et al. 2004, Tzanavari et al. 2007). Importantly, ZAG is secreted abundantly from mature adipocytes as a major adipokine which may have an autocrine/paracrine role in adipose tissue function (Bao et al. 2005, Mracek et al. 2009). ZAG expression in adipose tissue appears to be inversely linked to body fat mass. ZAG mRNA and protein content in adipose tissue are markedly increased in mice with cancer cachexia (Bing et al. 2004, 2006). In contrast, ZAG protein expression is reduced in white fat of neonatal rats at weaning when there is a rise in fat mass (Tzanavari et al. 2007). Furthermore, ZAG mRNA levels have been reported to be decreased in subcutaneous fat of obese women (Dahlman et al. 2005) and men (Marrades et al. 2008). Very recently, work from our group and by others has shown that ZAG mRNA is negatively correlated with body mass index and body fat mass in human subjects (Gong et al. 2009, Mracek et al. 2009, Selva et al. 2009). In addition to adipose tissue, the liver is also a major site of ZAG synthesis in rodents (Ueyama et al. 1994, Bing et al. 2004). However, the regulation of ZAG expression in adipose tissue and the liver and of the circulating levels in obesity remains to be established.
The aim of this study was to examine the effect of increased adiposity on ZAG gene and protein expression in adipose tissue and liver of obese (ob/ob) mice in comparison with their lean siblings (ob/+). Plasma ZAG protein levels were also determined in these mice. Finally, the study explored the effect of the pro-inflammatory cytokine TNFα on ZAG expression in adipocytes.
Materials and Methods
Animals
Nine-week-old male C57BL/6 obese (ob/ob; B6.V-Lepob/OlaHsd) and appropriate lean control (B6.V-Lepob/+Lep+OlaHsd) mice from Harlan (Bicester, UK) were housed at an ambient temperature of 22±1 °C under a 12 h light:12 h darkness cycle (lights on at 0700 h) and were fed ad libitum (CRM Diet, Labsure, Poole, UK). The final body weight of obese and lean mice (n=8 per group) was recorded as 39·2±0·7 vs 24·6±0·4 (g) (mean±s.e.m). Mice were killed by cervical dislocation. Adipose tissue (epididymal and subcutaneous) and liver were rapidly dissected and frozen in liquid nitrogen. Blood samples were collected and centrifuged to obtain plasma. The tissues and plasma were stored at −80 °C until analysis. Adipose tissue (epididymal and retroperitoneal) from 3-month-old male Zucker rats (lean: +/+ or fa/+ and obese: fa/fa) bred at the Rowett Research Institute (Aberdeen, Scotland) were kindly provided by Dr Varner Rayner. All the animal studies were carried out according to the UK Home Office Guidelines for the care and use of laboratory animals and in accordance with the provisions of the United Kingdom Animals (Scientific Procedures) Act 1986.
Cell culture
Simpson–Golabi–Behmel syndrome (SGBs) cells (kindly provided by Prof. M Wabitsch, University of Ulm, Germany) were maintained and cultured as described previously (Wabitsch et al. 2001). In brief, cells were cultured at 37 °C in a humidified atmosphere of 5% CO2/95% air. SGBS preadipocytes were maintained in DMEM/Ham's F12 (1:1) medium (Invitrogen) containing 10% (v/v) FCS (Sigma) and antibiotics (penicillin/streptomycin, Lonza, Tewkesbury, UK). Preadipocytes were seeded onto 12-well plates and grown until confluence. At confluence, cells were induced to differentiate (day 0) by incubation for 5 days in FCS-free medium containing 0·25 μM dexamethasone, 500 μM 3-isobutyl-1-methyl-xanthine, 10 nM insulin, 200 pM triiodothyronine (T3), 1 μM cortisol (all from Sigma) and 2 μM rosiglitazone (GlaxoSmithKline). After induction, cells were further maintained in feeding medium (containing 10 nM insulin, 1 μM cortisol and 200 pM T3, all from Sigma) until full differentiation. Differentiation into adipocytes was visualised under the microscope by observing the accumulation of lipid droplets. The differentiated cells at day 14 after the induction of differentiation were treated with 30 ng/ml recombinant human TNFα (Sigma) for 24 h. The control cells received no addition. For the time-course study of the effect of TNFα on ZAG expression in adipocytes, cells at day 14 post-differentiation were incubated in media containing TNFα (25 ng/ml) up to 48 h. No reagent was added to the media of control cells. Cells were collected at the time points of 0, 2, 4, 6, 8, 12, 24 and 48 h.
Histology
Liver samples from ob/ob and lean mice were fixed in 10% neutral formalin for 24 h, dehydrated in absolute ethanol, cleared in xylene and then embedded in paraffin. The paraffin was cut into 5-μm sections that were stained with Harris haematoxylin, counterstained with eosin, and then evaluated and photographed under light microscopy.
Real-time PCR for mRNA quantification
Total RNA was extracted from tissues and cells using TRI reagent (Sigma), and the RNA concentration was determined from the absorbance at 260 nm. First strand cDNA was reverse transcribed from 0·5 μg of total RNA using an iScript first strand synthesis kit (Bio-Rad) in a final volume of 10 μl.
