Summary
Mutations in α-synuclein and Leucine-rich repeat kinase 2 (LRRK2) are linked to autosomal dominant forms of Parkinson’s disease (PD). However, little is known about any potential pathophysiological interplay between these two PD-related genes. Here we show in transgenic mice that although over-expression of LRRK2 alone did not cause neurodegeneration, the presence of excess LRRK2 greatly accelerated the progression of neuropathological abnormalities developed in PD-related A53T α-synuclein transgenic mice. Moreover, we found that LRRK2 promoted the abnormal aggregation and somatic accumulation of α-synuclein in A53T mice, likely resulted from the impairment of microtubule dynamics, Golgi organization, and ubiquitin-proteasome pathway. Conversely, genetic ablation of LRRK2 preserved the Golgi structure, suppressed the aggregation and somatic accumulation of α-synuclein, and thereby delayed the progression of neuropathology in A53T mice. These findings demonstrate that over-expression of LRRK2 enhances α-synuclein-mediated cytotoxicity and suggest inhibition of LRRK2 expression as a potential therapeutic option for ameliorating α-synuclein-induced neurodegeneration.
Keywords: LRRK2, G2019S, α-synuclein, A53T, Golgi apparatus, microtubule, ubiquitin, mitochondria, aggregation, transgenic, knockout, Parkinson’s disease
Introduction
Parkinson’s disease (PD) is characterized pathologically by relatively selective loss of midbrain and brain stem catecholaminergic neurons and by deposition of α-synuclein (α-syn) aggregates in cell bodies and nerves (Nussbaum and Polymeropoulos, 1997). Since mutations in both α-syn and Leucine-rich repeat kinase 2 (LRRK2) cause familial forms of PD that resemble sporadic PD pathologically (Hardy et al., 2006), these genetic mutations provide valuable molecular tools to study the pathogenesis of PD. The etiology of PD remains elusive; however, it has been generally accepted that the formation of α-syn aggregates is a key step in the pathogenesis of PD (Trojanowski et al., 1998).
Mutations in the α-syn gene, including missense mutations (A53T and A30P) and gene duplication/triplication, lead to the development of early-onset familial PD (Hardy et al., 2006). Furthermore, α-syn is a main component of abnormal intracellular deposits known as Lewy bodies (LB) and Lewy neurites (LN) found in PD brains (Spillantini et al., 1997). While both wild-type (WT) and mutant α-syn form fibrillar aggregates, PD-related α-syn mutations greatly accelerate the formation of fibrils as compared to WT protein (Conway et al., 1998;Narhi et al., 1999). α-syn aggregates cause various cytotoxicity, including impairment of proteasomal and lysosomal activities, disruption of ER-Golgi traffic and microtubule-based transport, and dysfunction of mitochondria (Cooper et al., 2006;Cuervo et al., 2004;Gosavi et al., 2002;Lee et al., 2006;Tanaka et al., 2001). Because α-syn aggregates are detrimental to cells, many studies on PD therapy are focused on identifying elements that affect α-syn’s ability to form aggregates and fibrils (Savitt et al., 2006).
Mutations in LRRK2 have been linked to both familial and sporadic forms of PD (Paisan-Ruiz et al., 2004;Zabetian et al., 2005;Zimprich et al., 2004). LRRK2 protein, also known as Dardarin, contains multiple functional domains and may function as both an active GTPase and kinase (Li et al., 2007;West et al., 2005). The most common mutation of LRRK2 is the G2019S substitution at the conserved Mg++-binding motif within the kinase domain (Gilks et al., 2005;Nichols et al., 2005), which may increase the putative LRRK2 kinase activity (West et al., 2005). PD-related missense mutations are also found within the GTPase domain of LRRK2 (Paisan-Ruiz et al., 2004;Zimprich et al., 2004), which has been shown to physically interact with both α and β tubulin and may regulate the dynamics of microtubules in neurons (Gandhi et al., 2008;Gillardon, 2009). Although mutant LRRK2 is toxic when over-expressed in cultured cells (Greggio et al., 2006;Smith et al., 2006) and Drosophila (Imai et al., 2008;Liu et al., 2008), no apparent neuronal loss is observed in transgenic mice over-expressing PD-related LRRK2 R1441G and R144C mutants (Li et al., 2009;Tong et al., 2009). The pathogenic mechanism of PD-related LRRK2 mutants remains obscure.
To study the function of LRRK2 and how mutant LRRK2 causes neuron degeneration in vivo, we have generated LRRK2 knockout (LRRK2−/−) and transgenic mice expressing human WT, G2019S, or kinase domain-deletion LRRK2. Neither deletion nor over-expression of LRRK2 caused any overt gross neuropathological abnormalities in mutant mice. However, co-expression of WT, G2019S, or kinase domain-deletion LRRK2 with the PD-related A53T α-syn caused synergistic toxicity to neurons that accelerated the progression of α-syn-mediated neuropathology. Conversely, inhibition of LRRK2 expression reduced the aggregation and somatic accumulation of α-syn, and delayed the progression of α-syn-mediated neuropathology. Our findings suggest that LRRK2 may regulates A53T α-synuclein-mediated neuropathology through modulating the intracellular trafficking and accumulation of α-syn.
Results
Generation of LRRK2 and α-synuclein inducible transgenic mice
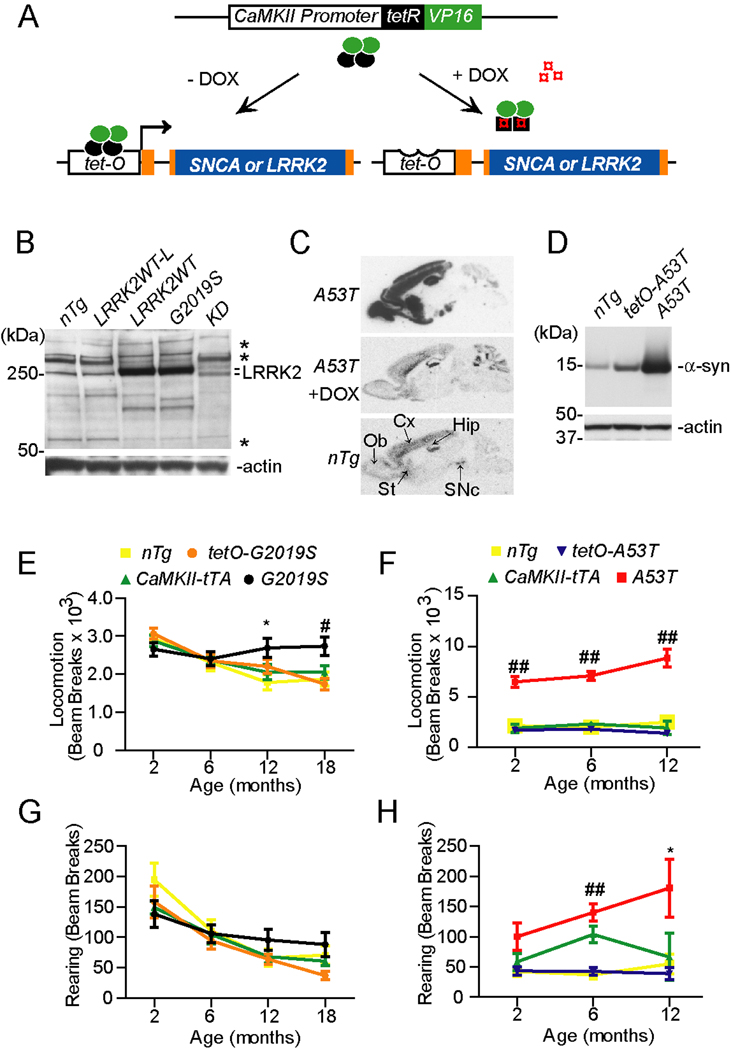
As described previously (Wang et al., 2008), we have generated LRRK2 inducible transgenic mice in which the expression of carboxyl-terminal hemagglutinin (HA)-tagged human wild-type (WT), G2019S, or kinase-deletion (KD) mutant LRRK2 was under the transcriptional control of tetracycline operator (tetO) (Figure 1A). To make the KD expression construct, we deleted residues from 1887 to 2102 of LRRK2, resulting in a complete deletion of its kinase domain. In parallel with the generation of LRRK2 inducible transgenic mice, we also used the same strategy to generate new lines of human α-synuclein (α-syn) A53T inducible transgenic mice (tetO-A53T). Given the expression pattern of LRRK2 (Galter et al., 2006) is similar to that of calcium/calmodulin-dependent protein kinase II-alpha (CaMKII) in the brain, we crossbred tetO-LRRK2 and tetO-A53T mice with a line of CaMKII promoter-controlled tetracycline transactivator (tTA) (CaMKII-tTA) mice (Mayford et al., 1996) to achieve high level expression of LRRK2 or α-syn in the forebrain regions. Accordingly, our previous in situ hybridization experiments showed that the expression of human LRRK2 was mainly detected at the olfactory bulb, cerebral cortex, hippocampus and striatum in the brain of tetO-LRRK2 and CaMKII-tTA double transgenic mice (Wang et al., 2008). For simplicity, we will later refer to the tetO-LRRK2 and CaMKII-tTA double transgenic mice as LRRK2WT, G2019S, and KD transgenic mice, respectively. We generated five independent lines of LRRK2WT, three independent lines of G2019S, and three independent lines of KD transgenic mice. We refer to the E3 line as the G2019S transgenic mice and the C74 line as the LRRK2 WT expression line (LRRK2WT) in later studies. In both G2019S and LRRKWT mice, the expression of LRRK2 protein was increased by about 8 to 16-fold compared to the endogenous one (Figure 1B, Supplemental Figure S1B). Additionally, we designate line C77 as the LRRK2 WT low expression line (LRRK2WT-L) and line D10 as the KD mice. In the brain homogenate of KD mice, a doublet of LRRK2-positive bands was detected in which the lower one likely represents the exogenous human LRRK2 with truncated kinase domain (Figure 1B). The expression level of exogenous human LRRK2 between LRRK2WT-L and KD transgenic mice was comparable, which was about 8 to 16-fold less than that of LRRK2WT mice (Supplemental Figure S1C). Therefore, the expression of LRRK2 was only modestly increased (< 1-fold) in the brain of LRRK2WT-L and KD mice as compared to nTg controls (Figure 1B). Q-RT-PCR was used to quantify the expression of exogenous LRRK2 in the brain of LRRK2WT-L and KD mice. The transcription of exogenous LRRK2 mRNA was about 5 and 15-fold increase in the brain of LRRK2WT-L and KD mice as compared to nTg controls (Supplemental Figure S1D). The rather lower accumulation of exogenous LRRK2 protein in the KD mouse brain may likely reflect the instability of this mutant protein.
Figure 1. Generation and behavioral characterization of LRRK2 andα-syn inducible transgenic mice.
(A) The schematic diagram shows the generation of α-syn and LRRK2 inducible transgenic mice using the “tet off” system.
(B) Western blot analysis shows LRRK2 expression in the brain of nTg, LRRK2WT-L, LRRK2WT, G2019S, and KD transgenic mice using a LRRK2 C-terminal antibody. Asterisks marked non-specific bands. LRRK2 appeared as doublet in KD sample.
(C) The expression pattern of A53T α-syn transgene in the brain by in situ hybridization using a P33-labeled human/mouse α-syn-specific oligo probe (upper panel). The expression of transgenic α-syn was suppressed by administering the animals with doxycycline (DOX)-containing feed for 4 wks (middle panel). The endogenous α-syn was also highly expressed by SNpc DA neurons (bottom panel). Ob: olfactory bulb; Cx: cortex; St: Striatum; Hip: hippocampus; SNpc: substantia nigra pars compacta.
(D) Western blots of α-syn expression in the brain of nTg, tetO-A53T, and A53T transgenic mice using an antibody recognizing both mouse and human α-syn.
(E, G) The nTg (n = 10), tetO-G2019S (n = 10), CaMKII-tTA (n = 14), and G2019S (n = 12) mice were subjected to Open-field tests. The ambulatory (E) and rearing activities (G) were quantified in the Open-field test. *p<0.05, #p<0.005
(F, H) A53T transgenic and control mice were subjected to Open-field test to evaluate their ambulatory (F) and rearing (H) activities. n =10 per genotype. *p<0.05, ##p<0.001
Under the same transcriptional activation of CaMKII promoter-controlled tTA, the expression pattern of human α-syn was the same as that of LRRK2 transgene in the brain (Figure 1C, upper panel). Administration of doxycycline (DOX) suppressed the expression of exogenous α-syn in this “tet-off” system (Figure 1C, middle panel). The expression level of human A53T α-syn protein was increased by about 30-fold as compared to endogenous protein (Figure 1D, Supplemental Figure S1B). Noticeably, under the CaMKII promoter only a small fraction (<5%) of midbrain DA neurons expressed the transgene (Supplemental Figures. S1E–G). Therefore, we focus our studies on the cortex and striatum where the exogenous α-syn and LRRK2 are widely expressed.
