Abstract
Silent cerebral infarctions (SCI) occur in up to 35% of children with sickle cell anemia (HbSS) but are rarely recognized during the initial 10–14 days when diffusion weighted magnetic resonance imaging can differentiate acute infarctions from remote events. We report acute SCI in 7 children with HbSS who had areas of restricted diffusion on MRI without persistent focal neurologic deficits. Four had acute SCI identified following acute anemic events. Our observations suggest that SCI are detectible in the acute phase, present with subtle neurologic symptoms, result in permanent neurologic injury, and may be caused by acute anemic events.
Introduction
Clinically overt stroke occurs in approximately 10% of children with sickle cell anemia (HbSS). In an additional 20–35%, magnetic resonance imaging (MRI) reveals areas of increased signal on T2-weighted images or fluid attenuated inversion recovery (FLAIR) images which are thought to represent smaller, subclinical infarcts.1 These so called silent cerebral infarcts (SCI) are poorly named, as these brain injuries are often associated with cognitive impairment,2–4 an increased risk for further silent or overt stroke,5 and subtle neurologic findings on detailed examination.6
The etiology of SCI in children with HbSS is unclear and likely multifactorial.7 Known risk factors for remote SCI include a history of frequent painful events, seizures, leukocytosis, and the Senegalese beta-globin haplotype.8 These differ from risk factors for clinically overt stroke, which include previous overt stroke or transient ischemic attack, low steady-state hemoglobin concentration, sickle cerebral vasculopathy, elevated transcranial Doppler ultrasound velocity, recent or recurrent acute chest syndrome, and acute anemic events.9–12 These risk factors for overt stroke may be associated with SCI as well.
Because they are clinically covert, SCI are almost always identified as remote events, found incidentally well after their onset. However, the temporal quality of the abnormal signal on diffusion-weighted MRI (DWI) allows acute infarcts to be distinguished from remote events. In acute stroke, the area of cytotoxic edema in the brain has a rapid decline in proton-diffusion capacity, which is visualized on DWI as a hyperintense signal with a corresponding area of decreased signal on the apparent diffusion coefficient (ADC) map. This abnormal signal on DWI becomes apparent in nearly all patients within 24 hours of stroke onset, but persists for only 10–14 days.13 The conventionally described T2-weighted and FLAIR abnormalities from stroke develop later and persist indefinitely. Detection of SCI during the acute phase, when there is an abnormal DWI signal, might provide further insight into their nature and risk factors. We therefore reviewed our experience with acute SCI detected by DWI in children with HbSS.
Patients and Methods
We report 7 children with HbSS, ages 3–11 years, who had acute SCI identified on MRI performed for clinical indications from 2003 to 2008 (Table). All were established patients in the Southwestern Comprehensive Sickle Cell Center. The Institutional Review Board of The University of Texas Southwestern Medical Center approved this analysis. MRI was obtained for clinical indications in all of the children. Our hospital instituted guidelines in 2003 to increase recognition and facilitate rapid diagnosis and treatment of pediatric stroke. These guidelines and recognition of these cases may have increased our clinical index of suspicion and detection of acute SCI.
Table.
Children with Sickle Cell Anemia and Acute Silent Cerebral Infarction
| Case | Age (years) |
Sex | Clinical Presentation | Hgb (g/dl) Presentation |
Hgb (g/dl) Baseline |
MRI | MRA | Follow up MRI |
|---|---|---|---|---|---|---|---|---|
| 1 | 5 | M | Transient L hemiparesis | 3.4 | 7.8 | Multiple bilateral deep white matter acute SCI |
Normal | Corresponding SCI |
| 2 | 3.5 | M | Transient ataxia | 7.1 | CT | Single acute SCI R deep white matter |
Abnormal | Corresponding SCI |
| 3 | 9.5 | F | Headache and transient gait abnormality 1 month after L hemisphere overt stroke |
8.4 | CT | Single acute SCI L precentral gyrus |
Abnormal | Not Done |
| 4 | 11 | F | Severe headache following transfusion for aplastic crisis |
2.7 | 8.3 | Multiple bilateral cortical and deep white matter acute SCI |
Abnormal | Corresponding SCI |
| 5 | 9 | M | Vertebral artery laceration from dog mauling |
3.4 | 7.1 | Bilateral watershed infarct | Normal | Not done |
| 6 | 4 | F | Altered mental status, ataxia, respiratory arrest from aplastic crisis |
2.2 | 7.2 | Acute SCI in R mesencephalon & lingula of R Occipital lobe |
Normal | Corresponding SCI |
| 7 | 6 | M | Asymptomatic. Fever and chest pain 2 weeks prior |
8.5 | 7.7 | Acute SCI in L temporal lobe | Abnormal | Not done |
Abbreviations: SCI= silent cerebral infarct, L=left, R=right, CT=chronic transfusion
In adult patients without HbSS, the definition of stroke, silent stroke, and transient ischemic attack (TIA) are in flux.14 With wider utilization of MRI, small strokes are now identified by DWI in patients presenting with clinical TIA.15 The classification of small remote infarcts detected on MRI in asymptomatic patients as overt or silent is dependent on patient recall of prior neurologic events. In pediatric patients, history of prior neurologic events is more difficult to obtain. Children with HbSS often present with nonspecific symptoms of fatigue or malaise and what appear to be focal neurologic deficits such as limping, weakness, or decreased use of an extremity, that are due to HbSS-related conditions such as splenic sequestration or vaso-occlusive crisis. In HbSS research, an operational distinction has been made between overt stroke and SCI16 with different risk factors identified and different treatment recommendations for the two categories. This dichotomy may be an oversimplification of a spectrum of ischemic injury.
