Abstract
PURPOSE This review summarizes the evidence regarding the efficacy of adjuvant steroids for pain reduction in acute pharyngitis.
METHODS We searched for randomized controlled trials, using MEDLINE, EMBASE, and the Cochrane Database of Systematic Reviews, published between 1966 and December 2008. Two reviewers assessed the quality of each retrieved article and summarized the data.
RESULTS Our review found 8 relevant randomized controlled trials (RCTs) with a total of 806 patients. There were 5 RCTs with adult patients and 3 with children. All RCTs found a statistically significant faster reduction of pain or complete pain relief from steroid use compared with placebo. The trials used different steroids (dexamethasone, betamethasone, prednisone), and most participants had received antibiotics at least initially. Analgesic medication, such as acetaminophen, was allowed in all studies, but this factor was not always controlled. No serious adverse side effects were reported.
CONCLUSIONS Steroids are effective in relieving pain in acute pharyngitis. Although no serious adverse effects were observed, the benefits have to be balanced with possible adverse drug effects. There are safe and effective over-the-counter medications to relieve throat pain. Most patients received concomitant antibiotics; however, reducing the prescription of antibiotics for generally benign upper respiratory tract infection is a public health goal. We therefore recommend further studies to establish both the safety of steroids without antibiotic coverage and the additional benefits of steroids when used with regular administration of over-the-counter analgesic medications.
Keywords: Pharyngitis, sore throat, steroids, adjuvant therapy, family practice
INTRODUCTION
Sore throat is a highly common condition. In a Scottish survey 31% of respondents reported at least 1 episode of sore throat within the last 12 months; most did not seek medical attention.1 Physicians frequently assume that patients seeking care expect a course of antibiotics. It has been shown, however, that pain relief is more important for patients, and patients who hope for antibiotics may in fact want treatment for pain.2 Thus, a major treatment goal for patients complaining of sore throat is to relieve pain and alleviate difficulties in swallowing.
Viral or bacterial infections that cause a sore throat generate pain through inflammation of the pharynx and the surrounding lymphatic tissue. Although antibiotic treatment may shorten the duration of symptoms in a bacterial throat infection (from 3.3 to 2.7 days), the benefits are considered moderate.3 Gargling, drinking warm liquids, and oral antipyretic or analgesic drugs are common supportive treatments available over the counter.4 The anti-inflammatory action of steroids might be effective to relieve symptoms caused by inflammation and has been studied in other upper respiratory tract infections. Steroids might, therefore, represent a useful clinical option to meet patients’ needs. Administering intramuscular or oral steroids for acute pharyngitis is not common practice; however, several randomized controlled trials, mainly from the United States and few other countries (Israel, Turkey), have studied the effectiveness of steroids.
We performed a systematic review of the effects of steroids as adjuvant therapy for acute pharyngitis in ambulatory patients and discuss the implications for practice.
METHODS
Data Sources
Our search was performed in the 3 following electronic bibliographic databases: MEDLINE, EMBASE, and Cochrane Database of Systematic Reviews. We included studies published between 1966 and December 31, 2008. The search algorithm with the keywords and MeSH terms used is available in Supplemental Appendix 1, available online at http://annfammed.org/cgi/content/full/8/1/58/DC1. Additionally, we searched through the reference list of the articles identified manually.
Study Selection
Eligibility Criteria
Our search included published randomized controlled trials (RCTs) that compared the administration of steroids as adjuvant therapy for sore throat in acute pharyngitis with a placebo. Steroids could be applied orally or intramuscularly. Furthermore, we did not place restrictions on eligibility according to drug dosing, duration of application, or publication language. We did not exclude specific populations or age-groups.
