Abstract
Spinal cord contusion produces a central lesion surrounded by a peripheral rim of residual white matter. Despite stimulation of NG2+ progenitor cell proliferation, the lesion remains devoid of normal glia chronically after spinal cord injury (SCI). To investigate potential cell-cell interactions of the predominant cells in the lesion at 3 days after injury, we used magnetic activated cell sorting to purify NG2+ progenitors and OX42+ microglia/macrophages from contused rat spinal cord. Purified NG2+ cells from the injured cord grew into spherical masses when cultured in defined medium with FGF2 plus GGF2. The purified OX42+ cells did not form spheroids and significantly reduced sphere growth by NG2+ cells in co-cultures. Conditioned medium from these OX42+ cells, unlike that from normal peritoneal macrophages or astrocytes also inhibited growth of NG2+ cells, suggesting inhibition by secreted factors. Expression analysis of freshly purified OX42+ cells for a panel of 6 genes for secreted factors showed expression of several that could contribute to inhibition of NG2+ cells. Further, the pattern of expression of four of these, TNFα, TSP1, TIMP1, MMP9, in sequential coronal tissue segments from a 2 cm length of cord centered on the injury epicenter correlated with the expression of Iba1, a marker gene for OX42+ cells, strongly suggesting a potential regional influence by activated microglia/macrophages on NG2+ cells in vivo after SCI. Thus, the non-replacement of lost glial cells in the central lesion zone may involve, at least in part, inhibitory factors produced by microglia/macrophages that are concentrated within the lesion.
Keywords: glial progenitors, NG2, macrophage, proliferation, spinal cord injury, cell culture, MACS
INTRODUCTION
Traumatic spinal cord injury (SCI) evokes a complex cascade of events with initial mechanical damage leading to secondary injury processes that contribute to further tissue loss and functional impairment (Hagg and Oudega 2006). Studies of clinically relevant rodent contusion injury models have described the patterns of secondary neuronal (Grossman et al. 2001) and axonal loss (Rosenberg and Wrathall 1997). There is also a massive glial cell loss in initially spared tissue, including a 50% or greater loss of oligodendrocytes from spared white matter by 1 or 2 days after injury (Grossman et al. 2001; Lytle and Wrathall 2007; Rabchevsky et al. 2007). This loss of oligodendrocytes and the associated axonal demyelination contribute to neurological dysfunction after SCI, as demonstrated by the beneficial effects of treatments that reduce oligodendrocyte loss (Rosenberg et al. 1999) or provide exogenous cells to enhance remyelination (Cao et al. 2005; Cummings et al. 2005; McDonald et al. 1999). However, SCI also results in the initiation of a number of processes that provide some degree of repair for the injured spinal cord (Beattie et al. 1997). One of these is stimulation of proliferation of endogenous glial progenitors that exist in the adult spinal cord and that express the NG2 proteoglycan (Horner et al. 2000).
During development, oligodendrocyte precursor cells (OPCs) express the NG2 proteoglycan which is down-regulated as they differentiate into mature oligodendrocytes. In the adult CNS there is also a significant population of NG2+ cells probably not all of which function as OPCs (Butt et al. 2002; Nishiyama 2007). After SCI, NG2+ cells in the spared tissue adjacent to the injury site begin to proliferate at a significantly higher rate than in uninjured spinal cord. Peak proliferation in spared ventral white matter is at three days after injury, but it continues at a high rate for at least a week and remains significantly above that in uninjured spinal cord for a month or more (Lytle et al. 2009; Lytle and Wrathall 2007; McTigue et al. 2001; Zai and Wrathall 2005). Cells that incorporate BrdU in the first week after SCI can be detected chronically at 6 weeks after injury and include oligodendrocytes and, to a lesser extent, astrocytes, as well as cells that express NG2 (Lytle and Wrathall 2007; Zai and Wrathall 2005). By 6 weeks or later after SCI, the density of oligodendrocytes in the spared ventral white matter is at or near normal (Rabchevsky et al. 2007; Rosenberg et al. 2005) and most axons at least in the ventral medial region of spared white matter appear to be remyelinated, albeit with abnormalities (Wrathall et al. 1998). However, in the central lesion area, virtually no oligodendrocyte-myelinated axons are present. Chronically, this region consists of empty-appearing cavities, accumulations of activated microglia/macrophages containing phagocytosed debris, and variable numbers of Schwann cell-myelinated axons (Lu and Wong 2007; Wrathall et al. 1998). The absence of oligodendrocytes in the central lesion chronically after SCI is strikingly evident in a recent study using the CNP-EGFP mouse (Supp. Fig. 1) (Lytle et al. 2009) in which all oligodendrocyte lineage cells are fluorescently labeled (Yuan et al. 2002).
