Abstract
There is increasing interest in gliogenesis as the relevance of glia to both brain development and pathology becomes better understood. However, little is known about this process. The use of multidimensional protein identification technology (MudPIT) to identify changes in phosphoprotein levels in rat neural precursor cells treated with cytokines or retinoic acid showed that phosphorylation of the catalytic subunit of phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K p110α) and dephosphorylation of the inositol phosphatase synaptojanin-1 were common to the gliogenic stimuli. While PI3K was found to be involved in both neuro- and astrogliogenesis, synaptojanin-1 was specifically involved in astrogliogenesis of neural precursor cells. The role of synaptojanin-1 in astrogliogenesis was further confirmed by analysis of neuron- and glia-specific markers in synaptojanin-1 knockout mouse brain. Additional experiments showed that the Sac1-like phosphatase domain of synaptojanin-1 is the responsible for the observed astrogliogenic effect. Our results strongly indicate that phosphatidylinositol metabolism plays a key role in astrogliogenesis. The relevance of our findings for Down’s syndrome pathology is discussed.
Keywords: synaptojanin-1, gliogenesis, retinoic acid, cytokine, Down’s syndrome
Introduction
Astrocytes are the major cell population in the brain and play an essential role in its development, normal function and pathology (1). However, the mechanisms underlying the generation of astrocytes remain little understood. Neurogenesis precedes gliogenesis during development (2) and embryonic neurons seem to regulate the onset of gliogenesis by the secretion of factors into the extracellular space (3). Probably the best known gliogenic factors are the interleukin-6 (IL-6) family of cytokines [i.e. leukemia inhibitory factor (LIF), IL-6, IL-11, oncostatin M, cardiotrophin-like cytokine/cytokine-like factor-1 (CLC/CLF), cardiotrophin-1 (CT-1) and ciliary neurotrophic factor (CNTF)] (3-7). The TGF-β superfamily of cytokines [i.e. transforming growth factor beta (TGF-β), activins, bone morphogenetic proteins (BMPs) and growth and differentiation factors (GDFs)] can enhance the gliogenic effect of IL-6 family members (5, 8). There is also evidence for a role for retinoic acid in gliogenesis, but its mechanism of action remains almost completely unknown, the retinoic acid receptor α (RARα) being the only identified mediator of this process (9, 10). Besides secreted factors, cell-to-cell contact also seems to be very relevant to gliogenesis, since activation of Notch pathway, which requires the binding of Notch receptors to their ligands in membranes of neighboring cells, promotes gliogenesis versus neurogenesis during development (11, 12).
Phosphorylation pathways play a key role in the transduction of signals from the extracellular medium to the ultimate cellular response, and thus they determine cell behavior and fate. Gliogenesis is not an exception, for the IL-6 family of cytokines binds to and activates its membrane receptors, which have tyrosine kinase activities. These receptors trigger the JAK/STAT phosphorylation pathway leading to the expression of the astroglial marker GFAP (3, 4, 7). The members of the TGF-β superfamily of cytokines also bind to and activate their membrane receptors, which in turn phosphorylate and activate Smad proteins. Smad proteins form heterodimers that strongly enhance STAT3-induced GFAP expression (5). Finally, although phosphorylation pathways are clearly involved in the effects of retinoic acid (13, 14), there is no report on the role of these pathways in retinoic acid-induced glial differentiation.
The goal of the present study is to identify kinases or phosphatases involved in gliogenesis. Our results led us to synaptojanin-1, an inositol phosphatase that plays an important role in synaptic vesicle recycling and clathrin-mediated endocytosis (15). We find that this phosphatase is involved in astrogliogenesis, but not in neurogenesis. A possible role for synaptojanin-1 in Down’s syndrome brain features can be deduced from our results and previously reported observations.
Results and discussion
Characterization of glial differentiation in the HCN-B27 clone of rat adult hippocampal precursor cells
HCN-B27 is a clone derived from the rat adult hippocampal cell line HCN (16). Unlike the original cell line, the B27 clone is able to grow in the absence of growth factors and laminin, but it still has neural precursor cell properties, for 95-99 % of cells express the neural precursor cell markers Musashi, Sox-2 and Nestin (Fig.1A, left, middle) and they are able to form neurospheres (Fig. 1A, right). In addition, they are able to differentiate into neurons and astrocytes (17). Glial differentiation can be induced by the IL-6 and the TGF-β families of cytokines, retinoic acid, fetal bovine serum (FBS) or combinations of these factors, with different efficiencies as determined by increases in the expression of the glia-specific protein GFAP and in the number of cells positive for this protein (Fig. 1B-C). In spite of the clear increase in the expression of GFAP upon gliogenic stimulation, HCN-B27 cells fail to express the mature glial markers S100β, GLAST and GLT-1 (two glutamate transporters), SPARC protein or CD44 under any experimental condition tested in the present article (Data not shown). On the other hand, the number of cells that are positive for precursor cell markers Nestin and Sox-2, as well as the expression levels of Sox-2, decreases upon exposure of the cells to most of the gliogenic stimuli (Fig. 1B-C). These data strongly indicate that gliogenic factors induce HCN-B27 precursor cells to leave their undifferentiated status and become immature astrocytes.
Figure 1.
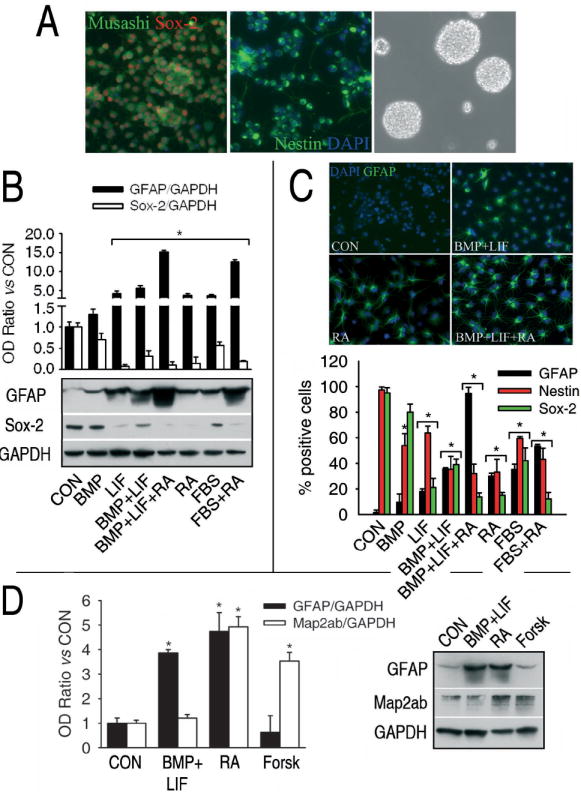
Glial differentiation in the HCN-B27 clone of adult rat hippocampal precursor cells. A, The B27 clone of hippocampal precursor cells expresses the neural precursor markers Sox-2 (left, red nuclear staining), Musashi (left, green cytoplasmic staining) and Nestin (middle, green cytoplasmic staining) and are able to form neurospheres when grown on plastic dishes without poly-L-ornithine nor laminin. Nuclei were stained with DAPI (blue). B, different combinations of gliogenic stimuli induce different degrees of glial differentiation, as indicated by the increase in the expression of the glial marker GFAP and the decrease in the expression of the precursor cell marker Sox-2. C, These results were confirmed by counting the cells that were positive for GFAP or precursor cell markers Nestin and Sox-2. Nuclei were stained with DAPI (blue). Cells were incubated for 4 days with the different drugs and factors; the same period of time was used in every experiment described below. The concentrations used here and in every experiment described below were BMP-2 (BMP), 50 ng/ml; LIF, 50 ng/ml; retinoic acid (RA), 1 μM; FBS, 5 % v/v. D, Cytokines only induce astrogliogenesis, retinoic acid induces both astrogliogenesis and neurogenesis, and forskolin (Forsk, 10 μM) only induces neurogenesis, as indicated by changes in the levels of the neuronal marker Map2ab and the astroglial marker GFAP. *, significant versus control, p<0.05.
