Abstract
Objective
In response to previously published findings of methylphenidate-induced chromosomal changes in children, this study was designed to determine whether methylphenidate- or amphetamine-based drugs induce chromosomal damage (structural aberrations, micronuclei, and sister chromatid exchanges) in peripheral blood lymphocytes of children with attention-deficit/hyperactivity disorder after 3 months of continuous treatment.
Method
Stimulant drug-naïve subjects, 6 to 12 years of age, in good overall health, and judged to be appropriate candidates for stimulant therapy based on rigorously diagnosed ADHD using DSM-IV criteria, were randomized into two open-label treatment groups (methylphenidate or mixed amphetamine salts). Each subject provided a blood sample before initiation of treatment and after 3 months of treatment. Pretreatment and posttreatment frequencies of chromosomal aberrations, micronuclei, and sister chromatid exchanges were determined for each subject.
Results
Sixty-three subjects enrolled in the study; 47 subjects completed the full 3 months of treatment, 25 in the methylphenidate group and 22 in the amphetamine group. No significant treatment-related increases were observed in any of the three measures of cytogenetic damage in the 47 subjects who completed treatment or the 16 subjects who did not.
Conclusions
Earlier findings of methylphenidate-induced chromosomal changes in children were not replicated in this study. These results add to the accumulating evidence that therapeutic levels of methylphenidate do not induce cytogenetic damage in humans. Furthermore, our results indicate that amphetamine-based products do not pose a risk for cytogenetic damage in children.
Keywords: stimulant drugs, micronuclei, chromosome aberrations, sister chromatid exchanges, cohort study
Methylphenidate (MPH) is the active ingredient in several frequently prescribed stimulant medications used in the treatment of attention-deficit/hyperactivity disorder (ADHD). Methylphenidate-based products have been prescribed for more than 50 years, and use of MPH has rapidly increased since 1990 as the number of children (and adults) diagnosed with ADHD has increased, along with the number of MPH-based drugs.1–4 Currently, millions of prescriptions are written annually for MPH in the United States alone. Another stimulant product, mixed amphetamine salts (MAS), was approved for the treatment of ADHD in 1996. Today, most new prescriptions for stimulants used to treat ADHD are written for either MPH-based or MAS-based products.5 The American Academy of Child and Adolescent Psychiatry recommends the use of stimulants as part of the frontline treatment for ADHD.6
Although the use of these products has been associated with a number of manageable adverse effects (appetite suppression, insomnia, nervousness, headache, and dry mouth) and concern has been raised over potential adverse cardiovascular effects, their overall safety profiles are good.7–11 However, the safety of MPH was abruptly challenged in 2005 by a report of increased frequencies of sister chromatid exchanges (SCE; indicative of DNA damage), structural chromosomal aberrations (CA), and micronuclei (MN; biomarkers of numerical and structural chromosomal changes) in lymphocytes of 12 pediatric patients with ADHD after 3 months of MPH-based drug therapy.12 That study raised concern among members of the medical community and families of children with ADHD receiving MPH-based therapy because increased frequencies of CAs and micronuclei in peripheral blood lymphocytes are associated with an increased risk of cancer.13,14 However, questions were raised regarding the study design and some of the reported findings, including the small sample size (12 children), a lack of critical details on blood sample processing and slide scoring, and the complete absence of SCE recorded in 6 of the 11 children analyzed for this endpoint.15 The absence of SCE was especially troubling because such an observation has never been reported before that study. In responding to these points, El-Zein et al.16 emphasized that their pilot study required replication and expansion to more conclusively define the genotoxic potential of MPH in humans. The reported observations of cytogenetic damage in children were unexpected because, before the El-Zein et al.12 report, there had been no clear evidence for MPH-induced genetic damage in standard mutation and chromosome damage studies conducted in bacterial and animal test systems in vitro or in vivo.17–20 More recent studies, prompted by the El-Zein et al.12 report, have shown no evidence for MPH-induced CA in human lymphocytes in vitro or MN in mouse erythrocytes after a single 250 mg/kg oral dose of MPH.21 Another study, designed to evaluate a single cytogenetic endpoint (MN) in lymphocytes of children with ADHD exposed to MPH for periods of 1 to 6 months, reported no MPH-related changes in the frequencies of micro-nucleated lymphocytes at any time point.22 The number of children evaluated in this study ranged from 30 (1-month sample) to 8 (6-month sample).
Despite the growing evidence suggesting that MPH does not cause genetic damage,23 the enormous public health significance of this issue requires additional investigation. The primary goal of the study reported here was to determine whether the earlier results,12 showing elevations in three measures of cytogenetic damage in children after 3 months of treatment with MPH, could be independently replicated in a well-designed carefully conducted study on a larger cohort of subjects with adequate statistical power for detecting an increase in MPH-associated cytogenetic damage. In addition, because of the widespread use of amphetamine-based drugs (mostly MAS) in the treatment of ADHD, the potential for MAS to induce cytogenetic damage in pediatric ADHD subjects was also evaluated.
METHOD
Participants
Subjects were children of either sex, ages 6 to 12 years, who met full criteria for ADHD, any subtype, as defined by the Diagnostic and Statistical Manual for the Classification of Mental Disorders, Fourth Edition (DSM-IV),24 and who had not previously been treated with stimulant medications. Participants were recruited via word of mouth, clinic referrals, and print advertisements. All clinical assessments and treatment took place at the Duke ADHD Program, Department of Psychiatry, Duke University Medical Center, Durham, NC. The parents or legal guardians of all of the subjects provided written informed consent. This study protocol was reviewed and approved by the institutional review boards of both Duke University Medical Center and the National Institute of Environmental Health Sciences.