G2019S inducible transgenic mice developed similar locomotor abnormalities as A53T transgenic mice
All lines of LRRK2WT, G2019S, and KD transgenic mice were viable and developed normally. G2019S mice appeared to gain less body weight compared to non-transgenic (nTg) and tetO-G2019S single transgenic mice starting at 12 months of age (Supplemental Figure S2A, p < 0.0001). However, no significant difference in body weight was found between G2019S and CaMKII-tTA mice (Supplemental Figure S2A). The motor functions of G2019S mice were examined by Rotarod and Open-field tests. G2019S mice performed normally in the Rotarod test (Supplemental Figure S2C). However, they displayed significantly increased ambulatory activities starting at 12 months of age (Figure 1E, p < 0.05). G2019S mice also showed a trend of increased rearing activities, but it did not reach the statistic significance (Figure 1G). In addition, there were no apparent motor phenotypes detected in LRRK2WT mice up to 6 months of age (Supplemental Figure S2F–H). By contrast, A53T transgenic mice weighted significantly less starting at 4 months of age (Supplemental Figure S2B, p < 0.0005), and began to display drastic increases of ambulatory activities at 2 months of age (Figure 1F, p < 0.0001) and elevated rearing activities at 6 months of age (Figure 1H, p < 0.05). Together, the motor behavioral studies suggest that over-expression of G2019S LRRK2 and A53T α-syn in the forebrain regions may induce a similar damage to the neural circuitry responsible for regulating motor activities.
A53T inducible transgenic mice developed progressive neuropathological abnormalities compared to G2019S transgenic mice
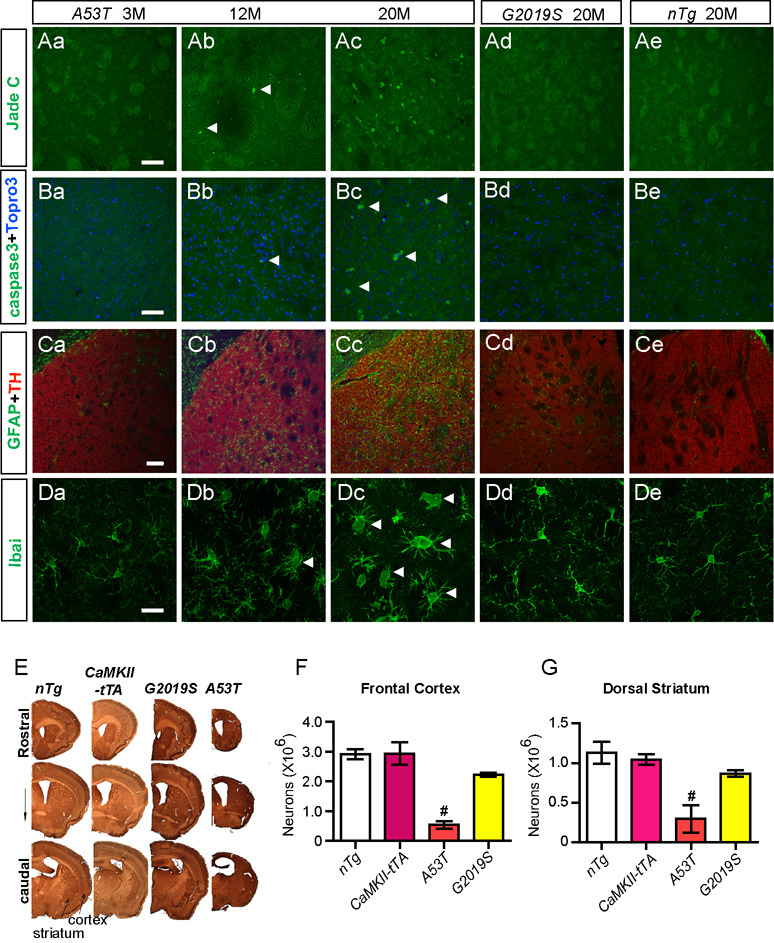
To determine if any neuropathological abnormalities developed in A53T and G2019S transgenic mice, we examined the brain sections of mutant and control animals for neurodegeneration and associated astrocytosis and microgliosis. The neurodegeneration was revealed by Jade C and cleaved-caspase 3 staining. The presence of reactive astrocytes was examined by staining for glial fibrillary acidic protein (GFAP). The morphology of microglia was visualized by staining for ionized calcium binding adaptor molecule-1 (Iba1). Both Jade C and c-caspase 3 staining were negative in the brain sections derived from 20-month old G2019S transgenic mice (Figures 2Ad, 2Bd; Table 1). Consistently, stereological studies revealed no significant changes in neuron counts at both the frontal cortex and dorsal striatum between G2019S and age-matched nTg mice (Figure 2E–G). In addition, no apparent increase of reactive astrocytosis or microglial activation was observed in the striatum and cortex of G2019S transgenic mice as compared to control nTg animals (Figure 2; Table 1 and Table 2). Similarly, no obvious gross neuropathological phenotypes were found in 12-month old LRRK2WT transgenic mice (Table 1, Supplemental Figures S2I–L).
Figure 2. A53T but not G2019S transgenic mice develop progressive neuropathology.
(Aa–Ae) Representative images show Jade C staining (arrowheads) in the striatum of A53T mice at 3 (a), 12 (b), and 20 (c) months of age, and of G2019S (d) and control nTg (e) mice at 20 months of age. Scale bar: 50 µm
(Ba–Be) Representative images show cleaved-caspase 3 (caspase3) staining (white arrowheads) in the striatum of A53T mice at 3 (a), 12 (b), and 20 (c) months of age, and of G2019S (d) and control nTg (e) mice at 20 months of age. Nuclei were stained with Topro 3 (blue). Scale bar: 50 µm
(Ca–Ce) Representative images reveal GFAP staining (green) in the striatum of A53T mice at 3 (a), 12 (b), and 20 (c) months of age, and of G2019S (d) and control nTg (e) mice at 20 months of age. The section was counter-stained with an antibody against tyrosine hydroxylase (TH) (red). Scale bar: 100 µm
(Da–De) Representative images show Iba1 staining (green) in the striatum of A53T mice at 3 (a), 12 (b), and 20 (c) months of age, and of G2019S (d) and control nTg (e) mice at 20 months of age. Scale bar: 20 µm
(E) Representative images display coronal sections co-stained with NeuN and TH across the striatum of 20-month old nTg, CaMKII-tTA, G2019S, and A53T mice.
(F–G) Bar graphs depict the numbers of neurons remained the frontal cortex (F) and dorsal striatum (G) of 20-month old nTg, CaMKII-tTA, G2019S, and A53T mice. #p < 0.005 vs. nTg
Table 1.
Neuronal degeneration in the striatum and cortex ofA53T and LRRK2 mutant mice
| Age | Genotype |
# of c. caspase 3+ Striatum |
Cortex |
# of Jade C+ Striatum |
Cortex |
|---|---|---|---|---|---|
| 1M | nTg | 0 | 0 | 0 | 0 |
| tTA | 0 | 0 | 0 | 0 | |
| A53T | 0 | 0 | 0 | 0 | |
| LRRK2WT-L | 0 | 0 | 0 | 0 | |
| LRRK2WT | 0 | 0 | 0 | 0 | |
| KD | 0 | 0 | 0 | 0 | |
| G2019S | 0 | 0 | 0 | 0 | |
| A53T/LRRK2WT-L | 1.4±0.2 | 2.4±0.2 | 3.4±0.5 | 5.2±0.5f | |
| A53T/LRRK2WT | 3.0±0.7 | 8.0±1.1 | 12.0±0.9b | 15.8±1.1b | |
| A53T/G2019S | 13.0±1.5b,c | 17.3±0.3 | 26.0±1.2b | 28.7±1.9b | |
| A53T/KD | 1.3±0.3 | 3.0±0.1 | 2.7±0.3 | 8.0±1.3e | |
| 12M | nTg (DOX) | 0 | 0 | 0 | 0 |
| A53T | 5.0±0.6 | 6.3±0.5 | 12.0±0.6 | 13.3±1.5 | |
| A53T/LRR2−/− | 0 | 0 | 0 | 0 | |
| A53T (DOX) | 0 | 0 | 0 | 0 | |
| LRRK2WT | 0 | 0 | 0 | 0 | |
| 20M | nTg* | 0 | 0 | 0 | 0 |
| A53T | 24.7±4.1 | 32±4.4 | 33.3±4.1 | 40.3±4.3 | |
| G2019S | 0 | 0 | 0 | 0 | |
| LRRK2−/− | 0 | 0 | 0 | 0 | |
The average numbers of c. caspase 3+ and Jade C+ cells in the striatum and cortex of each cohort of mice were counted by the event measurement tool of AxioVision (Zeiss). Two matched sections were analyzed for each mouse and three or more animals per genotype were used for each age group. The randomly selected sampling area was ~140, 000 µm2 per brain region for each animal. nTg* represents nTg mice from both transgenic and LRRK2−/− cohorts of mice. At 1 month of age, significantly more cleaved caspase 3 positive (c. caspase3+) neurons were revealed in the striatum and cortex of A53T/G2019S mice as compared to that of A53T (b, p < 0.0005) and A53T/LRRK2WT mice (c, p < 0.005). Additionally, more Jade C positive (Jade C+) cells were found in the striatum and cortex of A53T/LRRK2WT-L, A53T/LRRK2WT, A53T/KD and A53T/G2019S mice as compared to that of A53T mice (b, p < 0.0005; e, p < 0.01; f, p < 0.05). More Jade C+ cells were also detected in the striatum and cortex of A53T/G2019S mice as compared to that of A53T/LRRK2WT mice (b, p < 0.0005).
Table 2.
Activation of microglia in the striatum of A53T and LRRK2 mutant mice
| Age | Genotype | Ratio (%) | Size (µm2) |
|---|---|---|---|
| 1M | nTg | 0 | 31.77±0.46 |
| tTA | 0 | 28.95±1.63 | |
| A53T | 0 | 36.83±0.86 | |
| G2019S | 0 | 31.69±0.76 | |
| A53T/LRRK2WT-L | 5.88 | 44.40±3.73 | |
| A53T/LRRK2WT | 31.64 | 52.01±1.60a | |
| A53T/G2019S | 83.10 | 79.55±7.01b | |
| A53T/KD | 11.11 | 46.66±0.05 | |
| 12M | nTg (DOX) | 0 | 38.52±0.01c |
| A53T | 38.71 | 60.97±2.46 | |
| A53T/LRRK2−/− | 0 | 29.93±2.90d | |
| A53T (DOX) | 0 | 36.91±0.06c | |
| 20M | nTg* | 0 | 31.44±1.17 |
| A53T | 79.63 | 102.07±10.88e,f | |
| G2019S | 0 | 33.33±0.63 | |
| LRRK2−/− | 11.11 | 45.00±2.32f | |
The numbers of total and activated Iba1+ cells in the striatum were counted by the event measurement tool of AxioVision. The ratio of activated Iba1+ cells were calculated by dividing the number of enlarged Iba1+ cells with the total number of Iba1+ cells. The size of Iba1+ cell bodies were assessed by the outline function of AxioVision. Two matched sections were analyzed for each mouse and three or more animals per genotype were used for each age group. The area of sampled striatum was ~140, 000 µm2 for each animal. At 1 month of age, enlarged Iba1+ cells were only detected in A53T and LRRK2 double transgenic mice. While the average size of Iba1+ cell bodies appears larger in A53T and LRRK2 double transgenic mice, it reached statistically significance in only A53T/LRRK2WT and A53T/G2019S mice as compared to A53T mice (a, p < 0.02; b, p < 0.0005). At 12 months of age, more enlarged Iba1+ cells were detected in A53T with increasing size of cell bodies. In contrast, no apparent enlargement of Iba1+ cells was found in nTg, A53T/LRKK2−/−, or A53T (DOX) mice as compared to A53T mice (c, p < 0.005; d, p < 0.002). At 20 months of age, more and larger enlarged Iba1+ cells were found in A53T mice as compared to nTg and G2019S mice (e, p < 0.01), and 12-month old A53T mice (f, p < 0.05). Interestingly, more enlarged Iba1+ cells were detected in LRRK2−/− mice as compared to nTg mice (f, p < 0.05). nTg* represents nTg mice from both transgenic and LRRK2−/− cohorts of mice.
No apparent neuropathological abnormalities were detected in the striatum of 3-month old A53T mice (Figures 2Aa–2Da). Such neuropathology became obvious when A53T transgenic mice were examined at 12 and 20 months of age (Figure 2; Table 1 and Table 2). Widespread neurodegeneration was evident in the brains of 20-month old A53T transgenic mice (Figures 2Ac–2Bc, 2E). Massive neuronal loss in the frontal cortex (>80%) and dorsal striatum (>74%) of A53T mice was estimated by unbiased stereological approaches (Figures 2F–G). Therefore, A53T mice developed age-dependent, progressive neruodegeneration; whereas, G2019S mice did not show any obvious gross neuropathological phenotypes.