The most potent combinations of gliogenic factors are BMP-2, LIF and retinoic acid (around 90 % of the cells differentiated into astrocytes) or FBS plus retinoic acid (around 50 % differentiation). However, we used BMP-2 plus LIF or retinoic acid for the following experiments because they are the simplest conditions that induce a significant amount of differentiation, for BMP-2 alone or LIF alone are less potent, and FBS is an extraordinarily complex mixture of proteins, lipids and small molecules. While the combination of BMP-2 and LIF only induces glial differentiation, retinoic acid also induces neuronal differentiation as shown by increases in the neuron-specific protein Map2ab (Fig. 1D). In contrast, the adenylcyclase activator forskolin only induces neuronal differentiation (Fig. 1D).
Retinoic acid and cytokines induce many changes in the phosphoproteome of precursor cells
Factors with apparently very different mechanisms of action are able to cause the differentiation of precursor cells into the same cell type. For example, both retinoic acid and the IL-6 family of cytokines induce glial differentiation but they act through completely different receptors (4, 9). However, different initial stimuli for cell differentiation in the same cell type might share some common portions of the differentiation pathway. In order to identify possible common phosphorylation pathways involved in both retinoic acid- and cytokine-induced glial differentiation, the phosphoproteins from cells incubated with retinoic acid or BMP-2 and LIF for 4 days were isolated on metal affinity columns and then analyzed by multidimensional protein identification technology (MudPIT). MudPIT is a new, largely unbiased method for rapid and large-scale proteome analysis, based on coupling multidimensional liquid chromatography to tandem mass spectrometry analysis, which allows the identification of more than 200 proteins in a single run (18, 19). While the total proteome greatly exceeds this number, the analysis of a particular subset of proteins by means of this technique can give a large amount of information about a biological process at the protein level (20). We used this powerful tool for the first time to analyze the phosphoproteome during the differentiation of adult neural precursor cells.
There is a great increase in the number of phosphoproteins identified by MudPIT when cells are treated with retinoic acid or cytokines (Figure 2A, lower graph; supplementary Table I). Western blots of total proteins with an anti-phosphotyrosine antibody further support this observation (Figure 2A, upper picture). In order to have a general view of the samples, the data obtained where classified by means of the uniprot database (www.uniprot.org). The classification of the samples by their cellular localization or molecular function showed that not only the quantity of phosphoproteins changed upon gliogenic stimulation but also the quality of the samples (Figure 2B).
Figure 2.
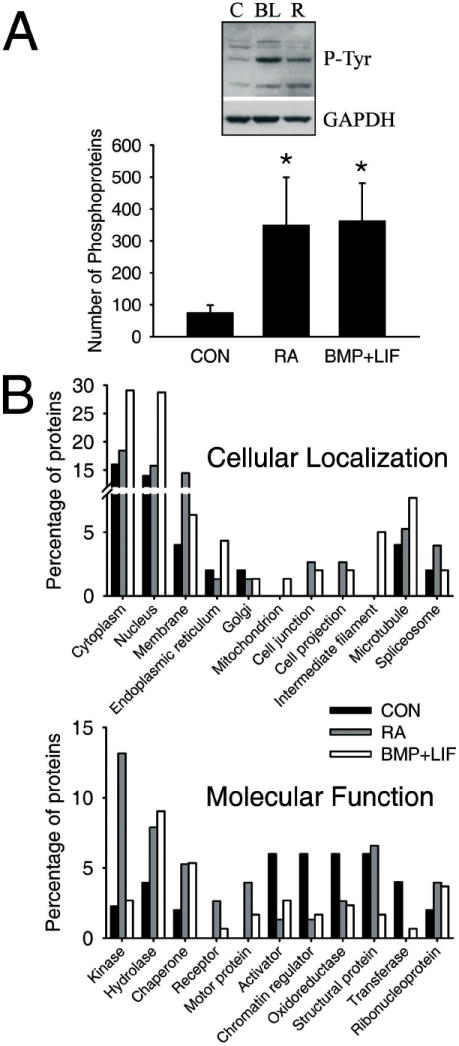
Retinoic acid and cytokines induce massive quantitative and qualitative changes in the phosphoproteome of precursor cells. A, Retinoic acid and cytokines induce an increase in the number of phosphoproteins identified by MudPIT (graphic, bottom) and also in the phosphorylation of some tyrosine residues (western blot, top). B, When the proteins identified by MudPIT were classified by means of the Uniprot database (www.uniprot.org) according to their cellular localization (top graphic) or their molecular function (bottom graphic), the results showed that there are changes in the constitution of the phosphoproteome of precursor cells after the incubation with retinoic or cytokines.
Since a large number of phosphoproteins were present in the MudPIT data from each sample (see Supplementary Table I for non-filtered results), we had to apply a series of filters in order to prioritize the analysis. First, proteins that were present in all groups were removed, for their phosphorylation is most likely constitutive and irrelevant to the differentiation process. Second, we only looked at kinases and phosphatases (Table I). Third, those kinases or phosphatases that appear in only one of the gliogenic conditions as well as in the control group, and those that only appear in the retinoic acid- or cytokine-treated groups, were considered events most likely to be specific to each of these processes. Fourth, we compared the phosphoproteins from cells treated with gliogenic stimuli with the phosphoproteins from cells treated with forskolin, which only induces nerve differentiation. The only two events that were common to retinoic acid and cytokines and not present in cells after incubation with forskolin were the phosphorylation of PI3K and the dephosphorylation of synaptojanin-1.
Table I.
Kinases and phosphatases (with their correspondent GI numbers) whose phosphorylation state changes after incubation with retinoic acid or cytokines.
| GI number | Common phosphorylations (in RA and BMP+LIF but not in CON) | Also Induced by Forsk | PI3K-dependent * |
|---|---|---|---|
| 109464719 | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha isoform | No | Yes |
| 109485997 | Mitogen-activated protein kinase kinase kinase kinase 4 isoform 1 (NIK) | Yes | No |
| Common dephosphorylations (in CON but not in RA or BMP+LIF) | |||
| 94400809 | Synaptojanin-1 (Synaptic inositol-1,4,5-trisphosphate 5-phosphatase 1) | No | Yes |
| 94370595 | [Segment 1 of 2] Traf2 and NCK-interacting kinase (TNIK) | Yes | Yes |
| 94377177 | Receptor-type tyrosine-protein phosphatase zeta precursor (R-PTP-zeta) | Yes | Yes |
| 109475468 | Inositol polyphosphate-5-phosphatase B | Yes | Yes |
| 109499493 | BMP2 inducible kinase | Yes | No |
| Phosphorylation events specific to RA (only in RA) | |||
| 3241856 | DNA-dependent protein kinase catalytic subunit | No | Yes |
| 109493692 | DNA-dependent protein kinase catalytic subunit (DNA-PK catalytic subunit) | No | Yes |
| 109494796 | DNA-dependent protein kinase catalytic subunit (DNA-PK catalytic subunit) | No | Yes |
| 4558873 | Testis-enriched protein tyrosine phosphatase | No | Yes |
| 5733095 | Tyrosine kinase TYK2 | No | Yes |
| 20988799 | Ptk2 protein (Focal adhesion kinase 1)(FAK1) | No | Yes |
| 109475613 | Mitogen-activated protein kinase kinase kinase 6 (ASK2) | No | Yes |
| 109478244 | Mitogen-activated protein kinase kinase kinase kinase 5 (GCKR) | No | Yes |
| 109494196 | Ephrin type-A receptor 6 precursor (EHK-2) | No | No |
| 109503199 | Interleukin-12 receptor beta-1 chain precursor (IL-12R-beta1) | No | No |
| 109503303 | Serine/threonine-protein kinase Nek1 (NimA-related protein kinase 1) | No | No |
| 109469632 | Inositol polyphosphate-5-phosphatase E | Yes | No |
| Dephosphorylation events specific to RA (present in CON and BMP+LIF but not in RA) | |||
| 94408289 | Insulin receptor substrate 4 | Yes | No |
| Phosphorylation events specific to BMP+LIF (only in BMP+LIF) | |||
| 203474 | Creatine kinase | No | N.D. |
| 12248185 | Nuclear ubiquitous casein kinase and cyclin-dependent kinase substrate | No | N.D. |
| 20151205 | Crystal structure of a transition state mimic of the catalytic subunit of cAMP-dependent protein kinase | No | N.D. |
| 27882143 | Wnk4 protein | No | N.D. |
| 109463815 | Pantothenate kinase 1 (Pantothenic acid kinase 1) (mPank1) (mPank) | No | N.D. |
| 114145515 | Protein kinase N3 | No | N.D. |
| Dephosphorylation events specific to BMP+LIF (present in CON and RA but not in BMP+LIF) | |||
| 109499587 | Ephrin type-A receptor 5 precursor (EHK-1) | No | N.D. |
Events prevented by LY292004, a specific PI3K inhibitor
N.D. Not determined
PI3K is involved in glial differentiation induced by retinoic acid or cytokines
The alpha catalytic subunit of PI3K is phosphorylated only after treatment with retinoic acid or cytokines (Table I), indicating that it is activated. The PI3K pathway was reported to be involved in cytokine-induced glial differentiation (6, 8), but this is the first time it is shown to be involved in retinoic acid-induced gliogenesis. Phosphorylation of Akt and mTOR, downstream mediators of the PI3K pathway, was observed after incubation with either retinoic acid or cytokines, confirming that the PI3K pathway is indeed activated by the gliogenic stimuli (Fig. 3A). In the case of the combination of cytokines, our results indicate that BMP-2 is responsible for this activation. In order to know if this activation is relevant to the differentiation induced by retinoic acid or cytokines we incubated the cells with these factors and with or without LY294002 (2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one, 10 μM). LY294002 is a highly potent and specific inhibitor of PI3K which acts on the ATP binding site of the enzyme. Inhibition of PI3K with LY294002 prevented glial differentiation induced by cytokines, and both glial and neuronal differentiation induced by retinoic acid as defined by both western blotting and cell counts of immunostained GFAP-positive cells (Figs. 3B-D).
