Abstract
Nephronophthisis (NPHP), a recessive cystic kidney disease, is the most frequent genetic cause of end-stage kidney disease in children and young adults. Positional cloning of nine genes (NPHP1-9) and functional characterization of their encoded proteins (nephrocystins) has contributed to a unifying theory that defines cystic kidney diseases as “ciliopathies”. The theory is based on the finding that all proteins mutated in cystic kidney diseases of humans or animal models are expressed in primary cilia or centrosomes of renal epithelial cells. Primary cilia are sensory organelles that connect mechanosensory, visual, and other stimuli to mechanisms of epithelial cell polarity and cell cycle control. Mutations in NPHP genes cause defects in signaling mechanisms that involve the non-canonical Wnt signaling pathway and the sonic hedgehog signaling pathway, resulting in defects of planar cell polarity and tissue maintenance. The ciliary theory explains the multiple organ involvement in NPHP, which includes retinal degeneration, cerebellar hypoplasia, liver fibrosis, situs inversus, and mental retardation. Positional cloning of dozens of unknown genes that cause NPHP will elucidate further signaling mechanisms involved. Nephrocystins are highly conserved in evolution, thus allowing the use of animal models to develop future therapeutic approaches.
Subjects: nephronophthisis, cystic kidney disease, planar cell polarity, wnt signaling, hedgehog signaling, ciliopathies
Clinical Features of Nephronophthisis
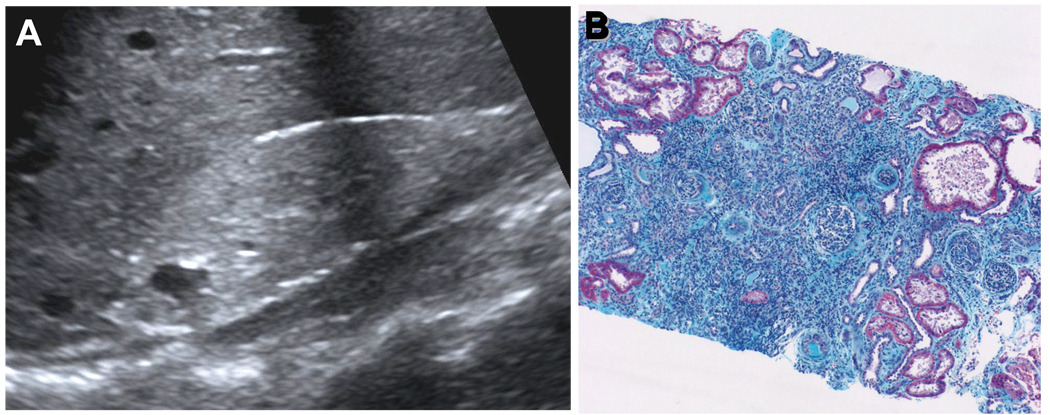
Nephronophthisis (NPHP) is an autosomal recessive cystic kidney disease that constitutes the most frequent genetic cause for end-stage kidney disease (ESKD) in the first 3 decades of life1–4. ESKD manifests at a median age 13 years. Initial symptoms are relatively mild, start around age 6 years, and consist of polyuria, polydipsia, secondary enuresis, and anemia 5. Regular fluid intake at nighttime is a characteristic feature of the patients’ history. Renal ultrasound reveals normal kidney size, increased echogenicity and corticomedullary cysts (Figure 1, A)6. Renal histology exhibits a characteristic triad of corticomedullary cysts, tubular basement membrane disruption, and tubulointerstitial nephropathy (Figure 1, B)7,8. Disease recurrence has never been reported in kidneys transplanted to NPHP patients9. NPHP is inherited in an autosomal recessive mode4. It was first described by Smith and Graham in 194510 and by Fanconi et al.11, who introduced the term "familial juvenile nephronophthisis". In NPHP, ESKD develops within the first 3 decades of life12–14. In contrast, infantile NPHP, which is characterized by mutations in NPHP2/inversin, leads to ESKD between birth and age 3 years14,15. In more than 10% of cases NPHP can be associated with extrarenal involvement, primarily including retinal degeneration (Senior-Loken syndrome), cerebellar vermis aplasia (Joubert syndrome), liver fibrosis, and cone-shaped epiphyses. These clinical features will be discussed below in light of the cilia/centrosome theory of NPHP. Over 300 cases of NPHP have been published in the literature7. It has been reported from virtually all regions of the world16. The incidence of the disease has been estimated as 9 patients/8.3 million17 in the United States or 1 in 50,000 live births in Canada7,18. In the North American pediatric end-stage renal disease population pooled data indicate a prevalence of approximately 5% of all children with ESKD19,20.
Figure 1. Morphology of nephronophthisis.
(A) Renal ultrasound demonstrates increased echogenicity, loss of corticomedullary differentiation, and the presence of corticomedullary cysts. In contrast to polycystic kidney disease kidneys are not enlarged. (B) Renal histology in NPHP shows the characteristic triad of renal tubular cysts, tubular membrane disruption, and tubulointerstitial cell infiltrates with interstitial fibrosis and periglomerular fibrosis (B is courtesy of D. Bockenhauer, London).
Cilia and Centrosomes: A Unifying Theory for Cystic Kidney Disease
Positional cloning has revealed nine genes that cause nephronophthisis if mutated21–32. These are monogenic recessive genes, implying that mutations in each single one is sufficient in itself to cause NPHP in a patient bearing mutations, indicating that their gene products are necessary for normal kidney function. Positional cloning thereby generated new insights into disease mechanisms of NPHP, and revealed that they are related to signaling mechanisms of primary cilia, centrosomes, and planar cell polarity1,25,33,34. The demonstration that nephrocystin-1 and inversin/NPHP-2 localize to primary cilia of renal tubular cells25 was among the first findings to support a new unifying theory of renal cystogenesis33. This theory states that proteins (“cystoproteins”) that are mutat35ed in renal cystic disease in humans, in mice, or zebrafish, are expressed in primary cilia, basal bodies, or centrosomes33,36. Basal bodies are the foundations from which cilia are assembled (Figure 2). After mitosis and cell division are completed, basal bodies derive from the mother centriole of the centriole pair that had organized the mitotic spindle in cell division. As cilia are formed from the basal body, the daughter centriole is placed on the side of the nucleus opposite to the basal body, thus specifying cell polarity. The structure and function of primary cilia and basal bodies is delineated in Figure 2.
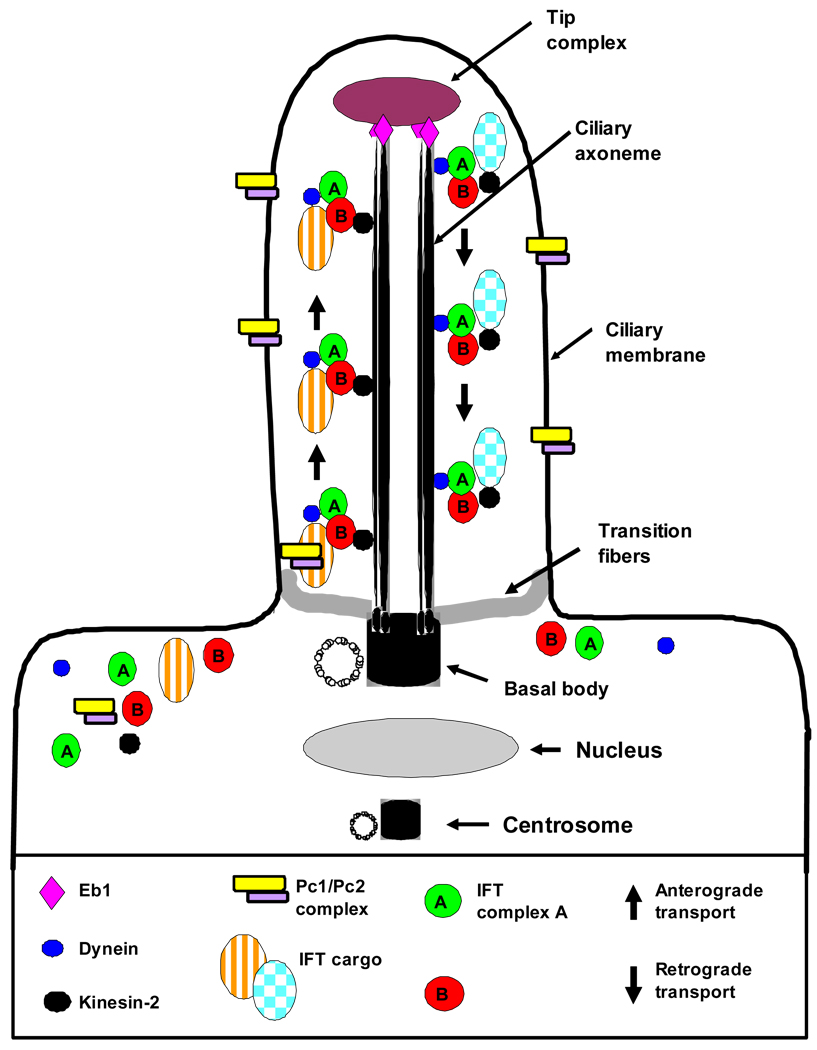
Figure 2. Cilia structure and intraflagellar transport.
The cilium is a hair-like structure that extends from the cell surface into the extracellular space. Virtually all vertebrate cell types can produce cilia. Cilia consist of a microtubule-based axoneme covered by a specialized plasma membrane. The axoneme has nine peripheral microtubule doublets. There may be two central microtubules (9+2 versus 9+0 axoneme). 9+2 cilia usually have dynein arms that link the microtubule doublets and are motile, while most 9+0 cilia lack dynein arms and are non-motile (“primary cilia”) with a few exceptions. The ciliary axoneme is anchored in the basal body, a microtubule-organizing center derived from the mother centriole. The transition zone at the junction of the basal body acts as a filter for the molecules that can pass into or out of the cilium. Nephrocystin-1 is localized at the transition zone of epithelial cells51. During ciliogenesis, cilia elongate from the basal body by the addition of new axonemal subunits to the distal tip, the plus end of the microtubules. Axonemal and membrane components are transported in raft macromolecular particles (complex A and B) by so-called intraflagellar transport (IFT) along the axonemal doublet microtubules135. Anterograde transport towards the tip is driven by heterotrimeric kinesin 2, which contains motor subunits Kif3a and Kif3b and a non-motor subunit. Mutations of Kif3a cause renal cysts and cerebellar vermis aplasia in mice136. Retrograde transport back to the cell body occurs via the motor protein cytoplasmic dynein 1B137 (modified from Bisgrove and Yost, 2006)138.
It is becoming apparent that primary cilia are highly conserved structures that sense of a wide variety of extracellular cues in a broad spectrum of epithelial tissues. There is a wide range of cues that can be received by specific ciliary receptors, including photosensation, mechanosensation, osmosensation, and olfactory sensation. In general, it seems that the pathogenesis of ciliopathies is based on an inability of epithelial cells to sense or process extracellular cues37.