The established definition of SCI in the literature is “an abnormal MRI of the brain with increased signal intensity in multiple T2-weighted images and no history or physical findings of a focal neurologic deficit lasting more than 24 hours.”17 For this report, we define acute SCI as an area of restricted diffusion on DWI in the absence of focal neurologic findings lasting longer than 24 hours. This is consistent with the definition of non-acute SCI used in other studies using T2-weighted or FLAIR MRI that lack the temporal specificity of DWI.16 When available, subsequent MRI studies obtained for other clinical indications were also reviewed.
Results
Symptoms at Presentation
Five of the 7 children (see Table) presented with transient diffuse neurologic signs including altered mental status in three, headache in two, and ataxia or altered gait in two. Only one child (case 1) presented with focal findings, a mild hemiparesis, which resolved within hours. This focal deficit was not reported by the family and was not initially noted in the emergency room. The only abnormality on neurological examination was an extensor plantar response on the left, which subsequently resolved. Case 4 had a severe headache with a pounding quality, photophobia, and phonophobia but normal mental status and no focal findings. MRI was performed to evaluate for hemorrhage, vascular abnormality, or stroke. Case 7 was asymptomatic, with acute SCI identified on MRI performed for evaluation of increased velocities noted on screening transcranial Doppler ultrasonography. Two (cases 2 and 3) had acute SCI after prior overt stroke.
Radiographic Findings
MRI showed areas of restricted diffusion in all cases with corresponding areas of decreased signal on the ADC map in 6 of 7 patients. Case 5, whose MRI was delayed until 9 days after presentation, had areas of restricted diffusion without ACD correlate. Absence of an ADC correlate in this case may reflect the subacute nature of injury on the delayed MRI. Cases 1, 2, 4, and 7 had discrete infarcts in a watershed distribution or in the deep white matter (Figure). Cases 3, 4, and 6 had acute SCI with cortical involvement. Follow-up MRI were obtained 2 – 36 months after the acute SCI for cases 1, 2, 4, and 6. All four had FLAIR abnormalities corresponding to the areas of prior restricted diffusion indicating permanent neurologic injury.
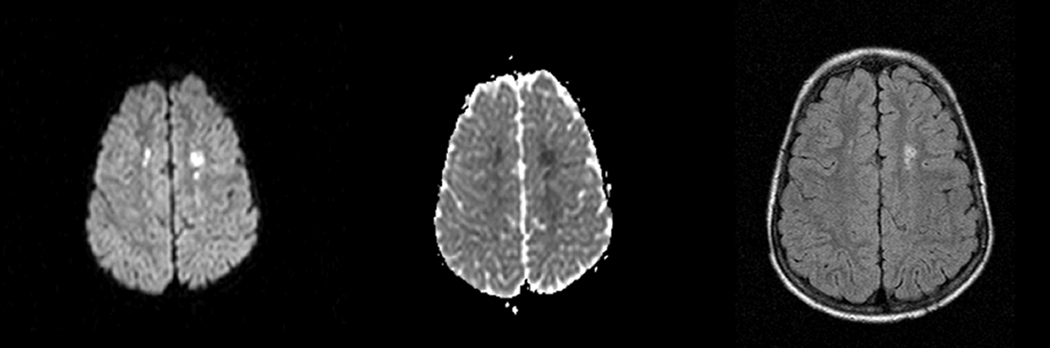
Figure.