Figure 3.
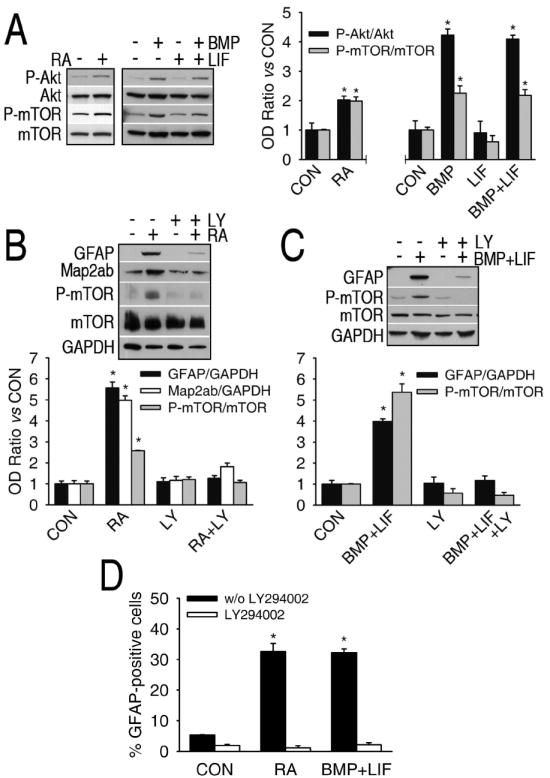
PI3K is involved in astrogliogenesis induced by retinoic acid or cytokines. A, The PI3K pathway is activated after incubation with cytokines (BMP+LIF) or retinoic acid (RA), as indicated by the increases in the phosphorylation of Akt and mTOR, two kinases downstream of PI3K. B-D, Incubation with the specific PI3K inhibitor LY294002 (LY, 10 μM) prevents glial differentiation by either retinoic acid (B) or cytokines (C), and neuronal differentiation by retinoic acid (B). Inhibition of PI3K by LY294002 is confirmed in all cases by the prevention of the increase in mTOR phosphorylation. The results obtained by western blot were confirmed by cell counting of GFAP-positive cells after the different treatments (D). *, significant versus control, p<0.05.
Given the critical role that PI3K plays in the differentiation-inducing effects of retinoic acid, we wanted to identify the important downstream substrates of this pathway. To this end, we extracted the phosphoproteins from cells treated with retinoic acid plus LY294002 and identified those changes in the phosphorylation state that are dependent on PI3K (i.e. prevented by LY294002) (Table I). It is noteworthy that around 65 % of the changes induced by retinoic acid are prevented by the PI3K inhibitor, either among the kinases and phosphatases or the whole phosphoprotein sample (Supplementary Table I), confirming that PI3K plays an important role in retinoic acid-induced differentiation and signaling. The dephosphorylation of synaptojanin-1 by retinoic acid was also prevented by LY294002, indicating that this event is downstream PI3K activation.
Synaptojanin-1 is involved in astrogliogenesis in vitro
Although synaptojanin-1 is mostly expressed in the synaptic terminals of neurons, our results indicate that it can also be expressed in precursor cells or to a lesser extent in primary cultures of astrocytes (Supplementary Figure 1). MudPIT results indicate that synaptojanin-1 is dephosphorylated by the gliogenic stimuli (Table I), and a previous report shows that the 5-phosphatase activity of synaptojanin-1 is activated by dephosphorylation and inactivated by phosphorylation on tyrosine residues (21). In order to confirm MudPIT results and know which specific residues were subject of dephosphorylation, HCN-B27 cells overexpressing synaptojanin-1 were incubated with or without retinoic acid or cytokines, the total proteins isolated and immunoprecipitated with an anti-synaptojanin-1 antibody, and blotted with antibodies against phosphotyrosine, phosphothreonine and phosphoserine (Figure 4A). No phosphorylation of synaptojanin-1 was detected on threonine residues under any of these circumstances, but a clear dephosphorylation of serine and tyrosine residues is observed after incubation with the gliogenic stimuli.
Figure 4.
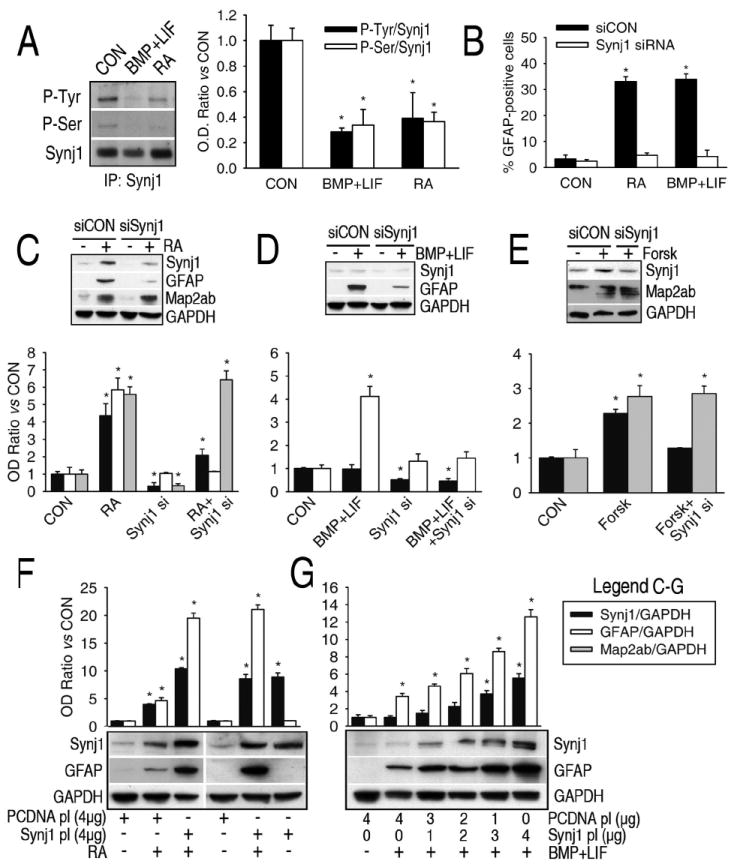
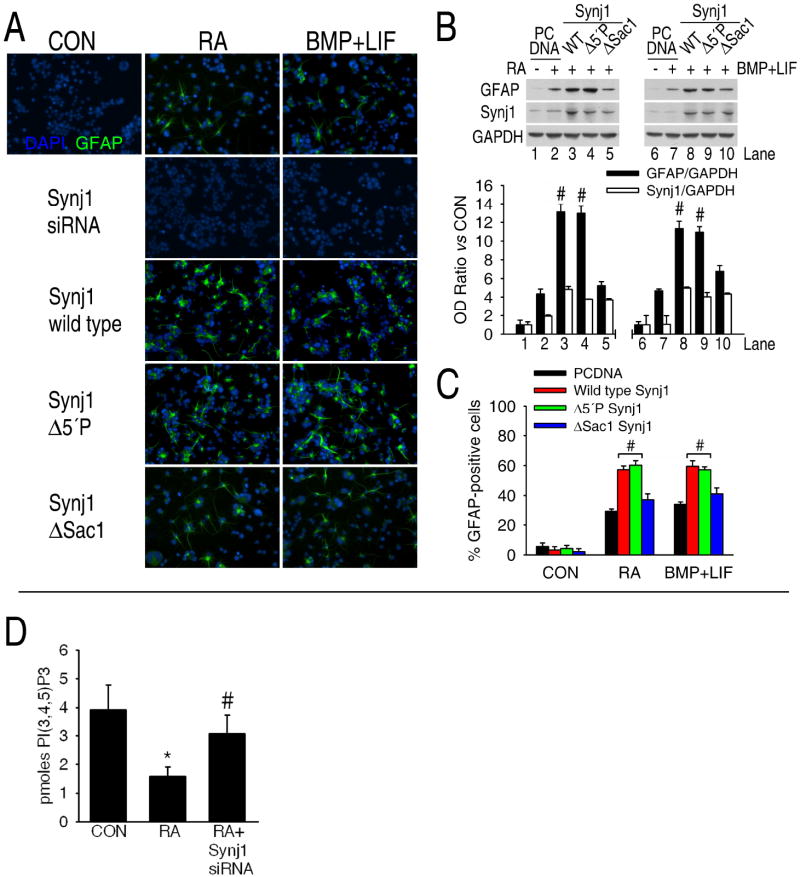
Synaptojanin-1 (Synj1) is involved in astrogliogenesis but not in neurogenesis in vitro. A, Serine and tyrosine residues of synaptojanin-1 are dephosphorylated after incubation of precursor cells with the gliogenic stimuli, as shown by western blots of phosphotyrosine and phosphothreonine antibodies on synaptojanin-1 immunoprecipitates. B-D, Downregulation of synaptojanin-1 expression by siRNA (10 nM, final concentration) prevents the increase in GFAP expression (western blot) and the number of astrocytes (immunocytochemistry) induced by either retinoic acid (B and C) or cytokines (B and D), but not the increase in Map2ab expression induced by retinoic acid (C). E, Forskolin induces a slight increase in synaptojanin-1 expression, and the inhibition of this increase by siRNA against synaptojanin-1 does not prevent the increase in Map2ab expression. F and G, Overexpression of synaptojanin-1 (1-4 μg of plasmid DNA per 106 cells was used for transfection) potentiates astrogliogenesis in the presence of retinoic acid (F) or cytokines (G) but it does not induce astrogliogenesis by itself (F).