Positional Cloning Reveals Nephronophthisis as a “Ciliopathy”
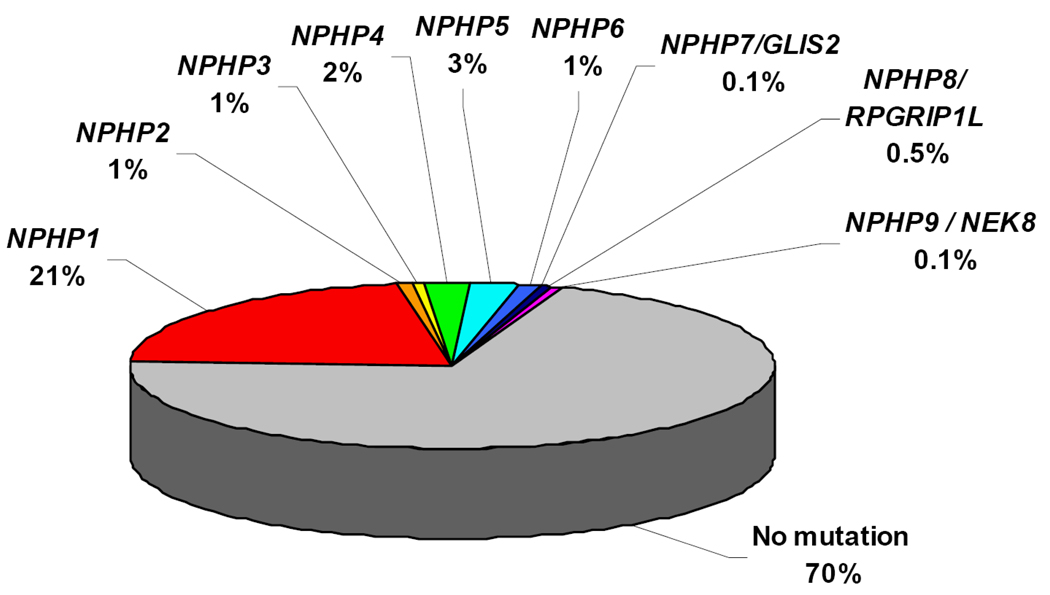
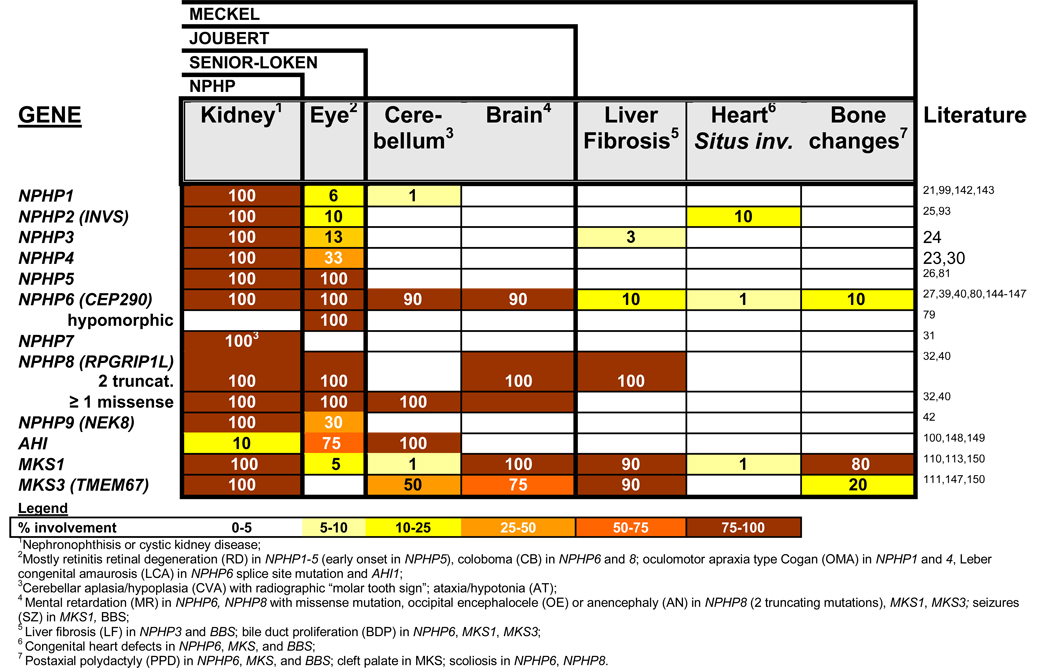
By positional cloning we and others have identified recessive mutations in nine different novel genes as causing NPHP: NPHP121,22, NPHP2/inversin25, NPHP324, NPHP423,30, NPHP538, NPHP6/CEP29027,39, NPHP7/GLIS231, NPHP8/RPGRIP1L32,40,41, and NPHP9/NEK842, defining NPHP types 1 through 9, respectively. This has made molecular genetic diagnostics possible (www.renalgenes.org). Homozygous deletions in the NPHP1 gene account for approximately 21% of all NPHP cases, whereas the other genes contribute less than 3% each (Figure 3). As determined in more than 1,000 families with NPHP, the causative genes are still unknown in about 70% of cases, indicating that further genes are involved in the pathogenesis of NPHP (Figure 3). Recently, evidence has been generated that more than one recessive gene may be mutated in individual patients with NPHP as has been proposed for the related disorder Bardet-Biedl syndrome (BBS)43. In this situation mutations in an additional NPHP gene may modify the clinical picture of NPHP in the direction of a more severe extrarenal involvement44,45. In the following, disease mechanisms of NPHP will be discussed in the context of the discovery of each of the NPHP1 through NPHP9 genes. We will describe how the resulting insights into the function of their gene products, the nephrocystins, helped refine the cilia/centrosome theory of renal cystic diseases.
Figure 3. Distribution of causative mutations in the NPHP1 – NPHP9 genes in a worldwide cohort of 1,079 families with NPHP.
Note that, whereas mutations in NPHP1 account for >21% of NPHP, all other genes are in the range of 3%.
NPHP1 locates to Cell Contacts and the Cilia Transition Zone
Mutations in NPHP1 were identified as causing juvenile nephronophthisis type 121,22. NPHP1 encodes nephrocystin-1, a protein that interacts with components of cell-cell and cell-matrix signaling, including p130Cas46, focal adhesion kinase 247, tensin, filamin A and B48,49. It is located at adherens junctions and focal adhesions of renal epithelial cells48,49, which are involved in cell-cell and cell-basement membrane contacts, respectively (Figure 4). It also interacts with the product of other nephronophthisis genes such as nephrocystin-2/inversin25, nephrocystin-324 and nephrocystin-423,50. More recently, it was shown that nephrocystin-1 is targeted to the transition zone of motile and primary cilia by the protein PACS-1 (phosphofurin acidic cluster sorting protein-1)51. This is initiated by casein kinase 2-mediated phosphorylation of three critical serine residues within a cluster of acidic amino acids in nephrocystin, leading to PACS-1 binding, and to colocalization of nephrocystin with PACS-1 at the base of cilia52.
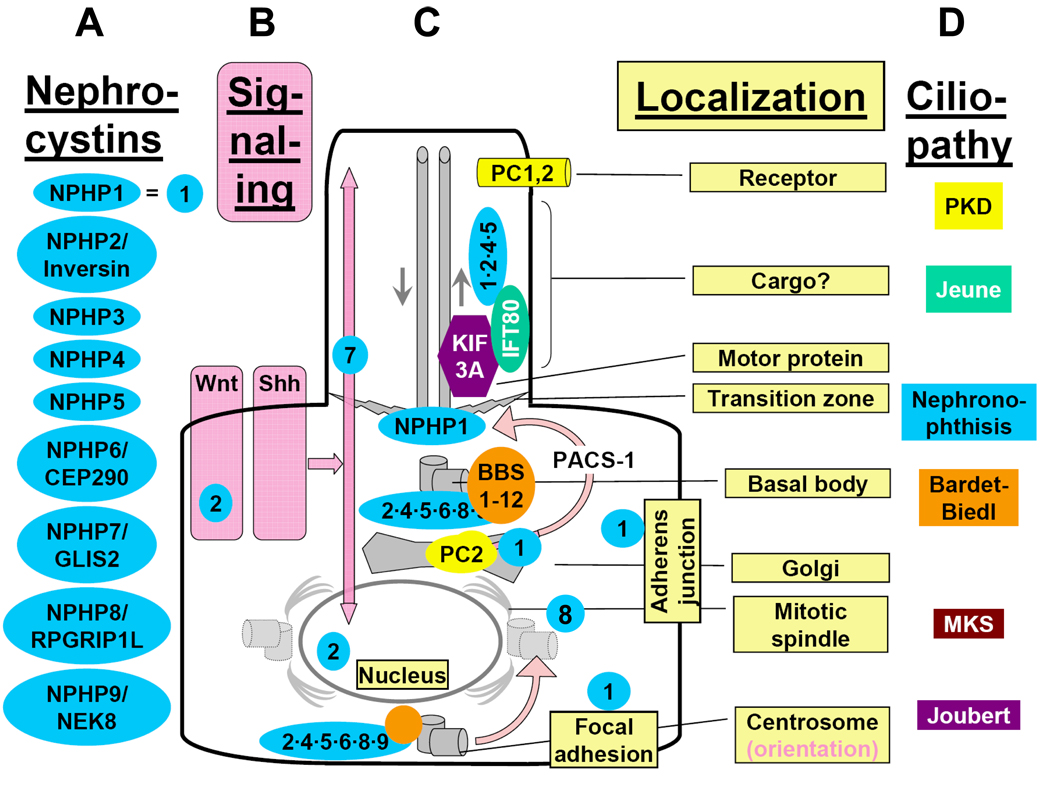
Figure 4. Subcellular localization of nephrocystins to primary cilia, basal bodies, the mitotic spindle, focal adhesions and adherens junctions, and functional interaction with other proteins mutated in renal “ciliopathies”.
"Cystoproteins” are proteins of genes mutated in cystic kidney diseases of humans, mice, or zebrafish. Depending on cell cycle stage, cystoproteins are localized at different subcellular organelles (shown in grey)27,139 including primary cilia, basal bodies, endoplasmic reticulum, the mitotic spindle, centrosomes, adherens junctions or focal adhesions. Arrows in the primary cilium indicate the direction of anterograde transport along the microtubule system mediated by kinesin-2 and retrograde transport by cytoplasmic dynein 1b. A) Most nephrocystins (blue) are located at cilia, the basal body, and centrosome in a cell cycle dependent manner. NPHP1 is also at the transition zone, focal adhesions and adherens junctions. B) Sensory cilia perceive and process cell external signals, and “cystoproteins” are involved in signaling mechanisms downstream of cilial signal recognition. Downstream of cilia (pink), Wnt signaling (Figure 6) and hedgehog signaling (Figure 8) play a role in planar cell polarity, which is mediated (C) partially through orientation of centrosomes and the mitotic spindle poles (Figure 7). D) Cilia-dependent mechanisms of planar cell polarity seem to be the central to the pathogenesis of the ciliopathies, the most prominent of which are listed on the right. Wht, the Wnti signaling pathway; Shh, the sonic hedgehog signaling pathway.
When NPHP1 was first identified21, we proposed a pathogenic hypothesis that tied nephrocystin-1 in with defects of cell-cell and cell-matrix signaling53,54. This was based on the finding that nephrocystin-1 contains an SH3 domain, localizes to adherens junctions and focal adhesions of renal epithelial cells, and interacts with integral components of these structures, such as p130CAS48,49. This “adherens junction/focal adhesion hypothesis” of NPHP pathogenesis53,54 has recently been partially reconciled with the “cilia/centrosome” hypothesis in an integrative hypothesis by showing that nephrocystin-4 in polarized epithelial cells colocalizes with β-catenin at cell-cell contact sites and to primary cilia, whereas in dividing cells it localizes to centrosomes50 (Figure 4).