Screening Process
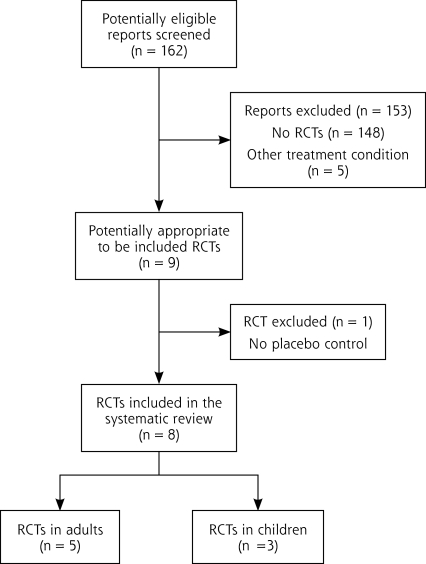
Our search strategy identified 162 citations. Two independent reviewers (K.K. and J.F.C.) separately analyzed the citation titles and abstracts using a predesigned form. We excluded titles and abstracts that clearly did not meet the inclusion criteria either regarding the participant (ambulatory patient with acute pharyngitis), type of intervention (steroids as adjuvant treatment), nature of outcome measure (pain reduction), or study type (RCT). We obtained full-text articles for those titles fulfilling inclusion criteria, and we were able to include 8 titles in our review. The 2 reviewers resolved disagreements by consensus and the reference details of excluded studies are available upon request from the authors.
RESULTS
We extracted 9 relevant RCTs and excluded 1 which was not placebo controlled.5 The search process is described in Figure 1▶. All but 1 study6 were conducted in emergency rooms. Most participants received at least antibiotics initially; concomitant use of other pain medication was allowed but generally not controlled. No serious side effects were reported. Five of the included trials were performed with adults (Table 1▶),6–10 and 3 were performed with children (Table 2▶).11–13 For a better overview we consider them separately in the following description.
Figure 1.
Literature search results.
RCT=randomized controlled trial.
Table 1.
Characteristics of the Included Randomized Controlled Trials in Adults
| Study, Year, Country | Setting (n) | Mean Age, y | Interventiona | Co-Intervention | Bacteriology | Main Outcomes | Main Results | Authors’ Conclusions |
|---|---|---|---|---|---|---|---|---|
| O’Brien et al,9 1993, USA | Educational (51) | 26.4 | 0 mg dexamethasone IM | Antibiotic: yes Analgesic: permitted, controlled |
None | 15-cm VAS (0–3.0) pain score at 24 h Time until onset of pain relief Time to complete pain relief |
6.1-h earlier onset of pain relief (6.3 h vs 12.4 h; P=.01) 20.4-h earlier pain free (15 h vs 35.4 h; P = .02) in the steroid arm |
“IM dexamethasone appears effective as an adjuvant to antibiotic therapy“ |
| Marvez-Valls et al,7 1998, USA | Educational (92) | 29.2 | 12 mg beta-methasone IM | Antibiotic: yes Analgesic: permitted, not controlled |
In 77 of 92 patients done | 10-cm VAS pain score at 24 h and 48 h Change in pain score at 24 h and 48 h Time until onset of pain relief Time to complete pain relief |
5-h earlier onset of pain relief (6.3 h vs 11.3 h; P=.005), 13.8-h earlier pain free (42.0 h vs 55.8 h; P = .013) in the steroid arm If GABHS positive: 28.2-h earlier pain free (28.8 h vs 57.0 h; P=.024) in the steroid arm |
“As an adjunct to antibiotic therapy, betamethasone appears to lessen time to relief of pain…most effective in streptococcal-positive pharyngitis” |
| Wei et al,8 2002, USA | Educational (118) | 28.1 | 10 mg dexamethasone IM/oral | Antibiotic: yes Analgesic: permitted, controlled |
Yes | Change in 10-cm VAS pain score after 12 h and 24 h Time until onset of pain relief Time to complete pain relief |
4-h earlier onset of pain relief (5.8 h IM/6 h oral vs 10.1 h placebo; P = .029) in the steroid arm | “Dexamethasone at a 10-mg …, significantly contributes to relief of pain” |
| Kiderman et al,6 2005, Israel | General practice (79) | 33.9 | 60 mg prednisone oral 1 or 2 d | Antibiotic: yes Analgesic: permitted, controlled |
In 73 of 79 patients done | 10-cm VAS pain score at 12 h, 24 h, 48 h, 72 h | Pain reduction at 12 h (3.9 vs 5.9 P <.001) and 24 h (2.4 vs 4.5; P=.002) in the steroid arm | “…adjuvant oral steroid therapy appears to be safe and more effective than placebo for symptom relief“ |
| Tasar et al,10 2008, Turkey | Educational (73) | 31.3 | 8 mg dexamethasone IM | Antibiotic: yes Analgesic: permitted, not controlled |
None | Time until onset of pain relief Time to complete pain relief |
11.8-h earlier onset of pain relief (8.1 h vs 19.9 h; P <.001) 24.8-h earlier pain free (28.9 h vs 53.7 h; P <.001) in the steroid arm |
“Single dose…dexamethasone therapy added to the standard treatment has a more rapid improvement of pain” |
Exp=experimental; GABHS=group A β-hemolytic streptococci, IM=intramuscular; VAS=visual analogue scale.
a All control groups received placebo.