Attention-deficit/hyperactivity disorder diagnosis was established in subjects following practice parameters of both the American Academy of Pediatrics and the American Academy of Child and Adolescent Psychiatry.25,26 Specifically, detailed developmental, medical, and behavioral history was gathered from caregivers. Standardized rating scales were obtained from both caregivers and teachers,27 and structured28 as well as semistructured diagnostic interviews were conducted with the caregiver(s) to evaluate DSM-IV ADHD criteria. Structured psychiatric interviews28 were also administered to caregivers to rule out the presence of comorbid conditions. All clinical evaluations were conducted by or supervised by a board-certified child and adolescent psychiatrist and a licensed clinical psychologist.
In addition to the requirements previously noted, all subjects had to, in the opinion of the clinical team, be good candidates for stimulant treatment. Subjects underwent a complete physical examination, including an electrocardiogram, before initiating medication. Children were excluded if they had received an x-ray of any sort in the previous 3 months (excluding routine dental x-rays), had comorbid conditions that would contraindicate treatment with stimulant medication, had clinically significant electrocardiogram readings, or, for females, had reached menarche. Recent x-ray exposures precluded enrollment because such exposures may elevate the frequency of chromosomal damage, thereby confounding the results.
Study Design
The medication regimen was a parallel-group open-label design. Subjects who met all inclusion criteria were randomized to receive treatment either with MPH or MAS products. The specific products were selected at the discretion of the primary pharmacotherapist (a board-certified child and adolescent psychiatrist) to mimic real-world clinical practice. Medication was obtained through local commercial pharmacies with funds from the study sponsor (NICHD/NIEHS). Four commercial products were used: Adderall and Adderall XR (Shire Pharmaceuticals Group plc, Wayne, PA), Concerta (McNeil Pediatrics, Titusville, NJ), and Ritalin LA (Novartis Pharmaceuticals, East Hanover, NJ). Per routine clinical practice, commonly used concomitant medications (antibiotics, cold/cough medications, analgesics, asthma medications, and vitamins) were allowed in this study. Concomitant medications were recorded along with the indication for use (e.g., bacterial infection, asthma, congestion, cough). Literature searches revealed no association with cytogenetic effects for any of the concomitant medications. These concomitant medications were subsequently considered in the data analyses. Compliance with prescribed ADHD medication regimen was assessed by parent report and through drug accountability logs completed at each clinic visit. Compliance was calculated as a percentage of prescribed doses for the period since the last visit.
Before medication initiation, a blood sample was taken to establish a baseline of cytogenetic endpoints in each subject. The subjects then underwent 4 weeks of dose titration in which they came for weekly clinic visits and met with the primary pharmacotherapist. Standardized parent and teacher rating scales were collected at each of these visits, and adverse events were reviewed. This phase was used to ensure that the subjects received an adequate dose and appropriate product. After completion of the 4-week dose-titration phase, the subjects returned to the clinic at monthly intervals (2 and 3 months after initiation of treatment). At these visits, doses were adjusted as needed based on clinical response and adverse events. A second blood sample was collected after 3 months of treatment. The medication regimen was designed to be consistent with how children are commonly treated in routine clinical practice.
The protocol permitted the subjects who failed to respond adequately or who showed intolerable side effects to switch treatment groups if the treating physician judged that this would be in the best clinical interest of the subject. In these cases, an interim blood sample for chromosomal analysis was collected before switching medication groups, and the subjects who completed a 90-day course of treatment on the new medication provided a third blood sample at study completion.
All of the subjects, regardless of when they left the study, were provided detailed counseling and medical referrals to ensure smooth transition of care.
Blood Culturing and Cytogenetic Analyses
Coded blood samples (approximately 10 mL per subject) in sodium heparin tubes were shipped at ambient temperature or with cool packs for overnight delivery to Covance Laboratories (Vienna, VA). Duplicate lymphocyte cultures were established for each endpoint (SCE, CA, and MN) from each sample on the day of receipt, along with concurrent positive control cultures. All slides were coded before analysis, so readers were unaware of treatment. To further blind the scorers to treatment status of the slides, slide scoring did not commence until a sufficient number of pretreatment and posttreatment blood samples had accumulated (approximately 5 months after start of study). Enrollments were ongoing over a period of 18 months, so pretreatment and posttreatment blood samples remained mixed during the course of the study, and slides were not scored in chronological order. Laboratory work was conducted using standard Good Laboratory Practices as specified in the U.S. Food and Drug Administration Guidelines,29 with adherence to a prepared protocol and/or its amendments, and a full quality assurance audit of the raw data and results.
CA Study
Whole-blood cultures were initiated in 15-mL centrifuge tubes (Falcon Tubes; Becton Dickinson, Franklin Lakes, NJ) by adding approximately 0.6 mL of heparinized blood into culture medium (RPMI 1640 [Mediatech, Herndon, VA]) for a final volume of 10.0 mL. Medium was supplemented with 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer (25 mol/L), 20% heat-inactivated fetal bovine serum (BioChemed, Winchester, VA), penicillin (100 U/mL), streptomycin (100 μg/mL), and L-glutamine (2 mol/L) (Mediatech). To stimulate mitosis, 2% phytohemagglutinin M (GIBCO, Grand Island, NY) was added. Cultures were incubated with loose caps at 37°C ± 2°C in a humidified 5% CO2 atmosphere for 52 hours; 0.1 μg/mL Colcemid (GIBCO) was added for the final 2 hours of incubation. Mitomycin C (Sigma-Aldrich, St. Louis, MO) (0.050, 0.075, and 0.1 μg/mL) served as the positive control.