LRRK2 accelerated the progression of A53T α-syn-mediated neuropathology
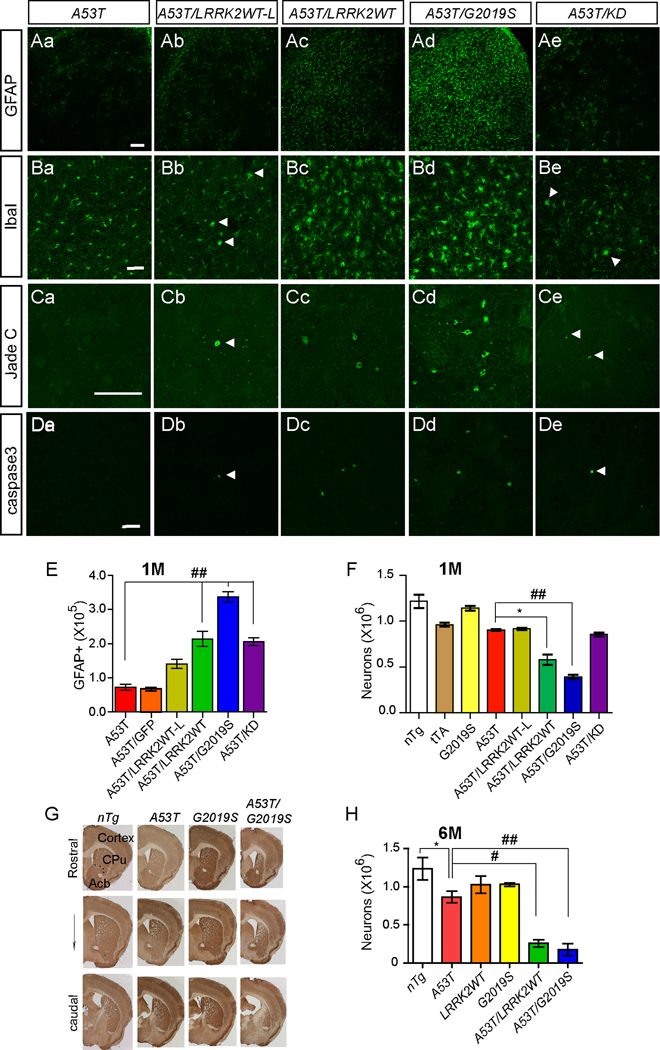
To test whether over-expression of LRRK2 affects A53T α-syn-mediated neurodegeneration, we generated A53T/LRRK2WT-L, A53T/LRRK2WT, A53T/G2019S and A53T/KD double transgenic mice and examined the progression of neuropathological alterations (Figure 3). Compared to 1-month old A53T single transgenic mice, the number of GFAP-positive astrocytes was significantly elevated in the striatum of age-matched A53T/LRRK2WT-L and A53T/LRRK2WT double transgenic mice (Figures 3A, 3E). In addition, more GFAP-positive astrocytes were found in the brain of A53T/LRRK2WT mice than A53T/LRRK2WT-L mice (Figures 3A, 3E), suggesting that the expression level of LRRK2 influences the progression of α-syn-mediated neuropathology. A similar increase of microglial activation was also observed in A53T/LRRK2WT-L and A53T/LRRK2WT double transgenic mice (Figures 3B, Table 2). Besides gliosis, neurodegeneration was also accelerated in the striatum of A53T/LRRK2WT-L and A53T/LRRK2WT transgenic mice (Figure 3, Table 1). Co-staining of caspase 3 with Ctip2, a specific marker for striatal medium-size spiny neurons (MSN) (Arlotta et al., 2008), revealed that most of these degenerating neurons were MSNs (Supplemental Figure S3). The prevalence of neurodegeneration in A53T/LRRK2WT double transgenic mice was also elevated in a LRRK2 dose-dependent fashion (Figure 3F, Table 1). Taken together, these findings demonstrate that over-expression of LRRK2 accelerates the progression of α-syn-mediated neuropathology.
Figure 3. LRRK2 accelerates the progression of A53 α-syn-mediated neuropathology.
(Aa–Ae) Representative images show GFAP staining in the striatum of 1-month old A53T (a), A53T/LRRK2WT-L (b), A53T/LRRK2WT (c), A53T/G2019S (d), and A53T/KD (e) mice. Scale bar: 100 µm
(Ba–Be) Representative images show Iba1 staining in the striatum of 1-month old A53T (a), A53T/LRRK2WT-L (b), A53T/LRRK2WT (c), A53T/G2019S (d), and A53T/KD (e) mice. Scale bar: 50 µm
(Ca–Ce) Representative images show Jade C staining in the striatum of 1-month old A53T (a), A53T/LRRK2WT-L (b), A53T/LRRK2WT (c), A53T/G2019S (d), and A53T/KD (e) mice. Scale bar: 20 µm
(Da–De) Representative images show caspase3 staining in the striatum of 1-month old A53T (a), A53T/LRRK2WT-L (b), A53T/LRRK2WT (c), A53T/G2019S (d), and A53T/KD (e) mice. Scale bar: 50 µm
(E–F) Bar graphs reveal the numbers of GFAP-positive (GFAP+, E) and NeuN-positive cells (F) in the dorsal striatum estimated by unbiased stereological methods. *p < 0.05, ##p < 0.001
(G) Representative images show coronal sections across the striatum of 6-month old nTg, A53T, G2019S, A53T/G2019S mice. The section was co-stained with NeuN and TH.
(H) Bar graph depicts the numbers of neurons remained the dorsal striatum (G) of 6-month old nTg, A53T, LRRK2WT, G2019S, A53T/LRRK2WT and A53T/G2019S mice. *p < 0.05, #p < 0.005, ##p < 0.001
Since the G2019S mutation in LRRK2 causes late onset PD, we decided to investigate whether the G2019S mutation would further enhance the neuropathology in A53T/G2019S mice as compared to A53T/LRRK2WT double transgenic mice. A significant exacerbation of astrocytosis, microgliosis, and neurodegeneration was observed in the striatum of A53T/G2019S mice as compared with age-matched A53T single transgenic animals (Figure 3; Table 1 and Table 2). When we compared the pathological phenotypes of 1-month old A53T/LRRK2WT and A53T/G2019S mice, we found the number of Jade C-positive and GFAP-positive cells, and the activation of microglia were significantly increased in the striatum of A53T/G2019S mice as compared to A53T/LRRK2WT animals (p < 0.0001, 0.005, and 0.002, respectively; Figure 3E; Table 1 and Table 2). However, when we compared the numbers of neurons remained in the striatum of A53T/LRRK2WT and A53T/G2019S mice, the difference was not statistically significant (p = 0.14, Figure 3F). To further investigate whether the kinase domain of LRRK2 is critical in regulating α-syn A53T-mediated neuropathology, we compared the progression of neuropathology between A53T/KD and A53T/LRRK2WT-L transgenic mice. As shown in Figure 3, the presence of kinase-deletion LRRK2 also accelerated A53T-mediated neuropathology to a similar extent as LRRK2WT-L (Figure 3 and Table 1–Table 2). These data suggest that the kinase domain of LRRK2 perhaps is not critical in modulating A53T-induced neuropathological abnormalities.
We further examined the neurodegeneration of A53T, A53T/LRRK2WT, and A53T/G2019S mice at 6 months of age (Figures 3G–H). While no obvious neuronal loss was observed in both LRRK2WT and G2019S mice, a significant reduction (30%) of striatal neurons was found in A53T single transgenic mice (Figures 3G–H, p < 0.02). More dramatic neuronal loss was detected in the dorsal striatum of A53T/LRRK2WT (80%) and A53T/G2019S (85%) mice (Figures 3G–H, p < 0.001 and 0.0001). However, the numbers of residual neurons in the dorsal striatum of A53T/LRRK2WT and A53T/G2019S mice were not statistically different (p = 0.45, Figure 3H). These observations from adult mice further support our earlier findings that the presence of excess WT and G2019S LRRK2 exacerbates A53T-mediated neurodegeneration and the expression level of LRRK2 rather the PD-related G2019S mutation perhaps is more important in accelerating A53T α-syn-mediated pathogenesis.
With the concern whether over-expression of any exogenous protein could accelerate α-syn-mediated neuropathology, we crossbred A53T mice with a line of green fluorescent protein (GFP) transgenic mice that selectively express GFP in striatal neurons (Gong et al., 2003). We did not observe any significant influence of GFP on the progression of neuropathology in A53T/GFP double transgenic mice (Figure 3E, Supplemental Figure S4). In addition, to test if LRRK2 selectively potentiates A53T-mediated neuropathology, we crossbred G2019S mice with amyloid precursor protein (APP) inducible transgenic mice in which an Alzheimer’s disease-related double mutant version of chimeric mouse/human APP is over-expressed 10 to 30-fold compared to the endogenous APP (Jankowsky et al., 2005). We found that LRRK2 did not accelerate APP-mediated astrocytosis and microgliosis in APP/G2019S double transgenic mice (Supplemental Figure S5). Together, these observations support a specific effect of LRRK2 on the pathogenesis of α-syn A53T mutation.
LRRK2 promoted the abnormal accumulation of α-syn in cell bodies
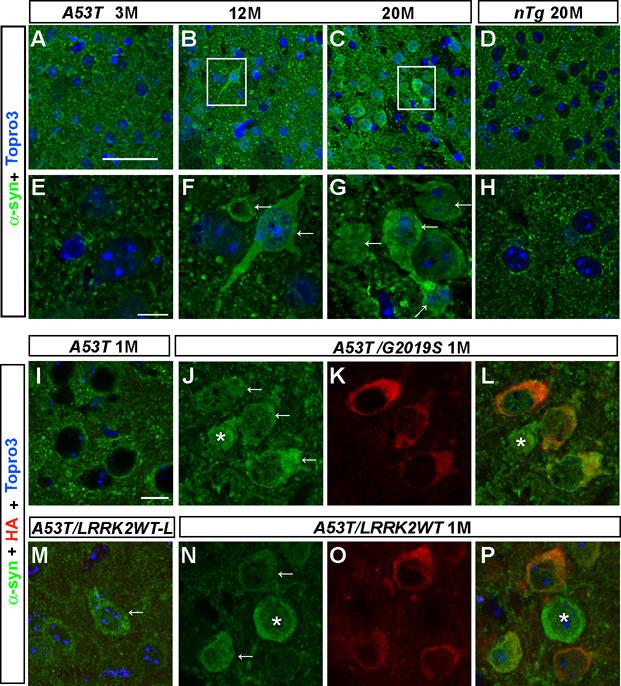
The α-syn staining is normally confined to axon terminals (Maroteaux et al., 1988), which was shown here as small puncta in the coronal striatal sections of 3-month old A53T mice and 20-month old nTg mice (Figure 4). Interestingly, a few neurons with strong α-syn staining in the cell body were detected in the brain sections of 12-month old A53T mice (Figures 4B, 4F). The occurrence of this abnormal somatic accumulation of α-syn was more prominent in the brain sections of 20-month old A53T mice (Figures 4C, 4G; Table 3), which was closely correlated with the progression of neurodegeneration in A53T mice, suggesting that the somatic accumulation of α-syn may trigger the pathogenic cascades leading to the cell death. We then investigated whether over-expression of LRRK2 affects the subcellular distribution of α-syn. No obvious α-syn staining was found in the cell body of striatal neurons in 1 month-old A53T mice (Figure 4I). However, co-expression of LRRK2 G2019S mutation greatly promoted the accumulation of α-syn in the soma of A53T/G2019S neurons in 1-month old mice (Figures 4J–L; Table 3). Similarly, increasing numbers of neurons with somatic accumulation of α-syn were detected in brain sections of 1-month old A53T/LRRK2WT-L, A53T/LRRK2WT and A53T/KD transgenic mice in a LRRK2 dose-dependent manner (Figure 4M–4P; Table 3). To investigate whether over-expression of LRRK2 G2019S mutation also promotes the accumulation of WT α-syn in the cell bodies, we examined the α-syn staining in brain sections from 20-month old G2019S mice and 1-month old human α-syn WT and G2019S double transgenic mice (α-synWT/G2019S). No apparent α-syn staining was detected in the soma of neurons in G2019S and α-synWT single transgenic mice (Supplemental Figure S6). By contrast, somatic staining of α-syn was apparent in neurons of 1-month old α-synWT/G2019S double transgenic mice (Supplemental Figure S6). These results suggest that the LRRK2-induced somatic accumulation of α-syn is independent of the presence of PD-related α-syn mutation but relies on the expression level of α-syn.
Figure 4. LRRK2 accelerates somatic accumulation of A53T α-syn in neurons.
(A–H) Representative images show α-syn staining (green) in striatal neurons of A53T mice at 3 (A), 12 (B), and 20 months of age (C), and nTg mice at 20 months of age (D). F and G represent enlarged images with the white boxes in B and C. Nuclei were stained with Topro 3 (blue). Scale bars: 50 µm (A–D); 10 µm (E–H).
(I–P) Representative images reveal α-syn staining (green) in striatal neurons of 1-month old A53T (I), A53T/G2019S (J, L), A53T/LRRK2WT-L (M), and A53T/LRRK2WT (N, P) mice. Human LRRK2 was stained with an anti-HA antibody (red, K–L, O–P). Nuclei were stained with Topro 3 (blue). Scale bar: 10 µm.
Table 3.
The prevalence of somatic accumulation of α-syn in the striatal neuron of A53T transgenic mice
| Age | Genotype | Ratio (%) |
|---|---|---|
| 1M | nTg | 0 |
| tTA | 0 | |
| A53T | 1.00 | |
| G2019S | 0 | |
| A53T/LRRK2WT-L | 6.19 | |
| A53T/LRRK2WT | 17.94d | |
| A53T/G2019S | 30.36b | |
| A53T/KD | 10.59a | |
| 12M | nTg | 0 |
| A53T | 23.48 | |
| A53T/LRRK2−/− | 2.13f | |
| A53T (DOX) | 0.69a | |
| 20M | nTg* | 0 |
| A53T | 76.99 | |
| G2019S | 0 | |
| LRRK2−/− | 0 | |
The ratio of striatal neurons displaying apparent somatic accumulation of α-syn was calculated from each cohort of mice. Two matched sections were analyzed for each mouse and three or more animals per genotype were used for each age group. The area of sampled striatum was ~140, 000 µm2 for each animal. nTg* represents nTg mice from both transgenic and LRRK2−/− cohorts of mice. At 1 month of age, significantly more neurons with apparent somatic accumulation of α-syn were detected in the striatum of A53T/LRRK2WT, A53T/G2019S, and A53T/KD mice as compared to A53T mice (a, p < 0.02; b, p < 0.0005; and d, p < 0.001). At 12 months of age, less neurons with somatic accumulation of α-syn was found in the striatum of A53T/LRKK2−/− and A53T (DOX) mice as compared to A53T mice (a, p < 0.02; f, p < 0.05).