Table 2.
Characteristics of the Included Randomized Controlled Trials in Children
| Study, Year, Country | Setting (n) | Mean Age, y | Interventiona | Co-Intervention | Bacteriology | Main Outcomes | Main Results | Authors’ Conclusions |
|---|---|---|---|---|---|---|---|---|
| Bulloch et al,11 2003, USA | Educational (184) | 9.7 | 10 mg dexamethasone oral | Antibiotic: yes if rapid antigen test positive for GABHS Analgesic: permitted, not controlled |
Yes | 10-cm VAS pain score at 24 h Change in pain score at 24 h Time until onset of pain relief Time to complete pain relief |
If GABHS positive: 5.5 h earlier onset of pain relief (6.0 h vs 11.5 h; P = .02) in the steroid arm | “…oral dexamethasone does not decrease the time to onset of clinically significant pain relief or the time to complete pain relief” |
| Olympia et al,12 2005, USA | Educational (125) | 11.4 | Maximum 10 mg dexamethasone (0.6 mg/kg) oral | Antibiotic: yes if rapid antigen test positive for GABHS Analgesic: permitted, |
Yes | Change in McGrath Facial Affective scale pain score at 24 h and 48 h Time until onset of pain relief Time to complete pain relief |
If GABHS positive: 5.1 h (95% CI, 0.5–10.8 h) earlier onset of pain relief (9.7 h vs 14.8 h) in the steroid arm | “Immunocompetent children with moderate to severe pharyngitis benefited from the use of oral dexamethasone” |
| Niland et al,13 2006, USA | Educational (84) | 8b | Maximum 10 mg dexamethasone (0.6 mg/kg) oral, 1 or 3 d | Antibiotic: yes Analgesic: permitted, controlled |
Yes | Time until onset of pain relief Time improvement of general condition Time to improvement of activity level in days |
1 d earlier onset of pain relief (1 d vs 2 d) in the steroid arm | “…children with acute uncomplicated GABHS pharyngitis who receive steroids as add-on treatment are likely to convalesce faster” |
CI=confidence interval; Exp=experimental; GABHS=group A β-hemolytic streptococci, VAS = visual analogue scale.
a All control groups received placebo.
b Median age.
Study Characteristics: Adult Trials
There were a total of 413 adult patients in 5 trials. The trials were heterogeneous in design and conduct. Four trials compared intramuscular steroid application with placebo. One had a third arm with oral steroids, and a further trial primarily compared oral application with placebo. The trials used different steroids (dexamethasone, betamethasone, prednisone) administered in doses of varying potency14 and for different time intervals, and different instruments were used for assessing pain. Four trials used the visual analogue scale (VAS) as a primary outcome,6–9 and 1 used a 15-cm scale instead of the conventional 10-cm scale.9 Four RCTs found a statistically significant earlier reduction of pain or complete pain relief ranging from 4 to 11.8 hours after administration and complete pain relief after 24.4 to 28.2 hours. One trial reported significantly better reduction of pain after 12 and 24 hours.6 The characteristics of the included trials are given in Table 1▶.
Study Characteristics: Pediatric Trials
There were a total of 393 children in 3 trials, with an average age of the children ranging between 8 and 11 years. All trials used an oral dexamethasone weight-adapted dose of 0.6 mg/kg up to a maximum 10 mg in a single dose given orally once at enrollment. In 1 study, a second intervention arm received dexamethasone for 3 days.13 Outcome measures were heterogeneous. One study used a color analogue scale,11 1 used a 9-point McGrath facial affective scale,12 and another used a 6-point Wong-Baker facial affective scale.13 All obtained a rapid streptococcal test; 2 trials were based on an antibiotic treatment decision as a result of the tests, and 1 trial included only children whose tests were positive. They used different visual analogue scales to assess symptoms. All RCTs found a statistically significantly earlier reduction in pain that ranged form 5.1 hour to 1 day. Dexamethasone treatment for 3 days was not superior to a single dose for any outcome.13 The characteristics of the included trials are displayed in Table 2▶.