NPHP2/Inversin Implicates Cilia and Planar Cell Polarity in Cystogenesis
On the basis of positional cloning14 and candidate gene data55,56 we identified mutations inhuman inversin (INVS) as the cause of infantile NPHP (type 2) with and without situs inversus25. The renal cystic changes of infantile nephronophthisis (NPHP type 2) combine clinical features of NPHP and of PKD57. We demonstrated that nephrocystin-1 and NPHP2/inversin interact with β-tubulin, which constitutes the microtubule axoneme of primary cilia, and that they are localized at primary cilia of renal tubular cells25. This was one of the first findings to support a unifying theory of renal cystogenesis1,33,36,58 which states that proteins (“cystoproteins”) which are mutated in renal cystic disease in humans, mice or zebrafish, are expressed in primary cilia, basal bodies, or centrosomes25,33. The interaction and co-localization to cilia and basal bodies of nephrocystin-1, inversin, and β-tubulin provided a functional link between the pathogenesis of NPHP, the pathogenesis of PKD, primary cilia function, and left-right axis determination25. Okada et al. had previously demonstrated that inversin is needed to position the cilia in cells of the ventral node59. Inversin was then shown to localize to different subcellular locations, in a cell cycle dependent manner (Figure 5). Specifically, it is found at the mitotic spindle in mitosis, at the midbody in cytokinesis, and in cilia, at the basal body and centrosome in interphase (Figure 5). All of these subcellular organelles are involved in regulation of planar cell polarity or the cell cycle (see below).
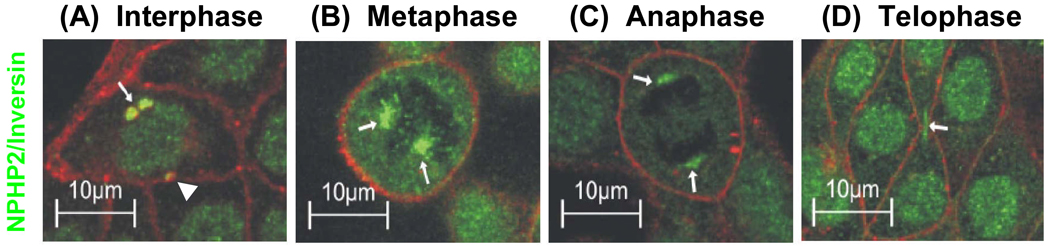
Figure 5. Inversin/NPHP2 localizes to cilia, centrosomes, and the mitotic spindle in a cell cycle dependent manner.
Inversin/NPHP2 is found in interphase (A) in the cilial axoneme (not shown), close to the centrioles of the basal body complex (arrow) and at the centrosome (arrow head). In metaphase (B) and anaphase (C) it is at the mitotic spindle (arrows), and in telophase (D) at the midbody (arrow) of the separating cells and in the nucleus. This reflects a centriole-associated function of inversin/NPHP2 throughout the cell cycle (from Morgan et al. Hum Mol Genet 11:3345, 2002).
A major breakthrough was made for the understanding of the pathogenesis of renal cystic diseases when Simons et al. demonstrated a role of inversin/NPHP2 in signaling mechanisms of planar cell polarity necessary to maintain normal tubular development and morphology60 as outlined in Figure 634,60. As a consequence of this model, when inversin is defective (as in NPHP type 2) the canonical Wnt pathway will prevail and disrupt apical-basolateral polarity of the renal epithelium34. Since planar cell polarity signaling is important for oriented cell division, it seems logical that Fisher et al. recently have been able to demonstrate abnormal orientation of the mitotic spindle in two different rodent models of cystic kidney disease61.
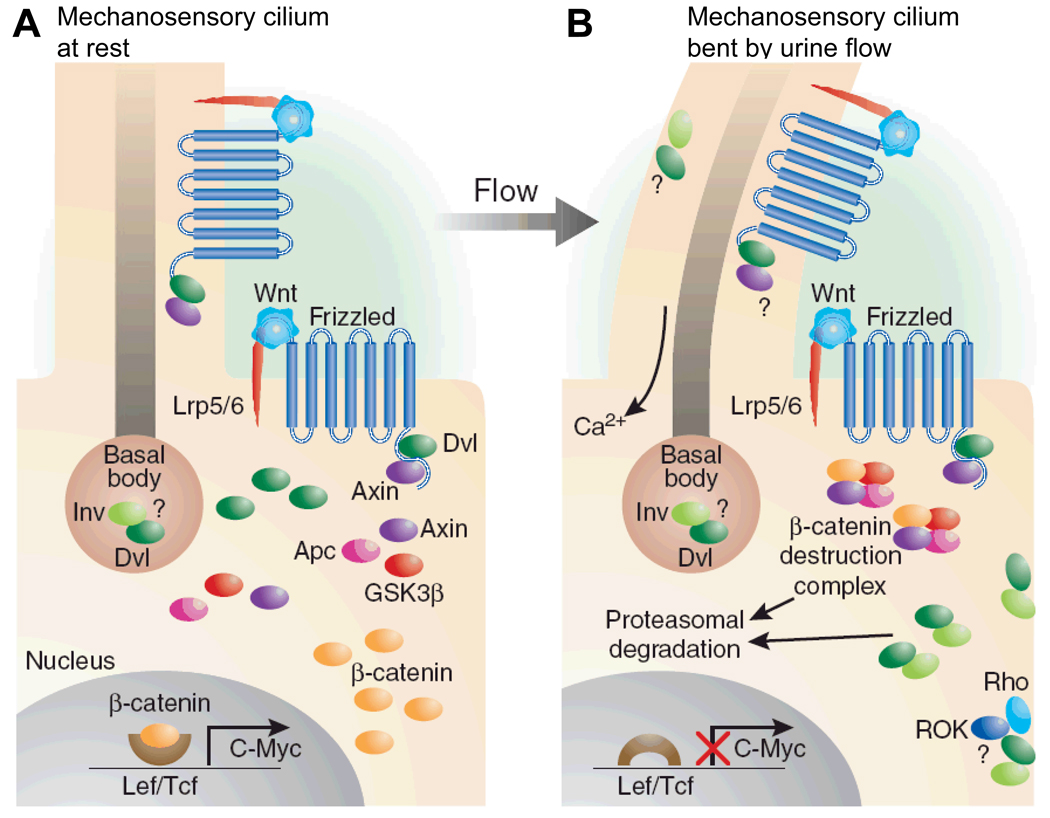
Figure 6. Inversin/NPHP2 mediates a switch from the canonical the non-canonical Wnt signaling pathway, which plays a role in planar cell polarity maintenance60.
(A) This cartoon of arenal tubular epithelial cell shows how Wnt signaling occurs primarily through β-catenin–dependent pathways in the absence of urine flow. Ligand binding by the frizzled receptor results in inactivation of the β-catenin destruction complex through the presence of disheveled (Dvl), increased β-catenin levels, and upregulation of effector gene expression of the canonical Wnt signaling pathway. (B) Stimulation of the primary cilium, e.g. by urine flow, results in increased expression of inversin (Inv), which then reduces levels of cytoplasmic Dvl by increasing its proteasomal degradation. This allows reassembly and activation of the β-catenin destruction complex, thereby switching from the canonical to the non-canonical Wnt signaling pathway. The model is consistent with the finding that overexpression of β-catenin (equivalent to canonical Wnt signaling) leads to renal cysts in a mouse model140 (from: Germino Nature Genet 37:455, 2005)34.
NPHP3: A Doorstep to Treatment?
By positional cloning we identified mutations in NPHP3 as responsible for adolescent nephronophthisis in a large Venezuelan kindred13,24. We demonstrated that mutations in the murine ortholog Nphp3 cause the renal cystic mouse mutant pcy24, which was demonstrated to be responsive to treatment with a vasopressin receptor antagonist62. Recently, it was shown that complete loss of Nphp3 function results in situs inversus, congenital heart defects, and embryonic lethality in mice63. In addition, truncating mutations of NPHP3 in humans can cause a broad clinical spectrum of early embryonic patterning defects that resembles Meckel syndrome. This included situs inversus, polydactyly, central nervous system malformations, structural heart defects, preauricular fistulas, and a wide range of congenital anomalies of the kidney and urinary tract63.
NPHP4 is Conserved in C. Elegans
Mutations in the novel gene NPHP4 were identified by homozygosity mapping and total genome search for linkage23,64,65. Nephrocystin-4, like inversin, localizes to primary cilia, basal bodies, centrosomes, and the cortical actin cytoskeleton50 (Figure 4). Nephrocystin-4 is conserved in C. elegans and expressed in ciliated head and tail neurons of the nematode66. Upon knockdown it exhibits a male mating phenotype, similar to the phenotype found upon knockdown of the polycystin-1 and polycystin-2 orthologs66. Localization of nphp-1 and nphp-4 to some of these ciliated neurons also overlaps with localization of the cystoprotein orthologs polycystin-1 (lov-1), polycystin-2 (pkd-2), and with many orthologs of Bardet-Biedl syndrome (BBS) proteins66,67 similar to what has been described for lov-1 and pkd-2 mutants68. These data have been recently refined for specific neuronal cell type69,70 and the necessity of nphp-1 and nphp-4 for morphologic integrity of ciliated neurons in C. elegans was demonstrated71,72. In addition, a role for nphp-4 in life span of the worm has been demonstrated73.
Evolutionary conservation of cystoproteins goes even further: Some cystoproteins have been conserved over more than 1.5 billion years of evolution from the unicellular organism Chlamydomonas Reinhardtii to vertebrates. Ch. Reinhardtii uses two motor cilia (flagella) for locomotion. Strikingly, nephrocystin-4 and at least six proteins mutated in BBS are conserved in Ch. Reinhardtii where they are part of its basal body proteome67,74. Defects of cystoprotein orthologs in Ch. Reinhardtii have deficient IFT and flagellar propulsion75.
NPHP5: The Senior-Loken Syndrome Gene
When the novel gene NPHP5 was identified as mutated in nephronophthisis type 526 all mutations detected were truncations of the encoded protein nephrocystin-5, and all patients had early-onset retinitis pigmentosa (Senior-Loken syndrome, SLSN). Nephrocystin-5 contains an IQ domain, which directly interacts with calmodulin38, and is in a complex with the retinitis pigmentosa GTPase regulator (RPGR), which when defective causes X-linked retinitis pigmentosa. Both, nephrocystin-5 and RPGR are localized in connecting cilia of photoreceptors and in primary cilia of renal epithelial cells26 (Figure 4). The fact that connecting cilia of photoreceptors are the structural equivalents of primary cilia of renal epithelial cells rendered an explanation for retinal involvement in the retinal-renal syndrome Senior-Loken syndrome.
Cystogenesis: Defective Regulation of Planar Cell Polarity?