MRI findings for case 1. DWI revealed multiple bilateral punctate areas of restricted diffusion in the deep white matter (left) with corresponding areas of decreased signal on ACD map (middle). FLAIR images obtained 3 years later (right) revealed corresponding areas of increased signal.
Clinical Setting
Four of the acute SCI occurred in the clinical setting of an acute anemic event, i.e., an exacerbation of the otherwise stable chronic anemia of HbSS. Case 5 presented with blood loss after a traumatic arterial laceration to a hemoglobin concentration of 3.4 g/dl. Cases 1, 4, and 6 presented with acute anemic events more common in patients with HbSS, i.e., aplastic crisis, with nadir hemoglobin concentrations between 2.2 and 3.4 g/dL. Their imaging studies revealed discrete or punctate unilateral or bilateral lesions, in a pattern typical of that observed in remote SCI in children with HbSS. Magnetic resonance angiography (MRA) was normal in 3 of these 4 cases associated with acute anemic events. Case 3 had acute SCI affecting the cortex, one month after a larger left hemisphere overt stroke and may represent infarction of an adjacent area of brain with tenuous blood supply.
Discussion
These observations suggest that SCI are detectible in the acute phase by DWI, that SCI onset may be associated with subtle, transient neurologic signs and symptoms, and that these acute SCI represent permanent brain injuries, not transient reversible changes or artifact. The presence of lesions typical for SCI on the follow-up imaging studies corresponding to the areas of restricted diffusion confirms a fundamental assumption about SCI: they do, in fact, represent ischemic lesions. They are not due to some other pathologic process such as demyelination, which would have a different pattern of evolution of signal characteristics on MRI.
The temporal quality of the DWI signal changes may assist in establishing a relationship between concurrent clinical events and the acute SCI. Our observation of acute SCI in the clinical setting of acute anemic events in 4 of 7 cases suggests that accelerated anemia may be an additional risk factor for SCI. In HbSS, the risk of overt stroke is increased in severe anemia, either acutely proximate to the stroke or in the steady state.12 The risk of overt stroke following an acute anemic event due to parvovirus B19 infection was 58 times greater than expected, suggesting a causal association.18 A prior report of acute SCI in HbSS also occurred in the setting of an acute anemic event.19 That patient was a 5 year old male who presented with a hemoglobin concentration of 2 g/dl. His MRI was similar to our case 1, with punctate areas of increased signal on DWI. We suspect that the rate of unrecognized acute SCI during acute anemic events could be similarly increased. Such events could lead to small areas of inadequate perfusion of brain tissue within the watershed distribution and result in small, clinically silent infarctions.
An increased index of suspicion for TIA or stroke is warranted for children with HbSS and increased education about stroke warning signs for patients and families could lead to increased and earlier diagnosis. Detection of these acute SCI has led to further evaluation and has altered the clinical management of several of these children. Increased vigilance for acute SCI in children with HbSS who present with subtle, transient neurologic symptoms is warranted. Unfortunately, the incidence of acute SCI cannot be determined from a retrospective review of our patients, because our clinical index of suspicion has increased over time with the identification of these cases. Prospective studies are needed to detect acute SCI in the appropriate clinical settings, such as acute anemic events and acute chest syndrome, which are known risk factors for overt stroke in HbSS. A better understanding of the etiologies of SCI in the population at highest risk, children with HbSS, may also provide insight into the problem of SCI in the general population. This might allow for the prevention or the amelioration of the neuro-cognitive sequelae of these not-so-silent strokes.
Acknowledgements
The authors were supported by the NHLBI Comprehensive Sickle Cell Center Program U54 HL 70588. MMD and CTQ were supported by the North and Central Texas Clinical and Translational Science Initiative from the NIH KL2 RR024983 and MMD is supported by the First American Real Estate Information Services, Inc. We thank John D. Nelson, M.D. and Michael R. DeBaun, M.D., M.P.H. for their valuable input.
Footnotes
Conflict of Interest
The authors have no conflicts of interest to report.