Secondary Outcome
Three studies, 2 in adults and 1 in children, recorded a missed day at work or at school.6,7,13 Marvez-Valls et al reported an average time off work of 0.4 days (SD ± 1.4) in the intervention group and 0.7 days (SD ± 1.4) in the nonintervention group, which was not significantly different.5 Kiderman et al stated that there was no significant difference in time taken off work between groups but did not provide any data.6 Niland et al reported that there was no significant difference in the number of days missed at school13 but fail to show the group average.
Subgroup Analysis
Five studies performed a subgroup analysis comparing pain relief in patients with or without proven bacterial infection.6–8,11,12 The reported findings are inconsistent. Three trials observed that patients with an identified bacterial pathogen seemed to benefit more from steroid administration than from placebo.6,7,11 Olympia et al reported that in patients without positive streptococcal antigen benefits were more pronounced with steroid administration than with placebo.12 As an explanation they suggest that children with streptococcal infection who received the placebo benefited from the antibiotics, which had an impact on the observed effectiveness of dexamethasone in the test subjects. Wei et al found steroids to be more effective if administered intramuscularly to patients with bacterial pathogen than if administered to patients without bacterial pathogen.8 In patients receiving oral steroids, however, those with a negative pathogen test result benefited more. These contradictory findings prompted the authors to conclude that the overall number of patients was too small to make any conclusions.
Quality Assessment of Included Trials
We assessed the quality of the included trials according to the Delphi list.15 Results are shown in Table 3▶. Most trials did not attempt an intention-to-treat analysis. In 4 trials the primary outcome was not clearly described, and a power calculation was not performed.
Table 3.
Quality Assessment of Included Trials
| Study, Year, Country | Random- ization Adequate | Primary Outcome Defined | Dropouts Described | Point Estimates/ Measures of Variability | Intention- to-Treat | Power Calculation Described | Double- Blinding | Eligibility Criteria Predefined |
|---|---|---|---|---|---|---|---|---|
| O’Brien et al,9 1993, USA | Yes | Unclear | Yes | Yes | No | No | Yes | Yes |
| Marvez-Valls et al,7 1998, USA | Yes | Yes | Yes (no drop-outs) | Yes | No | Yes | Yes | Yes |
| Wei et al, 2002,8 USA | Unclear | Unclear | Yes | Yes | No | No | Yes | Yes |
| Kidermann et al,6 2005, Israel | Yes | Yes | Yes (no drop-outs) | Yes | No | Yes | Yes | Yes |
| Tasar et al,10 2008, Turkey | Yes | No | Yes (no drop-outs) | Yes | No | No | Yes | Yes |
| Bulloch et al,11 2003, USA | Yes | No | Yes | Yes | No | Yes | Yes | Yes |
| Olympia et al,12 2005, USA | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Niland et al,13 2006, USA | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
DISCUSSION
The results of this systematic review show that steroids are effective for pain reduction in adult and pediatric patients complaining of sore throat. All RCTs found an earlier reduction in pain or complete pain relief after steroid administration compared with placebo and concluded that steroids are effective. No differences in missed days at work or at school were observed. These findings have potentially important implications for practice. Although the value of pain reduction for patients is undisputed, it is debatable that earlier pain relief of 4 hours to 1 day justifies the administration of steroids. Only Bulloch et al disputed the clinical relevance of steroids in managing symptoms of sore throat.11
Antibiotics have no analgesic properties, but the observed earlier pain reduction in patients with bacterial throat infection might be attributed to the medication’s specific antibacterial activity eventually reducing inflammation.1 Since it is conceivable that inflammation in patients with bacterial throat infection might be more responsive to steroid administration, some studies attempted a subgroup analysis comparing pain relief in patients with or without a proven bacterial infection.6–8,11,12 The results are inconsistent, and the available data are not sufficient to conclude that steroids are ineffective with viral pharyngitis.