NPHP6/CEP290: Centrosomes as a Central Hub for Planar Cell Polarity Regulation
We identified by positional cloning recessive truncating mutations in a novel gene NPHP6/CEP290 as the cause of NPHP type 6 and Joubert syndrome type 527. Its gene product nephrocystin-6/Cep29076 is part of the centrosomal proteome77. Like NPHP2/inversin (Figure 5) and NPHP4, NPHP6/CEP290 is expressed in centrosomes and the mitotic spindle in a cell-cycle dependent manner. We demonstrated that abrogation of NPHP6 function in zebrafish causes planar cell polarity defects and recapitulates the human phenotype of NPHP type 6, including renal cysts, retinal degeneration, and cerebellar defects27. Nephrocystin-6 modulates the activity of ATF4/CREB2, a transcription factor that may be implicated in cAMP-dependent renal cyst formation62. Interestingly, a 300-amino acid in-frame deletion of NPHP6/CEP290 caused retinal degeneration only, without renal or cerebellar involvement in the rds16 mouse model78. This is in accordance with the recent finding that a hypomorphic mutation of Nphp6/Cep290 represents the most frequent cause of Leber’s congenital amaurosis79. Mutations in NPHP6/CEP290 have been confirmed as causing JBTS with and without renal involvement 39. Furthermore, truncating mutations in NPHP6 were shown to cause Meckel-Gruber syndrome80.
Correct orientation of the mitotic spindle and centrosomes with respect to the longitudinal axis of the tubule is critical for proper apical-basolateral polarity (Figure 7). Non-canonical Wnt signaling (see Figure 6) is involved in these processes in renal tubular morphogenesis, when in rodents postnatally renal tubules still elongate. The structure that would result from disruption of the longitudinal growth would be a dilated tubule or cyst. Recently, evidence was generated for a role of planar cell polarity in renal cystic diseases61 by measuring orientation of the mitotic spindle through 3-D imaging of renal tubules. Comparison of the distribution of the mitotic angles in wild-type animals and rodent cystic kidney disease models revealed that mitotic angles of two rodent models of cystic kidneys, the HNF1 β-deficient mouse model and the pck rat model where clearly different from wild-type littermates61.
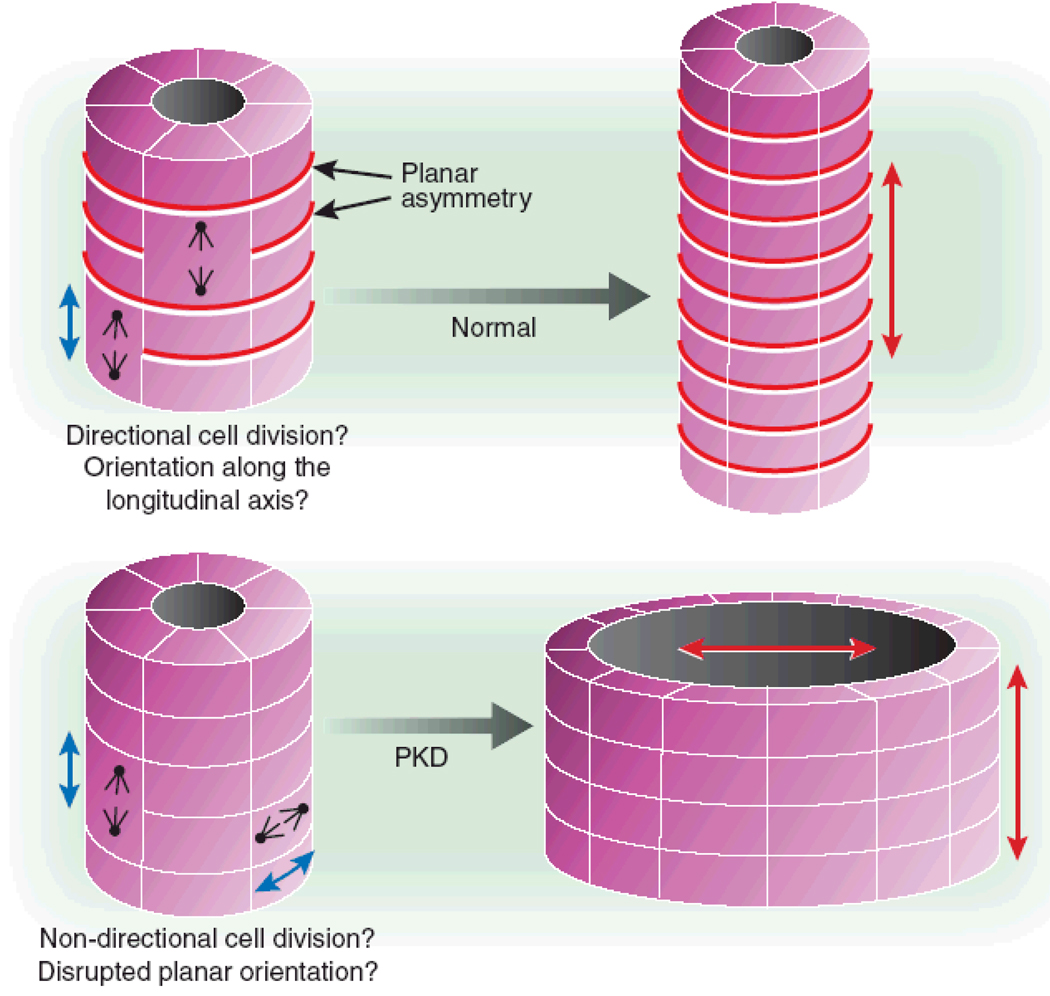
Figure 7. Defects of cystoproteins lead to disruption of planar cell polarity, and thereby to renal cysts through to malorientation of the centrosome or mitotic spindle complex.
Correct orientation of the mitotic spindle and centrosomes with respect to the longitudinal axis of the tubule is critical for proper planar cell polarity (i.e., the orientation of an epithelial cell layer in 3-dimensional space). Non-canonical Wnt signaling (see Figure 6) is involved in regulation of planar cell polarity during renal tubular morphogenesis, when in rodents 2 weeks post partum the tubules still elongate. The structure that would result from disruption of this longitudinal orientation is a dilated tubule or cyst (from Germino Nature Genet 37:455, 2005).
NPHP7/GLIS2: The link to Hedgehog Signaling
Recently, we identified in a Cree Indian kindred mutations in the NPHP7/GLIS2 gene, encoding the transcription factor Gli-similar protein 2 as the cause of NPHP type 7 (Figure 4)31. Starting at 8 weeks of age, Glis2 mutant mice showed severe renal atrophy and fibrosis resembling human nephronophthisis31. Differential gene expression studies on Glis2 mutant kidneys demonstrated that genes promoting epithelial-to-mesenchymal transition and fibrosis are upregulated in the absence of Glis231. Strikingly, there was also prominent apoptosis present in distal tubular segments of the kidney, which might provide an explanation why in PKD kidneys are enlarged with hyperproliferation prevailing, whereas in NPHP kidney size is reduced. GLIS2 is related to the GLI transcription factor these findings implicated the hedgehog pathway in the pathogenesis of cystic kidney diseases (Figure 8). It is a signaling pathway that controls, cell determination and tissue patterning during embryogenesis. The other known role of Hh is the maintenance of stem cells pools in post-embryonic tissues.
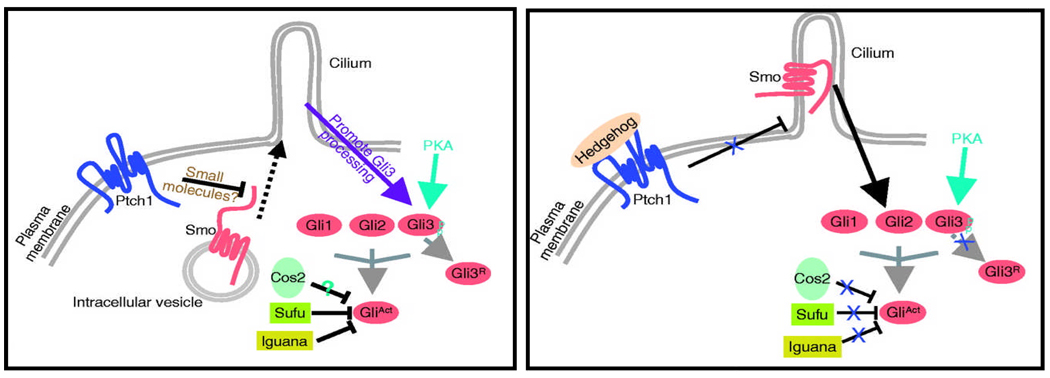
Figure 8. The hedgehog signaling pathway may be involved in renal cystogenesis.
In the hedgehog pathway of vertebrates, upon binding of the Shh ligand to the patched (Ptch1) receptor, repression of the smoothened (Smo) receptor to be inserted into the cilium membrane is relieved, and posttranslational modification of the Gli transcription factors within the cilium induces both their activator (GliAct) and repressor (GliR) functions. Several studies in mice have demonstrated that ciliary proteins are needed for hedgehog signaling. Recently, the related transcription factor Gli-similar 2 (GLIS2) was found to be mutated in NPHP type 7 (from: Huangfu & Anderson Development, 2005)141.
NPHP8: A Clinical Spectrum from Meckel Syndrome to Joubert Syndrome
Recently, missense and truncating mutations in the RPGRIP1L gene were identified by positional cloning as the cause of a Joubert syndrome-like phenotype (cerebro-oculo-renal syndrome, CORS) and Meckel syndrome (Figure 4)32. It was shown that defects in the mouse ortholog Rpgrip1l (Ftm) recapitulate the cerebral, renal and hepatic defects of CORS and Meckel syndrome. RPGRIP1L colocalized at the basal body and centrosomes with the protein products of both NPHP6 and NPHP432. RPGRIP1L missense mutations found in CORS individuals diminished the interaction between RPGRIP1L and nephrocystin-4. Missense mutations were seen in patients with Joubert syndrome32,40. These findings confirmed that there is a continuum for the multiorgan phenotypic abnormalities found in Meckel syndrome, Joubert syndrome/CORS, and nephronophthsisis on the basis of distinct mutations of identical genes (multiple allelism).
NPHP9: The Link From Cilia to Cell Cycle Control
We recently identified 3 different highly conserved amino acid changes in the gene NEK8 (never in mitosis kinase 8) as causing NPHP type 981. One of the mutations identified is positioned in the same RCC1 domain, in which the missense mutation causing the renal cystic mouse model jck is positioned82,83. Upon expression in medullary collecting duct cells all three mutant forms of NEK8 showed defects in ciliary and centrosomal localization to varying degrees, supporting the notion that mutations in NEK8 cause nephronophthisis (type 9)42 (Figure 4). As NEK8 plays a major role in cell cycle regulation, these data prove a direct link between a protein defective in renal cystic disease and the role of centrosomes for cell cycle regulation (Figure 4). In this context it is interesting that also for polycystin-1 and -2 signaling the renal cystic phenotype has been linked to cell growth regulation. Polycystin-1 expression activates the JAK-STAT pathway, thereby up regulating p21(waf1) and inducing cell cycle arrest in G0/G184. Cell cycle arrest required polycystin-2. Involvement of polycystin-1/2 signaling in the JAK/STAT pathway might explain how mutations of either gene can result in dysregulated growth84. Very recently, involvement of cell cycle regulation in renal cystic disease has been confirmed by demonstration that two mouse models of polycystic kidney disease (jck and cpk) can be efficiently treated with the cyclin-dependent kinase inhibitor roscovitine85.