Real-time PCR amplification was performed in a final volume of 12·5 μl, containing cDNA (equivalent to 10 ng of RNA), optimised concentrations of primers, TaqMan probe FAM-TAMRA and a master mix made from qPCR core kit (Eurogentec, Seraing, Belgium) using a Stratagene Mx3005P instrument. The sequences of primers and probes for mouse ZAG, leptin, TNFα and β-actin, and rat ZAG, leptin and β-actin, and human ZAG, adiponectin and β-actin were as described previously (Bing et al. 2004, 2006, Bao et al. 2005, Tzanavari et al. 2007). PCR amplification was performed in duplicate using 96-well plates, and the PCR cycling conditions were as follows: 95 °C for 10 min followed by 40 cycles (95 °C for 15 s and 60 °C for 1 min). Blank controls without cDNA were run in parallel. β-Actin was used as a reference gene. All samples were normalised to the β-actin values, and the results are expressed as fold changes of Ct value relative to controls using the formula (Schmittgen & Livak 2008).
Recombinant mouse ZAG protein
Recombinant mouse ZAG protein was used as a positive control for the detection of ZAG protein by western blotting. Full-length mouse Zag cDNA in the pCMV-SPORT6 vector (ImaGenes, Berlin, Germany) was recloned into pcDNA3.2 plasmid (Invitrogen) and was subsequently transfected into HEK-293 cells using Metafectene Pro reagent (Biontex, Planegg, Germany). Twenty-four hours after transfection, the cells were transferred into serum-free DMEM. Medium containing secreted ZAG protein was collected every day for four consecutive days. ZAG-conditioned medium was concentrated on Amicon Ultra 15 columns with a 30 k cut-off (Millipore, Watford, UK). Successful ZAG overexpression was confirmed by western blot analysis.
Western blotting
Protein samples were prepared from mouse white adipose tissue (WAT) and liver. Homogenates of these samples were prepared in isoosmotic buffer (250 mM sucrose, 10 mM Tris–HCl and 1 mM EDTA, pH 7·4), and the protein concentration was determined by the BCA method. For plasma ZAG detection, 0·5 μl of plasma was used for each sample. Tricine–SDS electrophoresis of the samples was performed on 10% polyacrylamide slab gels (Mini Protean Tetra, Bio-Rad) using the same aliquots of SDS-solubilised proteins (20–30 μg/slot). Proteins from the gel were blotted onto a nitrocellulose membrane (Hybond C Extra, GE Healthcare, Little Chalfont, UK) by wet transfer (Trans Blot, Bio-Rad) at 100 V for 1 h. The transfer of proteins onto the membrane was assessed by Ponceau S staining.
For immunodetection of ZAG, the membranes were blocked overnight at 4 °C with TBS (0·15 M NaCl and 20 mM Tris, pH=7·6) containing 0·1% Tween 20 and 5% non-fat milk (TBSTM) and were then incubated overnight at 4 °C with polyclonal goat anti-mouse ZAG antibody (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA; sc-11243) in TBSTM. The membranes were then incubated for 1 h using donkey anti-goat IgG (1:1000; R&D Systems, Abingdon, UK; HAF109) secondary antibody conjugated with HRP in TBSTM. For the detection of cytochrome oxidase subunit IV (COX IV), succinate dehydrogenase 70 kDa subunit (SDH 70), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and α-tubulin or β-actin (used as a loading control), the conditions were as follows: membranes were blocked with TBS containing 0·1% Tween 20 and 2% BSA (TBSTA) for 1 h and were incubated for 2 h with the primary monoclonal antibody diluted in TBSTA: anti-COX IV subunit of Complex IV (1:1000; Mito Sciences, Eugene, OR, USA; MS 407), anti-SDH 70 (1:1000; Mito Sciences, MS 204), anti-GAPDH (1:5000; Sigma), anti-α-tubulin (1:2000; Sigma) and anti-β-actin (1:2000; Sigma). Subsequently, membranes were incubated with secondary HRP-conjugated rabbit anti-mouse IgG (1:2000; Bio-Rad) diluted in TBSTA for 1 h. Signals were detected on film by chemiluminescence (West Pico kit, Perbio Science, Cramlington, UK). The size of the protein bands detected was estimated with PageRuler protein markers (Fermentas, York, UK). Plasma ZAG levels were normalised to total protein concentration.
Liver triglyceride measurement
The triglyceride levels in mouse liver were determined by a colorimetric method using a triglycerides assay kit from Randox (Antrim, UK) according to the manufacturer's instructions. Liver triglyceride levels are normalised to protein content.
Statistical analysis
Data are expressed as means±s.e.m. Differences between two groups were analysed by Student's unpaired t-test; differences were considered as statistically significant when P<0·05.