At harvest, cultures were centrifuged, the supernatant was discarded, and cells were swollen with 75 mol/L KCl, fixed in methanol and glacial acetic acid (3:1, vol/vol), dropped onto glass slides, and air-dried. Slides were stained with 5% Giemsa (Harleco EMD, Gibstown, NY), air-dried, and coverslipped. Cells with a modal chromosome number of 46 ± 2 were selected for scoring, except for heavily damaged cells in which an exact chromosome count was not possible. Two hundred metaphases were scored per subject (standard classifications of simple and complex chromatid and chromosome breaks, rearrangements, and exchanges were used). Mitotic index (percentage of cells in metaphase) was evaluated in 1,000 cells per subject.
SCE Study
Whole-blood cultures were initiated as previously described, with the addition of 25 μmol/L 5-bromo-2′-deoxyuridine (Sigma-Aldrich), and incubated for 72 hours, with Colcemid present for the final 2 hours. Mitomycin C (0.015 and 0.03 μg/mL) served as the positive control.
At harvest, slides were prepared as previously described, stained for at least 10 minutes with 5 μg/mL Hoechst 33258 (Acros Organics, Morris Plains, NJ) in phosphate buffer (pH 6.8; Mediatech), mounted in the same buffer, and exposed for 30 minutes at 60°C ± 5°C to black-light ultraviolet tubes for differential staining of sister chromatids. Slides were then rinsed, stained with 5% Giemsa, air-dried, and coverslipped. Fifty second-division metaphases were scored per subject.
MN Study
Density gradientisolated (Ficoll-Hypaque, density of approximately 1.077) lymphocytes were cultured as previously described in Falcon 5-mL polystyrene round-bottom culture tubes. Two-milliliter cultures with 106 lymphocytes per milliliter were established in RPMI 1640 supplemented as previously described, except that concanavalin A (type IV; Sigma-Aldrich) at a final concentration of 16 μg/mL was used instead of phytohemagglutinin. Cytochalasin B (3 μg/mL; Sigma-Aldrich) was added approximately 44 hours after culture initiation to block cytokinesis. Cultures were incubated for a total of 72 hours. Mitomycin C (0.00625, 0.0125, and 0.0250 μg/mL) was used as the positive control.
At harvest, cultures were centrifuged, the supernatant was discarded, and the cells were resuspended in phosphate-buffered saline; slides were prepared using a Cytospin 4 centrifuge. Air-dried slides were fixed in absolute methanol, stained with acridine orange (Sigma-Aldrich), prepared with a wet mount, and scored under a fluorescent microscope at a magnification of 1000×. Two thousand binucleated cells were analyzed per subject for frequency of cells with micronuclei.
Data Analysis
Because measures of chromosome damage were not normally distributed, nonparametric statistical methods were used to compare pretreatment with posttreatment and MPH with MAS.30 Within the MPH and MAS groups, the Wilcoxon signed ranks test, a nonparametric analog of the paired t test, was used to compare baseline measures of chromosome damage with the corresponding measures after 90 days of medication. These tests were one-tailed to test for an increase in indicators of chromosome damage after medication. Baseline and 90-day measures of chromosomal damage were compared between MPH and MAS groups using the Mann-Whitney U test, a nonparametric analog of the two-sample t test. Additionally, demographic characteristics of the MPH and MAS groups were compared using the Mann-Whitney U test (age), two-sample t test (body weight and height), or Fisher exact test/χ2 test (sex, race, and ADHD subtype). The tests comparing MPH and MAS groups were two-tailed. The relation between MPH and MAS doses on a body weight basis (milligrams per kilograms per day) to each of the three measures of cytogenetic damage was evaluated using Spearman correlations. Using this same approach, the correlation between cumulative dose for the 3-month exposure period and measures of cytogenetic damage was also evaluated.
The statistical analyses presented here are based on all subjects who completed 90 days of the study without switching medication. Baseline measurements and demographic characteristics of the subjects who dropped out of the study or switched medication during the study were compared with those who completed the study.
The target enrollment of 30 subjects in each group was based on the projected number of subjects needed to detect a doubling of any of the cytogenetic endpoints with at least 90% power at a 0.05 level of significance. Post hoc power analyses with actual sample sizes confirmed that, even with less-than-target enrollment, the power was at least 90% for detecting a doubling of each of the three cytogenetic endpoints within each treatment group because the variability of each endpoint was smaller than anticipated.