The major cells contributing to the massive inflammatory response after SCI are resident microglia and invading macrophages which peak between 3–7 days post-injury (Popovich et al. 1997). In the context of oligodendrogenesis in normal adult spinal cord, it has recently been reported that proliferation and differentiation of NG2+ progenitor cells can be potently influenced by activated macrophages (Schonberg et al. 2007). Macrophage activation stimulated by non-traumatic, microinjection of zymosan into the spinal cord inhibited NG2+ cell proliferation resulting in complete oligodendrocyte loss and lack of replacement. In contrast, activation by injection of lipopolysaccaride (LPS) increased NG2+ cell proliferation to an even greater extent than seen after lysolecithin-induced demyelination.
Studies have shown potential roles of factors secreted by microglia/macrophages on the survival of macroglial cells. For instance, Hamanoue et al (2004) showed that tumor necrosis factor (TNF-α) induces apoptosis of oligodendrocyte precursor cell lines. The effects of interactions between NG2+ cells and activated microglia/macrophages after SCI, however, have not previously been examined. We, therefore, used immunocytochemistry to examine the spatial distribution of these cell types in vivo in the first week after SCI and employed a newly developed procedure for purifying NG2+ cells and microglia/macrophages from the injured spinal cord (Yoo and Wrathall 2007) to study their interactions in vitro. To identify factors that could modulate the NG2+ cell responses, mRNA expression for a panel of 6 genes was compared for these purified microglia/macrogphages from the injured spinal cord to the patterns in tissue from normal and injured spinal cord and for control and experimentally activated peritoneal macrophages.
MATERIALS AND METHODS
Spinal Cord Injury
Adult female Sprague-Dawley rats weighing 220–260 g were subjected to an incomplete contusive SCI at the T8 level to create a “Mild” or “Moderate” SCI (Wrathall et al. 1985), as extensively characterized in previous reports. After surgery, rats were placed on a heating pad until fully recovered from anesthesia. Urine was expressed manually twice a day until reliable reflex bladder emptying was established. The experimental protocols were approved by the Georgetown University Animal Care and Use Committee.
Immunohistochemistry
To examine the distribution of NG2+ cells and microglia/macrophages at 3 days and 1 week after SCI, rats were reanesthetized and transcardially perfused with 0.1 M phosphate-buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde (PFA) in PBS. After post-fixation and cryopreservation, a 2.0-cm segment of spinal cord centered at the epicenter of the injury was sectioned at 10-µm thickness and thaw-mounted onto Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA). Sections representing sequential tissue locations were stained with Eriochrome-cyanine RC for myelinated white matter, hematoxylin for cell nuclei and eosin/phloxine to visualize cytoplasm. The lesion epicenter was defined as the location with the least amount of spared white matter. Longitudinal parasagittal sections were cut on a cryostat set at 14 µm intervals.
For immunolabeling, sections were incubated overnight at 4°C with a primary polyclonal rabbit antibody to NG2 (1:300; Chemicon, Temecula, CA) and monoclonal mouse anti-Cd11b (OX42, 1:300; Serotec, Raleigh, NC) and then with fluorescent-conjugated secondary antibodies (Alexa 555-conjugated goat anti-rabbit, 1:400; Alexa 488-conjugated goat anti-mouse, 1:400. Molecular Probes, Carlsbad, CA). Controls included sections from normal, uninjured spinal cord at the T8 level and sections of normal and injured cord subjected to similar processing without the primary antibody or secondary antibody. The latter was important to distinguish endogenous autofluorescence that can be present in macrophages with phagocytosed debris. Cell nuclei were labeled with TO-PRO-3 (1:1000, Invitrogen, Carlsbad, CA). Immunofluorescence was visualized by tile scan using a Zeiss LSM 5 confocal laser-scanning microscope.
Immunomagnetic Activated Cell Sorting (MACS)
Rats were anesthetized at the specified time after injury and the spinal cord was removed aseptically and placed in sterile ice-cold isolation solution containing Hanks’ Balanced Salt Solution (HBSS, Invitrogen, Carlsbad, CA), 1 mM HEPES (Invitrogen) and 25 µg/ml gentamycin (Invitrogen). Under a dissection microscope, the dura was removed, and a 2.0-cm spinal cord segment centered on the injury site (or equivalent thoracic region of an uninjured control) (Fig. 2A) was minced and then incubated in Minimal Essential Medium (MEM; Invitrogen) containing 0.25% trypsin (Invitrogen) and 50 U/ml DNase I (Sigma, St. Louis, MO) for 10 min at 37°C. After washing thoroughly with DMEM containing 10% Fetal Bovine Serum (FBS, Invitrogen), the tissue was triturated using a 1-ml micropipette tip and centrifuged for 10 min at 500 × g. The cell pellet was resuspended in 10 ml of isolation solution, and myelin was removed using a 30% pre-centrifuged gradient of Percoll (Amersham Pharmacia Biotech, Piscataway, NJ) with centrifugation at 30,000 × g for 45 min at 4°C. The cell layer between the white myelin layer and the red blood cell pellet was collected and washed once in cold isolation solution by centrifugation for 15 min at 500 × g. Finally, the cell pellet was resuspended in 500 µl PBS containing 0.5% BSA and 2 mM EDTA (MACS buffer).