Results
ZAG gene and protein expression in adipose tissue of ob/ob mice
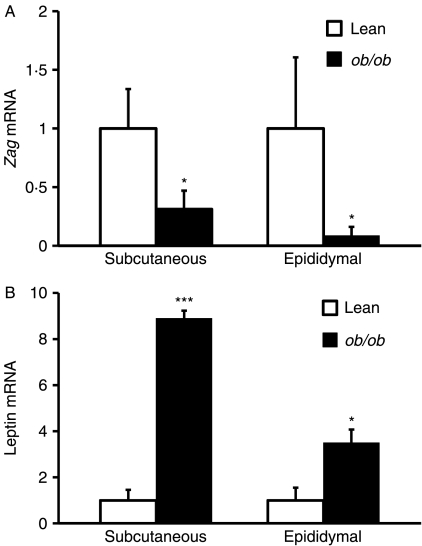
To examine whether ZAG expression in adipose tissue is altered in obesity, we first determined the relative Zag mRNA levels by real-time PCR in two major WAT depots (subcutaneous and epididymal) of ob/ob mice and their lean siblings. Zag mRNA levels were significantly reduced in both subcutaneous (fourfold, P<0·05) and epididymal (eightfold, P<0·05) fat depots of ob/ob mice compared with lean controls (Fig. 1A). In contrast, leptin mRNA levels, measured as a reference, were substantially elevated in both depots of ob/ob mice (subcutaneous: ninefold, P<0·001; epididymal: fourfold, P<0·05; Fig. 1B).
Figure 1.
Zag gene expression in adipose tissue of ob/ob and lean mice. Total RNA was extracted from subcutaneous and epididymal fat pads of lean (ob/+) and ob/ob mice, and mRNA levels of (A) Zag and (B) leptin were measured by real-time PCR and normalised to β-actin. Results are expressed as means±s.e.m. for groups of 6. *P<0·05, ***P<0·001 compared with lean controls.
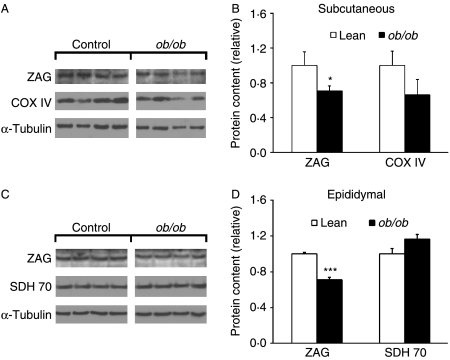
ZAG protein expression in adipose tissue of mice was assessed next by western blotting. Consistent with the data on Zag gene expression, there were decreases in ZAG protein levels in subcutaneous (−29%, P<0·05) and epididymal (−34%, P<0·001) fat of ob/ob mice relative to their lean controls (Fig. 2A and B). We also determined the levels of COX IV and SDH 70 protein as a measure of mitochondrial oxidative phosphorylation capacity in adipocytes (Choo et al. 2006). Protein expression of COX IV and SDH 70 in ob/ob mice was unchanged (both P>0·05; Fig. 2A and B) in the fat depots.
Figure 2.
ZAG protein expression in adipose tissue of ob/ob and lean mice. Protein was extracted from subcutaneous and epididymal fat of lean (ob/+) and ob/ob mice, and western blotting was used for protein expression of ZAG, COX IV, SDH 70 and α-tubulin. Representative western blot and quantification of protein expression normalised to α-tubulin in subcutaneous (A and B) and epididymal (C and D) fat. Data are means±s.e.m. for groups of 6. *P<0·05, ***P<0·001 compared with lean controls.
ZAG gene and protein expression in liver of ob/ob mice
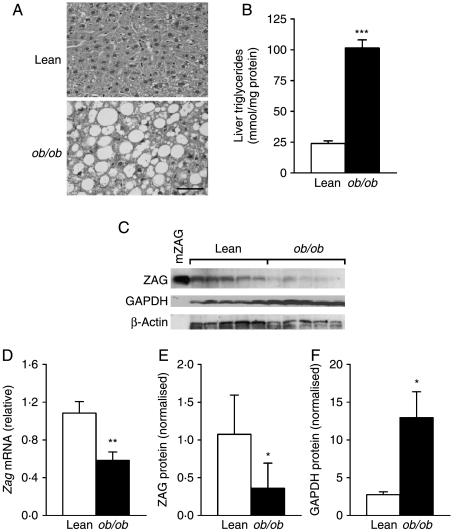
Liver histology revealed the typical features of steatosis (fatty liver) in ob/ob mice, which is characterised by the intracellular accumulation of multiple lipid droplets in hepatocytes (Fig. 3A). Concurrently, liver triglyceride content was significantly higher in obese mice than in lean siblings (4·2-fold, P<0·001; Fig. 3B). Since the liver is another major source of ZAG production, we further examined ZAG synthesis in liver of obese and lean mice. Zag mRNA levels were twofold lower (P<0·01) in ob/ob mice relative to lean mice (Fig. 3D). Consistent with the mRNA result, ZAG protein levels were 2·5-fold decreased in ob/ob mice compared with the lean controls (P<0·05; Fig. 3C and E). In contrast, GAPDH protein in the liver was increased by 4·8-fold (P<0·05) in obese animals (Fig. 3C and F).