The synaptojanin-1 gene is located on chromosome 21 in humans and the brains of patients with Down’s syndrome have higher levels of synaptojanin-1 expression (22). There is also an increase in the number and size of astrocytes in Down’s syndrome brain (23) as well as in the brains of Ts65Dn mice, a mouse model of Down’s Syndrome (24). These mice have a partial trisomy of chromosome 16 of a location containing the synaptojanin-1 gene (24). In contrast, synaptojanin-1 knockout mice are smaller than their littermate controls, indicating a general role for synaptojanin-1 in development (15). The brains of synaptojanin-1 knockout mice are also smaller. These differences in size appear postnatally, which is the stage of development when gliogenesis mainly occurs. Since astrocytes are the major cell population in the brain, the decrease in brain size might be caused by a diminished astroglial population. Therefore, we explored the possible relationship between synaptojanin-1 and astrogliogenesis.
Retinoic acid and forskolin, but not cytokines, induce an increase in synaptojanin-1 expression (Figs. 4C-E). Down-regulation of the expression of synaptojanin-1 by siRNA prevented glial differentiation induced by cytokines and retinoic acid, but not the neuronal differentiation induced by retinoic acid or forskolin (Figs. 4B-E and Fig. 5A), strongly indicating that synaptojanin-1 is exclusively involved in astrogliogenesis, but not in neurogenesis. Overexpression of synaptojanin-1 by itself does not induce glial differentiation, but it potentiates glial differentiation in the presence of gliogenic stimuli (Figs. 4F and 4G). The observations that 1) forskolin increases synaptojanin-1 expression but does not induce synaptojanin-1 dephosphorylation or gliogenesis; 2) cytokines induce synaptojanin-1 dephosphorylation and gliogenesis but they do not increase synaptojanin-1 expression; and 3) over-expression of synaptojanin-1 induces an increase in glial differentiation only in the presence of gliogenic stimuli strongly indicate that the critical point for inducing gliogenesis is not the increase in the expression of synaptojanin-1, but its activation.
Figure 5.
Potential role for phosphatidylinositol metabolism in astrogliogenesis. A-C, While overexpression of either wild type synaptojanin-1 (Synj1 WT) or a mutant lacking the 5-phosphatase activity (Synj1 Δ5’P) potentiates glial differentiation by retinoic acid or cytokines, overexpression of a mutant lacking the Sac1-like phosphatase activity (Synj1 ΔSac1) does not enhance it. Similar results were obtained by western blot (B) and immunocytochemistry (A and C). Nuclei were stained with DAPI (blue). #, Significant vs RA or BMP+LIF plus PCDNA. D, Retinoic acid induces a decrease in the intracellular concentration of PI(3,4,5)P3 which is almost completely recovered by the knockdown of synaptojanin-1. Phosphatidylinositols were extracted from 107 cells and assayed following manufacturer’s specifications. *, Significant vs CON; #, Significant vs RA.
A possible role for phosphatidylinositol metabolism in astrogliogenesis
Synaptojanin-1 has a dual phosphatase activity: a 5-phosphatase domain specifically removes a phosphate from the D-5 position of phosphoinositides and inositol polyphosphates; and a suppressor of actin 1 (Sac-1)-like domain has a 3-, 4-, and 5-phosphatase activity and therefore could catalyze a wider spectrum of reactions (25). At least the 5-phosphatase activity of synaptojanin-1 seems to be regulated by the dephosphorylation of tyrosine residues (21), and we observed that there is tyrosine and serine dephosphorylation of synaptojanin-1 after incubation with gliogenic stimuli (Fig. 4A). In order to know which of these phosphatase domains was responsible for the pro-gliogenic effect of synaptojanin-1, we overexpressed either wild type synaptojanin-1, a mutant lacking the 5-phosphatase activity or a mutant lacking the Sac1-like phosphatase activity (26) and analyzed their effect on retinoic acid-induced astrogliogenesis. We found that overexpression of either the mutant lacking Sac1-like phosphatase activity or the double mutant does not enhance gliogenesis as the overexpression of either the wild type or the 5-phosphatase mutant forms of synaptojanin-1 do (Figs. 5A-C). These results indicate that the Sac1-like phosphatase domain of synaptojanin-1 is responsible for the pro-gliogenic effect of synaptojanin-1.
Both PI3K and synaptojanin-1 are major regulators of inositol metabolism and signaling in the brain (27-29) and we found that they are involved in astrogliogenesis, indicating that inositol metabolism could play an important role in astrogliogenesis. The main substrate of PI3K in vivo is PI(4,5)P2, giving rise to PI(3,4,5)P3, and synaptojanin-1 is a major PI(3,4,5)P3 and PI(4,5)P2 5-phosphatase in the brain (15, 27). Accordingly, we observed that retinoic acid induce a decrease in PI(3,4,5)P3 levels and that this decrease is prevented by the knockdown of synaptojanin-1 (Figure 5D). Nevertheless, a direct hydrolysis of PI(3,4,5)P3 or PI(4,5)P2 is most likely catalyzed by the 5-phosphatase domain of synaptojanin-1, because phosphoinositides with phosphates in adjacent positions are poor substrates of the Sac1-like domain (25). On the other hand, the Sac1-like domain could regulate the levels of these phosphoinositides indirectly, by means of the hydrolysis of PI4P or PI5P, for example, to PI (25). The availability of PI4P or PI5P can be actually limiting for the generation of PI(3,4,5)P3 by PI3K, for PI(4,5)P2 is produced mainly by the phosphorylation of PI4P at position 5 or of PI5P at position 4 (30). Another possible mechanism could be the regulation of PI3P and/or PI(3,5)P2 levels, because PI3K can produce them by phosphorylation of PI or PI5P, respectively, and synaptojanin-1 Sac1-like domain could regulate their levels by catalyzing the dephosphorylation of PI(3,5)P2 to PI3P. However, more complex schemes for a role of phosphatidylinositol metabolism in astrogliogenesis, not necessarily centered on PI(3,4,5)P3 or PI(3,5)P2, cannot be ruled out.