Since most trials were undertaken in an emergency department setting, it is conceivable that these patients had more pain than average throat pain sufferers and that the results cannot be expected in a primary care setting. Another concern about the whether the reported findings can be generalized is the concomitant use of a medication to relieve throat pain. Paracetamol and ibuprofen are effective in reducing the symptoms of sore throat4 and were allowed in all trials. Only one-half of the trials reported and controlled for co-medication8,9,12,13; however, there was no difference between intervention and control groups. Because dosage and dosing intervals were neither reported nor standardized, a conclusion regarding the additional benefits of steroids added to over-the-counter medication cannot be drawn.
The benefits of any treatment have to be weighted against potential harms. No serious adverse side effects from steroid administration were reported. Only Wei et al reported 1 case of hiccup.8 Short-term oral steroid use is generally safe, but there have been numerous reports of associated avascular necrosis and a few cases of fatal varicellazoster in immunocompetent patients.13 Severe mood changes and psychotic reactions rarely occur unpredictably with short-term steroid use.16 A sample of 800 patients is too small to detect potentially deleterious effects.
Most trials used antibiotic coverage as part of their protocol. Safety of adjuvant steroids without antibiotic coverage remains to be established. It is an important goal to reduce the prescription of antibiotics for generally benign viral upper respiratory tract infections.17 There are controversies,18 and relevant national differences in guidelines concerning diagnosis and management of acute pharyngitis exist.19 In European countries, which mostly do not recommend routine testing for streptococcal infection with a rapid antigen test, the use of steroids for pain relief might not be safe.
All but 1 trial in adults and none in children used intramuscular application of steroids. Bioavailability of steroids is a higher with intramuscular administration than with oral administration.14 The 3-armed controlled trial of Wei et al found no difference between oral and intramuscular administration.8 A non–placebo-controlled study comparing oral and intramuscular administration not included in this review also found no difference.5 Oral administration of steroids seems to be as effective as intramuscular application. Given the complication associated with intramuscular injection, such as nerve damage,20 local infection, and necrosis,21 future studies should prefer oral application.
Advocating injections and use of prescription medication may promote further medicalization of this mostly benign self-limiting condition.
Limitations
There are some limitations to this systematic review. First, we cannot exclude publication bias in favor of studies finding steroids to be beneficial. Other limitations are related to the studies themselves. They used heterogeneous drugs, dosing and administration, and differing outcome measures. Although it would be possible to convert the scales, the reported outcomes cannot be compared, because measurements were done at different time intervals or patients were asked either to report the time of onset of pain relief or total pain relief. The reported values on the rating scale may be subject to recall bias, because enrolled patients did not always have a pain scale available at the time pain had to be reported according to the study protocol.7 One study reported that patients did not recall their previous pain level and needed a reminder.7 Accordingly, we did not attempt a meta-analysis. Future trials will require a consensus on a standard for measuring pain relief in sore throat.
Steroids are effective for relieving pain in acute pharyngitis. Although no serious adverse effects were observed, the benefits must be weighed against possible rare adverse drug effects and further medicalization of a condition for which most people do not seek medical attention. There are safe and effective over-the-counter medications to relieve throat pain. All patients received concomitant antibiotics; however, reducing prescription of antibiotics for frequently benign upper respiratory tract infections is a public health goal. We therefore recommend further studies to establish the safety of steroid use without antibiotic coverage and the added benefits of steroids when used with regular administration of over-the-counter analgesic medications.
Acknowledgments
We are grateful to Professor Michael M. Kochen for his helpful advice.