Figure 3.
Zag mRNA and protein expression in the liver of ob/ob and lean mice. (A) Histology of the liver sections stained with H&E (bar, 50 μm); (B) liver triglyceride content; and (C) representative western blot of ZAG and GAPDH protein, recombinant mouse ZAG (mZAG) used as a positive control. (D) Liver Zag mRNA levels were measured by real-time PCR and normalised to β-actin; (E) quantification of ZAG protein normalised to β-actin and (F) quantification of GAPDH protein normalised to β-actin. Data are means±s.e.m. for groups of 7. *P<0·05, **P<0·01 compared with lean controls.
Plasma ZAG levels in ob/ob mice
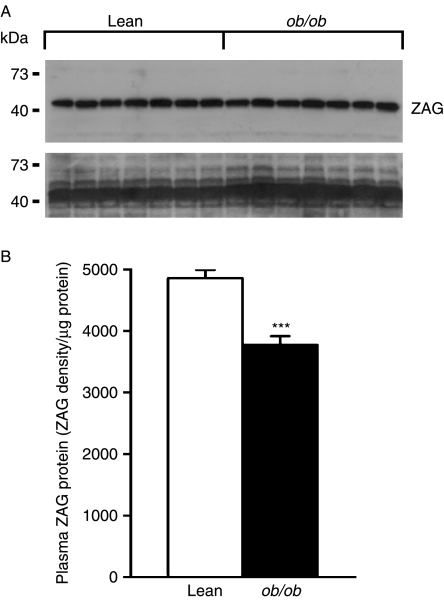
To examine whether the circulating levels of ZAG are altered in ob/ob mice, plasma ZAG protein was measured by western blotting. Plasma ZAG protein levels were lower (by 23%, P<0·001) in obese mice than in lean mice (Fig. 4A and B).
Figure 4.
Plasma ZAG protein content in ob/ob and lean mice. Plasma of 0·5 μl was used for western blotting analysis of ZAG protein. (A) Representative western blot of ZAG protein (upper panel), and total proteins assessed by Ponceau S staining of the membrane (lower panel); (B) quantification of ZAG protein, expressed as means±s.e.m. of signal density normalised by the total protein concentration relative to lean controls, n=7 per group. ***P<0·001 compared with lean controls.
ZAG expression in adipose tissue of Zucker rats
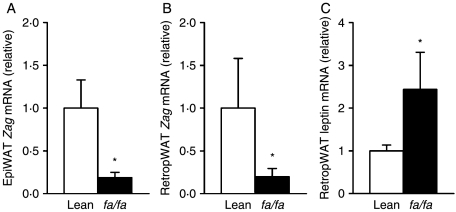
Consistent with the results in ob/ob mice, Zag mRNA levels were significantly reduced in both epididymal and retroperitoneal fat depots (by fivefold, P<0·05) in fa/fa Zucker rats relative to the lean controls (Fig. 5A and B). In contrast, leptin mRNA levels were elevated in retroperitoneal fat (by 2·5-fold, P<0·05) in fa/fa rats compared with their lean counterparts (Fig. 5C).
Figure 5.
Zag gene expression in adipose tissue of obese (fa/fa) and lean Zucker rats. Zag mRNA levels in epididymal (A) and retroperitoneal (B) fat, and leptin mRNA levels in retroperitoneal fat (C) of fa/fa and lean Zucker rats. mRNA levels were measured by real-time PCR and normalised to β-actin. Data are means±s.e.m. for groups of 6–8. *P<0·05 compared with lean controls.
Tnfα mRNA levels in adipose tissue and liver of ob/ob mice
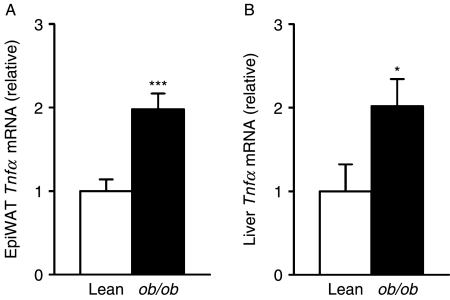
Since TNFα has a key role in obesity-related inflammation, we examined Tnfα gene expression in epididymal fat and the liver of ob/ob and lean mice. In contrast to the results for Zag, Tnfα mRNA levels were increased in both adipose tissue (twofold, P<0·001) and liver (twofold, P<0·05) in obese animals (Fig. 6A and B).
Figure 6.
Tnfα gene expression in adipose tissue and liver of ob/ob and lean mice. Total RNA was extracted from epididymal fat pad and liver of lean (ob/+) and ob/ob mice, and Tnfα mRNA levels in (A) epididymal adipose tissue and (B) liver were measured by real-time PCR and normalised to β-actin. Results are expressed as means±s.e.m. for groups of 6. *P<0·05, ***P<0·001 compared with lean controls.