The Ciliary Theory Explains Extrarenal Involvement of Eye, Brain, and Liver in NPHP
A prominent feature of NPHP is involvement of multiple organs (pleiotropy) outside the kidney. Defects in other organs are usually of degenerative or developmental nature (Figure 9). Specifically, NPHP may be associated with tapetoretinal degeneration (Senior-Loken syndrome86,87), cerebellar vermis aplasia (Joubert syndrome88,89), ocular motor apraxia type Cogan90, mental retardation27, liver fibrosis91, or cone-shaped epiphyses of the phalanges (Mainzer-Saldino syndrome92). Infantile NPHP type 225 can be associated with situs inversus15, retinitis pigmentosa93, or cardiac ventricular septal defect25. In some instances there appears to be a genotype/phenotype correlation regarding pleiotropy. For instance, there is involvement of the retina in all known cases with mutations of NPHP5 or NPHP6. In other instances, such as NPHP1 mutations, the molecular basis of eye involvement is unknown. The pleiotropy of NPHP has now found a potential explanation in the ciliary hypothesis of cystic kidney diseases (Figure 9). The extrarenal organ involvement in NPHP is discussed by organ system as follows:
Figure 9. Ciliopathies feature a broad spectrum of organ involvement, shown here for the nephronophthisis-related ciliopathies.
There is overlap between different syndromes: Exlusive kidney involvement is called nephronophthisis. Associated retinal degeneration in known as Senior-Loken syndrome. Involvement of the cerebellum represents Joubert syndrome. In the most severe form, Meckel syndrome, there are brain malformations, liver fibrosis, heart defects, polydactyly and perinatal mortality associated. It has recently become evident that the spectrum can vary by at least 2 mechanisms: First, multiple allelism, in which a hypomorphic mutation may cause a milder phenotype. For example, a splice site mutation of NPHP6 may cause Leber congenital optical atrophy (LCA) only. In another example, the presence of one non-truncating mutation in NPHP8 can rescue the phenotype from Meckel syndrome to Joubert syndrome. Secondly, NPHP genes can modify each other. For instance, NPHP6 and AHI1 modify recessive NPHP1 mutations to express a more severe phenotype147.
Retinal Involvement (Senior-Loken syndrome)
The renal-retinal involvement in Senior-Loken syndrome can be explained by the fact that the primary cilium of renal epithelial cells is a structural equivalent of the connecting cilium of photoreceptor cells in the retina94. We have shown that nephrocystin-5 and nephrocystin-6 are expressed in the connecting cilia of photoreceptors38,78.
Cerebellar Vermis Aplasia (Joubert Syndrome)
In Joubert syndrome (JBTS) NPHP is associated with coloboma of the eye, with aplasia/hypoplasia of the cerebellar vermis causing ataxia, and with the inconstant symptoms of psychomotor retardation, and episodic neonatal tachy/dyspnea88,89,95–97. The radiographic feature of JBTS on axial magnetic resonance brain imaging is the so-called “molar tooth sign” of the midbrain-hindbrain junction97,98. It is due to abnormal axonal decussation (nerve tract crossing) in the corticospinal tract and the superior cerebellar peduncles, as the basis of the motor and behavioral abnormalities of JBTS28. Ocular motor apraxia type Cogan, defined as the transient inability of horizontal eye movements in the first few years of life, may also be associated with JBTS. This symptom has been described in patients with mutations in the NPHP190,99 (“JBTS4”) and NPHP423 genes. Three different recessive genes, NPHP189,97,98, AHI100,101 (JBTS type 3), and NPHP639,76, have been found mutated in JBTS. Three further loci for JBTS have been identified: JBTS1 on chromosome 9q34.3102, JBTS2/CORS2 on chromosome 11p12-q13.3103. In addition, mutations of NPHP8/RPGRIP1L can cause JBTS if at least one mutation is not truncating32,40.
Liver Fibrosis
NPHP and the related disorder Bardet-Biedl syndrome (BBS) can be associated with periductal liver fibrosis (LF)91,104–106, as has been described for a patient with NPHP3 mutation24. Patients develop hepatomegaly and moderate portal fibrosis with mild bile duct proliferation. This pattern differs from that of classical congenital hepatic fibrosis, where biliary dysgenesis is prominent, and from hepatic involvement in ARPKD, Arima syndrome (cerebro-oculo-hepato-renal syndrome)107–109, and Meckel syndrome, which appears as bile duct proliferation. Bile duct involvement in these cystic kidney diseases may be explained by the ciliary theory, as the epithelial cells lining bile ducts (cholangiocytes) possess primary cilia.
Brain Malformations (Meckel Syndrome)
Meckel syndrome (MKS) features the association of renal cystic dysplasia with occipital encephalocele, polydactyly, and biliary digenesis (Figure 4). Two recessive genes have been identified, MKS1110 and MKS3111, and another gene locus, MKS2112, has been mapped. Recently, a Meckel-like phenotype has been described for truncating mutations of NPHP3 63, NPHP6/CEP29080, and NPHP8/RPGRIP1L32,40. Within the spectrum of NPHP-associated ciliopathies, MKS is the most severe, leading to perinatal mortality. Consequently, MKS represents the ciliopathy of the group that encompasses defects in most organs, and involvement is most severe and of developmental rather than degenerative nature. For instance, organ defects reveal cystic dysplasia rather than NPHP in the kidneys, microphthalmia of the eyes, bile duct dysgenesis in the liver, the occipital encephalocele in the brain, and bones are involved by postaxial polydactyly113. The notion that MKS is at the most pronounced end of the clinical spectrum, is supported by the finding that the presence of two truncating mutations in NPHP8/RPGRIP1L causes MKS, whereas one “mild” mutation (missense rather than truncating) may cause the less severe phenotype of JBTS32. In addition, the presence of 2 truncating mutations in NPHP6/CEP290 may cause an MKS-like phenotype (MKS4) 80.
Cardiac Defects and Situs inversus
In a patient with mutation ofNPHP2 we observed a ventricular septal defect as a congenital cardiac malformation15. Thus, the role of inversin for left-right axis specification which had been described in mice was confirmed in humans55,56. The patient with situs inversus also had a cardiac ventricular septal defect, which may be viewed as a “heterotaxy” (left-right orientation) phenotype caused by the same mechanism114 that lead to situs inversus in this patient. We confirmed the phenotypic combination of cystic kidney disease, situs inversus, and cardiac septal defect on the basis of inversin mutations is observed in humans, mice, and zebrafish25. The PKD2 gene, mutations in which cause autosomal dominant PKD was shown to also represent a gene that regulates left-right axis determination, acting upstream of Nodal, Ebaf, Leftb and Pitx2114,115.
Skeletal Defects
Multiple disease variants that are associated with NPHP include skeletal defects, strongly suggesting a role of primary cilia function in skeletal development. These include Jeune syndrome (asphyxiating thoracic dysplasia)116–119, Ellis van Creveld syndrome120, RHYNS syndrome (retinitis pigmentosa, hypopituitarism, NPHP, skeletal dysplasia)121, Meckel-Gruber syndrome110,111, and Sensenbrenner syndrome (cranioectodermal dysplasia)122,123. The association of NPHP with cone-shaped epiphyses of the phalanges (type 28 and 28A) is known as Mainzer-Saldino syndrome, and occurred in patients who also had retinal degeneration and cerebellar ataxia92. Interestingly, mutations in the ortholog of the intraflagellar transport protein IFT80 of Ch. Reinhardtii was found to be the cause of Jeune syndrome124 (Figure 4), which emphasized again the strong evolutionary conservation of “ciliopathy genes”.
Bardet-Biedl and Alstrom Syndromes
Bardet-Biedl syndrome (BBS) exhibits renal histology similar to NPHP125,126 (Figure 4). Positional cloning of recessive genes mutated in BBS has revealed that the molecular relation between NPHP and BBS may lie in coexpression of the respective gene products in primary cilia, basal bodies, or centrosomes of renal epithelial cells33. Alstrom syndrome exhibits some phenotypic overlap with BBS (NPHP, retinitis pigmentosa, deafness, obesity, and diabetes mellitus without mental defect, polydactyly, or hypogonadism)127. The single underlying recessive gene, ALMS1, encodes a novel protein that is a molecular component of the centrosome77,128–130. This finding, together with the finding that BBS proteins localize to centrosomes, confirms the role of centrosomal proteins in cystic kidney diseases that are associated with diabetes, obesity and retinitis pigmentosa131,132. Obesity, which is part of the clinical spectrum of the ciliopathies BBS and ALMS. Interestingly, in the Bbs6 knockout mouse model obesity was associated with hyperphagia and decreased activity of the mice133.
Therapeutic Approaches to NPHP
Currently there is no effective prophylaxis or treatment available for NPHP other than supportive treatment once chronic renal failure has developed, and dialysis and transplantation for terminal renal failure. Gattone et al. have recently shown that the renal cystic phenotype of pcy mice, which is the equivalent of human NPHP type 3 can be strongly mitigated or even reversed by treatment with the vasopressin V2 receptor antagonist OPC3126062. Similar results were obtained using a pkd2 mouse model134. This effect is thought to be mediated by a reduction in intracellular cAMP levels27. An important future challenge will be the development of therapies that capitalizes on what we have learnt about the biology of NPHP and other cystic diseases of the kidney.