Figure 2.
Schematic presentation of the procedure for rapid and concurrent purification of OX42+ or NG2+ cells from adult rat spinal cord. A: A 2-cm spinal cord is dissected from each laminectomy control rat and from injured rats at 3 days or up to 12 weeks after SCI. B: A cartoon diagram showing the MACS-purification procedures.
MACS was applied to purify NG2+ and OX42+ cells from the normal or injured spinal cord (Yoo and Wrathall 2007). The cell suspension obtained after Percoll gradient centrifugation was incubated with the OX42 antibody (IgG2a, 1:100; Serotec) for 15 min on ice (Fig. 2B). After washing with MACS buffer, the cell pellet was resuspended in 80 µl MACS buffer and incubated with 20 µl of rat anti-mouse IgG2a+b MicroBeads (Miltenyi Biotec, Auburn, CA) for 10 min at 4°C, followed by washing once with MACS buffer. The cell pellet was resuspended in 500 µl of MACS buffer and applied onto a premoistened MACS column which had been placed in the magnetic field of the MACS separator. The column was washed with MACS buffer, and the bound OX42+ cells were then collected by removing the column from the magnet and passing 1 ml of the buffer through the column using a plunger, according to the manufacturer’s instructions, to produce the OX42 elute. The pass-through fluids containing cells that had not bound to the column were also harvested and washed once by centrifugation. This cell pellet was resuspended and incubated with rabbit anti-NG2 polyclonal antibody (IgG, 1:100; Chemicon) for 1 h at room temperature with shaking. After washing in MACS buffer, the pellet was resuspended in 80 µl of MACS buffer mixed with 20 µl of goat anti-rabbit IgG MicroBeads (Miltenyi Biotec) and incubated for 15 min at 4°C. The cell suspension was washed by centrifugation, resuspended in MACS buffer and then applied onto a new pre-moistened column in the magnetic field of the MACS separator. The magnetically labeled NG2+ cells were collected by flushing the column with MACS buffer after the column was removed from the separator to produce the NG2 elute. The pass-through fluids were also harvested, washed once by centrifugation and designated the final elute.
Primary Free-Floating Sphere Cultures
Cell suspensions were counted by hemocytometer using trypan blue exclusion to estimate cell yield and viability, then plated onto uncoated 96-well plates at a density of 2,000 viable cells/well in DMEM/F12/1×B27 supplemented with 1% FBS and/or growth factors as described in results. The growth factors tested include basic fibroblast growth factor (FGF2, 20 ng/ml, Peprotech, Rocky Hill, NJ), recombinant human glial growth factor 2 (GGF2, 200 ng/ml, Accorda Therapeutics, NY), and murine epidermal growth factor (EGF, 20 ng/ml, Peprotech). Cultures were maintained at 37°C with 5% CO2 atmosphere and one-third of the medium changed twice a week. In the absence of a substrate that supported adhesion, cells started forming spheres after 3–4 days in vitro. Free-floating spheres in each well were characterized as ≤50 µm, 50–100 µm, or ≥100 µm in diameter and counted using an inverted phase contrast microscope at 7, 14, and 21 days in vitro (Lu and Wong 2007). For estimating the total number of cells that had formed spheres in a well, the floating spheres were collected after shaking at 100 rpm for 10 min (Orbital Shaker, ArmaLab, LLC, Bethesda, MD) and washed in divalent cation-free PBS. Each pellet of spheres was incubated in 0.025% trypsin for 10 min at 37°C. Any cells that remained in the well were washed once with a divalent cation-free PBS and incubated in 0.025% trypsin for 10 min at 37°C. The enzymatically dissociated cells were combined, counted by hemocytometer, and the total cells from each well were calculated.
To examine the properties of spheres, individual spheres after 2–3 weeks in culture were transferred with a pipette onto PDL-coated glass coverslips in DMEM/F12/1×B27 medium supplemented with 1% FBS. After 2 h in such adherent culture conditions, the coverslips were fixed and processed for immunocytochemistry with rabbit anti-NG2 (1:400, Chemicon) and a mouse anti-nestin (rat-401, 1:50, Developmental Studies Hybridoma Bank, Iowa City, IA).