RESULTS
Sample Characteristics and Subject Demographics
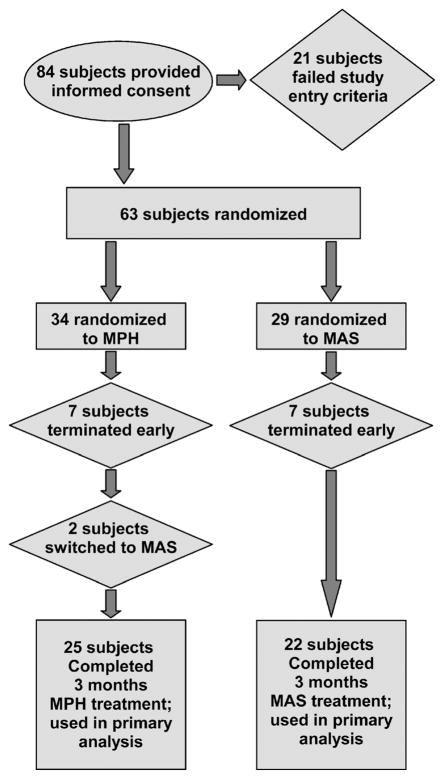
Figure 1 provides an overview of subject disposition throughout the study. Specifically, 84 subjects provided written informed consent; 21 did not meet all inclusion/exclusion criteria, yielding an overall enrollment of 63 subjects. Fourteen enrolled subjects withdrew before completing the full 90 days of treatment (because of adverse effects, lack of efficacy, or lost to follow-up/withdrawal of consent). Forty-seven subjects completed the full 90 days of treatment (25 for MPH and 22 for MAS), and 2 subjects switched medications and completed 90 days of treatment on the new medication.
Fig. 1.
Disposition flowchart of subject enrollment and retention. MPH = methylphenidate; MAS = mixed amphetamine salts.
Table 1 summarizes the characteristics of the sample population. There were no significant differences between subjects in the MAS and MPH groups with regard to age, sex, race, body weight, height, or ADHD subtype (all p > 0.30). The groups also showed similar ADHD symptom levels at screening and termination, indicating that the two groups were of comparable severity, and both responded equally well to the pharmacotherapy.
TABLE 1.
Description of the Study Participants
| MPH (n = 25) | MAS (n = 22) | Total (n = 47) | Test Statistic and p | |
|---|---|---|---|---|
| Age, mean (SD), y | 8.9 (1.9) | 8.4 (1.3) | 8.7 (1.7) | W(22, 25) = 489.5, p = .41 |
| Age group, y | χ21 = 0.50, p = .48 | |||
| 6–9 | 17 | 17 | 34 | |
| 10–12 | 8 | 5 | 13 | |
| Sex | χ21 = 0.99, p = .32 | |||
| Female | 9 | 5 | 14 | |
| Male | 16 | 17 | 33 | |
| Race | Fisher exact test, p = .45 | |||
| African American | 8 | 10 | 18 | |
| Hispanic | 1 | 2 | 3 | |
| White | 16 | 10 | 26 | |
| Body weight, mean (SD), kg | 37.6 (11.3) | 34.7 (10.5) | 35.2 (10.9) | t45 = −0.92, p = .36 |
| Height, mean (SD), cm | 137.7 (11.7) | 133.5 (9.8) | 134.8 (11.0) | t45 = −1.31, p = .20 |
| ADHD subtype | Fisher exact test, p = .83 | |||
| Hyperactive-Impulsive | 1 | 1 | 2 | |
| Inattentive | 11 | 12 | 23 | |
| Combined | 12 | 9 | 21 | |
| Not otherwise specified | 1 | 0 | 1 | |
| DSM-IV ADHD-Rating Scale score at screening, mean (SD) | 35.3 (6.4) | 36.6 (7.7) | 36.0 (7.1) | |
| DSM-IV ADHD-Rating Scale score at end of the study, mean (SD) | 8.7 (3.5) | 8.5 (4.8) | 8.6 (4.2) |
Note: MPH = methylphenidate; MAS = mixed amphetamine salts; n = number of subjects; SD = standard deviation; W = Mann-Whitney U test statistic; t = two-sample t statistic.
In the group of 47 subjects who completed the study, 4 commercial products were used: Adderall and Adderall XR, and Ritalin LA. Doses, number of subjects per product category/dose, and average dosage for each of the two treatment groups are presented in Table 2. Compliance with the prescribed medication regimen was excellent, with 96% of prescribed doses administered.
TABLE 2.
End of Study Dose of MPH or MAS
| MPH |
MAS |
||||
|---|---|---|---|---|---|
| Product | Dose (mg/day) | No. of subjects | Product | Dose (mg/day) | No. of subjects |
| Concerta | 18 | 3 | Adderall | 5 | 1 |
| 27 | 11 | Adderall XR | 5 | 3 | |
| 36 | 7 | 10 | 10 | ||
| 54 | 3 | 15 | 4 | ||
| Ritalin LA | 30 | 1 | 20 | 2 | |
| 25 | 2 | ||||
| Mean dose (SD) | 31.8 (10.4) | 12.3 (5.9) | |||
| Mean dose in mg/kg body weight/day (SD) and range | 0.92 (0.53); 0.33–2.78 | 0.37 (0.17); 0.16–0.78 | |||
Note: MPH = methylphenidate; MAS = mixed amphetamine salts.