Synaptojanin-1 is involved in astrogliogenesis in vivo
To confirm our in vitro observations, the expression of glial and neuronal markers in synaptojanin-1 knockout mouse brain was analyzed. The expression of the neuronal markers Map2ab, beta III tubulin, synaptotagmin and synaptophysin does not change in synaptojanin-1 knockout mice vs controls (Fig. 6A). On the other hand, the expression of the glial markers GFAP, GLT1, GLAST, SPARC and CD44 is down-regulated in knockout mice compared to wild type mice, while the expression of the glial marker S100β remain unchanged (Fig. 6A). These results could indicate that synaptojanin-1 knockout mice brains have less astrocytes than their littermate controls or that the total number of astrocytes is the same but they are not normal. We find the first possibility more likely for 5 out of 6 glial markers were decreased and, in spite of the general agreement that it is a glial marker, S100β is not only found in astrocytes but also in oligodendrocytes and neurons (31, 32). In fact, all of the astroglial markers are found in other cell types (33-36), but in the case of GFAP, in addition to astrocytes, it is only found in radial glia or precursor cells, which are either not present in the adult brain or found in a very small number. Furthermore, whether adult nerve precursor cells actually express GFAP is still a matter of discussion (16, 37). Whether there are changes in the number or in the quality of astrocytes, it is clear that astrogliogenesis is not normal in synaptojanin-1 knockout mice, supporting our observations in vitro that synaptojanin-1 is involved in astrogliogenesis. The fact that no change is observed in the neuronal markers indicates that neurogenesis is normal in the knockout mice, further supporting our in vitro results.
Figure 6.
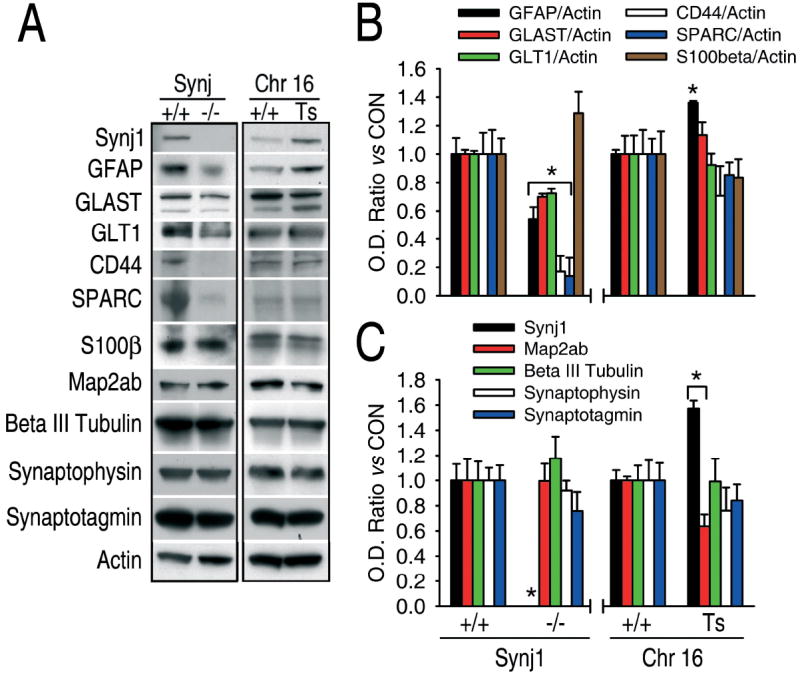
Synaptojanin-1 levels are directly related to astroglial content in vivo. A, Representative western blots of total proteins extracted from brains of synaptojanin-1 knockout mice, Ts65Dn mice and their respective littermate controls. On the left, synaptojanin-1 knockout mouse brain (Synj1, -/-) shows lower levels of the astroglial markers GFAP, GLAST, GLT1, CD44 and SPARC than the brain of littermate controls (Synj1, +/+), but no changes are observed in the expression of the astroglial marker S100β and the neuronal markers Map2ab, beta III tubulin, synaptophysin and synaptotagmin. On the right, Ts65Dn mouse brain (Chr 16, Ts) shows higher levels of synaptojanin-1 expression and the astroglial marker GFAP than the brain of littermate controls (Chr16, +/+), but Map2ab expression is decreased and no changes were observed in the expression of any other neuronal or glial markers. B and C, Densitometric and statistic analyses of the experiments described in A. *, significant versus control, p<0.05.
Finally, given that 1) Ts65Dn mice have a partial trisomy of chromosome 16 containing synaptojanin-1 gene and 2) it has been previously shown that Ts65Dn mouse astrocytes are larger than normal and more abundant, it was predicted that both the expression levels of synaptojanin-1 and glial markers would be elevated in this model of Down’s syndrome. Figures 6B and 6C show that Down’s syndrome mouse brain has a 50 % increase in synaptojanin-1 expression and 36 % increase in GFAP expression. Although the expression of the other glial markers tested did not change significantly, our results still support the original observation that astrocytes are more abundant and larger in Ts65Dn mouse brain, which was exclusively based on immunohistochemistry with GFAP (38). The fact that no other glial marker is altered indicates the existence of an anomalous gliogenesis that can be easily explained by the great genetic complexity underlying Down’s syndrome. The expression of the neuronal markers beta III tubulin, synaptophysin and synaptotagmin does not change, but a slight decrease in Map2ab expression is observed (Fig. 6C).
The above data indicate that synaptojanin-1 is involved in astrogliogenesis but not in neurogenesis, and offer a plausible link between the increase in synaptojanin-1 expression and the increased GFAP reactivity in Down’s syndrome brain (22, 23). Together with published results, the data support a key role for synaptojanin-1 and inositolphosphate homeostasis in brain development and the histopathological features of Down’s syndrome brain (28). Most brain pathologies are classically associated with neuronal dysfunction or neuronal loss. However, astrocytes are essential for neuronal function, survival and differentiation (1, 39), and growing evidence indicates that they might be involved in neuronal dysfunction or damage (1). In this sense, individuals with Alexander’s disease, a disorder caused by mutations in the GFAP gene, have defects in both glial and neuronal function, severe loss of white matter and very often mental retardation, the hallmark of Down’s syndrome (40). Very recently, synaptojanin-1 has been shown to contribute to the cognitive deficits observed in mouse models of Down’s syndrome (28). Little is known about the molecular determinants of Down’s syndrome pathology, but our data support a possible role for astrocytes or astrogliogenesis in this disease.
Material and Methods
Materials
Cell culture plastics were acquired from Falcon (Becton Dickinson, Franklin Lakes, NJ, USA) and culture media, supplements and additives were obtained from Invitrogen (Carlsbad, CA, USA), unless otherwise indicated. Retinoic acid and forskolin were purchased from Sigma (St. Louis, MO, USA). Human recombinant BMP-2 was obtained from R&D systems (Minneapolis, MN, USA) and LIF from Chemicon (Temecula, CA, USA). LY294002 was obtained from LC Laboratories (Woburn, MA, USA). “Milo” antibody against Rat synaptojanin-1 was a generous gift from Dr. Peter McPherson (McGill University, Montreal, Canada) and antibodies against glutamate transporters GLT1 and GLAST were a generous gift from Dr. Jeffrey D. Rothstein (Johns Hopkins University, Baltimore, MD). Wild type rat synaptojanin-1 expression plasmid and synaptojanin-1 knockout and control littermate mouse brains were a kind gift from Dr. Pietro Di Camilli (Yale University School of Medicine, New Haven, CT, USA.). Expression plasmids for synaptojanin-1 mutants lacking 5-phosphatase and Sac1-like phosphatase activity were a kind gift from Dr. Timothy A. Ryan (Weill Medical College, Cornell University, New York, NY, USA). The siRNA against rat synaptojanin-1 and siRNA control were obtained from Dharmacon (Chicago, IL, USA). Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) was used for synaptojanin-1 siRNA and plasmid lipofection following the manufacturer’s instructions. ELISA kit for PI(3,4,5)P3 determination was purchased from Echelon Biosciences Incorporated (Salt Lake City, UT, USA) and used according to manufacturer’s instructions.