Conflicts of interest: none reported
REFERENCES
- 1.Hannaford PC, Simpson JA, Bisset AF, Davis A, McKerrow W, Mills R. The prevalence of ear, nose and throat problems in the community: results from a national cross-sectional postal survey in Scotland. Fam Pract. 2005;22(3):227–233. [DOI] [PubMed] [Google Scholar]
- 2.van Driel ML, De Sutter A, Deveugele M, et al Are sore throat patients who hope for antibiotics actually asking for pain relief? Ann Fam Med. 2006;4(6):494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Mar CB, Glasziou PP, Spinks AB. Antibiotics for sore throat. Cochrane Database Syst Rev. 2006;18(4):CD000023. [DOI] [PubMed] [Google Scholar]
- 4.Thomas M, Del Mar C, Glasziou P. How effective are treatments other than antibiotics for acute sore throat? Br J Gen Pract. 2000; 50(459):817–820. [PMC free article] [PubMed] [Google Scholar]
- 5.Marvez-Valls EG, Stuckey A, Ernst AA. A randomized clinical trial of oral versus intramuscular delivery of steroids in acute exudative pharyngitis. Acad Emerg Med. 2002;9(1):9–14. [DOI] [PubMed] [Google Scholar]
- 6.Kiderman A, Yaphe J, Bregman J, Zemel T, Furst AL. Adjuvant prednisone therapy in pharyngitis: a randomised controlled trial from general practice. Br J Gen Pract. 2005;55(512):218–221. [PMC free article] [PubMed] [Google Scholar]
- 7.Marvez-Valls EG, Ernst AA, Gray J, Johnson WD. The role of beta-methasone in the treatment of acute exudative pharyngitis. Acad Emerg Med. 1998;5(6):567–572. [DOI] [PubMed] [Google Scholar]
- 8.Wei JL, Kasperbauer JL, Weaver AL, Boggust AJ. Efficacy of single-dose dexamethasone as adjuvant therapy for acute pharyngitis. Laryngoscope. 2002;112(1):87–93. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien JF, Meade JL, Falk JL. Dexamethasone as adjuvant therapy for severe acute pharyngitis. Ann Emerg Med. 1993;22(2):212–215. [DOI] [PubMed] [Google Scholar]
- 10.Tasar A, Yanturali S, Topacoglu H, Ersoy G, Unverir P, Sarikaya S. Clinical efficacy of dexamethasone for acute exudative pharyngitis. J Emerg Med. 2008;35(4):363–367. [DOI] [PubMed] [Google Scholar]
- 11.Bulloch B, Kabani A, Tenenbein M. Oral dexamethasone for the treatment of pain in children with acute pharyngitis: a randomized, double-blind, placebo-controlled trial. Ann Emerg Med. 2003;41(5):601–608. [DOI] [PubMed] [Google Scholar]
- 12.Olympia RP, Khine H, Avner JR. Effectiveness of oral dexamethasone in the treatment of moderate to severe pharyngitis in children. Arch Pediatr Adolesc Med. 2005;159(3):278–282. [DOI] [PubMed] [Google Scholar]
- 13.Niland ML, Bonsu BK, Nuss KE, Goodman DG. A pilot study of 1 versus 3 days of dexamethasone as add-on therapy in children with streptococcal pharyngitis. Pediatr Infect Dis J. 2006;25(6):477–481. [DOI] [PubMed] [Google Scholar]
- 14.Brunton LL. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill Higher Education; 2005.
- 15.Verhagen AP, de Vet HC, de Bie RA, et al The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51(12):1235–1241. [DOI] [PubMed] [Google Scholar]
- 16.Richards RN. Side effects of short-term oral corticosteroids. J Cutan Med Surg. 2008;12(2):77–81. [DOI] [PubMed] [Google Scholar]
- 17.Tan T, Little P, Stokes T; Guideline Development Group. Antibiotic prescribing for self limiting respiratory tract infections in primary care: summary of NICE guidance. BMJ. 2008;337:a437. [DOI] [PubMed] [Google Scholar]
- 18.Centor RM, Allison JJ, Cohen SJ. Pharyngitis management: defining the controversy. J Gen Intern Med. 2007;22(1):127–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthys J, De Meyere M, van Driel ML, De Sutter A. Differences among international pharyngitis guidelines: not just academic. Ann Fam Med. 2007;5(5):436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramtahal J, Ramlakhan S, Singh K. Sciatic nerve injury following intramuscular injection: a case report and review of the literature. J Neurosci Nurs. 2006;38(4):238–240. [DOI] [PubMed] [Google Scholar]
- 21.Luton K, Garcia C, Poletti E, Koester G. Nicolau Syndrome: three cases and review. Int J Dermatol. 2006;45(11):1326–1328. [DOI] [PubMed] [Google Scholar]