Peritoneal Macrophages
To obtain peritoneal macrophages, rats were injected with sodium periodate (5 mM; 5 ml) intraperitoneally once a day for 3 days. At 24 h after the last injection, the rats were euthanized, and the peritoneal cavity opened. Sterile, pre-warmed (37°C) PBS containing 0.5 ml heparin (20 ml) was added to the cavity, and the belly was gently massaged to mix. Using a sterile, blunt ended glass tube the peritoneal fluid was gently removed from the cavity and placed into a tube at 4°C. The cavity was washed a second time with PBS, and the cells removed to a 50 ml centrifuge tube on ice. If red blood cells were present, they were removed by exposing cells to hypotonic media for 30 sec. The suspended cells were pelleted, and the pellet resuspended in DMEM containing 10% FBS, 2 mM glutamine and 100 U/ml pen/strep. Cells were then plated at high density into 6 well plates and allowed to adhere for 6 h. Cells were washed with fresh media and treated with either LPS (100 ng/ml) or IL-4 (80 ng/ml) diluted in media or remained untreated for an additional 15 h, to activate macrophages by the classical (LPS) or a major alternative (IL-4) pathway (Gordon 2003; Martinez et al. 2008). Media were then removed, and cells were scraped into RLT buffer (Qiagen, Valencia, CA) with 1% β-mercaptoethanol.
Astrocyte Cultures
Primary astrocytes were cultured from the cerebral cortices of 1- to 2-day-old SD rat as described (Jakovcevski et al. 2007). The cells were grown in DMEM/F12/10% FBS at 37°C in a CO2 incubator. The culture medium was changed 2–3 times weekly. At confluence (days 7 or 8 after plating), the flasks were shaken at 200 rpm for 6 h at 37°C to remove microglia. On days 8–10, the cultures were continuously shaken at 200 rpm and 37°C to remove oligodendroglia and the adherent astrocytes were subcultured.
Conditional Media
The conditioned media were generated from OX42+ cells purified from injured spinal cord, normal peritoneal macrophages, and astrocytes. Cells were plated onto a 25 cm2-cell culture flask at a density of 10,000–20,000 cells/cm2 in DMEM/F12/1% FBS and maintained for 1 week. At the end of 7 days, the medium was collected by centrifuging at 2,000×g for 15 min and filter-sterilized. The resulting medium was combined with an equal volume of fresh DMEM/F12 medium to produce the conditioned medium (CM).
RNA Extraction and Real-time PCR
To investigate factors produced by the microglia/macrophages from the injured spinal cord that could influence the growth of NG2+ cells, a series of 5 preparations of MACS purified OX42+ cells from 5 different rats at 3 days after SCI were harvested, immediately frozen at −80°C and used to prepare RNA. The RNA from the 5 preparations was combined and cDNA prepared for analysis by quantitative RT PCR. Controls for this study included normal female rat peritoneal macrophages and these cells activated by injection of IL-4 or LPS, 3 preparations of each.
RNA was also extracted from sequential 2 mm thick coronal tissue segments from a 2 cm length of cord centered on the injury epicenter after 3 days of SCI and from equivalent segments of uninjured controls (n = 5 rats per group) to compare the expression levels of the six genes correlated with that of Iba1, a marker gene for microglia/macrophages.
Total RNA was extracted using the Qiagen RNeasy kit (Qiagen, Valencia, CA). RNA concentrations were determined and cDNA produced using the cDNA archive kit (Applied Biosystems, Foster City, CA). Real-time PCR was performed using the TaqMan Gene Expression assay kit (Applied Biosystems) according to the manufacturer’s instructions and as previously described (Colton et al. 2006). The expression of each gene was normalized to 18S rRNA, and results were shown as fold change versus the normal rat macrophages (n = 3) in the study of purified cells, and normal spinal cord (n = 4) for the study of spinal cord tissue. The following genes were analyzed: 18S (ID#Hs99999901_s1), tissue inhibitor of matrix metaloprotease-1 (TIMP1, ID# Rn01430875_g1), matrix metaloprotease- 9 (MMP9, ID# Rn00675895_g1), nerve growth factor (NGF, ID# Rn0153872_m1), insulin-like growth factor-1 (IGF1, ID# Rn00710306_m1), TNFα (ID# Rn99999017_m1), thrombospondin (TSP, ID# Mm01335418_m1), and ionized binding calcium adapter molecule 1 (Iba1, ID# Rn00574125_q1).
Statistical Analysis
All tissue culture experiments were repeated at least three times using independent culture preparations from different sets of rats. Data are presented as group mean values with standard errors of the mean (SEM) where n equals the number of separate culture preparations from individual animals, or when indicated, show data from representative individual studies where n equals the numbers of wells or coverslips analyzed. Statistical analysis was conducted by one-way analysis of variance followed by post hoc comparisons using Student’s t test with Bonferroni corrections, or Dunett’s test to compare injured samples to controls. The threshold value for significance was 0.05.