Time-dependent effects of TNFα on Zag gene expression in differentiated adipocytes
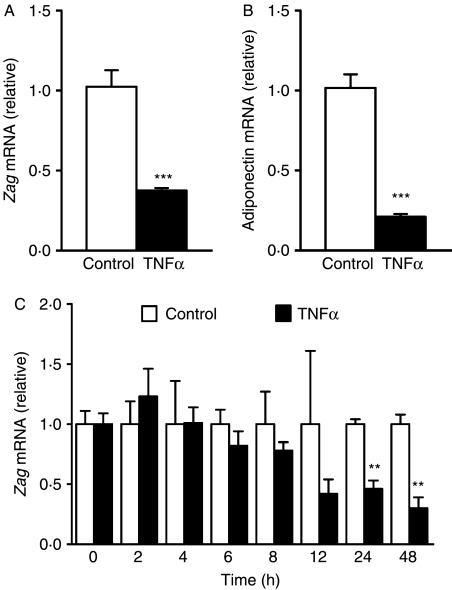
The effect of TNFα on Zag expression was further studied in differentiated SGBS adipocytes. Consistent with our previous observation with a higher dose of TNFα (50 ng/ml; Bao et al. 2005), addition of TNFα (30 ng/ml) for 24 h led to a reduction in mRNA levels of Zag (threefold, P<0·001; Fig. 7A) and adiponectin (fourfold, P<0·001; Fig. 7B), with the latter being used as a reference gene. We then examined whether the inhibitory effect of TNFα on Zag expression is time-dependent. As shown in Fig. 7C, the effect of TNFα on Zag mRNA appeared to be chronic rather than acute; the reduction in Zag mRNA was progressive, but it was only significant after incubation with TNFα for 24 (P<0·01) and 48 h (P<0·01).
Figure 7.
Effects of TNFα on Zag expression in adipocytes. SGBS cells were harvested at day 14 after the induction of differentiation and were incubated for 24 h in media containing TNFα (30 ng/ml). Control cells received no addition. (A) Zag and (B) adiponectin mRNA levels were measured by real-time PCR, normalised to β-actin and expressed relative to the controls. Data are means±s.e.m. for groups of 6. **P<0·01 compared with controls. (C) Time course of the effect of TNFα on Zag mRNA levels in adipocytes. SGBS cells were differentiated from preadipocytes, and at day 14 post-induction, they were incubated in media containing TNFα (25 ng/ml) up to 48 h. No reagent was added to the media of control cells. Zag mRNA levels were measured by real-time PCR. Data are means±s.e.m. for groups of 3. **P<0·01, ***P<0·001 compared with controls.
Discussion
In the present study, a significant difference in Zag gene and its protein expression in white fat between lean and genetically obese mice has been demonstrated. We found that Zag mRNA levels are markedly reduced in subcutaneous and epididymal adipose tissue of ob/ob mice. In contrast, mRNA levels of the mutated leptin are significantly higher in the two depots of ob/ob mice as reported previously (Frederich et al. 1995, Trayhurn et al. 1995), reflecting the greater tissue mass and perhaps a compensatory response to the mutation. Consistent with the gene expression data, we further demonstrated that ZAG protein expression is decreased in epididymal and subcutaneous fat of ob/ob mice. In agreement with our findings, a recent study has shown that Zag gene expression was also reduced in epididymal adipose tissue of obese mice fed with a high-fat diet (HFD; Gong et al. 2009). Furthermore, in the present study, we demonstrate that in addition to mouse models of obesity, Zag mRNA levels in epididymal and retroperitoneal fat are significantly decreased in obese (fa/fa) Zucker rats. Collectively, these observations suggest a possible link between increased adiposity and the downregulation of Zag expression in adipose tissue.
Obesity is associated with alterations in basal lipolysis, impaired antilipolytic action of insulin and reduced lipoprotein lipase activity (Hauner et al. 2001). Furthermore, the substantial decrease in the expression and function of β1- and β3-adrenoreceptors in adipose tissue in ob/ob mice has been suggested to be a reason for the impaired lipid mobilisation in response to β-adrenergic agonists (Robidoux et al. 2004). The repression of ZAG synthesis in obese animals might be related to its function in modulating lipid metabolism (Bing & Trayhurn 2008). It has been shown that ZAG treatment selectively reduces body fat in obese and normal mice in vivo and stimulates lipolysis in vitro (Hirai et al. 1998, Bing et al. 2002). It was proposed that ZAG may act as an alternative β-adrenergic agonist by signalling through β3-adrenoceptors in rodents (Hirai et al. 1998, Russell et al. 2004). The role of ZAG in lipid mobilisation has been supported by a recent study of ZAG-knockout mice which were susceptible to weight gain on a HFD, and this probably results from a decrease in adipocyte lipolysis (Rolli et al. 2007). In addition, mRNA levels of hormone-sensitive lipase were elevated in adipose tissue from ZAG-overexpressing transgenic mice which exhibit decreased body weight and epididymal fat (Gong et al. 2009). Therefore, reduced ZAG synthesis in adipose tissue of ob/ob mice observed in the present study may contribute to an impairment of lipid mobilisation.