The staining of LRRK2 seemed to partially overlap with that of α-syn in the soma (Figures 4L, 4P). However, co-immunoprecipitation experiments failed to pull down LRRK2 and α-syn together from brain homogenates of A53T/G2019S and A53T/LRRK2WT mice (unpublished data). In addition, a few neurons, which showed obvious α-syn staining in the soma, were lack of substantial expression of LRRK2 transgene (asterisk, Figures 4L, 4P). These observations suggest that LRRK2 may not directly bind to α-syn and prevent its trafficking to the axon terminals.
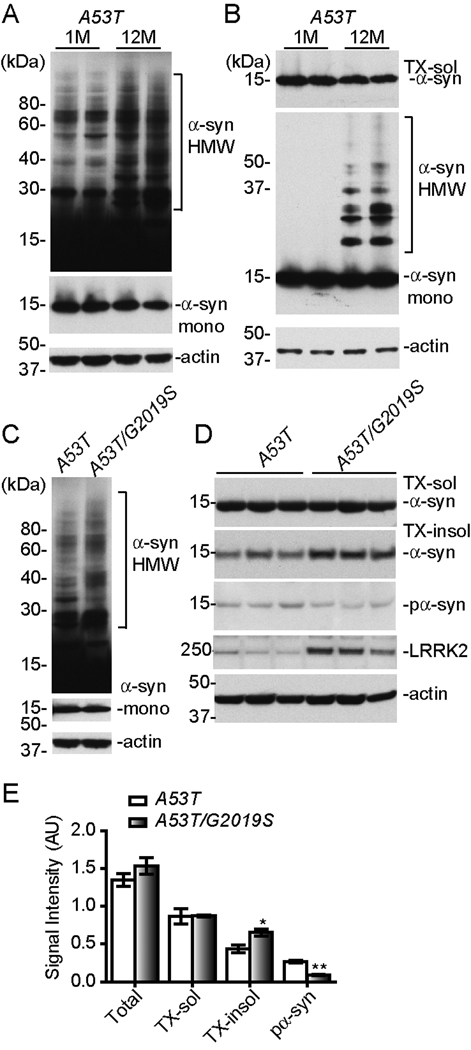
LRRK2 promoted the formation of α-syn aggregates
To examine if increased somatic accumulation of α-syn correlates with the formation of α-syn aggregates, we compared the levels of α-syn protein in total and sequentially detergent-extracted fractions of brain homogenates from 1 and 12-month old A53T transgenic mice. The formation of α-syn aggregates was detected as α-syn-positive high molecular weight (HMW) bands in the total brain homogenates of A53T mice (Figure 5). Interestingly, the intensity of HMW bands was significantly enhanced in the samples of 12-month old A53T mice as compared to 1-month old animals (Figure 5A). We then examined the presence of α-syn aggregates in the Triton X100-insoluble (TX-insol) fractions of brain extracts. While the level of Triton X100-soluble (TX-sol) α-syn was comparable between these two age groups, a significant increase of TX-insol HMW α-syn was found in the samples of 12-month old A53T mice (Figure 5B). These data indicate the accumulation of α-syn in the soma may favor the formation of α-syn aggregates or vise versa.
Figure 5. LRRK2 promotes the formation of α-syn Aggregates.
(A–B) Western blots show high molecular weight (HMW) bands of α-syn in the total (A) and sequentially detergent-extracted (B) brain homogenates of A53T transgenic mice at 1 and 12 months of age. The middle panel in (A) shows the monomeric α-syn (α-syn mono) under lower exposure.
(C) Western blots show α-syn-positive HMW bands in the total brain homogenates of A53T single and A53T/G2019S double transgenic mice at 1 month of age. The middle panel in (C) shows the monomeric α-syn with shorter exposure.
(D) Western blots show phosphorylated α-syn (pα-syn) in the total and monomeric α-syn in sequentially detergent-extracted fractions of brain homogenates of A53T single and A53T/G2019S double transgenic mice at 1 month of age.
(E–F) Bar graphs compare the levels of α-syn and pα-syn in different fractions of brain homogenates (E) of A53T and A53T/G2019S transgenic mice at 1 month of age. *p<0.05, **p<0.01
Since over-expression of LRRK2 promoted the somatic accumulation of α-syn, we reasoned that LRRK2 may also promote the aggregation of α-syn. We therefore compared the level of HMW α-syn in the total brain lysates of 1-month old A53T and age-matched A53T/G2019S transgenic mice. A modest increase of HMW α-syn was detected in the A53T/G2019S samples (Figure 5C). Similarly, significantly more α-syn was detected in the TX-insoluble fraction of brain homogenates from A53T/G2019S transgenic mice (Figures 5D–E, p = 0.033). Together, these observations indicate that G2019S LRRK2 promotes the formation of α-syn aggregates in neurons.
To test if α-syn is a potential substrate of LRRK2’s kinase activity, we examined the phosphorylation of α-syn in total brain homogenates derived from one-month old A53T and A53T/G2019S transgenic mice (Figures 5D–E). Unexpectedly, we observed a reduction of α-syn phosphorylation at serine 129 in the brain lysate of double transgenic mice compared with that of A53T single transgenic animals (p = 0.001). This observation indicates that α-syn is unlikely to be a physiological substrate of LRRK2’s kinase activity in vivo. Secreted α-syn is also implied in the pathogenesis of PD (Lee et al., 2005). To investigate if LRRK2 affects the secretion of α-syn, we measured the level of α-syn in cerebral spinal fluid (CSF) by ELISA. We found no significant alteration of secreted α-syn in A53T/G2019S double transgenic mice as compared to A53T single transgenic mice (data not shown).
Co-expression of LRRK2 and A53T led to severe fragmentation of Golgi complex in neurons
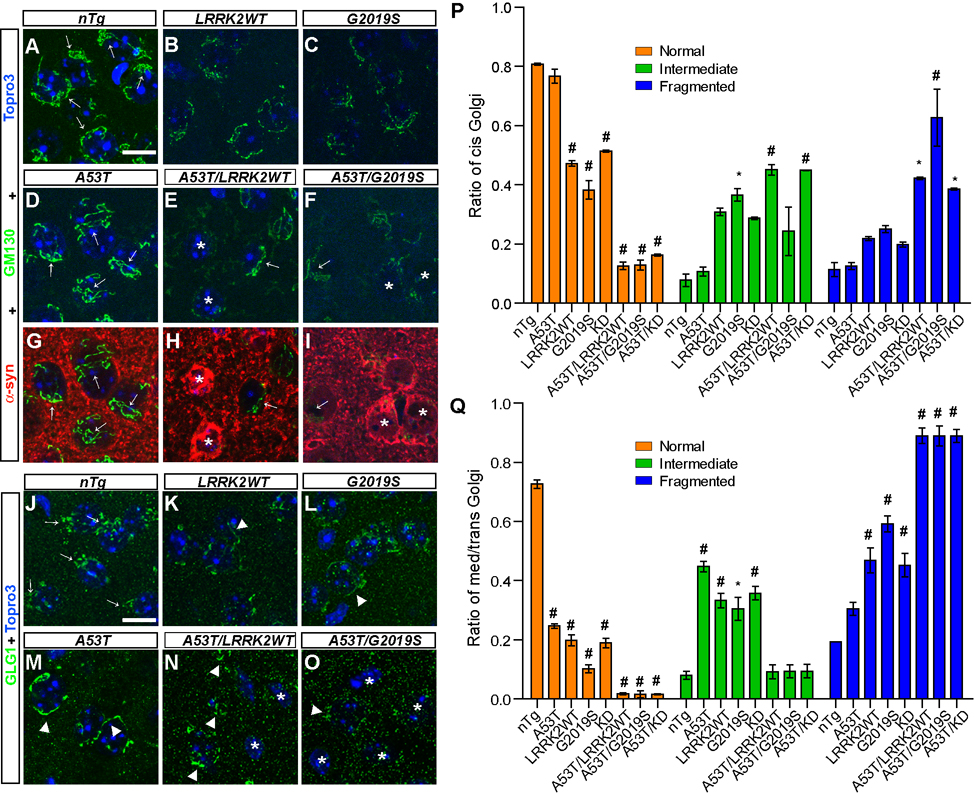
Recent studies indicate that over-expression of either WT or PD-related mutant α-syn disrupts ER-Golgi traffic and causes Golgi fragmentation (Cooper et al., 2006;Gosavi et al., 2002). Interestingly, LRRK2 is associated with Golgi apparatus (Biskup et al., 2006). We therefore decided to examine the ER and Golgi structures in neurons of A53T and LRRK2 single and double transgenic mice. There was no apparent change of ER structure in these neurons as revealed by calnexin staining (data not shown). However, the structure of Golgi complex was drastically altered in neurons of 1-month old A53T/LRRK2 double transgenic mice (Figure 6). The morphology of Golgi apparatus was examined by GM130 and GLG1 staining, which recognizes cis and medial/trans-Golgi apparatus respectively. GM130 staining revealed tubular structures largely stacked at one side of neurons in 1-month old nTg and A53T mice (arrows, Figures 6A, 6D), which was classified as “Normal” Golgi. The GM130-positive tubules, however, appeared thinner and fragmented in neurons of LRRK2WT and G2019S mice (Figures 6B–C). When A53T was co-expressed with either WT or G2019S LRRK2, severe fragmentation of cis-Golgi was found (asterisk, Figures 6E–F), which was designed as “Fragmented” Golgi thereafter; and the partially fragmented Golgi was referred as “Intermediate” Golgi. Interestingly, the degree of Golgi apparatus fragmentation was correlated with the somatic accumulation of α-syn in these neurons (asterisks, Figures 6H–I). By contrast, neurons that lacked substantial α-syn staining in the soma displayed fairly normal appearance of cis-Golgi network (arrows, Figures 6E–F, Figures 6H–I). The prevalence of cis-Golgi fragmentation was quantified, which showed a significant decrease of “Normal” Golgi in LRRK2 single and A53T/LRRK2 double transgenic neurons as compared to nTg controls (Figure 6P). Meanwhile, the ratio of “Fragmented” Golgi was significantly elevated in neurons of A53T/LRRK2 double transgenic mice (Figure 6P). We also examined the integrity of cis-Golgi network in striatal neurons of 6-month old animals and observed a similar fragmentation of GM-130-posotive Golgi apparatus in LRRK2WT, G2019S, and A53T/LRRK2 double transgenic neurons (Supplemental Figure S7). Interestingly, a significant increase of Golgi fragmentation was observed in neurons of 6-month old A53T mice (Supplemental Figure S7).
Figure 6. α-syn and LRRK2 cause synergistic damage to Golgi apparatus.
(A–I) Representative images show GM130 staining (arrows, green) in the striatum of nTg (A), LRRK2WT (B), G2019S (C), A53T (D, G), A53T/LRRK2WT (E, H) and A53T/G2019S (F, I) mice at 1 month of age. Co-staining of GM130 (green) and α-syn (red) was shown in the striatum of A53T (G), A53T/LRRK2WT (H) and A53T/G2019S (I) mice. Neurons displaying somatic accumulation of α-syn were marked with asterisks. Normal GM130 staining was pointed by arrows in neurons. Nuclei were labeled by Topro 3 staining (blue). Scale bar: 10 µm
(J–O) Representative images show GLG1 staining (green) in the striatum of nTg (J), LRRK2WT (K), G2019S (L), A53T (M), A53T/LRRK2WT (N) and A53T/G2019S (O) mice at 1 month of age. Normal GLG1 staining was pointed by arrows in neurons; and abnormal tubular and fragmented GLG1 staining was marked by arrowheads. Neurons with complete fragmentation of Golgi were marked with asterisk. Nuclei were labeled by Topro 3 staining (blue). Scale bar: 10 µm.
(P–Q) Bar graphs quantify the morphological changes of cis- (P) and trans- (Q) Golgi in neurons (> 300 neurons and ≥ 3 mice per genotype). *p < 0.05, #p < 0.0005
We then checked the structural integrity of medial/trans- Golgi in neurons of A53T and LRRK2 mutant mice. In control nTg mice, GLG1 staining revealed the “Normal” perinuclear and polarized localization of Golgi stacks in neurons (arrows, Figure 6J). However, GLG1-staining was significantly altered in LRRK2WT, G2019, and A53T neurons and was shown as tubular structures scattered around the nucleus (arrowheads, Figures 6K–M). In A53T/LRRK2WT and A53T/G2019S neurons, with a few exceptions (arrowheads, Figures 6N–O,), the GLG1-staining was dispersed as small puncta or became indistinguishable from the background staining (asterisks, Figures 6N–O). We classified these dispersed structures as “Fragmented” Golgi. The ratio of “Fragmented” trans-Golgi was calculated, which was significantly increased in A53T and LRRK2 single and double transgenic neurons as compared to nTg controls (Figure 6Q). Increased fragmentation of Golgi apparatus was also observed in neurons of KD and A53T/KD mice (Figures 6P–Q). Together these observations demonstrate that over-expression of LRRK2 and α-syn severely disrupted the structure of Golgi apparatus, which may impair the ER/Golgi trafficking and contribute to the somatic accumulation of α-syn.