Cytogenetic Analyses
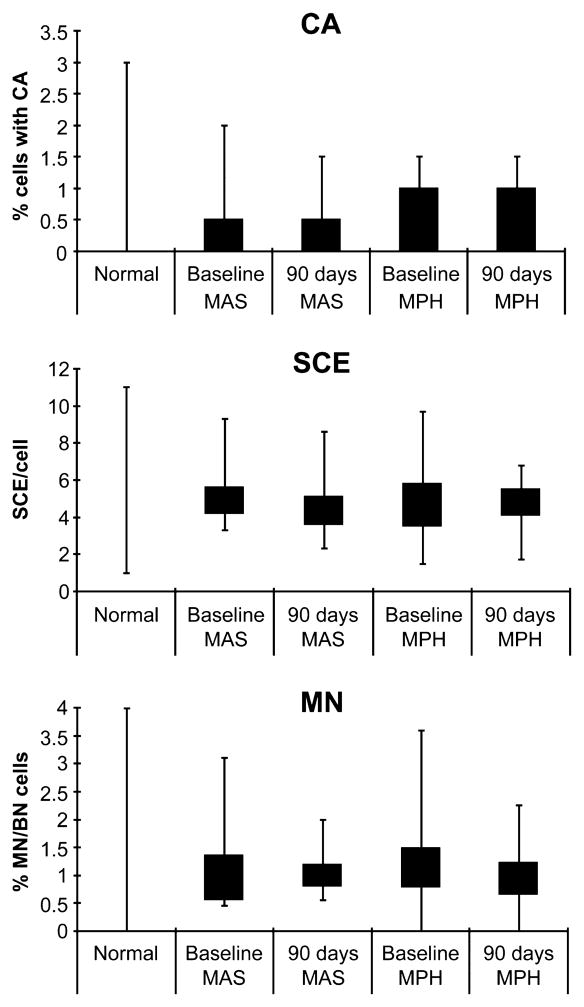
The pretreatment and posttreatment values of CA, SCE, and MN for all subjects were within expected ranges31,32 (Fig. 2). No significant treatment-related increases were detected in the frequencies of SCE/cell or the frequencies of cells with CA or MN in either medication group among subjects who completed the entire 90-day treatment period (Table 3). In addition, no differences were noted in the cytogenetic measures between subjects treated with MPH and those receiving MAS (Table 4). Furthermore, none of the three cytogenetic endpoints varied by age, medication group, sex, body weight, height, race, or ADHD subtype (all p > .06). Final dose/body weight was not correlated with any of the three cytogenetic endpoints (p > .35 for the MAS group, and p > .20 for the MPH group). Additional analyses revealed no associations between chromosomal damage indicators and either concomitant medications or medical indications for taking concomitant medications such as allergies, asthma, head cold, and cough. Measurements of SCE/cell variability within each group did not change after 3 months of treatment (Wilcoxon signed ranks tests, MPH: D(25) = −9.5, p = 0.60; MAS: D(20) = −58.0, p = .99), suggesting that neither drug product increased the frequency of highly damaged cells per subject. These analyses were repeated on data from all subjects, regardless of whether they completed the 90-day treatment period or switched medication, and no treatment-related effects on any of the endpoints of cytogenetic damage were observed (data not shown).
Fig. 2.
Measures of cytogenetic damage for subjects in this study compared with the published normal ranges31,32 in control populations. Box represents data falling within the 25th to the 75th percentiles. Whiskers extend to the minimum and maximum observed values. CA = chromosomal aberration; SCE = sister chromatid exchanges; MN/BN = micronucleated binucleate; MAS = mixed amphetamine salts; MPH = methylphenidate.
TABLE 3.
Pretreatment and Terminal Measures of Cytogenetic Damage in Subjects Treated With MPH or MAS for 3 Months
| Drug | Measure | n | Pretreatmenta | Terminala | pb |
|---|---|---|---|---|---|
| MPH | % cells with CA | 25 | 0.48 (0.09) | 0.46 (0.09) | .54 |
| Mean SCE/cell | 25 | 4.70 (0.36) | 4.83 (0.24) | .37 | |
| % MN/BN cells | 19 | 1.20 (0.17) | 0.96 (0.10) | .83 | |
| MAS | % cells with CA | 22 | 0.43 (0.13) | 0.46 (0.09) | .43 |
| Mean SCE/cell | 20 | 5.17 (0.36) | 4.41 (0.33) | .97 | |
| % MN/BN cells | 15 | 1.22 (0.21) | 1.04 (0.09) | .70 |
Note: n = number of subjects included in the analysis; MPH = methylphenidate; MAS = mixed amphetamine salts; CA = chromosomal aberration; SCE = sister chromatid exchange; MN/BN = micronucleated binucleate.
Mean (SE).
One-sided Wilcoxon signed rank p values.
TABLE 4.
Comparison of Pretreatment and Terminal Cytogenetic Damage End-points Between MPH and MAS Groups
| MAS |
MPH |
MAS vs. MPH |
|||
|---|---|---|---|---|---|
| Sample | Measure | Mean (SE) | Mean (SE) | Mann-Whitney U test statistic (nMAS, nMPH)a | pb |
| Baseline | % cells with CA | 0.43 (0.13) | 0.48 (0.09) | 485.0 (22, 25) | .33 |
| Mean SCE/cell | 5.17 (0.36) | 4.70 (0.36) | 533.5 (21, 25) | .38 | |
| % MN/BN cells | 1.22 (0.21) | 1.20 (0.17) | 309.0 (17, 20) | .68 | |
| Terminal | % cells with CA | 0.46 (0.09) | 0.46 (0.09) | 527.5 (22, 25) | 1.00 |
| Mean SCE/cell | 4.41 (0.33) | 4.83 (0.24) | 423.0 (21, 25) | .12 | |
| % MN/BN cells | 1.04 (0.09) | 0.96 (0.10) | 461.5 (20, 24) | .80 | |
Note: CA = chromosomal aberration; SCE = sister chromatid exchanges; MN/BN = micronucleated binucleate; SE = standard error; MAS = mixed amphetamine salts; MPH = methylphenidate.
n, number of subjects analyzed for the endpoint.