References
- 1.Steen RG, Emudianughe T, Hankins GM, et al. Brain Imaging findings in Pediatric Patients with Sickle Cell Disease. Radiology. 2003;228(1):216–225. doi: 10.1148/radiol.2281020943. [DOI] [PubMed] [Google Scholar]
- 2.DeBaun MR, Schatz J, Siegel MJ, et al. Cognitive screening examinations for silent cerebral infarcts in sickle cell disease. Neurology. 1998;50(6):1678–1682. doi: 10.1212/wnl.50.6.1678. [DOI] [PubMed] [Google Scholar]
- 3.Schatz J, Brown RT, Pascual JM, et al. Poor school and cognitive functioning with silent cerebral infarcts and sickle cell disease. Neurology. 2001;56(8):1109–1111. doi: 10.1212/wnl.56.8.1109. [DOI] [PubMed] [Google Scholar]
- 4.White DA, Moinuddin A, McKinstry RC, et al. Cognitive Screening for silent cerebral infarction in children with sickle cell disease. J Pediatr Hematol Oncol. 2006;28(3):166–169. doi: 10.1097/01.mph.0000203720.45448.ea. [DOI] [PubMed] [Google Scholar]
- 5.Miller ST, Macklin EA, Pegelow CH, et al. Silent infarction as a risk factor for overt stroke in children with sickle cell anemia: A report from the Cooperative Study of Sickle cell disease. J Pediatrics. 2001;139(3):385–390. doi: 10.1067/mpd.2001.117580. [DOI] [PubMed] [Google Scholar]
- 6.Mercuri E, Faundez JC, Roberts I. Neurological ‘soft’ signs may identify children with sickle cell disease who are at risk for stroke. Eur J Pediatr. 1995;154:150–156. [PubMed] [Google Scholar]
- 7.DeBaun MR, Derdeyn CP, McKinstry RC. Etiology of Strokes in Children with Sickle Cell Anemia. MRDD Research Reviews. 2006;12:192–199. doi: 10.1002/mrdd.20118. [DOI] [PubMed] [Google Scholar]
- 8.Kinney TR, Sleeper LA, Wang WC, et al. Silent Cerebral Infarcts in Sickle Cell Anemia: A Risk Factor Analysis. Pediatrics. 1999;103:640–645. doi: 10.1542/peds.103.3.640. [DOI] [PubMed] [Google Scholar]
- 9.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular Accidents in Sickle Cell Disease: Rates and Risk Factors. Blood. 1998;91(1):288–294. [PubMed] [Google Scholar]
- 10.Adams R, McKie V, Nichols F, et al. The use of transcranial ultrasonography to predict stroke in sickle cell disease. N Engl J Med. 1992;326(9):605–610. doi: 10.1056/NEJM199202273260905. [DOI] [PubMed] [Google Scholar]
- 11.Balkaran B, Char G, Morris JS, et al. Stroke in a cohort of patients with homozygous sickle cell disease. J Pediatr. 1992;120(3):360–366. doi: 10.1016/s0022-3476(05)80897-2. [DOI] [PubMed] [Google Scholar]
- 12.Quinn CT, Miller ST. Risk Factors and prediction of outcomes in children and adolescents who have sickle cell anemia. Hematol Oncol Clin N Am. 2004;18:1339–1354. doi: 10.1016/j.hoc.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Muir KW, Buchan A, von Kummer R, et al. Imaging of acute stroke. Lancet Neurol. 2006;5:755–768. doi: 10.1016/S1474-4422(06)70545-2. [DOI] [PubMed] [Google Scholar]
- 14.Das RR, Seshadri S, Beiser AS, et al. Prevalence and correlates of silent cerebral infarcts in the Framingham Offspring Study. Stroke. 2008;39:2929–2935. doi: 10.1161/STROKEAHA.108.516575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oppenheim C, Lamy C, Touze E, et al. Do Transient Ischemic Attacks with Diffusion-Weighted Imaging Abnormalities Correspond to Brain Infarctions? AJNR. 2006;27:1782–1787. [PMC free article] [PubMed] [Google Scholar]
- 16.Kirkham FJ, Lerner NB, Noetzel M, et al. Trials in sickle cell disease. Pediatr Neurol. 2006;34:450–458. doi: 10.1016/j.pediatrneurol.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Buchanan GR, DeBaun MR, Quinn CT, Steinberg MH. Sickle Cell Disease. Hematology (Am Soc Hematol Educ Program) 2004:35–47. doi: 10.1182/asheducation-2004.1.35. [DOI] [PubMed] [Google Scholar]
- 18.Wierenga KJ, Serjeant GR. Cerebrovascular complications and parvovirus infection in homozygous sickle cell disease. J Pediatr. 2001;139(3):438–442. doi: 10.1067/mpd.2001.117070. [DOI] [PubMed] [Google Scholar]
- 19.Zimmerman RA. MRI/MRA evaluation of sickle cell disease of the brain. Pediatr Radiol. 2005;35:249–257. doi: 10.1007/s00247-005-1420-z. [DOI] [PubMed] [Google Scholar]