Cell cultures and animals
The HCN rat adult hippocampal precursor cell line was kindly provided by Drs. J. Ray and F. H. Gage (The Salk Institute for Biological Studies, La Jolla, CA). The B27 clone was isolated from these cells in our laboratory as previously described (17). HCN-B27 cells were maintained in Neurobasal medium plus 1 mM glutamine, 1X penicillin/streptomycin mixture and 1X B27 supplement (minus antioxidants) (Invitrogen, Carlsbad, CA, USA). For experiments, cells were seeded into polyornithine-coated dishes in DMEM/Ham F12 1:1 medium (Omega Scientific, Tarzana, CA, USA) plus 1 mM glutamine, 1X penicillin/streptomycin and 1X N2 supplement (Invitrogen, Carlsbad, CA, USA).
All procedures for animal studies adhered to the Guide for the Care and Use of Laboratory Animals and were approved by the Salk Institute Animal Care and Use Committee. Male Ts65Dn mice and their littermate controls were obtained from The Jackson Laboratory (Bar Harbor, ME, USA), and were housed in a temperature- and humidity-controlled environment on a 12 h–12 h light–dark cycle with free access to food and water. One month-old mice were anesthetized with 500 μl of chloral hydrate 7 % and perfused with KPBS plus protease inhibitors (Roche diagnostics, Mannheim, Germany) for 5 minutes. Brains were extracted, immediately frozen on dry ice and stored at -80°C for posterior analysis.
Western blot, immunoprecipitation and immunocytochemistry
Cells or brains were lysed with RIPA buffer [HEPES 50 mM pH 7.0, NaCl 150 mM, NP-40 1% v/v, sodium dodecyl sulfate 0.1% w/v, deoxycholic acid 0.5 % w/v, PMSF 0.1 mM, dithiotreitol 1 mM, cocktail of protease inhibitors, EDTA-free (Roche diagnostics, Mannheim, Germany) and cocktail of phosphatase inhibitors II 0.01% v/v (Sigma, St. Louis, MO, USA)], sonicated and centrifuged at 10,000 × g for 10 min at 4 °C. Supernatants were collected and the protein concentration was quantified by the Dc protein assay (Bio-rad, Hercules, CA, USA). Fifty micrograms of protein were loaded and run on 12 % acrylamide pre-cast gels (Bio-rad, Hercules, CA, USA) and transferred to PVDF membranes for blotting. Antibodies against the following proteins were used: GFAP 1:2000 from Chemicon (Temecula, CA, USA); Actin and Map2ab (1:1000) from Sigma (St. Louis, MO, USA); mTOR, phospho-mTOR (Ser2448), Akt and phospho-Akt (Ser473) (1:1000) from Cell Signaling technologies (Danvers, MA, USA); GAPDH (1:10000) from Labfrontier (Republic of South Korea); Neuronal class III beta-tubulin (beta III tubulin, antigen Tuj1, 1:2000) from Covance (Berkeley, CA, USA); GLAST (1:100); GLT1 (1:10000); S100β (1:1000) from DAKO (Carpinteria, CA, USA; secreted protein acidic and rich in cysteine (SPARC, 1:50) from Developmental Studies Hybridoma Bank (University of Iowa, Iowa, USA); Synaptophysin and synaptotagmin (1:1000) from Roche diagnostics (Mannheim, Germany) and Milo synaptojanin-1 antibody (1:200). Immunoblots were revealed by enhanced chemoluminescence (Pierce, Rockford, IL, USA) and their optic density was quantified with the Scion image free software (www.scioncorp.com).
For immunoprecipitation, the protocol supplied by Cell signaling was followed using the Milo antibody against rat synaptojanin-1. After immunoprecipitation, western blots of the samples were carried out as described above and blotted with the antibodies against rat synaptojanin-1, phosphoserine, phosphothreonine (Qiagen, Valencia, CA, USA) and phosphotyrosine (Cell Signaling technologies, Danvers, MA, USA).
For immunocytochemistry, cells were sequentially fixed wit h 4 % w/v paraformaldehyde for 20 min, washed once with PBS, permeabilized with 0.5 % Triton-X in PBS for 10 min and washed 3 times with PBS before blocking in 3 % bovine serum albumin in PBS for 1 hour. Cells were then incubated with primary antibodies against GFAP (1:1000), Musashi (1:200), Sox-2 (1:200) or Nestin (1:200) (All from Chemicon, Temecula, CA, USA) in blocking solution overnight at 4 °C. After removal of the primary antibody, cells were sequentially washed 3 times with 0.5 % Triton-X in PBS (10 min each); incubated with the appropiate goat anti-rabbit or anti-mouse secondary antibodies conjugated with Fluor 488 or Texas red (1:1000, Invitrogen, Carlsbad, CA, USA) in blocking solution for 1 hour at room temperature; washed again 3 times with 0.5 % Triton-X in PBS (10 min each); incubated for 20 min with a 1 μg/ml DAPI solution in PBS; and washed twice with PBS (10 min each). Pictures were taken with a Hamamatsu digital camera (Bridgewater, NJ, USA) coupled to a Leica fluorescence microscope (Allendale, NJ, USA).
Phosphoprotein isolation and Multidimensional protein identification (MudPIT)
Phosphoproteins from cells were isolated by means of the Phosphoprotein Isolation kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. Briefly, following cell lysis, lysates were passed through metal affinity columns and the eluates containing the phosphoproteins were collected. The phosphoprotein eluates were then passed through Sephadex G-25 buffer exchange columns (GE Healthcare, Buckinghamshire, UK) following the manufacturer’s directions, in order to remove salts and small molecules that would interfere with MudPIT analysis. Ten μg of phosphoproteins per sample were reduced with DTT (20mM) in 6 M urea followed by alkylation with iodoacetamide. After 8-fold dilution with 50 mM ammonium bicarbonate, trypsin (1 μg) was added and digestion was allowed to proceed overnight. The samples were desalted using Bond-Elut cartridges (50 mg, Varian, Palo Alto, CA). The peptide eluate was concentrated to dryness in a vacuum centrifuge and reconstituted in 0.1 % aqueous formic acid. Tandem (strong cation exchange/ reversed phase) capillary columns were packed in house. First, a capillary with integrated spray tip (75 um I.D., 10 um tip, New Objective, Woburn, MA) was packed with C-18 reversed phase material (Zorbax SB-C18, 5 um particle size, Agilent, Santa Clara, CA) to a length of 8 cm. This was followed by high pressure packing with strong cation exchange material (Polysulfoethyl A, 5 um particle size, Poly LC, Columbia, MD) to a length of 4 cm. Protein digests were then bomb-loaded onto the column (Nanobaume Capillary Column Packer, Western Fluids, Wildomar, CA). The column was subsequently connected to a nano-flow HPLC (Eksigent, Livermore, CA). To perform the multi-dimensional chromatography, ten pH steps were employed, each followed by an acetonitrile gradient. The pH step buffers (pH 2.5, 3.0, 3.5, 4.0, 4.5, 5.0, 5.5, 6.0 & 8, Column Technology, Fremont, CA) were loaded onto the column using an autosampler and a 3 μl loop. The reversed phase elution was achieved by of a linear gradient of 0-60 % acetonitrile in 0.1 % formic acid was used to elute the peptides within 60 minutes at a flow rate of 300 nl/min. The eluate was introduced into a Thermo LTQ-Orbitrap mass spectrometer (ThermoFisher, Waltham, MA) via a nano-spray source. Mass spectrometric analysis was conducted by recording precursor ion scans at a resolution of 60,000 in the Orbitrap Fourier-transform analyzer followed by MS/MS scans of the top 5 ions in the linear ion trap (cycle time approx. 1 s). An active exclusion window of 90 s was employed. Data were analyzed using the Mascot algorithm (Matrix Science, London, UK). This approach gave us lists of 200-300 phosphoproteins per sample, which was compared between the samples using the Scaffold program (Proteome Software, Portland, OR) on a Sorcerer Solo platform (SageN, San Jose, CA). Filters to reduce the number of possible target proteins were applied as described in the Results and Discussion section.
Statistic analysis
Graphs show the mean ± standard error of three independent experiments. Data were analyzed with one way analysis of variance followed by the Tukey post hoc test. Significance was accepted when p<0.05.