Two-sided p values.
DISCUSSION
The present study found no evidence of changes in any of the three cytogenetic endpoints examined in the lymphocytes of children treated with MPH- or MAS-based products continuously for 3 months. Thus, earlier reported findings regarding the potential for MPH to induce chromosomal changes associated with increased risk for cancer in pediatric patients with ADHD were not replicated.12 Our results add to a growing body of evidence that therapeutic levels of MPH do not induce chromosomal damage in humans.19,21,22
Our findings should not be interpreted as categorical proof of the long-term safety of stimulants for the treatment of ADHD. Given the paucity of studies that have evaluated the physical and behavioral effects of extended treatment with stimulants, continued work in this area is imperative.33 As previously noted, concern has been raised over potential cardiovascular risks associated with stimulant treatment, and some studies have reported an increased risk for smoking and other substance abuse, although other studies have disputed these claims.7,11,34,35 Therefore, continued close monitoring of a wide range of endpoints is needed to inform clinical practice on the safety of long-term stimulant use for the treatment of ADHD.
Genetic alterations are associated with increased risks for cancer.13,14 A cancer bioassay conducted by the National Toxicology Program in rats and mice showed no evidence for tumor induction in rats exposed to MPH in drinking water for 2 years, but increases in hepatocellular neoplasms occurred in male and female mice exposed to the highest dose tested (500 ppm MPH in drinking water, equivalent to approximately 60–80 mg MPH per kilograms per day).20 It should be noted that the strain of mouse used in this bioassay (B6C3F1) is prone to the development of spontaneous hepatocellular neoplasms, and the incidences recorded in the MPH-dosed mice were within historic control ranges. In contrast to the studies in mice, no evidence for an increased risk for cancer in humans has been found, although studies are limited in number and scope. A recent study reporting no evidence for a moderate or strong increase in a variety of cancers among 35,400 MPH users adds to the safety profile of MPH36 and extends the negative findings reported in an earlier study of cancer incidence in a small population of 529 individuals chronically exposed to MPH.37
The present study extends the cytogenetic literature in several important ways. First, the sample size was larger than those in previous reports. Data were obtained from 25 children exposed for 3 months to MPH, which is twice the sample size of the original El-Zein et al.12 study and nearly 20% larger than the sample size in the recent Walitza et al.22 study that evaluated a single endpoint, lymphocyte MN frequency, in children exposed to MPH for periods of 1 to 6 months. In accord with the results of the present study, Walitza et al.22 found no MPH-related changes in the frequencies of micronucleated lymphocytes at any of the time points investigated. Second, cytogenetic data were provided on a comparably sized group of children treated with MAS, a stimulant product used at least as frequently as MPH for the treatment of ADHD and for which no chromosomal damage data were available. Thus, we were able to provide information on the potential for induced chromosomal damage in children exposed to two different chemical classes of therapeutics. Third, the rigorous characterization of all subjects in this study makes generalizing the results to other ADHD sample populations defensible. Finally, the flexible dosing regimen used in the present study is comparable to the frequent dose changes that are observed in routine clinical practice, and therefore, both the sample population and the medication exposure in this study were consistent with real-world clinical experience.
Despite its several strengths, the present study is not without limitations. First, the sample size in this study, although sufficient to detect a biologically meaningful twofold increase in cytogenetic abnormalities with at least 90% power, was still relatively small. Thus, future studies may continue to monitor these and related genetic damage endpoints in larger study groups. Another limitation in the present study was the 3-month exposure period selected to replicate the El-Zein et al.12 protocol. Typically, individuals with ADHD are exposed to stimulant drugs for longer periods.38 Thus, assessment of cytogenetic endpoints after longer exposure periods would provide data to further evaluate the potential for therapeutic doses of MPH to induce cytogenetic damage in patients with ADHD. In addition, the cytogenetic endpoints investigated in the present study were measured in a cell population (lymphocytes) that does not normally divide. Thus, studies in stimulant-exposed children measuring chromosomal or gene changes in other cell types (e.g., rapidly dividing proerythrocytes) may be warranted.39 Finally, we restricted enrollment to children who were 6 to 12 years of age to match the age group investigated in the El-Zein et al.12 study. Although there is little reason to suspect that the pattern of findings would be different for younger or older children, or adults, generalizations can be made only to this age range.
In summary, the present study provides new evidence that treatment of pediatric patients with ADHD with MPH- or MAS-based products for 3 months does not result in increased levels of cytogenetic damage in the form of CA, MN, or SCE in peripheral blood lymphocytes. The present study did not replicate findings in a previous report of adverse chromosomal effects of MPH in children with ADHD.12 Future studies may be directed toward addressing some of the limitations previously noted, particularly the assessment of other genetic endpoints in MPH- or MAS-exposed populations.
Acknowledgments
This research was supported by the NIH/NICHD Best Pharmaceuticals for Children Act pediatric drug development program and the Intramural Research Program of the National Toxicology Program of the National Institute of Environmental Health Sciences.