Acknowledgements
This research was supported by grants from the National Institutes of Health to F.H. (R01-DK69274, R01-DK68306, R01-DK064614). F.H. is the Frederick G. L. Huetwell Professor, a Doris Duke Distinguished Clinical Scientist, and an Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Hildebrandt F, Otto EA. Primary cilia: a unifying pathogenic concept for cystic kidney disease? Nature Reviews Genetics. 2005 doi: 10.1038/nrg1727. [DOI] [PubMed] [Google Scholar]
- 2.Smith C, Graham J. Congenital medullary cysts of the kidneys with severe refractory anemia. Am J Dis Child. 1945;69:369–377. [Google Scholar]
- 3.Fanconi G, Hanhart E, Albertini A. Die familiäre juvenile Nephronophthise. Hel Pediatr Acta. 1951;6:1–49. [PubMed] [Google Scholar]
- 4.Hildebrandt F. Juvenile nephronophthisis. In: Barratt TM, Avner ED, Harmon WE, editors. Pediatric nephrology. Baltimore: Williams & Wilkins; 1999. [Google Scholar]
- 5.Ala-Mello S, Kivivuori SM, Ronnholm KA, Koskimies O, Siimes MA. Mechanism underlying early anaemia in children with familial juvenile nephronophthisis. Pediatr Nephrol. 1996;10:578–581. doi: 10.1007/s004670050164. [DOI] [PubMed] [Google Scholar]
- 6.Blowey DL, Querfeld U, Geary D, Warady BA, Alon U. Ultrasound findings in juvenile nephronophthisis. Pediatr Nephrol. 1996;10:22–24. doi: 10.1007/BF00863431. [DOI] [PubMed] [Google Scholar]
- 7.Waldherr R, Lennert T, Weber HP, Fodisch HJ, Scharer K. The nephronophthisis complex. A clinicopathologic study in children. Virchows Arch A Pathol Anat Histol. 1982;394:235–254. doi: 10.1007/BF00430668. [DOI] [PubMed] [Google Scholar]
- 8.Zollinger HU, et al. Nephronophthisis (medullary cystic disease of the kidney). A study using electron microscopy, immunofluorescence, and a review of the morphological findings. Helv Paediatr Acta. 1980;35:509–530. [PubMed] [Google Scholar]
- 9.Steel BT, Lirenman DS, Battie CW. Nephronophthisis. Am J Med. 1980;68:531–538. doi: 10.1016/0002-9343(80)90299-5. [DOI] [PubMed] [Google Scholar]
- 10.Smith CH, Graham JB. Congenital medullary cysts of the kidneys with severe refractory anemia. Am J Dis Child. 1945;69:369–377. [Google Scholar]
- 11.Fanconi G, et al. Die familiäre juvenile Nephronophthise. Helv Paediatr Acta. 1951;6:1–49. [PubMed] [Google Scholar]
- 12.Hildebrandt F, et al. Molecular genetic identification of families with juvenile nephronophthisis type 1: rate of progression to renal failure. APN Study Group. Arbeitsgemeinschaft fur Padiatrische Nephrologie. Kidney Int. 1997;51:261–269. doi: 10.1038/ki.1997.31. [DOI] [PubMed] [Google Scholar]
- 13.Omran H, et al. Identification of a new gene locus for adolescent nephronophthisis, on chromosome 3q22 in a large Venezuelan pedigree. Am J Hum Genet. 2000;66:118–127. doi: 10.1086/302705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haider NB, Carmi R, Shalev H, Sheffield VC, Landau D. A Bedouin kindred with infantile nephronophthisis demonstrates linkage to chromosome 9 by homozygosity mapping. Am J Hum Genet. 1998;63:1404–1410. doi: 10.1086/302108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Otto EA, et al. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat Genet. 2003;34:413–420. doi: 10.1038/ng1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleinknecht C. The inheritance of nephronophthisis. In: Spitzer A, Avner ED, editors. Inheritance of Kidney and Urinary Tract Diseases. Vol. 9. Boston: Kluwer Academic Publishers; 1989. p. 464. [Google Scholar]
- 17.Potter DE, et al. Treatment of end-stage renal disease in children: a 15-year experience. Kidney Int. 1980;18:103–109. doi: 10.1038/ki.1980.115. [DOI] [PubMed] [Google Scholar]
- 18.Pistor K, et al. Children with chronic renal failure in the Federal Republic of Germany: II. Primary renal diseases, age and intervals from early renal failure to renal death. Arbeitsgemeinschaft fur Padiatrische Nephrologie. Clin Nephrol. 1985;23:278–284. [PubMed] [Google Scholar]
- 19.Warady BA, et al. Renal transplantation, chronic dialysis, and chronic renal insufficiency in children and adolescents. The 1995 Annual Report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Nephrol. 1997;11:49–64. doi: 10.1007/s004670050232. [DOI] [PubMed] [Google Scholar]
- 20.Avner ED. Medullary cystic disease and medullary sponge kidney. In: Greenberg A, editor. Primer on kidney diseases. Boston: Academic Press; 1994. [Google Scholar]
- 21.Hildebrandt F, et al. A novel gene encoding an SH3 domain protein is mutated in nephronophthisis type 1. Nat Genet. 1997;17:149–153. doi: 10.1038/ng1097-149. [DOI] [PubMed] [Google Scholar]
- 22.Saunier S, et al. A novel gene that encodes a protein with a putative src homology 3 domain is a candidate gene for familial juvenile nephronophthisis. Hum Mol Genet. 1997;6:2317–2323. doi: 10.1093/hmg/6.13.2317. [DOI] [PubMed] [Google Scholar]
- 23.Mollet G, et al. The gene mutated in juvenile nephronophthisis type 4 encodes a novel protein that interacts with nephrocystin. Nat Genet. 2002;32:300–305. doi: 10.1038/ng996. [DOI] [PubMed] [Google Scholar]
- 24.Olbrich H, et al. Mutations in a novel gene, NPHP3, cause adolescent nephronophthisis, tapeto-retinal degeneration and hepatic fibrosis. Nat Genet. 2003;34:455–459. doi: 10.1038/ng1216. [DOI] [PubMed] [Google Scholar]
- 25.Otto EA, et al. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat Genet. 2003;34:413–420. doi: 10.1038/ng1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Otto E, Loeys B, Khanna H, Hellemans J, Sudbrak R, Fan S, Muerb U, O'Toole JF, Helou J, Attanasio M, Utsch B, Sayer JA, Lillo C, Jimeno D, Coucke P, De Paepe A, Reinhardt R, Klages S, Tsuda M, Kawakami I, Kusakabe T, Omran H, Imm A, Tippens M, Raymond PA, Hill J, Beales P, He S, Kispert A, Margolis B, Williams DS, Swaroop A, Hildebrandt F. A novel ciliary IQ domain protein, NPHP5, is mutated in Senior-Loken syndrome (nephronophthisis with retinitis pigmentosa), and interacts with RPGR and calmodulin. Nat Genet. 2005 doi: 10.1038/ng1520. [DOI] [PubMed] [Google Scholar]
- 27.Sayer JA, et al. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet. 2006 doi: 10.1038/ng1786. [DOI] [PubMed] [Google Scholar]
- 28.Ferland RJ, et al. Abnormal cerebellar development and axonal decussation due to mutations in AHI1 in Joubert syndrome. Nat Genet. 2004;36:1008–1013. doi: 10.1038/ng1419. [DOI] [PubMed] [Google Scholar]
- 29.Dixon-Salazar T, et al. Mutations in the AHI1 gene, encoding jouberin, cause Joubert syndrome with cortical polymicrogyria. Am J Hum Genet. 2004;75:979–987. doi: 10.1086/425985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otto E, et al. A gene mutated in nephronophthisis and retinitis pigmentosa encodes a novel protein, nephroretinin, conserved in evolution. Am J Hum Genet. 2002;71:1167–1171. doi: 10.1086/344395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Attanasio M, et al. Loss of GLIS2 causes nephronophthisis in humans and mice by increased apoptosis and fibrosis. Nat Genet. 2007;39:1018–1024. doi: 10.1038/ng2072. [DOI] [PubMed] [Google Scholar]
- 32.Delous M, et al. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat Genet. 2007;39:875–881. doi: 10.1038/ng2039. [DOI] [PubMed] [Google Scholar]
- 33.Watnick T, Germino G. From cilia to cyst. Nat Genet. 2003;34:355–356. doi: 10.1038/ng0803-355. [DOI] [PubMed] [Google Scholar]
- 34.Germino GG. Linking cilia to Wnts. Nat Genet. 2005;37:455–457. doi: 10.1038/ng0505-455. [DOI] [PubMed] [Google Scholar]
- 35.Arts HH, et al. Mutations in the gene encoding the basal body protein RPGRIP1L, a nephrocystin-4 interactor, cause Joubert syndrome. Nat Genet. 2007;39:882–888. doi: 10.1038/ng2069. [DOI] [PubMed] [Google Scholar]
- 36.Igarashi P, Somlo S. Genetics and pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2002;13:2384–2398. doi: 10.1097/01.asn.0000028643.17901.42. [DOI] [PubMed] [Google Scholar]
- 37.Benzing T, Walz G. Cilium-generated signaling: a cellular GPS? Curr Opin Nephrol Hypertens. 2006;15:245–249. doi: 10.1097/01.mnh.0000222690.53970.ca. [DOI] [PubMed] [Google Scholar]
- 38.Otto EA, et al. Nephrocystin-5, a ciliary IQ domain protein, is mutated in Senior-Loken syndrome and interacts with RPGR and calmodulin. Nat Genet. 2005;37:282–288. doi: 10.1038/ng1520. [DOI] [PubMed] [Google Scholar]
- 39.Valente EM, et al. Mutations in CEP290, which encodes a centrosomal protein, cause pleiotropic forms of Joubert syndrome. Nat Genet. 2006;38:623–625. doi: 10.1038/ng1805. [DOI] [PubMed] [Google Scholar]
- 40.Wolf MT, et al. Mutational analysis of the RPGRIP1L gene in patients with Joubert syndrome and nephronophthisis. Kidney Int. 2007;72:1520–1526. doi: 10.1038/sj.ki.5002630. [DOI] [PubMed] [Google Scholar]
- 41.Roepman R, et al. The retinitis pigmentosa GTPase regulator (RPGR) interacts with novel transport-like proteins in the outer segments of rod photoreceptors. Hum Mol Genet. 2000;9:2095–2105. doi: 10.1093/hmg/9.14.2095. [DOI] [PubMed] [Google Scholar]
- 42.Otto EA, et al. NEK8 mutations affect ciliary and centrosomal localization and may cause nephronophthisis. J Am Soc Nephrol. 2008;19:587–592. doi: 10.1681/ASN.2007040490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beales PL, et al. Genetic interaction of BBS1 mutations with alleles at other BBS loci can result in non-Mendelian Bardet-Biedl syndrome. Am J Hum Genet. 2003;72:1187–1199. doi: 10.1086/375178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoefele J, et al. Evidence of oligogenic inheritance in nephronophthisis. J Am Soc Nephrol. 2007;18:2789–2795. doi: 10.1681/ASN.2007020243. [DOI] [PubMed] [Google Scholar]
- 45.Badano JL, et al. Heterozygous mutations in BBS1, BBS2 and BBS6 have a potential epistatic effect on Bardet-Biedl patients with two mutations at a second BBS locus. Hum Mol Genet. 2003;12:1651–1659. doi: 10.1093/hmg/ddg188. [DOI] [PubMed] [Google Scholar]
- 46.Otto E, et al. Nephrocystin: gene expression and sequence conservation between human, mouse, and Caenorhabditis elegans. J Am Soc Nephrol. 2000;11:270–282. doi: 10.1681/ASN.V112270. [DOI] [PubMed] [Google Scholar]
- 47.Benzing T, et al. Nephrocystin interacts with Pyk2, p130(Cas), and tensin and triggers phosphorylation of Pyk2. Proc Natl Acad Sci U S A. 2001;98:9784–9789. doi: 10.1073/pnas.171269898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Donaldson JC, et al. Crk-associated substrate p130(Cas) interacts with nephrocystin and both proteins localize to cell-cell contacts of polarized epithelial cells. Exp Cell Res. 2000;256:168–178. doi: 10.1006/excr.2000.4822. [DOI] [PubMed] [Google Scholar]