RESULTS
Distribution of NG2+ Cells and Microglia/macrophages in situ After SCI
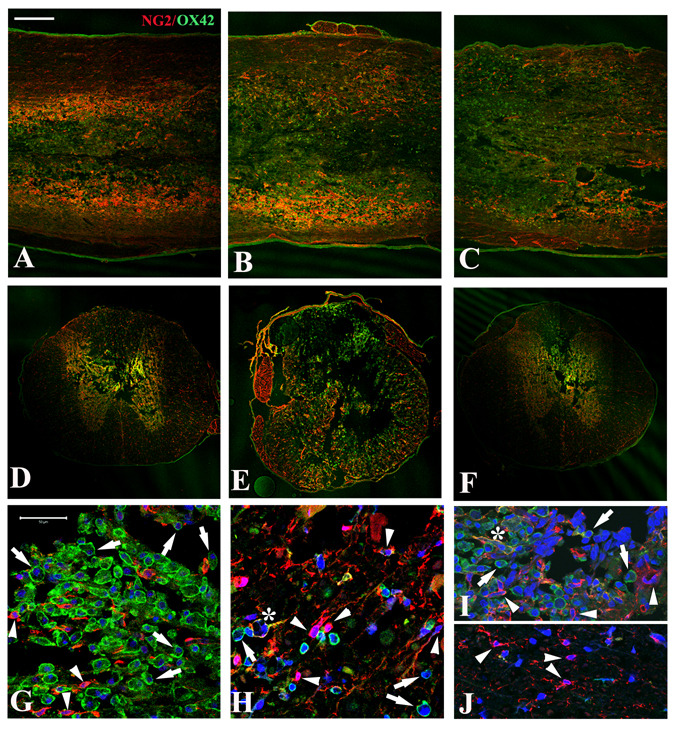
At 3 days after spinal cord contusion, large numbers of OX42+ activated microglia and macrophages and NG2+ cells were evident in a 2 cm length of cord centered on the injury site (Fig. 1). Longitudinal and coronal sections double stained with OX42 and NG2 antibodies showed the microglia/macrophages were most concentrated in the central lesion, extending from rostral (Fig. 1A) to the lesion epicenter (Fig. 1B) and caudal (Fig. 1C) over the entire 2 cm length of spinal cord. NG2 labeling tended to be more evident in cells located in the preserved tissue surrounding the central lesion, particularly in a border zone, immediately adjacent to the central lesion (Fig. 1). Confocal images of coronal sections showed evidence of many OX42+ macrophages at the injury epicenter and also in the tapered lesion area rostral and caudal to it (Figs. 1E, G, I), confirming that macrophage invasion was extensive by 3 days after the injury. OX42-labeled cells in the lesion area were typically grouped in clusters (Fig. 1G). OX42-labeled cells were also present in preserved spinal cord tissue surrounding the lesion area (Fig. 1H), but at a lower apparent density.
Figure 1.
Distribution of NG2+ cells and microglia/macrophages in situ at 3 days after spinal cord injury. A–C: Horizontal sections through the dorsal funiculus of injured spinal cords show strong immunoreactivity with NG2 (red) and OX42 (green) antibodies at the injury epicenter (B) as well as rostral (A) and caudal (C) to it. D–F: Coronal sections at the injury epicenter (E) and 5 mm rostral (D) or caudal (F) to it show that OX42+ cells are concentrated in the lesion with NG2+ cells more evident in the surrounding spared tissue, especially in the spared tissue bordering the lesion. Scale bar for A–F = 500 µm. G–J: At higher magnification, the distribution of OX42+ and NG2+ cells in different regions is seen more clearly. In the lesion epicenter (G), numerous OX42+ cells (green, arrows) are present in clusters as well as some NG2+ cells. Both types of cells are present in the residual lateral white matter at the epicenter (H), but with more NG2+ cells (red, arrowheads) and fewer OX2+ cells (green, arrows). Occasional NG2+/OX42+ cells (H) are also present (yellow, *). At 4 mm rostral to the epicenter, the tapering dorsal-central lesion area (I) contains both OX42+ and NG2+ cells similar to the lesion at the epicenter (G), while only NG2+ cells appear in the preserved ventral white matter (J). Scale bars for G–J = 50 µm.
Many NG2+ cells were observed at 3 days after injury in the lesion areas and also in preserved white matter and gray matter (Figs. 1D–F, H, J), consistent with results of previous studies in both rats and mice (Lytle et al. 2009; Lytle and Wrathall 2007; Zai and Wrathall 2005). Some of the OX42-labeled cells in the lesion area expressed the NG2 proteoglycan although they were morphologically characterized as activated macrophages/microglia. In addition, we found that NG2+/P75+ Scwhann cells were rarely present at 3 days after SCI (data not shown) although many can be seen in the lesion area by 2 weeks and chronically after injury.