The authors thank Dr. Gregory Erexson, George Stojhovic, and Kelley Boyer for establishing, coordinating, and conducting the cytogenic analysis. The authors also thank the Duke ADHD Program psychologists for help in recruitment and enrollment, the Program’s clinical trials team for assistance with the evaluation process during the screening period, and the Program’s research assistants for invaluable help in the conduct of the study. The authors also thank Drs. Raymond Tice and Andrew Kligerman for fruitful discussions during preparation of this manuscript.
Footnotes
Clinical trial registration information—Genetic Measurements in Blood Cells of Children Taking Adderall or Methylphenidate. URL: http://clinicaltrials.gov. Unique identifier: NCT00341029.
Disclosure: Dr. Kollins received research support and/or honoraria/consulting fees from the following sources during the conduct of this study: Athenagen, Eli Lilly, Psychogenics, Pfizer, New River Pharmaceuticals, Shire Pharmaceuticals, National Institute on Drug Abuse, National Institute of Mental Health, National Institute of Neurological Disorders and Stroke, and Environmental Protection Agency. Dr. Chrisman received honoraria and was on the speakers’ bureaus for Shire Pharmaceuticals and McNeil-PPC. The other authors report no conflicts of interest.
References
- 1.Safer DJ. Are stimulants overprescribed for youths with ADHD? Ann Clin Psychiatry. 2000;12:55–62. doi: 10.1023/a:1009031211900. [DOI] [PubMed] [Google Scholar]
- 2.Safer DJ, Malever M. Stimulant treatment in Maryland public schools. Pediatrics. 2000;106:533–539. doi: 10.1542/peds.106.3.533. [DOI] [PubMed] [Google Scholar]
- 3.Zito JM, Safer DJ, dosReis S, Magder LS, Gardner JF, Zarin DA. Psychotherapeutic medication patterns for youths with attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med. 1999;153:1257–1263. doi: 10.1001/archpedi.153.12.1257. [DOI] [PubMed] [Google Scholar]
- 4.Zito JM, Safer DJ, dosReis S, Gardner JF, Boles M, Lynch F. Trends in the prescribing of psychotropic medications to preschoolers. JAMA. 2000;283:1025–1030. doi: 10.1001/jama.283.8.1025. [DOI] [PubMed] [Google Scholar]
- 5.Castle L, Aubert RE, Verbrugge RR, Khalid M, Epstein RS. Trends in medication treatment for ADHD. J Atten Disord. 2007;10:335–342. doi: 10.1177/1087054707299597. [DOI] [PubMed] [Google Scholar]
- 6.Dulcan MK, Benson RS. AACAP Official Action. Summary of the practice parameters for the assessment and treatment of children, adolescents, and adults with ADHD. J Am Acad Child Adolesc Psychiatry. 1997;36:1311–1317. doi: 10.1097/00004583-199709000-00033. [DOI] [PubMed] [Google Scholar]
- 7.Biederman J, Spencer TJ, Wilens TE, Prince JB, Faraone SV. Treatment of ADHD with stimulant medications: response to Nissen perspective in the New England Journal of Medicine. J Am Acad Child Adolesc Psychiatry. 2006;45:1147–1150. doi: 10.1097/01.chi.0000227883.88521.e6. [DOI] [PubMed] [Google Scholar]
- 8.Donner R, Michaels MA, Ambrosini PJ. Cardiovascular effects of mixed amphetamine salts extended release in the treatment of school-aged children with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61(5):706–712. doi: 10.1016/j.biopsych.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Faraone SV. Stimulant therapy in the management of ADHD: mixed amphetamine salts (extended release) Expert Opin Pharmacother. 2007;8:2127–2134. doi: 10.1517/14656566.8.13.2127. [DOI] [PubMed] [Google Scholar]
- 10.Greenhill LL, Halperin JM, Abikoff H. Stimulant medications. J Am Acad Child Adolesc Psychiatry. 1999;38:503–512. doi: 10.1097/00004583-199905000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Nissen SE. ADHD drugs and cardiovascular risk. N Engl J Med. 2006;354:1445–1448. doi: 10.1056/NEJMp068049. [DOI] [PubMed] [Google Scholar]
- 12.El-Zein RA, Abdel-Rahman SZ, Hay MJ, et al. Cytogenetic effects in children treated with methylphenidate. Cancer Lett. 2005;230:284–291. doi: 10.1016/j.canlet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Bonassi S, Znaor A, Norppa H, Hagmar L. Chromosomal aberrations and risk of cancer in humans: an epidemiologic perspective. Cytogenet Genome Res. 2004;104:376–382. doi: 10.1159/000077519. [DOI] [PubMed] [Google Scholar]
- 14.Bonassi S, Znaor A, Ceppi M, et al. An increased micronucleus frequency in peripheral blood lymphocytes predicts the risk of cancer in humans. Carcinogenesis. 2007;28:625–631. doi: 10.1093/carcin/bgl177. [DOI] [PubMed] [Google Scholar]
- 15.Preston RJ, Kollins SH, Swanson JM, et al. Comments on ‘Cytogenetic effects in children treated with methylphenidate’ by El-Zein et al. Cancer Lett. 2005;230:292–294. doi: 10.1016/j.canlet.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 16.El-Zein RA, Hay MJ, Lopez MS, et al. Response to comments on ‘Cytogenetic effects in children treated with methylphenidate’ by El-Zein et al. Cancer Lett. 2006;231(1):146–148. doi: 10.1016/j.canlet.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B, Zeiger E. Salmonella mutagenicity tests: II. Results from the testing of 270 chemicals. Environ Mutagen. 1986;8(Suppl 7):1–119. [PubMed] [Google Scholar]