Over-expression of LRRK2 perturbed the dynamics of microtubule assembly in LRRK2 and A53T/LRRK2 transgenic mice
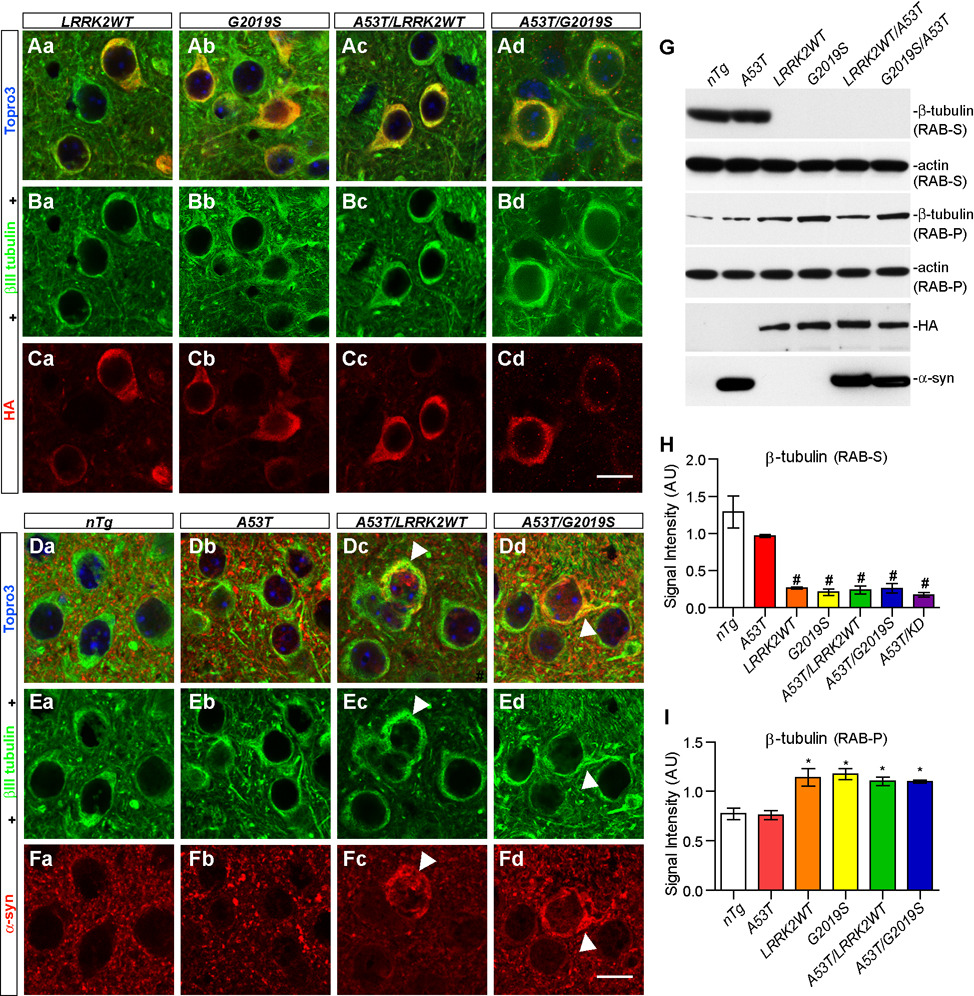
Microtubule and microtubule-based intracellular transport play a critical role in maintaining the structure and function of Golgi apparatus (Thyberg and Moskalewski, 1999). LRRK2 has been shown to physically interact with both α and β tubulin through its GTPase domain (Gandhi et al., 2008;Gillardon, 2009). Consistently, we found that both WT and G2019S LRRK2 co-stained with βIII tubulin in the soma and proximal processes of striatal neurons in 1 and 6-month old LRRK2 single and A53T/LRRK2 double transgenic mice (Figure 7; Supplemental Figure S8). A significant overlap of somatic α-syn and βIII tubulin staining was also observed in neurons of 6-month old A53T single transgenic mice (Supplemental Figure S8) as well as in neurons of 1 and 6-month old A53T/LRRK2WT and A53T/G2019S double transgenic mice (Figures 7Dc–Dd, Supplemental Figure S8). These observations were consistent with the previous report that prefibrillar cytoplasmic α-syn interacts with tubulin in cell cultures (Lee et al., 2006).
Figure 7. Over-expression of LRRK2 impairs the dynamics of microtubules.
(A–C) Representative images show βIII tubulin (green) and HA (red) staining in the striatum of LRRK2WT (Aa, Ba, and Ca), G2019S (Ab, Bb, and Cb), A53T/LRRK2WT (Ac, Bc, and Cc) and A53T/G2019S (Ad, Bd, and Cd) mice at 1 month of age. Nuclei were labeled by Topro 3 staining (blue). Scale bar: 10 µm.
(D–F) Representative images show βIII tubulin (green) and α-syn (red) staining in the striatum of nTg (Da, Ea, and Fa), A53T (Db, Eb, and Fb), A53T/LRRK2WT (Dc, Ec, and Fc) and A53T/G2019S (Dd, Ed, and Fd) mice at 1 month of age. The abnormal somatic accumulation of α-syn and βIII tubulin was marked by arrowheads. Nuclei were labeled by Topro 3 staining (blue). Scale bar: 10 µm.
(G) Western blots of β-tubulin in RAB buffer-soluble supernatant (RAB-S) and insoluble pellet (RAB-P) fractions of brain homogenates from various transgenic mice at 1 month of age and age-matched nTg controls.
(H–I) Bar graphs quantify the levels of β- tubulin in RAB-S (H) and RAB-P (I) fractions of brain homogenates of transgenic mice at 1 month of age and age-matched nTg controls. *p < 0.05, ##p < 0.001
To reveal the functional impact of LRRK2 over-expression on the dynamics of microtubule organization, we compared the level of β-tubulin in both the Reassembly High-salt Buffer (RAB)-soluble and insoluble fractions of mouse brain homogenates from 1-month old A53T, LRRK2WT and G2019S single, and A53T/LRRK2 double transgenic animals as well as nTg controls (Figures 7G–I). RAB buffer is generally used to extract intracellular free tubulin and cold-labile microtubules from brain homogenates (Weingarten et al., 1975). A dramatic reduction of β-tubulin was observed in the RAB-soluble (RAB-S) fraction of brain homogenates in LRRK2WT, G2019S, and A53T/LRRK2 transgenic mice as compared to nTg controls and A53T single transgenic mice (Figures 7G–H). Concomitantly, more β-tubulin was found in the RAB-insoluble pellets (RAB-P) of LRRK2 over-expressing mouse brains as compared to nTg and A53T samples (Figures 7G, 7I). The total level of β-tubulin, meanwhile, was comparable among all different genotypes (Supplemental Figure S9). The distribution of α-tubulin followed the same pattern as β-tubulin in the RAB-extracted brain homogenates from LRRK2 transgenic mice (data not shown). Taken together, these findings are consistent with previous in vitro assays that over-expression of LRRK2 may enhance the polymerization of tubulin in cells (Gillardon, 2009), suggesting that the impairment of microtubule assembly may affect the organization of microtubule network in the cell, resulting in the fragmentation of Golgi apparatus.
Over-expression of LRRK2 and A53T impaired ubiquitin-proteasome system in both A53T and LRRK2 transgenic mice
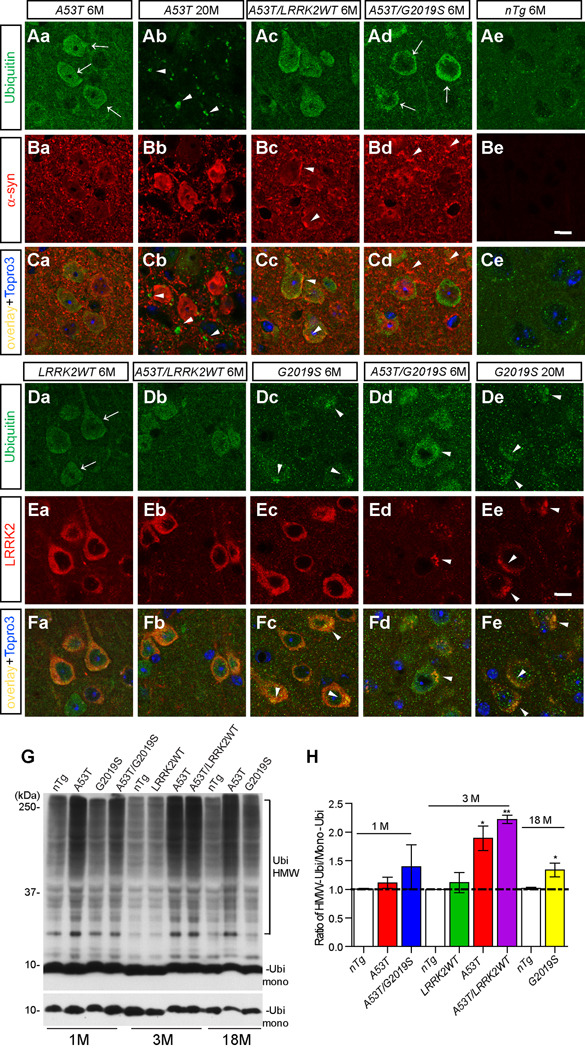
α-syn aggregates have been shown previously to impair the proteasomal and lysosomal activities that leads to accumulation of ubiquinated proteins in cells (Cuervo et al., 2004;Tanaka et al., 2001). Consistently, an elevation of ubiquitin (Ubi) staining was observed in the soma and nucleus of cortical neurons from 6-month old A53T mice (arrows, Figure 8Aa) but not from nTg controls (Figure 8Ae). The Ubi staining was partially overlapped with α-syn (Figure 8Ca). The pattern of Ubi staining was altered in 20-month old A53T mice, which displayed a punctuated staining pattern at neuron processes with no apparent co-localization with α-syn staining (arrowheads, Figures 8Ab, 8Cb). In the presence of excess WT LRRK2, a slightly more α-syn and Ubi-positive clusters were observed in brain sections of 6-month old A53T/LRRK2WT mice (arrowheads, Figure 8Bc). Interestingly, the presence of G2019S seemed to alter the Ubi-staining pattern to a more perinuclear location in neurons of A53T/G2019S mice (arrow, Figure 8Ad).
Figure 8. Over-expression of A53T and LRRK2 impairs the UPS activities in neurons.
(A–C) Representative images show Ubi staining (arrows, green) in the cortex of A53T mice at 6 (Aa) and 20 (Ab) months of age, and A53T/LRRK2WT (Ac), A53T/G2019S (Ad), and nTg (Ae) mice at 6 months of age. Images from Ba to Be display corresponding α-syn staining (red); while images from Ca to Ce show the overlay of Ubi and α-syn staining. Nuclei were labeled by Topro 3 staining (blue). Scale bar: 10 µm
(D–F) Representative images show Ubi staining (arrows, green) in the cortex of LRRK2WT (Da) and A53T/LRRK2WT (Db) mice at 6 months of age; G2019S (Dc) and A53T/G2019S (Dd) mice at 6 and G2019S mice at 20 (De) months of age. Images from Ea to Ee display corresponding LRRK2 staining (red); whilst images from Fa to Fe show the overlay of Ubi and LRRK2 staining. Nuclei were labeled by Topro 3 staining (blue).
(G) Western blots show Ubi-positive HMW bands in the total brain homogenates of nTg, A53T, G2019S, and A53T/G2019S mice at 1 month of age; nTg, LRRK2WT, A53T, and A53T/LRRK2WT mice at 3 months of age; and nTg, A53T, and G2019S mice at 18 months of age. The bottom panel in (G) shows the level of monomeric Ubi (Ubi mono) with shorter exposure.
(H) Bar graph shows the ratio of HMW/mono-Ubi in the total brain homogenates of mice with different genotype at 1, 3, 18 months of age. *p<0.05, **p<0.01
We then examined the Ubi staining in LRRK2 transgenic mice. The level of Ubi staining was increased in cortical neurons of 6-month old LRRK2WT and G2019S mice (Figure 8D). Interestingly, the occurrence of Ubi-positive clusters was more prominent in G2019S neurons (arrowheads, Figure 8D). These Ubi-positive clusters appeared co-stained with LRRK2 in G2019S neurons (arrowheads, Figure 8F). This phenomenon became more apparent in neurons of 20-month old G2019S mice in which LRRK2 and Ubi staining were tightly co-localized as clusters (arrowheads, Figure 8Fe). Moreover, the presence of A53T α-syn accelerated the clustering of LRRK2 staining in neurons of A53T/G2019S mice (arrowheads, Figures 8Ed, 8Fd) as compared to age-matched G2019S mice (Figures 8Dc, 8Fc). In contrast, no obvious LRRK2-positive clusters were found in neurons of 6-month old LRRK2WT and A53T/LRRK2WT mice (Figures 8Da–b, 8Fa–b). Taken together, these observations indicate that G2019S LRRK2 may further inhibit UPS activities, resulting in more frequent formation of LRRK2 and Ubi-positive aggregates in neurons of aged G2019S mice (Figures 8De, 8Ee). Our studies also indicate that the presence of A53T α-syn and G2019S LRRK2 may cause synergistic impairment of ubiquitin-proteasome system (UPS) activities, which further accelerates the formation of LRRK2/Ubi-positive aggregates in neurons of A53T/G2019S double transgenic mice (Figures 8Dd, 8Ed).