A similar distribution of NG2+ and OX42+ cells was seen in sections of spinal cord at 7 days after SCI. However, although the total NG2-immunoreactivity was at least as high, there appeared to be fewer NG2+ cells and even more OX42 immunoreactive cells in the overt lesion areas (data not shown). Based on these observations, cultures were prepared from 2 cm lengths of spinal cord centered on the injury epicenter (or an equivalent length of tissue from uninjured controls). The two major cell populations stimulated by SCI, NG2+ cells and OX42+ cells, were purified and cultured separately, and their interaction investigated in co-culture.
Free-Floating Sphere Formation from Purified NG2+ Cells
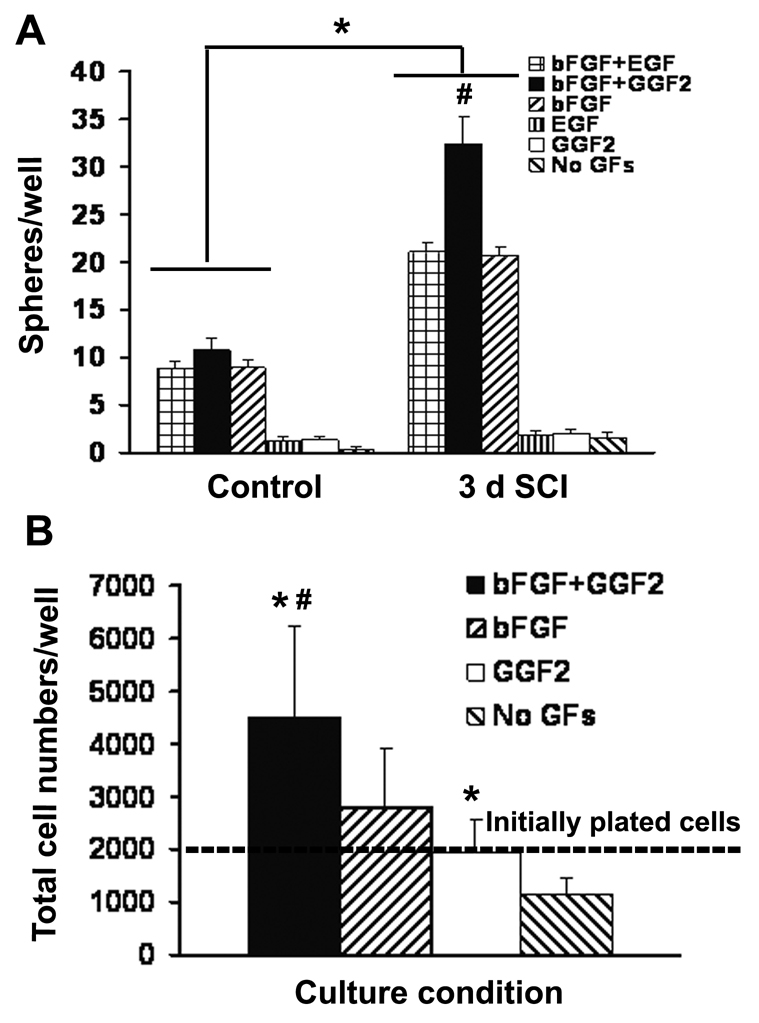
To examine the proliferative capacity of NG2+ cells from the normal and injured adult spinal cord, we set up free floating sphere cultures from MACS purified cells. We first examined whether defined culture medium (DMEM/F12/1×B27) would permit growth of spheres from purified NG2+ cells obtained from injured or control spinal cord in the absence of exogenous growth factors. After 1 week in culture, we did not see spheres formed in either group (Fig. 3A), suggesting that the culture medium was insufficient to support growth of spheres. As it is known that growth factors such as FGF2 and/or GGF2 are important for sustaining isolated progenitors (Frost et al. 2003; Shihabuddin et al. 1997; Yoo and Wrathall 2007), we investigated the effect of adding different exogenous growth factors on sphere formation from the purified cells. Culture of NG2+ cells with DMEM/F12/1×B27 supplemented by FGF2 supported sphere formation. Supplements of GGF2 or EGF alone were not effective. When we combined FGF2 with EGF, there was no additional effect on growth of NG2+ cells from the uninjured spinal cord. However, the addition of both FGF2 and GGF2 to cultures of NG2+ cells from injured rat spinal cord resulted in significant (about 60%) greater sphere formation than that with FGF2 alone or FGF2 plus EGF (Fig. 3A). This combination was used in subsequent studies.
Figure 3.