- 49.Donaldson JC, Dise RS, Ritchie MD, Hanks SK. Nephrocystin-conserved domains involved in targeting to epithelial cell-cell junctions, interaction with filamins, and establishing cell polarity. J Biol Chem. 2002;277:29028–29035. doi: 10.1074/jbc.M111697200. [DOI] [PubMed] [Google Scholar]
- 50.Mollet G, et al. Characterization of the nephrocystin/nephrocystin-4 complex and subcellular localization of nephrocystin-4 to primary cilia and centrosomes. Hum Mol Genet. 2005;14:645–656. doi: 10.1093/hmg/ddi061. [DOI] [PubMed] [Google Scholar]
- 51.Fliegauf M, et al. Nephrocystin specifically localizes to the transition zone of renal and respiratory cilia and photoreceptor connecting cilia. J Am Soc Nephrol. 2006;17:2424–2433. doi: 10.1681/ASN.2005121351. [DOI] [PubMed] [Google Scholar]
- 52.Schermer B, et al. Phosphorylation by casein kinase 2 induces PACS-1 binding of nephrocystin and targeting to cilia. Embo J. 2005;24:4415–4424. doi: 10.1038/sj.emboj.7600885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hildebrandt F. Identification of a gene for nephronophthisis. Nephrol Dial Transplant. 1998;13:1334–1336. doi: 10.1093/ndt/13.6.1334. [DOI] [PubMed] [Google Scholar]
- 54.Hildebrandt F, Otto E. Molecular genetics of nephronophthisis and medullary cystic kidney disease. J Am Soc Nephrol. 2000;11:1753–1761. doi: 10.1681/ASN.V1191753. [DOI] [PubMed] [Google Scholar]
- 55.Mochizuki T, et al. Cloning of inv, a gene that controls left/right asymmetry and kidney development. Nature. 1998;395:177–181. doi: 10.1038/26006. [DOI] [PubMed] [Google Scholar]
- 56.Morgan D, et al. Inversin, a novel gene in the vertebrate left-right axis pathway, is partially deleted in the inv mouse. Nat Genet. 1998;20:149–156. doi: 10.1038/2450. [DOI] [PubMed] [Google Scholar]
- 57.Gagnadoux MF, Bacri JL, Broyer M, Habib R. Infantile chronic tubulo-interstitial nephritis with cortical microcysts: variant of nephronophthisis or new disease entity? Pediatr Nephrol. 1989;3:50–55. doi: 10.1007/BF00859626. [DOI] [PubMed] [Google Scholar]
- 58.Nauli SM, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003 doi: 10.1038/ng1076. Advanced Online Publication. [DOI] [PubMed] [Google Scholar]
- 59.Okada Y, et al. Abnormal nodal flow precedes situs inversus in iv and inv mice. Mol Cell. 1999;4:459–468. doi: 10.1016/s1097-2765(00)80197-5. [DOI] [PubMed] [Google Scholar]
- 60.Simons M, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fischer E, et al. Defective planar cell polarity in polycystic kidney disease. Nat Genet. 2006;38:21–23. doi: 10.1038/ng1701. [DOI] [PubMed] [Google Scholar]
- 62.Gattone VH, 2nd, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med. 2003;9:1323–1326. doi: 10.1038/nm935. [DOI] [PubMed] [Google Scholar]
- 63.Bergmann C, et al. Loss of nephrocystin-3 function can cause embryonic lethality, Meckel-Gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. Am J Hum Genet. 2008;82:959–970. doi: 10.1016/j.ajhg.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Otto E, et al. A Gene Mutated in Nephronophthisis and Retinitis Pigmentosa Encodes a Novel Protein, Nephroretinin, Conserved in Evolution. Am J Hum Genet. 2002;71 doi: 10.1086/344395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schuermann MJ, et al. Mapping of gene loci for nephronophthisis type 4 and Senior-Loken syndrome, to chromosome 1p36. Am J Hum Genet. 2002;70:1240–1246. doi: 10.1086/340317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wolf MT, et al. Expression and phenotype analysis of the nephrocystin-1 and nephrocystin-4 homologs in Caenorhabditis elegans. J Am Soc Nephrol. 2005;16:676–687. doi: 10.1681/ASN.2003121025. [DOI] [PubMed] [Google Scholar]
- 67.Badano JL, Teslovich TM, Katsanis N. The centrosome in human genetic disease. Nat Rev Genet. 2005;6:194–205. doi: 10.1038/nrg1557. [DOI] [PubMed] [Google Scholar]
- 68.Barr MM, et al. The Caenorhabditis elegans autosomal dominant polycystic kidney disease gene homologs lov-1 and pkd-2 act in the same pathway. Curr Biol. 2001;11:1341–1346. doi: 10.1016/s0960-9822(01)00423-7. [DOI] [PubMed] [Google Scholar]
- 69.Bae YK, et al. General and cell-type specific mechanisms target TRPP2/PKD-2 to cilia. Development. 2006;133:3859–3870. doi: 10.1242/dev.02555. [DOI] [PubMed] [Google Scholar]
- 70.Jauregui AR, Barr MM. Functional characterization of the C. elegans nephrocystins NPHP-1 and NPHP-4 and their role in cilia and male sensory behaviors. Exp Cell Res. 2005;305:333–342. doi: 10.1016/j.yexcr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 71.Bae YK, Lyman-Gingerich J, Barr MM, Knobel KM. Identification of genes involved in the ciliary trafficking of C. elegans PKD-2. Dev Dyn. 2008 doi: 10.1002/dvdy.21531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jauregui AR, Nguyen KC, Hall DH, Barr MM. The Caenorhabditis elegans nephrocystins act as global modifiers of cilium structure. J Cell Biol. 2008;180:973–988. doi: 10.1083/jcb.200707090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Winkelbauer ME, Schafer JC, Haycraft CJ, Swoboda P, Yoder BK. The C. elegans homologs of nephrocystin-1 and nephrocystin-4 are cilia transition zone proteins involved in chemosensory perception. J Cell Sci. 2005;118:5575–5587. doi: 10.1242/jcs.02665. [DOI] [PubMed] [Google Scholar]
- 74.Mykytyn K, Sheffield VC. Establishing a connection between cilia and Bardet-Biedl Syndrome. Trends Mol Med. 2004;10:106–109. doi: 10.1016/j.molmed.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 75.Efimenko E, et al. Analysis of xbx genes in C. elegans. Development. 2005;132:1923–1934. doi: 10.1242/dev.01775. [DOI] [PubMed] [Google Scholar]
- 76.Sayer JA, et al. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet. 2006;38:674–681. doi: 10.1038/ng1786. [DOI] [PubMed] [Google Scholar]
- 77.Andersen JS, et al. Proteomic characterization of the human centrosome by protein correlation profiling. Nature. 2003;426:570–574. doi: 10.1038/nature02166. [DOI] [PubMed] [Google Scholar]
- 78.Chang B, et al. In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum Mol Genet. 2006;15:1847–1857. doi: 10.1093/hmg/ddl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.den Hollander AI, et al. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am J Hum Genet. 2006;79:556–561. doi: 10.1086/507318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baala L, et al. Pleiotropic effects of CEP290 (NPHP6) mutations extend to Meckel syndrome. Am J Hum Genet. 2007;81:170–179. doi: 10.1086/519494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Otto EA, et al. Mutation analysis in nephronophthisis using a combined approach of homozygosity mapping, CEL I endonuclease cleavage, and direct sequencing. Hum Mutat. 2008;29:418–426. doi: 10.1002/humu.20669. [DOI] [PubMed] [Google Scholar]
- 82.Sohara E, et al. Nek8 regulates the expression and localization of polycystin-1 and polycystin-2. J Am Soc Nephrol. 2008;19:469–476. doi: 10.1681/ASN.2006090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu S, et al. A defect in a novel Nek-family kinase causes cystic kidney disease in the mouse and in zebrafish. Development. 2002;129:5839–5846. doi: 10.1242/dev.00173. [DOI] [PubMed] [Google Scholar]
- 84.Bhunia AK, et al. PKD1 induces p21(waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell. 2002;109:157–168. doi: 10.1016/s0092-8674(02)00716-x. [DOI] [PubMed] [Google Scholar]
- 85.Bukanov NO, Smith LA, Klinger KW, Ledbetter SR, Ibraghimov-Beskrovnaya O. Long-lasting arrest of murine polycystic kidney disease with CDK inhibitor roscovitine. Nature. 2006 doi: 10.1038/nature05348. [DOI] [PubMed] [Google Scholar]
- 86.Senior B, Friedmann AI, Braudo JL. Juvenile familial nephropathy with tapetoretinal degeneration: a new oculorenal dystrophy. Am J Ophthalmol. 1961;52:625–633. doi: 10.1016/0002-9394(61)90147-7. [DOI] [PubMed] [Google Scholar]
- 87.Loken AC, Hanssen O, Halvorsen S, Jolster NJ. Hereditary renal dysplasia and blindness. Acta Paediatr. 1961;50:177–184. doi: 10.1111/j.1651-2227.1961.tb08037.x. [DOI] [PubMed] [Google Scholar]
- 88.Saraiva JM, Baraitser M. Joubert syndrome: a review. Am J Med Genet. 1992;43:726–731. doi: 10.1002/ajmg.1320430415. [DOI] [PubMed] [Google Scholar]
- 89.Valente EM, et al. Distinguishing the four genetic causes of jouberts syndrome-related disorders. Ann Neurol. 2005;57:513–519. doi: 10.1002/ana.20422. [DOI] [PubMed] [Google Scholar]
- 90.Saunier S, et al. Large deletions of the NPH1 region in Cogan syndrome (CS) associated with familial juvenile nephronophthisis (NPH) Am J Hum Genet. 1997;61:A346. [Google Scholar]
- 91.Boichis H, Passwell J, David R, Miller H. Congenital hepatic fibrosis and nephronophthisis. A family study. Q J Med. 1973:221–233. [PubMed] [Google Scholar]
- 92.Mainzer F, Saldino RM, Ozonoff MB, Minagi H. Familial nephropathy associated with retinitis pigmentosa, cerebellar ataxia and skeletal abnormalities. Am J Med. 1970;49:556–562. doi: 10.1016/s0002-9343(70)80051-1. [DOI] [PubMed] [Google Scholar]
- 93.O'Toole JF, Otto E, Frishberg Y, F H. Retinitis Pigmentosa and Renal Failure in a Patient with Mutations in Inversin. J Am Soc Nephrol. 2004;15:215A. doi: 10.1093/ndt/gfl088. [DOI] [PubMed] [Google Scholar]
- 94.Pazour GJ, et al. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Joubert M, Eisenring JJ, Robb JP, Andermann F. Familial agenesis of the cerebellar vermis. A syndrome of episodic hyperpnea, abnormal eye movements, ataxia, and retardation. Neurology. 1969;19:813–825. doi: 10.1212/wnl.19.9.813. [DOI] [PubMed] [Google Scholar]
- 96.Parisi MA, Dobyns WB. Human malformations of the midbrain and hindbrain: review and proposed classification scheme. Mol Genet Metab. 2003;80:36–53. doi: 10.1016/j.ymgme.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 97.Gleeson JG, et al. Molar tooth sign of the midbrain-hindbrain junction: occurrence in multiple distinct syndromes. Am J Med Genet. 2004;125A:125–134. doi: 10.1002/ajmg.a.20437. discussion 117. [DOI] [PubMed] [Google Scholar]
- 98.Castori M, et al. NPHP1 gene deletion is a rare cause of Joubert syndrome related disorders. J Med Genet. 2005;42:e9. doi: 10.1136/jmg.2004.027375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Betz R, et al. Children with ocular motor apraxia type Cogan carry deletions in the gene (NPHP1) for juvenile nephronophthisis. J Pediatr. 2000;136:828–831. [PubMed] [Google Scholar]
- 100.Utsch B, et al. Identification of the first AHI1 gene mutations in nephronophthisis-associated Joubert syndrome. Pediatr Nephrol. 2006;21:32–35. doi: 10.1007/s00467-005-2054-y. [DOI] [PubMed] [Google Scholar]