In parallel with immunohistological studies, we also checked the levels of ubiquitinated proteins in brain homogenates of A53T, LRRK2WT, G2019S, A53T/LRRK2WT, and A53T/G2019S mice by Western blots (Figure 8G). A significant elevation of ubiquitination was observed in the brain homogenate of 3-month old A53T and A53T/LRRK2WT mice as compared to age-matched nTg, LRRK2WT, and G2019S mice (Figure 8H). The level of ubiquitination, however, was only moderately increased in the brain samples of 18-month old G2019S mice compared to age-matched nTg controls (Figures 8G–H).
LRRK2 exacerbated mitochondrial structural and functional abnormalities in neurons of A53T transgenic mice
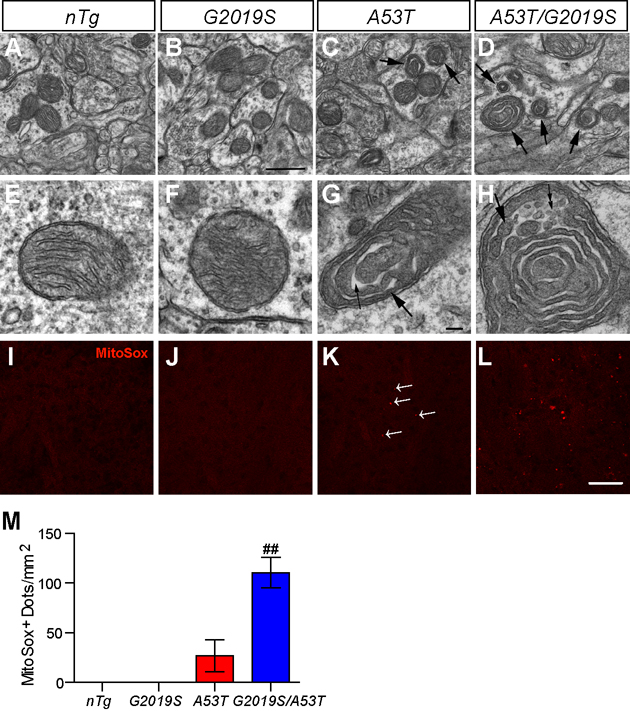
Mitochondrial dysfunction has been shown in cell and mouse models over-expressing PD-related mutant α-syn (Hsu et al., 2000;Martin et al., 2006). We examined the morphology of mitochondria in striatal neurons of 1-month old mice by EM and found distinctive structural changes in some of the mitochondria in A53T and A53T/G2019S neurons. Compared to nTg and G2019S samples, the matrix of these abnormal mitochondria was denser (large arrows in Figures 9C–D, 9G–H), and the cristae became widened (small arrows in Figure 9G). The frequency of such abnormal mitochondria was 52/1000 µm2 in striatal area of A53T mice and ~3.5 times higher in A53T/G2019S double transgenic mice (184/1000 µm2); whereas virtually none was found in age-matched nTg or G2019S transgenic mice (Figures 9A–D). The abnormal mitochondria were preferentially distributed in dendrites/soma, with only a few in axon terminals (unpublished data). The mitochondrial structural abnormalities were also confirmed by immunostaining with an antibody against cytochrome c oxidase subunit I, a mitochondrial inner membrane protein (supplemental Figure S10).
Figure 9. LRRK2 exacerbates α-syn-induced mitochondrial structural and functional abnormalities.
(A–H) Representative EM images show mitochondria in the striatum of nTg (A, E), G2019S (B, F), A53T (C, G), and A53T/G2019S (D, H) transgenic mice at 1 month of age. The abnormal mitochondria were marked by arrows (C, D). The dense matrix of the abnormal mitochondria in G and H often appear “sausage-like” with multiple constrictions (large arrows in G, H) and concentric in arrangement, and occasionally became vesiculated (small double arrows in H). N = 2 per genotype. Scale bar: 0.5 µm (A–D); 0.1 µm (E–H). (I–L) Representative images show the MitoSox Red staining in the striatum of nTg (I), G2019S (J), A53T (K), and A53T/G2019S (L) mice at 1 month of age. Scale bar: 50 µm. (M) Bar graph shows the quantification of MitoSox Red staining shown in I–L. ##p < 0.001
To address whether the morphological abnormalities affect the function of mitochondria in striatal neurons of 1-month old A53T and A53T/G2019S transgenic mice, we infused the animal with MitoSox Red, a fluorescent dye for detecting the surplus of mitochondrial superoxide released to the matrix (Robinson et al., 2006). MitoSox Red signal was easily detected in the brain of A53T/G2019S transgenic mice and was more prominent in the dorsal lateral striatum (Figures 9L–M), where most neurodegeneration was found. In contrast, only a few MitoSox Red-positive neurons were found in the stratum of A53T mice (arrowhead, Figure 9K) and no positive staining was detected in nTg or G2019S transgenic mice (Figures 9I–J). These results suggest that co-expression of α-syn and LRRK2 cause synergistic toxicity to mitochondria.
Inhibition of LRRK2 expression ameliorated α-syn-mediated neuropathology
Since over-expression of LRRK2 accelerated the progression of α-syn-induced neuropathological abnormalities, we decided to investigate whether inhibition of LRRK2 expression was able to alleviate the pathogenesis of mutant α-syn. To inhibit LRRK2 expression, we generated LRRK2 knockout (LRRK2−/−) mice (Supplemental Figure S11). LRRK2−/− mice were viable, fertile, and displayed no obvious motor behavioral phenotypes (Supplemental Figure S12). No apparent elevation of reactive astrocytosis, neurodegeneration, or somatic accumulation of α-syn was found in brains of 20-month old LRRK2−/− mice (Supplemental Figure S12; Table 1–Table 3). Furthermore, the levels of α-syn in Triton-X100 insoluble fraction appeared comparable between LRRK2+/+ and LRRK2−/− mice, indicating no apparent α-syn aggregates were formed in LRRK2−/− mouse brains (data not shown). Compared to littermate controls, only a moderate increase of activated microglia (Supplemental Figure S12; Table 2) was observed in the brain of 20-month old LRRK2−/− mice.
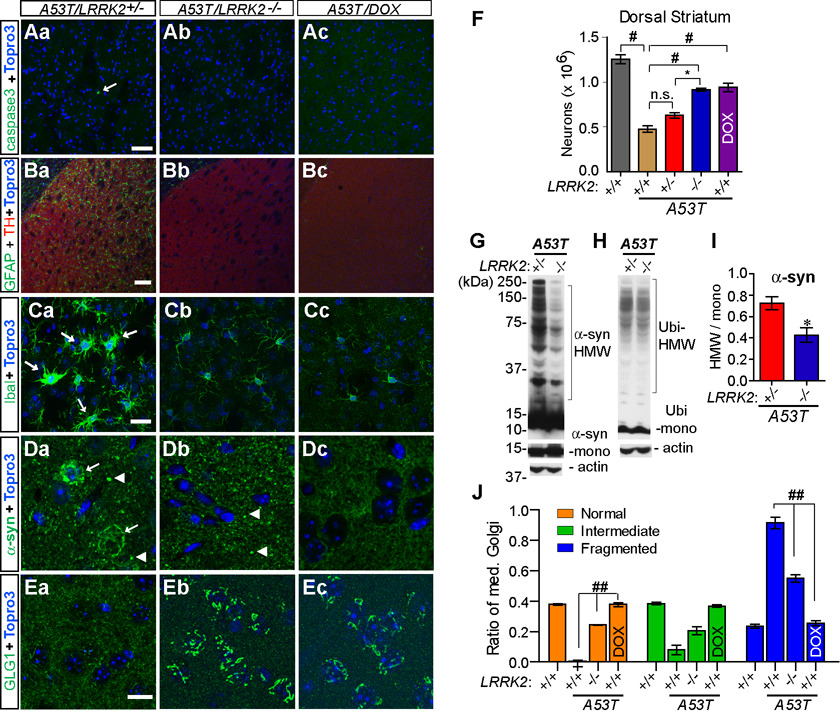
To generate A53T mice in the LRRK2 knockout background (A53T/LRRK2−/−), we intercrossed tetO-A53T/LRRK2+/− and CaMKII-tTA/LRRK2+/− mice to get A53T/LRRK2−/−and littermate control mice. The neuropathology of A53T/LRRK2−/− mice and littermate controls was examined at 6 and 12 months of age (Supplemental Figure S13; Figure 10). Similar to A53T/LRRK2+/+ mice, 12-month old A53T/LRRK2+/− mice also displayed significant neurodegeneration, astrocytosis, microgliosis, somatic accumulation of α-syn and severe fragmentation of Golgi apparatus in the striatum (Figure 10). In contrast, no apparent neurodegeneration was found in the striatum of age-matched littermate A53T/LRRK2−/− mice (Figure 10Ab; Table 1). The number of residual neurons in the striatum of 12-month old A53T/LRRK2−/− is also significantly more as compared to age-matched A53T/LRRK2+/− and A53T/LRRK2+/+ mice (Figure 10F). Moreover, no significant elevation of astrocytosis, microgliosis, or somatic accumulation of α-syn was detected in the striatum of 12-month old A53T/LRRK2−/− mice (Figure 10; Table 2 and Table 3). GLG1 staining revealed normal morphology and distribution of Golgi apparatus in neurons of A53T/LRRK2−/− mice (Figures 10Eb, 10J). Similar rescuing effects were also observed in the brain of 12-month old A53T/LRRK2+/+ mice treated with doxycycline (A53T/DOX) (Figure 10). Stereological analyses further revealed significant reduction of neurodegeneration in A53T/DOX mice as compared to age-matched A53T/LRRK2+/+ mice (Figure 10F). In addition to immunohistological analyses, Western blots revealed a significant decrease of HMW α-syn in the total brain homogenates of A53T/LRRK2−/− mice as compared to age-matched A53T/LRRK2+/− mice (Figures 10G, 10I). By contrast, only a moderate decrease of HMW-Ubi was observed in A53T/LRRK2−/− samples (Figure 10G), which did not reach statistical significance by the densitometry analysis. Together these findings demonstrated that inhibition of LRRK2 expression successfully ameliorated α-syn-mediated neuropathological abnormalities in A53T transgenic mice.
Figure 10. Inhibition of LRRK2 delays the progression of A53T α-syn-mediated neuropathology and reduces the somatic accumulation and aggregation of α-syn.
(Aa–Ec) Representative images show caspase3 (Aa–Ac), GFAP (Ba–Bc), Iba1 (Ca–Cc), α-syn (Da–Dc), and GLG1 (Ea–Ec) staining in the striatum of A53T/LRRK2+/− and littermate A53T/LRRK2−/− mice, and A53T/DOX mice at 12 months of age. The caspase3-positive neuron (Aa), activated microglia (Ca) and somatic accumulation of α-syn (Da) were pointed by arrows. The enlarged and α-syn-positive nerve terminals were labeled with arrowhead (Da and Db). The striatum was outlined by TH staining (red, Ba–Bc). Nuclei were labeled by Topro 3 staining (blue). Scale bar: 100 µm (Ba–Bc), 50 µm (Aa–Ac), 20 µm (Ca–Cc), 10 µm (Da–Ec).
(F) Bar graph depicts the numbers of neurons remained the dorsal striatum of 12-month old LRRK2+/+, A53T/LRRK2+/+, A53T/LRRK+/−, A53T/LRRK2−/−, and A53T/DOX mice. *p < 0.05, #p < 0.001
(G–H) Western blots show HMW α-syn (G) and Ubi-positive bands (H) in the total homogenates from 12 month-old A53T/LRRK2+/− and A53T/LRRK2−/− mice.
(I) Densitometry analyses revealed significant reduction of HMW α-syn in the brain homogenates of 12-month old A53T/LRRK2−/− mice (n=3) compared to age-matched A53T/LRRK+/− mice (n= 4). *p < 0.05
(J) Quantification of trans-Golgi morphology in striatal neurons (≥ 300 per genotype) of mutant and control mice (≥ 3 per genotype) at 12 months of age. ##p < 0.0001
Discussion
Mutations in α-syn or LRRK2 lead to typical PD-like neuropathological features such as the formation of α-syn-containing cytoplasmic inclusion, Lewy bodies (LB) (Hardy et al., 2006). LRRK2-immunoreactivity is also associated with LB (Higashi et al., 2007;Zhu et al., 2006). The expression of α-syn and LRRK2 appears co-regulated in the mouse striatum (Westerlund et al., 2008). These early studies indicate a potential pathophysiological interplay between α-syn and LRRK2. To systematically investigate whether α-syn and LRRK2 act synergistically in the pathogenesis of PD, we generated and characterized a series of compound transgenic mice over-expressing PD-related A53T α-syn mutant with various forms of LRRK2. Here we show that LRRK2 regulated the progression of neuropathological abnormalities induced by A53T α-syn. Over-expression of either wild-type or PD-associated G2019S LRRK2 greatly accelerated the progression of A53T α-syn-mediated neurodegeneration. At the cellular level, over-expression of LRRK2 impaired microtubule dynamics and caused Golgi fragmentation, which we suspect might exacerbate A53T α-syn-induced cytotoxicity via promoting the abnormal somatic accumulation of α-syn. By contrast, genetic ablation of LRRK2 maintained the normal organization of Golgi complex, reduced the aggregation and somatic accumulation of A53T α-syn, and thereby significantly delayed the progression of A53T α-syn-induced neuropathology.