Effects of exogenous growth factors on sphere formation from purified NG2+ cells. Data are shown from a representative preparation of cells from tissue at 3 days after SCI and uninjured spinal cord. A: The total sphere number after 7 days in vitro is increased by the addition of FGF2 to the defined media. The combination of FGF2 and GGF2 further increases sphere production from NG2+ cells isolated from the injured spinal cord. Data represent mean ± SEM for n = 4 culture wells in each group. B: After 2 weeks in culture, the spheres in each culture well are harvested, dissociated with trypsin and counted by hemocytometer to estimate the total numbers of cells per well which increased with medium containing FGF2 and GGF2. Data represent mean ± SEM for n = 4 culture wells in each group. *Significantly different from uninjured controls (A) or medium without growth factors (B); #significantly different from any other condition.
To see whether increased sphere numbers was related to increased survival and/or proliferation of NG2+ cells in the presence of exogenous growth factors, we counted total cells per well after trypsinization (Fig. 3B). In the absence of growth factors, NG2+ cell survival was compromised. With GGF2 alone, the yield at 2 weeks was similar to the number of plated cells. With FGF2, the total cells were about 1.4 fold of the initially plated numbers, while the combination of FGF2 and GGF2 raised the total cell numbers to 2.3 fold, indicating that FGF2 plus GGF2 promotes higher proliferation of NG2+ progenitors from the injured spinal cord.
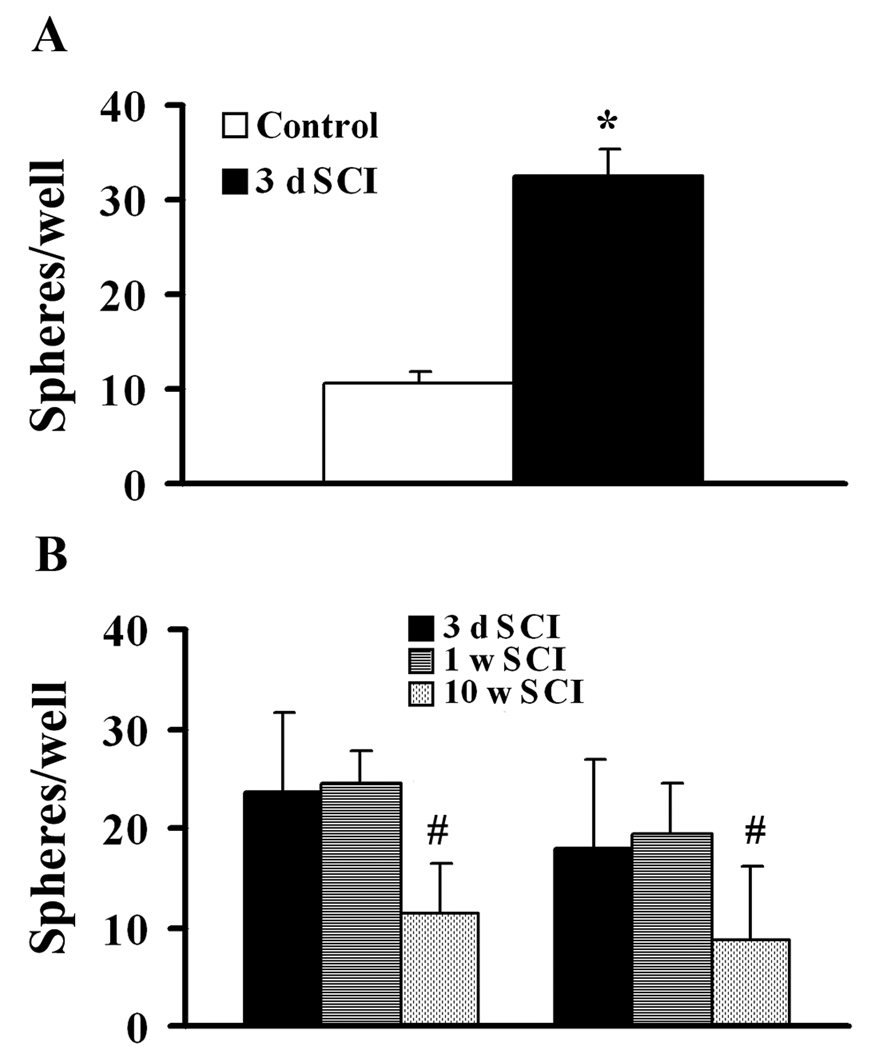
To determine whether spinal cord contusion stimulates the production of NG2+ cells with an increased potential for sphere formation, NG2+ cells were obtained from either injured or normal spinal cord and placed in culture at the same initial density. After 7 days of free-floating culture with media supplemented with FGF2 and GGF2, the total sphere numbers from NG2+ cells purified from tissue at 3 days after injury was 3.3 fold higher than that of an equivalent number of NG2 cells from the laminectomy control group (Fig. 4A), demonstrating that acute spinal cord injury stimulates the capacity of NG2+ progenitors to proliferate as spheres in vitro. As shown in Fig. 4B, there was no significant difference in sphere formation for NG2+ cells purified from tissue at 3 or 7 days after injury as evaluated after either 2 or 3 weeks in culture. However, although there were still twice the normal number of NG2+ cells in tissue from 10 weeks after SCI (data not shown), the capacity of these NG2+ cells for sphere formation was significantly reduced compared to animals at 3–7 days after SCI (Fig. 4B).