Another interesting finding of this study is the demonstration of a decrease in Zag expression, both mRNA and protein, in the fatty liver of ob/ob mice. Liver is another major site for ZAG synthesis in rodents (Ueyama et al. 1994, Bing et al. 2004). It is of interest that Zag mRNA levels have been reported to be increased in the liver of mice with cancer cachexia (Bing et al. 2004), a condition associated with significantly decreased adiposity. Liver steatosis in ob/ob mice, as shown in the present study and previously (Shimomura et al. 1999), has been suggested to be the consequence of impaired lipid mobilisation and enhanced lipogenesis. Given the role of ZAG as an LMF, the fall of ZAG synthesis in the liver of obese mice may contribute to the hepatic accumulation of triglycerides. In addition to alterations in lipid metabolism, there are significant disturbances in liver glucose metabolism in ob/ob animals. A previous study has shown that hyperglycaemia in ob/ob mice is characterised by decreased phosphoenolpyruvate carboxykinase mRNA and increased Gapdh mRNA levels in the liver (Ferber et al. 1994). We show here an enhanced expression of GAPDH protein in the liver of ob/ob mice, which further supports a dysregulation of hepatic glucose metabolism in obesity.
In the present study, plasma ZAG protein content was decreased in obese ob/ob mice, and a similar decline was observed in HFD-fed obese mice (Gong et al. 2009). Concomitantly, recent studies in humans have reported reductions in serum ZAG levels in obese patients (Gong et al. 2009, Selva et al. 2009). However, a study by Stejskal et al. (2007) has found no differences in serum ZAG concentrations between obese patients with the metabolic syndrome and otherwise healthy controls. These results suggest that adipose tissue- and liver-derived ZAG may be important locally through its autocrine/paracrine actions. The production of ZAG in other tissues would influence its circulating levels, and also the clearance of ZAG in the circulation might be altered in obesity.
Although ZAG expression has been shown to be inversely related to adiposity, its regulation in obesity remains to be established. Obesity, characterised by a state of chronic low-grade inflammation, is associated with upregulation of pro-inflammatory cytokines (Yudkin et al. 1999). TNFα has been indicated as a key player in the inflammatory processes (Hotamisligil et al. 1993), and it alters the production of adipokines, such as inhibiting adiponectin whilst stimulating the synthesis of pro-inflammatory adipokines (Degawa-Yamauchi et al. 2005, Wang & Trayhurn 2006). In the present study, we demonstrate that contrary to Zag expression, Tnfα mRNA levels are increased in both adipose tissue and liver in ob/ob mice. The rise in Tnfα with decreased Zag expression may imply a role for TNFα in the lowered ZAG production in obesity. This notion is supported by the in vitro study that shows an inhibitory effect of TNFα on Zag gene expression in differentiated adipocytes, which is consistent with our previous observation (Bao et al. 2005). Therefore, TNFα could be a negative regulator of ZAG synthesis in adipose tissue. To further characterise the nature of the inhibitory effect of TNFα on Zag expression, we assessed the changes over a time course. Our results reveal a chronic effect of TNFα on ZAG in adipocytes as the apparent reduction of Zag mRNA occurs at 24 and 48 h following TNFα treatment. Taken together, these results suggest that repression of ZAG expression in adipose tissue and possibly in the liver of obese mice might be the consequence of increased TNFα in the obese state.
In conclusion, the present study shows that Zag mRNA and its protein expression in adipose tissue is downregulated in ob/ob mice. It also demonstrates that in the fatty liver of obese mice, both Zag mRNA levels and protein content are reduced. Furthermore, circulating ZAG levels are decreased in obese mice. In contrast, Tnfα mRNA levels are elevated in adipose tissue and liver of ob/ob mice. Treatment of TNFα in vitro represses ZAG expression in differentiated adipocytes. It is suggested that reduced ZAG production may advance the susceptibility to lipid accumulation in adipose tissue and liver in obesity, and this could be at least in part attributable to the inhibitory effect of TNFα.
Declaration of interest
The authors declare that there are no conflicts of interest that would prejudice the impartiality of the research reported here.
Funding
PT is a member of COST BM0602. This work was supported by the Liverpool University R&D Fund, the Biotechnology and Biological Sciences Research Council (BBE015379) and the Medical Research Council (87972).
Acknowledgements
We are grateful to Prof. Martin Wabitsh (University of Ulm, Germany) for the provision of SGBS adipocytes. We thank Dr Vernon Rayner (Rowett Research Institute, Aberdeen, Scotland) for providing adipose tissue of Zucker rats. We also thank Dr Qi Ding and Mrs Evelyn Beckett for expert technical assistance.