Supplementary Material
Synaptojanin-1 is expressed at very low levels in astrocytes. (A) Synaptojanin-1 mRNA is present in astrocytes and PC12 cells (Lane 1, PC12 cells; Lanes 2-5, primary cultures of astrocytes), but the protein is present only at very low amounts and cannot be detected by western blot (B) unless the samples are enriched by means of immunoprecipitation of synaptojanin-1 (C). We carried out the immunoprecipitations starting with equal amounts of protein (200 μg), and we found that astrocytes isolated from embryonic day 18 (E18) brains have more synaptojanin-1 than astrocytes isolated from newborn brains, indicating that astrocytes may lose synaptojanin-1 while they are maturating. *, significant versus E18 astrocytes, p<0.05.
Total RNA was isolated from primary cultures of astrocytes and rat PC12 pheochromocytoma cells using Trizol reagent (Invitrogen, USA) according to the manufacturer’s instructions and its concentration determined by spectrophotometry. Reverse transcription reactions were performed using 1 μg of total RNA in reverse transcription buffer [10mM DTT, 20 μM each of dATP, dCTP, dGTP, and dTTP, and 1 μM of oligo(dT)15-18]. The solution was heated to 65°C for 5 min and cooled to 37°C for 10 min, and then 25 units of AMV reverse transcriptase (New England Biolab, USA) were added and the reaction mixture was incubated at 42°C for 1 hour. The PCR reaction master mixture contained forward and reverse primers (10-20 pmol each), dNTPs (200 μM each as final concentration), 1x PCR buffer, Taq DNA polymerase (0.5 units) (Takara, Japan), and 1 μl of the reverse transcription reaction as the source of cDNA. The rat synaptojanin primer set used for PCR reactions was: Synj-1(3590): CAC CAC AGA GGC CAC CTC CAC CTT CA (nt3590-3125); Synj-2: CTA GCT GCA CCA TAT CCA GAA GGT CC (nt3810-3835) (GeneBank NM_053476). The Actin primer set was purchased from Stratagene (USA). Amplification was performed at 94°C for 40 sec, 60°C for 1 min, and 72°C for 1 min, for 35 cycles. A PCR reaction of water with primers was also conducted as a negative control. After amplification, samples were run on 1.2% agarose gel and visualized by ethidium bromide staining.
Phosphoproteins (sorted by their correspondent GI numbers, from smaller to larger) identified by MudPIT analysis in all groups (control, retinoic acid, BMP+LIF, retinoic acid + LY294002 and forskolin) before any filter was applied (See Results and Discussion section).
Acknowledgments
Work supported by a grant to FH from the Instituto de Salud Carlos III/Centro Superior de Investigaciones Científicas (SALK06/02) and grants to PM, DS and WF from the NIH (AG025337) and to La Jolla Interdisciplinary Neuroscience Center (NIH NS057096). Authors sincerely thank Drs. Pietro de Camilli, Peter McPherson, Timothy A. Ryan, Jeffrey D. Rothstein, Jasodhara Ray and Fred H. Gage for the useful material they kindly provided. We also thank Jessica Read and William Low for technical assistance and Dr. Karsten Schmidt for help with MS data analysis.
References
- 1.Croisier E, Graeber MB. Glial degeneration and reactive gliosis in alpha-synucleinopathies: the emerging concept of primary gliodegeneration. Acta Neuropathol (Berl) 2006;112(5):517–30. doi: 10.1007/s00401-006-0119-z. [DOI] [PubMed] [Google Scholar]
- 2.Qian X, Shen Q, Goderie SK, He W, Capela A, Davis AA, et al. Timing of CNS cell generation: a programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron. 2000;28(1):69–80. doi: 10.1016/s0896-6273(00)00086-6. [DOI] [PubMed] [Google Scholar]
- 3.Barnabe-Heider F, Wasylnka JA, Fernandes KJ, Porsche C, Sendtner M, Kaplan DR, et al. Evidence that embryonic neurons regulate the onset of cortical gliogenesis via cardiotrophin-1. Neuron. 2005;48(2):253–65. doi: 10.1016/j.neuron.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 4.Bonni A, Sun Y, Nadal-Vicens M, Bhatt A, Frank DA, Rozovsky I, et al. Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science. 1997;278(5337):477–83. doi: 10.1126/science.278.5337.477. [DOI] [PubMed] [Google Scholar]
- 5.Nakashima K, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, Kawabata M, et al. Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science. 1999;284(5413):479–82. doi: 10.1126/science.284.5413.479. [DOI] [PubMed] [Google Scholar]
- 6.Hermanson O, Jepsen K, Rosenfeld MG. N-CoR controls differentiation of neural stem cells into astrocytes. Nature. 2002;419(6910):934–9. doi: 10.1038/nature01156. [DOI] [PubMed] [Google Scholar]
- 7.Rajan P, McKay RD. Multiple routes to astrocytic differentiation in the CNS. J Neurosci. 1998;18(10):3620–9. doi: 10.1523/JNEUROSCI.18-10-03620.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajan P, Panchision DM, Newell LF, McKay RD. BMPs signal alternately through a SMAD or FRAP-STAT pathway to regulate fate choice in CNS stem cells. J Cell Biol. 2003;161(5):911–21. doi: 10.1083/jcb.200211021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goncalves MB, Boyle J, Webber DJ, Hall S, Minger SL, Corcoran JP. Timing of the retinoid-signalling pathway determines the expression of neuronal markers in neural progenitor cells. Dev Biol. 2005;278(1):60–70. doi: 10.1016/j.ydbio.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Wohl CA, Weiss S. Retinoic acid enhances neuronal proliferation and astroglial differentiation in cultures of CNS stem cell-derived precursors. J Neurobiol. 1998;37(2):281–90. doi: 10.1002/(sici)1097-4695(19981105)37:2<281::aid-neu7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 11.Chambers CB, Peng Y, Nguyen H, Gaiano N, Fishell G, Nye JS. Spatiotemporal selectivity of response to Notch1 signals in mammalian forebrain precursors. Development. 2001;128(5):689–702. doi: 10.1242/dev.128.5.689. [DOI] [PubMed] [Google Scholar]
- 12.Taylor MK, Yeager K, Morrison SJ. Physiological Notch signaling promotes gliogenesis in the developing peripheral and central nervous systems. Development. 2007;134(13):2435–47. doi: 10.1242/dev.005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bastien J, Plassat JL, Payrastre B, Rochette-Egly C. The phosphoinositide 3-kinase/Akt pathway is essential for the retinoic acid-induced differentiation of F9 cells. Oncogene. 2006;25(14):2040–7. doi: 10.1038/sj.onc.1209241. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Carballo G, Moreno L, Masia S, Perez P, Barettino D. Activation of the phosphatidylinositol 3-kinase/Akt signaling pathway by retinoic acid is required for neural differentiation of SH-SY5Y human neuroblastoma cells. J Biol Chem. 2002;277(28):25297–304. doi: 10.1074/jbc.M201869200. [DOI] [PubMed] [Google Scholar]
- 15.Cremona O, Di PG, Wenk MR, Luthi A, Kim WT, Takei K, et al. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell. 1999;99(2):179–88. doi: 10.1016/s0092-8674(00)81649-9. [DOI] [PubMed] [Google Scholar]
- 16.Ray J, Gage FH. Differential properties of adult rat and mouse brain-derived neural stem/progenitor cells. Mol Cell Neurosci. 2006;31(3):560–73. doi: 10.1016/j.mcn.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Schubert D, Klar A, Park M, Dargusch R, Fischer WH. F-spondin promotes nerve precursor differentiation. J Neurochem. 2006;96(2):444–53. doi: 10.1111/j.1471-4159.2005.03563.x. [DOI] [PubMed] [Google Scholar]
- 18.MacCoss MJ, McDonald WH, Saraf A, Sadygov R, Clark JM, Tasto JJ, et al. Shotgun identification of protein modifications from protein complexes and lens tissue. Proc Natl Acad Sci U S A. 2002;99(12):7900–5. doi: 10.1073/pnas.122231399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Licklider LJ, Thoreen CC, Peng J, Gygi SP. Automation of nanoscale microcapillary liquid chromatography-tandem mass spectrometry with a vented column. Anal Chem. 2002;74(13):3076–83. doi: 10.1021/ac025529o. [DOI] [PubMed] [Google Scholar]