PD is clinically characterized as dyskinesia, resting tremor, rigidity, and abnormal posture. The lack of obvious PD-like behavioral phenotypes in our G2019S and A53T mutant mice might be attributed to the scarce expression of exogenous LRRK2 and α-syn transgenes in midbrain dopaminergic (DA) neurons under the CaMKII promoter. Therefore, our present A53T and G2019S transgenic mice are not ideal for studying the dysfunction of DA neurons. Nevertheless, they may serve as useful tools to investigate the pathogenic mechanisms of PD-related mutant LRRK2 and α-syn in vivo. Currently, we are in the process of generating new lines of mice, which over-express G2019S LRRK2 or A53T α-syn in midbrain DA neurons. It would be interesting to determine whether G2019S LRRK2 and A53T α-syn have the same synergistic toxic effect when co-expressed in DA neurons.
PD is pathologically characterized by the presence of α-syn-containing inclusion bodies in the perinuclear area (Spillantini et al., 1997). However, in normal neurons, α-syn is typically enriched at axon terminals where it is associated with synaptic vesicles (Maroteaux and Scheller, 1991;Tao-Cheng, 2006). Like other synaptic vesicle proteins, the secretion of α-syn is mediated by the ER-Golgi network and transported to the axonal terminals by microtubule-based motor proteins (Roy et al., 2008). Therefore, the formation of somatic inclusion of α-syn may result from the dysfunction of ER/Golgi trafficking and microtubule-based axonal transport (Cooper et al., 2006;Gosavi et al., 2002). As an extension of these previous in vitro observations, we found a significant increase of fragmented Golgi apparatus in neurons of 6 and 12-month old A53T α-syn transgenic mice, which is correlated with an increased prevalence of neurons with somatic accumulation of α-syn. Interestingly, over-expression of either WT or G2019S LRRK2 greatly promoted the abnormal somatic accumulation of both the WT and A53T α-syn in neurons. LRRK2 has been shown to associate with Golgi complex (Biskup et al., 2006). While we did not observe any substantial co-staining of LRRK2 and Golgi in LRRK2 transgenic neurons (data not shown), we found that over-expression of LRRK2 caused significant fragmentation of Golgi complex. Moreover, co-expression of LRRK2 and α-syn led to more severe and synergistic fragmentation of Golgi apparatus, which was tightly correlated with the augmentation of α-syn accumulation in the soma. By contrast, inhibition of LRRK2 expression prevented the disintegration of Golgi complex in A53T neurons and suppressed the accumulation of α-syn in cell bodies. Together, our present study reveals a novel function of LRRK2 in maintaining the normal organization of Golgi apparatus and further demonstrates that the dysfunction of ER-Golgi-mediated protein/vesicle trafficking may contribute significantly to α-syn-induced pathogenesis in PD.
In mammalian cells, Golgi apparatus is composed of multiple layers of cisternal stacks juxtaposed with microtubule organization center in the vicinity of the nucleus and microtubules play an important role in maintaining the organization of Golgi complex (Thyberg and Moskalewski, 1999). In line with this notion, Lee and colleagues recently showed that α-syn co-aggregates with microtubules and impairs microtubule-dependent vesicle trafficking, which is proposed as a potential molecular mechanism underlying α-syn-induced Golgi fragmentation in cell cultures (Lee et al., 2006). Interestingly, LRRK2 has been shown to physically interact with both α and β-tubulin through its GTPase domain (Gandhi et al., 2008;Gillardon, 2009). Various microtubule-associated proteins, including tau, regulate the stability and dynamics of microtubule network (Valiron et al., 2001). In line with this notion, a recent report suggests that G2019S LRRK2 preferentially phosphorylates β-tubulin purified from brain tissues and enhances the assembly of microtubules in the presence of other microtubule-associated proteins (Gillardon, 2009). In contrast, the level of free tubulin is significantly increased in the brain extract of LRRK2−/− mice (Gillardon, 2009). In agreement with these early observations, we found that the level of RAB-insoluble tubulin was significantly elevated in the brain homogenate of LRRK2 transgenic mice as compared with nTg controls and A53T single transgenic mice; whereas, the level of RAB-soluble tubulin was dramatically decreased in the brain homogenate of LRRK2 single and A53T/LRRK2 double transgenic mice. While the total levels of α and β-tubulin in the brain homogenates were not affected in LRRK2 transgenic mice, the altered ratio of RAB-soluble vs. RAB-insoluble tubulin in LRRK2 over-expressing neurons may reflect a depletion of the free pool subunits and thereby a significant enhancement of tubulin polymerization. Previous studies demonstrated that the treatment of taxol, a microtubule stabilizer, leads to redistribution of microtubules in the cell and fragmentation of Golgi apparatus into areas of cells rich in microtubules (Wehland et al., 1983). Therefore, we suspect a similar sequence of events occurred in neurons over-expressing LRRK2 in which LRRK2 increases of the ability of tubulin to polymerize, disrupts the normal microtubule organization, and as the consequence, alters the normal distribution of Golgi complex. The fragmentation of Golgi apparatus per se may not cause overt cytotoxicity, but it could impair the efficiency of coordinated vesicle trafficking in mammalian cells (Thyberg and Moskalewski, 1999), which may explain why over-expression of LRRK2 alone did not cause any neurodegeneration albeit apparent Golgi fragmentation; while together with A53T α-syn, LRRK2 dramatically accelerated the abnormal somatic accumulation of α-syn and associated cell loss. Together with previous in vitro studies (Gandhi et al., 2008;Gillardon, 2009), our findings indicate that LRRK2 is a stabilizer of microtubule assembly in cells and over-expression of LRRK2 promotes the additional polymerization of tubulin in neurons, which we suspect might lead to the fragmentation of Golgi apparatus and exacerbate α-syn-induced ER/Golgi trafficking defects and other cytotoxicities.
The increased accumulation of α-syn in the soma may favor the formation of α-syn aggregates, a key factor underlying its toxicity to neurons (Conway et al., 1998; Narhi et al., 1999). Concurrent with somatic accumulation of α-syn, more HMW and detergent-insoluble α-syn was detected in the brain homogenate of aged A53T and A53T/G2019S mice. Previous studies indicate that α-syn aggregates cause proteasome impairment (Tanaka et al., 2001), which may lead to the accumulation of ubiquitinated proteins in neurons. Consistently, the levels of ubiquitinated proteins were up-regulated in A53T and aged G2019S mice. Moreover, G2019S LRRK2 mutant protein was sequestered as ubiquitin-positive clusters in neurons of aged animals. We have shown earlier that the degradation of LRRK2 is primarily through the proteasomal pathway (Wang et al., 2008). The accumulating of G2019S mutant and ubiquitin-positive protein aggregates in aged mice indicate that over-expression of LRRK2, especially the G2019S mutant, may impair the UPS activities in neurons. Moreover, the presence of A53T α-syn seemed to further damage the UPS activity and accelerate the sequestration of LRRK2 G2019S aggregates. Although the UPS activities were apparently impaired in neurons of A53T mice, the dysfunction of UPS activities might not play a main role in A53T-mediated neuropathogenesis in our mouse model, since the inhibition of LRRK2 expression, which dramatically delayed the progression of neurodegeneration in A53T mice, only moderately reduced the accumulation of HMW ubiquitinated proteins. These observations further support the notion that the dysfunction of Golgi and microtubule-based molecule/vesicle trafficking is likely a main pathogenic route of α-syn and LRRK2-mediated neurodegeneration.
Extensive efforts have been devoted to identify the potential physiological substrates of LRRK2’s kinase activities (Imai et al., 2008;Jaleel et al., 2007), although no formal link has been established between the LRRK2’s kinase activities and the pathogenesis of PD. To further address whether the putative protein kinase domain of LRRK2 is critical in regulating α-syn-mediated neuropathology, we generated LRRK2 kinase-deletion (KD) inducible transgenic mice and crossbred these mice with A53T transgenic mice. Over-expression of LRRK2 KD mutant also caused Golgi fragmentation and the impairment of microtubule dynamics. When co-expressed with A53T α-syn, LRRK2 KD mutant promoted the somatic accumulation of α-syn A53T mutant and accelerated A53T-mediated neuropathology to a similar extent as LRRK2 WT protein. These data suggest that the kinase domain of LRRK2 is likely not critical in accelerating A53T–induced neuropathological abnormalities. Instead, the GTPase domain of LRRK2 may play a more important role in LRRK2-induced damage to microtubules and Golgi apparatus through its direct association with microtubules. It will be interesting to evaluate the role of LRRK2 GTPase domain in regulating the stability of microtubule network and ER/Golgi trafficking in neurons and its contribution to A53T α-syn-mediated neurodegeneration.
In summary, we have revealed a novel function of LRRK2 in regulating the intracellular trafficking and accumulation of α-syn in neurons. Our data suggest that excessive amount of LRRK2 or its mutants may impair the structure and function of Golgi complex and microtubule-based transport, resulting in abnormal somatic accumulation of α-syn, which may contribute to the accelerated progression of neuropathology induced by α-syn A53T mutant. Generally, pathogenic proteins of human origin need to be overly expressed in order to reproduce the desired behavioral and pathological phenotypes in transgenic mouse models, which also raise concerns on disease-unrelated cytotoxicity. While an 8 to 16-fold increase of LRRK2 protein expression in LRRK2WT and G2019S mice greatly exacerbated α-syn A53T-mediated cytotoxicity, a moderate increase of LRRK2 expression in both LRRK2WT-L and KD mice also enabled to promote α-syn A53T-induced neurodegeneration. These findings suggest that the synergistic cytotoxicity induced by co-expression of α-syn A53T and LRRK2 could not be simply attributed to the excess expression of LRRK2. Instead, our study indicates that LRRK2 may process an intrinsic function in regulating the progression of α-syn A53T-mediated neuropathological abnormalities. In support of this notion, genetic inhibition of LRRK2 expression in α-syn A53T transgenic mice significantly reduced the fragmentation of Golgi complex and somatic accumulation of α-syn in neurons, and effectively delayed the progression of α-syn A53T-mediated neuropathological abnormalities. These results also suggest that inhibition of LRRK2 expression may provide an applicable therapeutic strategy to ameliorate α-syn-induced neurodegeneration in PD or other related neurodegenerative diseases.
Experimental Procedures
Generation of LRRK2 and α-synuclein Inducible Transgenic Mice and LRRK2−/− mice
The generation of human WT and KD LRRK2, as well as WT and A53T α-syn inducible transgenic mice was followed the same protocol as described previously for the development of G2019S LRRK2 transgenic mice (Wang et al., 2008). The LRRK2−/− mice were generated through deletion the second coding exon of LRRK2 (Parisiadou et al., 2009, in press). All mouse work follows the guidelines approved by the Institutional Animal Care and Use Committees of the National Institute of Child Health and Human Development.
Immunohistochemistry and Light Microscopy
The detailed information about immunohistochemical procedures was provided in Supplemental Materials. Three or more animals per genotype and age group were used for each study. Fluorescence images were captured using a laser scanning confocal microscope (LSM 510; Zeiss, Thornwood, NJ). The paired images in all the figures were collected at the same gain and offset settings. When post collection processing was done, it was applied uniformly to all paired images. The images of α-syn staining were presented as a single optic layer after acquired in z-series stack scans at 0.8 µm intervals.
Stereology
According to stereotaxic coordinates (3rd edition, Keith B.J. Franklin and George Paxinos), series of coronal sections across the dorsal striatum were processed for TH and GFAP as well as TH and NeuN co-staining. Three or more animals per genotype and age group were used for each study. The numbers of GFAP and NeuN-positive cells was assessed using Stereo Investigator 8 (MicroBrightField Inc, Williston, VT). The sampling scheme was designed to have coefficient of error (CE) less than 10% in order to get reliable results. All stereological analyses were performed under the 100 × objective of a Zeiss Axio microscope (Imager A1).
Extraction of tubulin from mouse brains
As described previously (Ishihara et al., 1999), freshly dissected mouse brains were homogenized in 2ml/g of RAB Hi-Salt buffer [0.1M morpholineethanesulfonic acid (MES), 1mM EGTA, 0.75M NaCl, 0.02M NaF, 0.5mM MgSO4, 1mM PMSF, 100mM EDTA)] and protease inhibitors cocktail and centrifuged at 50, 000 × g for 1 hour. The pellets (RAB-P) were saved and the RAB-extractable supernatants were boiled for 5 min and then chilled on ice for another 5 min. In turn, they were centrifuged at 10, 000 × g for 20 min at 4°C. Both the pellets and the supernatants (RAB-S) were saved. After protein concentration was determined using the BCA assay kit (Pierce), equal amount of proteins from RAB-P and RAB-S fractions was analyzed by Western Blot.
Statistical Analysis
Statistical analysis was performed using Graphpad Prism 5 (Graphpad Software Inc. La Jolla, CA) and StatView program (SAS Institute Inc.). Data are presented as means ± SEM. Statistical significances were determined by comparing means of different groups using t-test or ANOVA followed by Post Hoc Tukey HSD test.
Supplementary Material
Acknowledgements
This work was supported in part by the intramural research programs of National Institute on Aging, National Human Genome Research Institute (NHGRI), National Institute of Mental Health (NIMH), and National Institute of Neurological Disorders and Stroke (NINDS) at the National Institutes of Health and the Henry Jackson Foundation. We thank the NHGRI and NIMH transgenic mouse facilities for blastocystic and pronuclear injections; the NINDS DNA Sequence Facility for sequencing DNA constructs; Dr. David Borchelt for kindly providing the tetO expression vector; Drs. Darren Moore, Valina Dawson, Ted Dawson (the Johns Hopkins University School of Medicine) and Jean-Marc Taymans (Universiteit Leuven) for kindly providing LRRK2 antibodies; Drs. John Hardy and Andy Singleton for their helpful suggestions; and the NIH Fellows Editorial Board for editing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: There is no competing financial interest of all researchers involved in this work.