Footnotes
(T Mracek and D Gao contributed equally to this work)
References
- Bao Y, Bing C, Hunter L, Jenkins JR, Wabitsch M, Trayhurn P. Zinc-alpha2-glycoprotein, a lipid mobilizing factor, is expressed and secreted by human (SGBS) adipocytes. FEBS Letters. 2005;579:41–47. doi: 10.1016/j.febslet.2004.11.042. [DOI] [PubMed] [Google Scholar]
- Bing C, Trayhurn P. Regulation of adipose tissue metabolism in cancer cachexia. Current Opinion in Clinical Nutrition and Metabolic Care. 2008;11:201–207. doi: 10.1097/MCO.0b013e3282f948e2. [DOI] [PubMed] [Google Scholar]
- Bing C, Russell ST, Beckett EE, Collins P, Taylor S, Barraclough R, Tisdale MJ, Williams G. Expression of uncoupling proteins-1, -2 and -3 mRNA is induced by an adenocarcinoma-derived lipid-mobilizing factor. British Journal of Cancer. 2002;86:612–618. doi: 10.1038/sj.bjc.6600101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing C, Bao Y, Jenkins J, Sanders P, Manieri M, Cinti S, Tisdale MJ, Trayhurn P. Zinc-alpha2-glycoprotein, a lipid mobilizing factor, is expressed in adipocytes and is up-regulated in mice with cancer cachexia. PNAS. 2004;101:2500–2505. doi: 10.1073/pnas.0308647100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing C, Russell S, Becket E, Pope M, Tisdale MJ, Trayhurn P, Jenkins JR. Adipose atrophy in cancer cachexia: morphologic and molecular analysis of adipose tissue in tumour-bearing mice. British Journal of Cancer. 2006;95:1028–1037. doi: 10.1038/sj.bjc.6603360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgi W, Schmid K. Preparation and properties of Zn-alpha 2-glycoprotein of normal human plasma. Journal of Biological Chemistry. 1961;236:1066–1074. [PubMed] [Google Scholar]
- Choo HJ, Kim JH, Kwon OB, Lee CS, Mun JY, Han SS, Yoon YS, Yoon G, Choi KM, Ko YG. Mitochondria are impaired in the adipocytes of type 2 diabetic mice. Diabetologia. 2006;49:784–791. doi: 10.1007/s00125-006-0170-2. [DOI] [PubMed] [Google Scholar]
- Dahlman I, Kaaman M, Olsson T, Tan GD, Bickerton AS, Wahlen K, Andersson J, Nordstrom EA, Blomqvist L, Sjogren A, et al. A unique role of monocyte chemoattractant protein 1 among chemokines in adipose tissue of obese subjects. Journal of Clinical Endocrinology and Metabolism. 2005;90:5834–5840. doi: 10.1210/jc.2005-0369. [DOI] [PubMed] [Google Scholar]
- Degawa-Yamauchi M, Moss KA, Bovenkerk JE, Shankar SS, Morrison CL, Lelliott CJ, Vidal-Puig A, Jones R, Considine RV. Regulation of adiponectin expression in human adipocytes: effects of adiposity, glucocorticoids, and tumor necrosis factor alpha. Obesity Research. 2005;13:662–669. doi: 10.1038/oby.2005.74. [DOI] [PubMed] [Google Scholar]
- Ferber S, Meyerovitch J, Kriauciunas KM, Kahn CR. Vanadate normalizes hyperglycemia and phosphoenolpyruvate carboxykinase mRNA levels in ob/ob mice. Metabolism. 1994;43:1346–1354. doi: 10.1016/0026-0495(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Frederich RC, Lollmann B, Hamann A, Napolitano-Rosen A, Kahn BB, Lowell BB, Flier JS. Expression of ob mRNA and its encoded protein in rodents. Impact of nutrition and obesity. Journal of Clinical Investigation. 1995;96:1658–1663. doi: 10.1172/JCI118206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong FY, Zhang SJ, Deng JY, Zhu HJ, Pan H, Li NS, Shi YF. Zinc-alpha2-glycoprotein is involved in regulation of body weight through inhibition of lipogenic enzymes in adipose tissue. International Journal of Obesity. 2009;33:1023–1030. doi: 10.1038/ijo.2009.141. [DOI] [PubMed] [Google Scholar]
- Hauner H, Skurk T, Wabitsch M. Cultures of human adipose precursor cells. Methods in Molecular Biology. 2001;155:239–247. doi: 10.1385/1-59259-231-7:239. [DOI] [PubMed] [Google Scholar]
- Hirai K, Hussey HJ, Barber MD, Price SA, Tisdale MJ. Biological evaluation of a lipid-mobilizing factor isolated from the urine of cancer patients. Cancer Research. 1998;58:2359–2365. [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Marrades MP, Martinez JA, Moreno-Aliaga MJ. ZAG, a lipid mobilizing adipokine, is downregulated in human obesity. Journal of Physiology and Biochemistry. 2008;64:61–66. doi: 10.1007/BF03168235. [DOI] [PubMed] [Google Scholar]