- 101.Parisi MA, et al. AHI1 mutations cause both retinal dystrophy and renal cystic disease in Joubert syndrome. J Med Genet. 2005 doi: 10.1136/jmg.2005.036608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Saar K, et al. Homozygosity mapping in families with Joubert syndrome identifies a locus on chromosome 9q34.3 and evidence for genetic heterogeneity. Am J Hum Genet. 1999;65:1666–1671. doi: 10.1086/302655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Keeler LC, et al. Linkage analysis in families with Joubert syndrome plus oculo-renal involvement identifies the CORS2 locus on chromosome 11p12-q13.3. Am J Hum Genet. 2003;73:656–662. doi: 10.1086/378206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Delaney V, Mullaney J, Bourke E. Juvenile nephronophthisis, congenital hepatic fibrosis and retinal hypoplasia in twins. Q J Med. 1978;47:281–290. [PubMed] [Google Scholar]
- 105.Proesmans W, Van Damme B, Macken J. Nephronophthisis and tapetoretinal degeneration associated with liver fibrosis. Clin Nephrol. 1975;3:160–164. [PubMed] [Google Scholar]
- 106.Rayfield EJ, McDonald FD. Red and blonde hair in renal medullary cystic disease. Arch Intern Med. 1972;130:72–75. [PubMed] [Google Scholar]
- 107.Kumada S, et al. Renal disease in Arima syndrome is nephronophthisis as in other Joubert-related Cerebello-oculo-renal syndromes. Am J Med Genet A. 2004;131:71–76. doi: 10.1002/ajmg.a.30294. [DOI] [PubMed] [Google Scholar]
- 108.Satran D, Pierpont ME, Dobyns WB. Cerebello-oculo-renal syndromes including Arima, Senior-Loken and COACH syndromes: more than just variants of Joubert syndrome. Am J Med Genet. 1999;86:459–469. [PubMed] [Google Scholar]
- 109.Chance PF, et al. Clinical nosologic and genetic aspects of Joubert and related syndromes. J Child Neurol. 1999;14:660–666. doi: 10.1177/088307389901401007. discussion 669-72. [DOI] [PubMed] [Google Scholar]
- 110.Kyttala M, et al. MKS1, encoding a component of the flagellar apparatus basal body proteome, is mutated in Meckel syndrome. Nat Genet. 2006;38:155–157. doi: 10.1038/ng1714. [DOI] [PubMed] [Google Scholar]
- 111.Smith UM, et al. The transmembrane protein meckelin (MKS3) is mutated in Meckel-Gruber syndrome and the wpk rat. Nat Genet. 2006;38:191–196. doi: 10.1038/ng1713. [DOI] [PubMed] [Google Scholar]
- 112.Roume J, et al. A gene for Meckel syndrome maps to chromosome 11q13. Am J Hum Genet. 1998;63:1095–1101. doi: 10.1086/302062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Khaddour R, et al. Spectrum of MKS1 and MKS3 mutations in Meckel syndrome: a genotype-phenotype correlation. Mutation in brief #960. Online. Hum Mutat. 2007;28:523–524. doi: 10.1002/humu.9489. [DOI] [PubMed] [Google Scholar]
- 114.McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell. 2003;114:61–73. doi: 10.1016/s0092-8674(03)00511-7. [DOI] [PubMed] [Google Scholar]
- 115.Pennekamp P, et al. The ion channel polycystin-2 is required for left-right axis determination in mice. Curr Biol. 2002;12:938–943. doi: 10.1016/s0960-9822(02)00869-2. [DOI] [PubMed] [Google Scholar]
- 116.Donaldson MD, Warner AA, Trompeter RS, Haycock GB, Chantler C. Familial juvenile nephronophthisis, Jeune's syndrome, and associated disorders. Arch Dis Child. 1985;60:426–434. doi: 10.1136/adc.60.5.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jeune M, Beraud C, Carron R. Dystrophie thoracique asphyxiante de caractere familial. [Asphyxiating thoracic dystrophy with familial characteristics.] Arch Fr Pediatr. 1955;12:886–891. [PubMed] [Google Scholar]
- 118.Amirou M, et al. Successful renal transplantation in Jeune syndrome type 2. Pediatr Nephrol. 1998;12:293–294. doi: 10.1007/s004670050456. [DOI] [PubMed] [Google Scholar]
- 119.Sarimurat N, Elcioglu N, Tekant GT, Elicevik M, Yeker D. Jeune's asphyxiating thoracic dystrophy of the newborn. Eur J Pediatr Surg. 1998;8:100–101. doi: 10.1055/s-2008-1071131. [DOI] [PubMed] [Google Scholar]
- 120.Moudgil A, et al. Nephronophthisis associated with Ellis-van Creveld syndrome. Pediatr Nephrol. 1998;12:20–22. doi: 10.1007/s004670050395. [DOI] [PubMed] [Google Scholar]
- 121.Di Rocco M, et al. Retinitis pigmentosa, hypopituitarism, nephronophthisis, and mild skeletal dysplasia (RHYNS): a new syndrome? Am J Med Genet. 1997;73:1–4. doi: 10.1002/(sici)1096-8628(19971128)73:1<1::aid-ajmg1>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 122.Tsimaratos M, Sarles J, Sigaudy S, Philip N. Renal and retinal involvement in the Sensenbrenner syndrome. Am J Med Genet. 1998;77:337. [PubMed] [Google Scholar]
- 123.Costet C, et al. Pigmentosum retinis and tubulo-interstitial nephronophtisis in Sensenbrenner syndrome: a case report. J Fr Ophtalmol. 2000;23:158–160. [PubMed] [Google Scholar]
- 124.Beales PL, et al. IFT80, which encodes a conserved intraflagellar transport protein, is mutated in Jeune asphyxiating thoracic dystrophy. Nat Genet. 2007;39:727–729. doi: 10.1038/ng2038. [DOI] [PubMed] [Google Scholar]
- 125.Alton DJ, McDonald P. Urographic findings in the Bardet-Biedl syndrome, formerly the Laurence-Moon-Biedl syndrome. Radiology. 1973;109:659–663. doi: 10.1148/109.3.659. [DOI] [PubMed] [Google Scholar]
- 126.Green JS, et al. The cardinal manifestations of Bardet-Biedl syndrome, a form of Laurence-Moon-Biedl syndrome. N Engl J Med. 1989;321:1002–1009. doi: 10.1056/NEJM198910123211503. [DOI] [PubMed] [Google Scholar]
- 127.Marshall JD, et al. New Alstrom syndrome phenotypes based on the evaluation of 182 cases. Arch Intern Med. 2005;165:675–683. doi: 10.1001/archinte.165.6.675. [DOI] [PubMed] [Google Scholar]
- 128.Hearn T, et al. Subcellular localization of ALMS1 supports involvement of centrosome and basal body dysfunction in the pathogenesis of obesity, insulin resistance, and type 2 diabetes. Diabetes. 2005;54:1581–1587. doi: 10.2337/diabetes.54.5.1581. [DOI] [PubMed] [Google Scholar]
- 129.Collin GB, et al. Mutations in ALMS1 cause obesity, type 2 diabetes and neurosensory degeneration in Alstrom syndrome. Nat Genet. 2002;31:74–78. doi: 10.1038/ng867. [DOI] [PubMed] [Google Scholar]
- 130.Hearn T, et al. Mutation of ALMS1, a large gene with a tandem repeat encoding 47 amino acids, causes Alstrom syndrome. Nat Genet. 2002;31:79–83. doi: 10.1038/ng874. [DOI] [PubMed] [Google Scholar]
- 131.Alstrom CH, Hallgren B, Nilsson LB, Asander H. Retinal degeneration combined with obesity, diabetes mellitus and neurogenous deafness: a specific syndrome (not hitherto described) distinct from the Laurence-Moon-Biedl syndrome. A clinical endocrinological and genetic examination based on a large pedigree. Acta Psychiat Neurol Scand. 1959;34 suppl. 129:1–35. [PubMed] [Google Scholar]
- 132.Goldstein JL, Fialkow PJ. The Alstrom syndrome. Report of three cases with further delineation of the clinical, pathophysiological, and genetic aspects of the disorder. Medicine (Baltimore) 1973;52:53–71. doi: 10.1097/00005792-197301000-00003. [DOI] [PubMed] [Google Scholar]
- 133.Fath MA, et al. Mkks-null mice have a phenotype resembling Bardet-Biedl Syndrome. Hum Mol Genet. 2005 doi: 10.1093/hmg/ddi123. [DOI] [PubMed] [Google Scholar]
- 134.Torres VE, et al. Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med. 2004;10:363–364. doi: 10.1038/nm1004. [DOI] [PubMed] [Google Scholar]
- 135.Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci U S A. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lin F, et al. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci U S A. 2003;100:5286–5291. doi: 10.1073/pnas.0836980100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pazour GJ, Dickert BL, Witman GB. The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J Cell Biol. 1999;144:473–481. doi: 10.1083/jcb.144.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bisgrove BW, Yost HJ. The roles of cilia in developmental disorders and disease. Development. 2006;133:4131–4143. doi: 10.1242/dev.02595. [DOI] [PubMed] [Google Scholar]
- 139.Nurnberger J, Bacallao RL, Phillips CL. Inversin forms a complex with catenins and N-cadherin in polarized epithelial cells. Mol Biol Cell. 2002;13:3096–3106. doi: 10.1091/mbc.E02-04-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Saadi-Kheddouci S, et al. Early development of polycystic kidney disease in transgenic mice expressing an activated mutant of the beta-catenin gene. Oncogene. 2001;20:5972–5981. doi: 10.1038/sj.onc.1204825. [DOI] [PubMed] [Google Scholar]
- 141.Huangfu D, et al. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- 142.Hildebrandt F, et al. Establishing an algorithm for molecular genetic diagnostics in 127 families with juvenile nephronophthisis. Kidney Int. 2001;59:434–445. doi: 10.1046/j.1523-1755.2001.059002434.x. [DOI] [PubMed] [Google Scholar]
- 143.Parisi MA, et al. The NPHP1 gene deletion associated with juvenile nephronophthisis is present in a subset of individuals with Joubert syndrome. Am J Hum Genet. 2004;75:82–91. doi: 10.1086/421846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Brancati F, et al. CEP290 mutations are frequently identified in the oculo-renal form of Joubert syndrome-related disorders. Am J Hum Genet. 2007;81:104–113. doi: 10.1086/519026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Helou J, et al. Mutation analysis of NPHP6/CEP290 in patients with Joubert syndrome and Senior-Loken syndrome. J Med Genet. 2007;44:657–663. doi: 10.1136/jmg.2007.052027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Frank V, et al. Mutations of the CEP290 gene encoding a centrosomal protein cause Meckel-Gruber syndrome. Hum Mutat. 2008;29:45–52. doi: 10.1002/humu.20614. [DOI] [PubMed] [Google Scholar]
- 147.Baala L, et al. The Meckel-Gruber syndrome gene, MKS3, is mutated in Joubert syndrome. Am J Hum Genet. 2007;80:186–194. doi: 10.1086/510499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Parisi MA, et al. AHI1 mutations cause both retinal dystrophy and renal cystic disease in Joubert syndrome. J Med Genet. 2006;43:334–339. doi: 10.1136/jmg.2005.036608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kroes HY, et al. DNA analysis of AHI1, NPHP1 and CYCLIN D1 in Joubert syndrome patients from the Netherlands. Eur J Med Genet. 2008;51:24–34. doi: 10.1016/j.ejmg.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 150.Consugar MB, et al. Molecular diagnostics of Meckel-Gruber syndrome highlights phenotypic differences between MKS1 and MKS3. Hum Genet. 2007;121:591–599. doi: 10.1007/s00439-007-0341-3. [DOI] [PubMed] [Google Scholar]