- 20.Florens L, Washburn MP, Raine JD, Anthony RM, Grainger M, Haynes JD, et al. A proteomic view of the Plasmodium falciparum life cycle. Nature. 2002;419(6906):520–6. doi: 10.1038/nature01107. [DOI] [PubMed] [Google Scholar]
- 21.Irie F, Okuno M, Pasquale EB, Yamaguchi Y. EphrinB-EphB signalling regulates clathrin-mediated endocytosis through tyrosine phosphorylation of synaptojanin 1. Nat Cell Biol. 2005;7(5):501–9. doi: 10.1038/ncb1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arai Y, Ijuin T, Takenawa T, Becker LE, Takashima S. Excessive expression of synaptojanin in brains with Down syndrome. Brain Dev. 2002;24(2):67–72. doi: 10.1016/s0387-7604(01)00405-3. [DOI] [PubMed] [Google Scholar]
- 23.Mito T, Becker LE. Developmental changes of S-100 protein and glial fibrillary acidic protein in the brain in Down syndrome. Exp Neurol. 1993;120(2):170–6. doi: 10.1006/exnr.1993.1052. [DOI] [PubMed] [Google Scholar]
- 24.Antonarakis SE, Lyle R, Dermitzakis ET, Reymond A, Deutsch S. Chromosome 21 and down syndrome: from genomics to pathophysiology. Nat Rev Genet. 2004;5(10):725–38. doi: 10.1038/nrg1448. [DOI] [PubMed] [Google Scholar]
- 25.Guo S, Stolz LE, Lemrow SM, York JD. SAC1-like domains of yeast SAC1, INP52, and INP53 and of human synaptojanin encode polyphosphoinositide phosphatases. J Biol Chem. 1999;274(19):12990–5. doi: 10.1074/jbc.274.19.12990. [DOI] [PubMed] [Google Scholar]
- 26.Mani M, Lee SY, Lucast L, Cremona O, Di PG, De CP, et al. The dual phosphatase activity of synaptojanin1 is required for both efficient synaptic vesicle endocytosis and reavailability at nerve terminals. Neuron. 2007;56(6):1004–18. doi: 10.1016/j.neuron.2007.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woscholski R, Finan PM, Radley E, Totty NF, Sterling AE, Hsuan JJ, et al. Synaptojanin is the major constitutively active phosphatidylinositol-3,4,5-trisphosphate 5-phosphatase in rodent brain. J Biol Chem. 1997;272(15):9625–8. doi: 10.1074/jbc.272.15.9625. [DOI] [PubMed] [Google Scholar]
- 28.Voronov SV, Frere SG, Giovedi S, Pollina EA, Borel C, Zhang H, et al. Synaptojanin 1-linked phosphoinositide dyshomeostasis and cognitive deficits in mouse models of Down’s syndrome. Proc Natl Acad Sci U S A. 2008;105(27):9415–20. doi: 10.1073/pnas.0803756105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L, Liu F, Ross AH. PTEN regulation of neural development and CNS stem cells. J Cell Biochem. 2003;88(1):24–8. doi: 10.1002/jcb.10312. [DOI] [PubMed] [Google Scholar]
- 30.Carricaburu V, Lamia KA, Lo E, Favereaux L, Payrastre B, Cantley LC, et al. The phosphatidylinositol (PI)-5-phosphate 4-kinase type II enzyme controls insulin signaling by regulating PI-3,4,5-trisphosphate degradation. Proc Natl Acad Sci U S A. 2003;100(17):9867–72. doi: 10.1073/pnas.1734038100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vives V, Alonso G, Solal AC, Joubert D, Legraverend C. Visualization of S100B-positive neurons and glia in the central nervous system of EGFP transgenic mice. J Comp Neurol. 2003;457(4):404–19. doi: 10.1002/cne.10552. [DOI] [PubMed] [Google Scholar]
- 32.Steiner J, Bernstein HG, Bielau H, Berndt A, Brisch R, Mawrin C, et al. Evidence for a wide extra-astrocytic distribution of S100B in human brain. BMC Neurosci. 2007;8:2. doi: 10.1186/1471-2202-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mendis DB, Brown IR. Expression of the gene encoding the extracellular matrix glycoprotein SPARC in the developing and adult mouse brain. Brain Res Mol Brain Res. 1994;24(14):11–9. doi: 10.1016/0169-328x(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 34.Plachez C, Martin A, Guiramand J, Recasens M. Astrocytes repress the neuronal expression of GLAST and GLT glutamate transporters in cultured hippocampal neurons from embryonic rats. Neurochem Int. 2004;45(7):1113–23. doi: 10.1016/j.neuint.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 35.Plachez C, Danbolt NC, Recasens M. Transient expression of the glial glutamate transporters GLAST and GLT in hippocampal neurons in primary culture. J Neurosci Res. 2000;59(5):587–93. doi: 10.1002/(SICI)1097-4547(20000301)59:5<587::AID-JNR1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 36.Rampon C, Weiss N, Deboux C, Chaverot N, Miller F, Buchet D, et al. Molecular mechanism of systemic delivery of neural precursor cells to the brain: assembly of brain endothelial apical cups and control of transmigration by CD44. Stem Cells. 2008;26(7):1673–82. doi: 10.1634/stemcells.2008-0122. [DOI] [PubMed] [Google Scholar]
- 37.Seri B, Garcia-Verdugo JM, McEwen BS, varez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21(18):7153–60. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holtzman DM, Santucci D, Kilbridge J, Chua-Couzens J, Fontana DJ, Daniels SE, et al. Developmental abnormalities and age-related neurodegeneration in a mouse model of Down syndrome. Proc Natl Acad Sci U S A. 1996;93(23):13333–8. doi: 10.1073/pnas.93.23.13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kornyei Z, Gocza E, Ruhl R, Orsolits B, Voros E, Szabo B, et al. Astroglia-derived retinoic acid is a key factor in glia-induced neurogenesis. FASEB J. 2007 doi: 10.1096/fj.06-7756com. [DOI] [PubMed] [Google Scholar]
- 40.Quinlan RA, Brenner M, Goldman JE, Messing A. GFAP and its role in Alexander disease. Exp Cell Res. 2007;313(10):2077–87. doi: 10.1016/j.yexcr.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Synaptojanin-1 is expressed at very low levels in astrocytes. (A) Synaptojanin-1 mRNA is present in astrocytes and PC12 cells (Lane 1, PC12 cells; Lanes 2-5, primary cultures of astrocytes), but the protein is present only at very low amounts and cannot be detected by western blot (B) unless the samples are enriched by means of immunoprecipitation of synaptojanin-1 (C). We carried out the immunoprecipitations starting with equal amounts of protein (200 μg), and we found that astrocytes isolated from embryonic day 18 (E18) brains have more synaptojanin-1 than astrocytes isolated from newborn brains, indicating that astrocytes may lose synaptojanin-1 while they are maturating. *, significant versus E18 astrocytes, p<0.05.
Total RNA was isolated from primary cultures of astrocytes and rat PC12 pheochromocytoma cells using Trizol reagent (Invitrogen, USA) according to the manufacturer’s instructions and its concentration determined by spectrophotometry. Reverse transcription reactions were performed using 1 μg of total RNA in reverse transcription buffer [10mM DTT, 20 μM each of dATP, dCTP, dGTP, and dTTP, and 1 μM of oligo(dT)15-18]. The solution was heated to 65°C for 5 min and cooled to 37°C for 10 min, and then 25 units of AMV reverse transcriptase (New England Biolab, USA) were added and the reaction mixture was incubated at 42°C for 1 hour. The PCR reaction master mixture contained forward and reverse primers (10-20 pmol each), dNTPs (200 μM each as final concentration), 1x PCR buffer, Taq DNA polymerase (0.5 units) (Takara, Japan), and 1 μl of the reverse transcription reaction as the source of cDNA. The rat synaptojanin primer set used for PCR reactions was: Synj-1(3590): CAC CAC AGA GGC CAC CTC CAC CTT CA (nt3590-3125); Synj-2: CTA GCT GCA CCA TAT CCA GAA GGT CC (nt3810-3835) (GeneBank NM_053476). The Actin primer set was purchased from Stratagene (USA). Amplification was performed at 94°C for 40 sec, 60°C for 1 min, and 72°C for 1 min, for 35 cycles. A PCR reaction of water with primers was also conducted as a negative control. After amplification, samples were run on 1.2% agarose gel and visualized by ethidium bromide staining.
Phosphoproteins (sorted by their correspondent GI numbers, from smaller to larger) identified by MudPIT analysis in all groups (control, retinoic acid, BMP+LIF, retinoic acid + LY294002 and forskolin) before any filter was applied (See Results and Discussion section).