- McDermott LC, Freel JA, West AP, Bjorkman PJ, Kennedy MW. Zn-alpha2-glycoprotein, an MHC class I-related glycoprotein regulator of adipose tissues: modification or abrogation of ligand binding by site-directed mutagenesis. Biochemistry. 2006;45:2035–2041. doi: 10.1021/bi051881v. [DOI] [PubMed] [Google Scholar]
- Mracek T, Ding Q, Tzanavari T, Kos K, Pinkney J, Wilding J, Trayhurn P, Bing C. The adipokine zinc-alpha2-glycoprotein is downregulated with fat mass expansion in obesity. Clinical Endocrinology. 2009 doi: 10.1111/j.1365-2265.2009.03658.x. DOI: 10.1111/j.1365-2265.2009.03658.x. [DOI] [PubMed] [Google Scholar]
- Robidoux J, Martin TL, Collins S. Beta-adrenergic receptors and regulation of energy expenditure: a family affair. Annual Review of Pharmacology and Toxicology. 2004;44:297–323. doi: 10.1146/annurev.pharmtox.44.101802.121659. [DOI] [PubMed] [Google Scholar]
- Rolli V, Radosavljevic M, Astier V, Macquin C, Castan-Laurell I, Visentin V, Guigne C, Carpene C, Valet P, Gilfillan S, et al. Lipolysis is altered in MHC class I zinc-alpha(2)-glycoprotein deficient mice. FEBS Letters. 2007;581:394–400. doi: 10.1016/j.febslet.2006.12.047. [DOI] [PubMed] [Google Scholar]
- Russell ST, Zimmerman TP, Domin BA, Tisdale MJ. Induction of lipolysis in vitro and loss of body fat in vivo by zinc-alpha2-glycoprotein. Biochimica et Biophysica Acta. 2004;1636:59–68. doi: 10.1016/j.bbalip.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Sanchez LM, Chirino AJ, Bjorkman P. Crystal structure of human ZAG, a fat-depleting factor related to MHC molecules. Science. 1999;283:1914–1919. doi: 10.1126/science.283.5409.1914. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nature Protocols. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Selva DM, Lecube A, Hernandez C, Baena JA, Fort JM, Simo R. Lower zinc-{alpha}2-glycoprotein production by adipose tissue and liver in obese patients unrelated to insulin resistance. Journal of Clinical Endocrinology and Metabolism. 2009;94:4499–4507. doi: 10.1210/jc.2009-0758. [DOI] [PubMed] [Google Scholar]
- Shimomura I, Bashmakov Y, Horton JD. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. Journal of Biological Chemistry. 1999;274:30028–30032. doi: 10.1074/jbc.274.42.30028. [DOI] [PubMed] [Google Scholar]
- Stejskal D, Karpisek M, Reutova H, Stejskal P, Kotolova H, Kollar P. Determination of serum zinc-alpha-2-glycoprotein in patients with metabolic syndrome by a new ELISA. Clinical Biochemistry. 2007;41:313–316. doi: 10.1016/j.clinbiochem.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Tada T, Ohkubo I, Niwa M, Sasaki M, Tateyama H, Eimoto T. Immunohistochemical localization of Zn-alpha 2-glycoprotein in normal human tissues. Journal of Histochemistry and Cytochemistry. 1991;39:1221–1226. doi: 10.1177/39.9.1918940. [DOI] [PubMed] [Google Scholar]
- Todorov PT, McDevitt TM, Meyer DJ, Ueyama H, Ohkubo I, Tisdale MJ. Purification and characterization of a tumor lipid-mobilizing factor. Cancer Research. 1998;58:2353–2358. [PubMed] [Google Scholar]
- Trayhurn P, Thomas ME, Duncan JS, Rayner DV. Effects of fasting and refeeding on ob gene expression in white adipose tissue of lean and obese (oblob) mice. FEBS Letters. 1995;368:488–490. doi: 10.1016/0014-5793(95)00719-p. [DOI] [PubMed] [Google Scholar]
- Tzanavari T, Bing C, Trayhurn P. Postnatal expression of zinc-alpha2-glycoprotein in rat white and brown adipose tissue. Molecular and Cellular Endocrinology. 2007;279:26–33. doi: 10.1016/j.mce.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Ueyama H, Naitoh H, Ohkubo I. Structure and expression of rat and mouse mRNAs for Zn-alpha 2-glycoprotein. Journal of Biochemistry. 1994;116:677–681. doi: 10.1093/oxfordjournals.jbchem.a124579. [DOI] [PubMed] [Google Scholar]
- Wabitsch M, Brenner R.E, Melzner I, Braun M, Moller P, Heinze E, Debatin K.M, Hauner H. Characterization of a human preadipocyte cell strain with high capacity for adipose differentiation. International Journal of Obesity and Related Metabolic Disorders. 2001;25:8–15. doi: 10.1038/sj.ijo.0801520. [DOI] [PubMed] [Google Scholar]
- Wang B, Trayhurn P. Acute and prolonged effects of TNF-alpha on the expression and secretion of inflammation-related adipokines by human adipocytes differentiated in culture. Pflügers Archiv: European Journal of Physiology. 2006;452:418–427. doi: 10.1007/s00424-006-0055-8. [DOI] [PubMed] [Google Scholar]
- Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]