- 18.Galloway SM, Armstrong MJ, Reuben C, et al. Chromosome aberrations and sister chromatid exchanges in Chinese hamster ovary cells: evaluations of 108 chemicals. Environ Mol Mutagen. 1987;10(Suppl 10):1–175. doi: 10.1002/em.2850100502. [DOI] [PubMed] [Google Scholar]
- 19.Teo SK, San RH, Wagner VO, et al. D-Methylphenidate is non-genotoxic in in vitro and in vivo assays. Mutat Res. 2003;537:67–79. doi: 10.1016/s1383-5718(03)00053-6. [DOI] [PubMed] [Google Scholar]
- 20.National Toxicology Program. U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health. Research Triangle Park: U.S. Department of Health and Human Services; 1995. Toxicology and Carcinogenesis Studies of Methylphenidate Hydrochloride (CAS no. 298-59-9) in F344/N Rats and B6C3F1 Mice (Feed studies) Technical Report Series No. 439. [PubMed] [Google Scholar]
- 21.Sutter W, Martus HJ, Elhajouji A. Methylphenidate is not clastogenic in cultured human lymphocytes and in the mouse bone-marrow micronucleus test. Mutat Res. 2006;607:153–159. doi: 10.1016/j.mrgentox.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Walitza S, Werner B, Romanos M, Warnke A, Gerlach M, Stopper H. Does methylphenidate cause a cytogenetic effect in children with attention deficit hyperactivity disorder? Environ Health Perspect. 2007;115:936–940. doi: 10.1289/ehp.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobson-Kram D, Mattison D, Shelby M, Slikker W, Tice R, Witt K. Methylphenidate and chromosome damage. Cancer Lett. 2008;260:216–218. doi: 10.1016/j.canlet.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR. 4. Washington: American Psychiatric Association; 2000. [Google Scholar]
- 25.Dulcan M. Practice parameters for the assessment and treatment of children, adolescents, and adults with attention-deficit/hyper-activity disorder. American Academy of Child and Adolescent Psychiatry. J Am Acad Child Adolesc Psychiatry. 1997;36(Suppl 10):85S–121S. doi: 10.1097/00004583-199710001-00007. [DOI] [PubMed] [Google Scholar]
- 26.American Academy of Pediatrics. Clinical practice guideline: diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder. Pediatrics. 2000;105:1158–1170. doi: 10.1542/peds.105.5.1158. [DOI] [PubMed] [Google Scholar]
- 27.Conners CK. Conners Rating Scales—Revised. North Tonawanda: Multi-Health Systems; 1997. Technical Manual. [Google Scholar]
- 28.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 29.FDA Guidelines, Good Laboratory Practice, Part 58. Title 21. Code of Federal Regulations.
- 30.Conover WJ. Practical Nonparametric Statistics. New York: John Wiley & Sons; 1971. [Google Scholar]
- 31.Bender MA, Preston RJ, Leonard RC, Pyatt BE, Gooch PC. Chromosomal aberration and sister-chromatid exchange frequencies in peripheral blood lymphocytes of a large human population sample. II. Extension of age range. Mutat Res. 1989;212:149–154. doi: 10.1016/0027-5107(89)90065-1. [DOI] [PubMed] [Google Scholar]
- 32.Neri M, Ceppi M, Knudsen LE, et al. Baseline micronuclei frequency in children: estimates from meta- and pooled analyses. Environ Health Perspect. 2005;113:1226–1229. doi: 10.1289/ehp.7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Volkow ND, Insel TR. What are the long-term effects of methylphenidate treatment? Biol Psychiatry. 2003;54:1307–1309. doi: 10.1016/j.biopsych.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 34.Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil. 1998;31:533–544. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- 35.Mick E, Biederman J, Faraone SV. Comment on Lambert and Hartsough (1998) J Learn Disabil. 2000;33:314. doi: 10.1177/002221940003300401. author reply 314–316. [DOI] [PubMed] [Google Scholar]
- 36.Oestreicher N, Friedman GD, Jiang SF, Chan J, Quesenberry C, Jr, Habel LA. Methylphenidate use in children and risk of cancer at 18 sites: results of surveillance analyses. Pharmacoepidemiol Drug Saf. 2007;16:1268–1272. doi: 10.1002/pds.1519. [DOI] [PubMed] [Google Scholar]
- 37.Selby JV, Friedman GD, Fireman BH. Screening prescription drugs for possible carcinogenicity: eleven to fifteen years of follow-up. Cancer Res. 1989;49:5736–5747. [PubMed] [Google Scholar]
- 38.Wu EQ, Birnbaum HG, Zhang HF, Ivanova JI, Yang E, Mallet D. Health care costs of adults treated for attention-deficit/hyperactivity disorder who received alternative drug therapies. J Manag Care Pharm. 2007;13:561–569. doi: 10.18553/jmcp.2007.13.7.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Witt KL, Cunningham CK, Patterson KB, et al. Elevated frequencies of micronucleated erythrocytes in infants exposed to zidovudine in utero and postpartum to prevent mother-to-child transmission of HIV. Environ Mol Mutagen. 2007;48:322–329. doi: 10.1002/em.20266. [DOI] [PMC free article] [PubMed] [Google Scholar]