Reference List
- 1.Arlotta P, Molyneaux BJ, Jabaudon D, Yoshida Y, Macklis JD. Ctip2 controls the differentiation of medium spiny neurons and the establishment of the cellular architecture of the striatum. J.Neurosci. 2008;28:622–632. doi: 10.1523/JNEUROSCI.2986-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biskup S, Moore DJ, Celsi F, Higashi S, West AB, Andrabi SA, Kurkinen K, Yu SW, Savitt JM, Waldvogel HJ, Faull RL, Emson PC, Torp R, Ottersen OP, Dawson TM, Dawson VL. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann.Neurol. 2006;60:557–569. doi: 10.1002/ana.21019. [DOI] [PubMed] [Google Scholar]
- 3.Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat.Med. 1998;4:1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 4.Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, Liu K, Xu K, Strathearn KE, Liu F, Cao S, Caldwell KA, Caldwell GA, Marsischky G, Kolodner RD, Labaer J, Rochet JC, Bonini NM, Lindquist S. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 6.Galter D, Westerlund M, Carmine A, Lindqvist E, Sydow O, Olson L. LRRK2 expression linked to dopamine-innervated areas. Ann.Neurol. 2006;59:714–719. doi: 10.1002/ana.20808. [DOI] [PubMed] [Google Scholar]
- 7.Gandhi PN, Wang X, Zhu X, Chen SG, Wilson-Delfosse AL. The Roc domain of leucine-rich repeat kinase 2 is sufficient for interaction with microtubules. J.Neurosci.Res. 2008 doi: 10.1002/jnr.21622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilks WP, Abou-Sleiman PM, Gandhi S, Jain S, Singleton A, Lees AJ, Shaw K, Bhatia KP, Bonifati V, Quinn NP, Lynch J, Healy DG, Holton JL, Revesz T, Wood NW. A common LRRK2 mutation in idiopathic Parkinson’s disease. Lancet. 2005;365:415–416. doi: 10.1016/S0140-6736(05)17830-1. [DOI] [PubMed] [Google Scholar]
- 9.Gillardon F. Leucine-rich repeat kinase 2 phosphorylates brain tubulin-beta isoforms and modulates microtubule stability - a point of convergence in Parkinsonian neurodegeneration? J.Neurochem. 2009 doi: 10.1111/j.1471-4159.2009.06235.x. [DOI] [PubMed] [Google Scholar]
- 10.Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- 11.Gosavi N, Lee HJ, Lee JS, Patel S, Lee SJ. Golgi fragmentation occurs in the cells with prefibrillar alpha-synuclein aggregates and precedes the formation of fibrillar inclusion. J.Biol.Chem. 2002;277:48984–48992. doi: 10.1074/jbc.M208194200. [DOI] [PubMed] [Google Scholar]
- 12.Greggio E, Jain S, Kingsbury A, Bandopadhyay R, Lewis P, Kaganovich A, van der Brug MP, Beilina A, Blackinton J, Thomas KJ, Ahmad R, Miller DW, Kesavapany S, Singleton A, Lees A, Harvey RJ, Harvey K, Cookson MR. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol.Dis. 2006;23:329–341. doi: 10.1016/j.nbd.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Hardy J, Cai H, Cookson MR, Gwinn-Hardy K, Singleton A. Genetics of Parkinson's disease and parkinsonism. Ann.Neurol. 2006;60:389–398. doi: 10.1002/ana.21022. [DOI] [PubMed] [Google Scholar]
- 14.Higashi S, Biskup S, West AB, Trinkaus D, Dawson VL, Faull RL, Waldvogel HJ, Arai H, Dawson TM, Moore DJ, Emson PC. Localization of Parkinson’s disease-associated LRRK2 in normal and pathological human brain. Brain Res. 2007;1155:208–219. doi: 10.1016/j.brainres.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 15.Hsu LJ, Sagara Y, Arroyo A, Rockenstein E, Sisk A, Mallory M, Wong J, Takenouchi T, Hashimoto M, Masliah E. alpha-synuclein promotes mitochondrial deficit and oxidative stress. Am.J.Pathol. 2000;157:401–410. doi: 10.1016/s0002-9440(10)64553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imai Y, Gehrke S, Wang HQ, Takahashi R, Hasegawa K, Oota E, Lu B. Phosphorylation of 4E-BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila. EMBO J. 2008;27:2432–2443. doi: 10.1038/emboj.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishihara T, Hong M, Zhang B, Nakagawa Y, Lee MK, Trojanowski JQ, Lee VM. Age-dependent emergence and progression of a tauopathy in transgenic mice overexpressing the shortest human tau isoform. Neuron. 1999;24:751–762. doi: 10.1016/s0896-6273(00)81127-7. [DOI] [PubMed] [Google Scholar]
- 18.Jaleel M, Nichols RJ, Deak M, Campbell DG, Gillardon F, Knebel A, Alessi DR. LRRK2 phosphorylates moesin at threonine-558: characterization of how Parkinson's disease mutants affect kinase activity. Biochem.J. 2007;405:307–317. doi: 10.1042/BJ20070209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jankowsky JL, Slunt HH, Gonzales V, Savonenko AV, Wen JC, Jenkins NA, Copeland NG, Younkin LH, Lester HA, Younkin SG, Borchelt DR. Persistent amyloidosis following suppression of Abeta production in a transgenic model of Alzheimer disease. PLoS.Med. 2005;2:e355. doi: 10.1371/journal.pmed.0020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee HJ, Khoshaghideh F, Lee S, Lee SJ. Impairment of microtubule-dependent trafficking by overexpression of alpha-synuclein. Eur.J.Neurosci. 2006;24:3153–3162. doi: 10.1111/j.1460-9568.2006.05210.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J.Neurosci. 2005;25:6016–6024. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Tan YC, Poulose S, Olanow CW, Huang XY, Yue Z. Leucine-rich repeat kinase 2 (LRRK2)/PARK8 possesses GTPase activity that is altered in familial Parkinson's disease R1441C/G mutants. J.Neurochem. 2007 doi: 10.1111/j.1471-4159.2007.04743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Liu W, Oo TF, Wang L, Tang Y, Jackson-Lewis V, Zhou C, Geghman K, Bogdanov M, Przedborski S, Beal MF, Burke RE, Li C. Mutant LRRK2(R1441G) BAC transgenic mice recapitulate cardinal features of Parkinson's disease. Nat.Neurosci. 2009;12:826–828. doi: 10.1038/nn.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Z, Wang X, Yu Y, Li X, Wang T, Jiang H, Ren Q, Jiao Y, Sawa A, Moran T, Ross CA, Montell C, Smith WW. A Drosophila model for LRRK2-linked parkinsonism. Proc.Natl.Acad.Sci.U.S.A. 2008 doi: 10.1073/pnas.0708452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maroteaux L, Campanelli JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J.Neurosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maroteaux L, Scheller RH. The rat brain synucleins; family of proteins transiently associated with neuronal membrane. Brain Res.Mol.Brain Res. 1991;11:335–343. doi: 10.1016/0169-328x(91)90043-w. [DOI] [PubMed] [Google Scholar]
- 27.Martin LJ, Pan Y, Price AC, Sterling W, Copeland NG, Jenkins NA, Price DL, Lee MK. Parkinson's disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J.Neurosci. 2006;26:41–50. doi: 10.1523/JNEUROSCI.4308-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- 29.Narhi L, Wood SJ, Steavenson S, Jiang Y, Wu GM, Anafi D, Kaufman SA, Martin F, Sitney K, Denis P, Louis JC, Wypych J, Biere AL, Citron M. Both familial Parkinson's disease mutations accelerate alpha-synuclein aggregation. J.Biol.Chem. 1999;274:9843–9846. doi: 10.1074/jbc.274.14.9843. [DOI] [PubMed] [Google Scholar]
- 30.Nichols WC, Pankratz N, Hernandez D, Paisan-Ruiz C, Jain S, Halter CA, Michaels VE, Reed T, Rudolph A, Shults CW, Singleton A, Foroud T. Genetic screening for a single common LRRK2 mutation in familial Parkinson's disease. Lancet. 2005;365:410–412. doi: 10.1016/S0140-6736(05)17828-3. [DOI] [PubMed] [Google Scholar]
- 31.Nussbaum RL, Polymeropoulos MH. Genetics of Parkinson's disease. Hum.Mol.Genet. 1997;6:1687–1691. doi: 10.1093/hmg/6.10.1687. [DOI] [PubMed] [Google Scholar]
- 32.Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der BM, Lopez dM, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, de Silva R, Lees A, Marti-Masso JF, Perez-Tur J, Wood NW, Singleton AB. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 33.Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, Hagen TM, Murphy MP, Beckman JS. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc.Natl.Acad.Sci.U.S.A. 2006;103:15038–15043. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roy S, Winton MJ, Black MM, Trojanowski JQ, Lee VM. Cytoskeletal requirements in axonal transport of slow component-b. J.Neurosci. 2008;28:5248–5256. doi: 10.1523/JNEUROSCI.0309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savitt JM, Dawson VL, Dawson TM. Diagnosis and treatment of Parkinson disease: molecules to medicine. J.Clin.Invest. 2006;116:1744–1754. doi: 10.1172/JCI29178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith WW, Pei Z, Jiang H, Dawson VL, Dawson TM, Ross CA. Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat.Neurosci. 2006;9:1231–1233. doi: 10.1038/nn1776. [DOI] [PubMed] [Google Scholar]
- 37.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka Y, Engelender S, Igarashi S, Rao RK, Wanner T, Tanzi RE, Sawa A, Dawson L, Dawson TM, Ross CA. Inducible expression of mutant alpha-synuclein decreases proteasome activity and increases sensitivity to mitochondria-dependent apoptosis. Hum.Mol.Genet. 2001;10:919–926. doi: 10.1093/hmg/10.9.919. [DOI] [PubMed] [Google Scholar]
- 39.Tao-Cheng JH. Activity-related redistribution of presynaptic proteins at the active zone. Neuroscience. 2006;141:1217–1224. doi: 10.1016/j.neuroscience.2006.04.061. [DOI] [PubMed] [Google Scholar]
- 40.Thyberg J, Moskalewski S. Role of microtubules in the organization of the Golgi complex. Exp.Cell Res. 1999;246:263–279. doi: 10.1006/excr.1998.4326. [DOI] [PubMed] [Google Scholar]
- 41.Tong Y, Pisani A, Martella G, Karouani M, Yamaguchi H, Pothos EN, Shen J. R1441C mutation in LRRK2 impairs dopaminergic neurotransmission in mice. Proc.Natl.Acad.Sci.U.S.A. 2009;106:14622–14627. doi: 10.1073/pnas.0906334106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trojanowski JQ, Goedert M, Iwatsubo T, Lee VM. Fatal attractions: abnormal protein aggregation and neuron death in Parkinson's disease and Lewy body dementia. Cell Death.Differ. 1998;5:832–837. doi: 10.1038/sj.cdd.4400432. [DOI] [PubMed] [Google Scholar]
- 43.Valiron O, Caudron N, Job D. Microtubule dynamics. Cell Mol.Life Sci. 2001;58:2069–2084. doi: 10.1007/PL00000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L, Xie C, Greggio E, Parisiadou L, Shim H, Sun L, Chandran J, Lin X, Lai C, Yang WJ, Moore DJ, Dawson TM, Dawson VL, Chiosis G, Cookson MR, Cai H. The chaperone activity of heat shock protein 90 is critical for maintaining the stability of leucine-rich repeat kinase 2. J.Neurosci. 2008;28:3384–3391. doi: 10.1523/JNEUROSCI.0185-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wehland J, Henkart M, Klausner R, Sandoval IV. Role of microtubules in the distribution of the Golgi apparatus: effect of taxol and microinjected anti-alpha-tubulin antibodies. Proc.Natl.Acad.Sci.U.S.A. 1983;80:4286–4290. doi: 10.1073/pnas.80.14.4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc.Natl.Acad.Sci.U.S.A. 1975;72:1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.West AB, Moore DJ, Biskup S, Bugayenko A, Smith WW, Ross CA, Dawson VL, Dawson TM. Parkinson's disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc.Natl.Acad.Sci.U.S.A. 2005;102:16842–16847. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Westerlund M, Ran C, Borgkvist A, Sterky FH, Lindqvist E, Lundstromer K, Pernold K, Brene S, Kallunki P, Fisone G, Olson L, Galter D. Lrrk2 and alpha-synuclein are co-regulated in rodent striatum. Mol.Cell Neurosci. 2008;39:586–591. doi: 10.1016/j.mcn.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 49.Zabetian CP, Samii A, Mosley AD, Roberts JW, Leis BC, Yearout D, Raskind WH, Griffith A. A clinic-based study of the LRRK2 gene in Parkinson disease yields new mutations. Neurology. 2005;65:741–744. doi: 10.1212/01.wnl.0000172630.22804.73. [DOI] [PubMed] [Google Scholar]
- 50.Zhu X, Siedlak SL, Smith MA, Perry G, Chen SG. LRRK2 protein is a component of Lewy bodies. Ann.Neurol. 2006;60:617–618. doi: 10.1002/ana.20928. [DOI] [PubMed] [Google Scholar]
- 51.Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.