Figure 4.
Spinal cord injury stimulates sphere forming potential of NG2+ cells. A: NG2+ cells from tissue at 3 d SCI produce more spheres than an equal number of NG2+ cells from uninjured adult spinal cords. B: The sphere forming potential of NG2+ cells is similar at 3 and 7 days but decreases chronically by 10 weeks after SCI. N = 5 preparations. *Significantly different from control; #significantly different from the other groups.
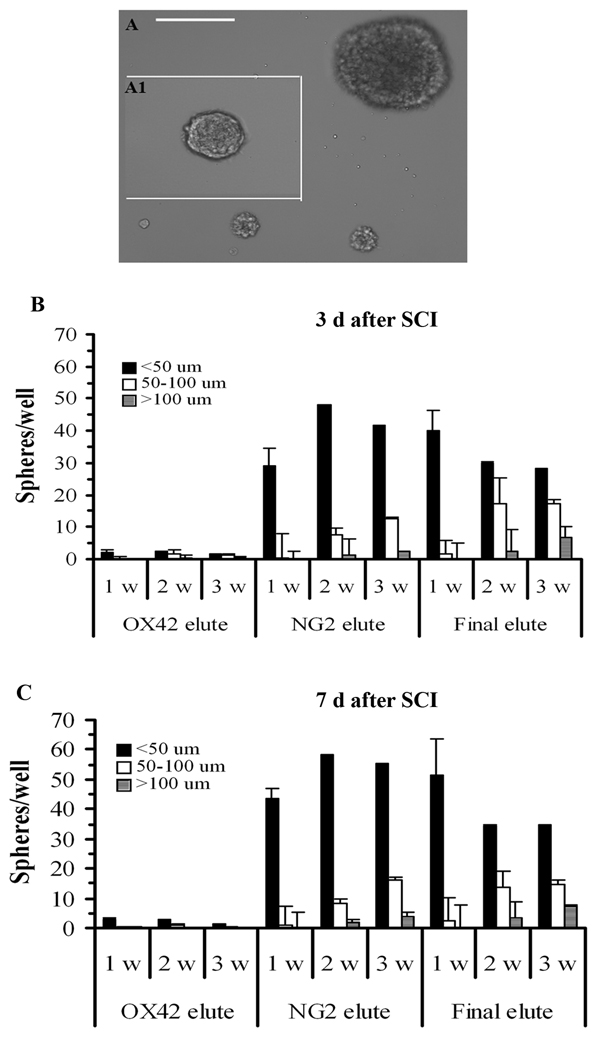
Comparison of Sphere Formation from the OX42, NG2, and Final Elutes
We compared sphere forming capacity of cells from the different MACS elutes. In the NG2-elute, spheres were observed by 3–4 days in vitro and increased to about 35 per well by 1 week (Fig. 5). The major sphere size at 1 week was ≤50 µm in diameter. With further culture, the number and size of spheres increased and enlarged, and larger size spheres (≥100 µm in diameter) appeared at 2 and 3 weeks in culture. Fig. 5A shows the appearance of different size spheres from a 2 week culture. Under the same culture conditions, only a few (≤3 per well) very small “spheres” were ever found in cultures from the OX42-elute. Interestingly, many, about 30–50 spheres per well, were found in cultures from the final-elute in which none of the cells were OX42+ and only a few (≤4 %) of the cells were NG2+ (Fig. 5B). More medium and large size spheres were observed at 2–3 weeks compared with the NG2-elute, suggesting that the NG2+ cell is not the only cell type in the injured spinal cord that can generate spheres. We also confirmed that there is no significant difference between 3 and 7 days after injury in terms of sphere formation capacity of the NG2+ cells (Fig. 5C).
Figure 5.
Comparison of sphere formation from cells in the OX42, NG2 and final elutes from MACS of cells from spinal cords at 3 and 7 days after SCI. A: Phase contrast micrographs showing different size spheres after 2 weeks culture. Scale bar = 100 µm. B: Cells in the NG2 elute and the final elute in preparations from 3 day SCI tissue form spheres that increase in size and numbers over 1–3 weeks in culture. C: Similar results are seen for cells purified from tissue at 7 days after SCI. Spheres are characterized as ≤50 µm, 50–100 µm, ≥100 µm in diameter and counted using an inverted microscope at days 7, 14, and 21 in vitro. N = 5.
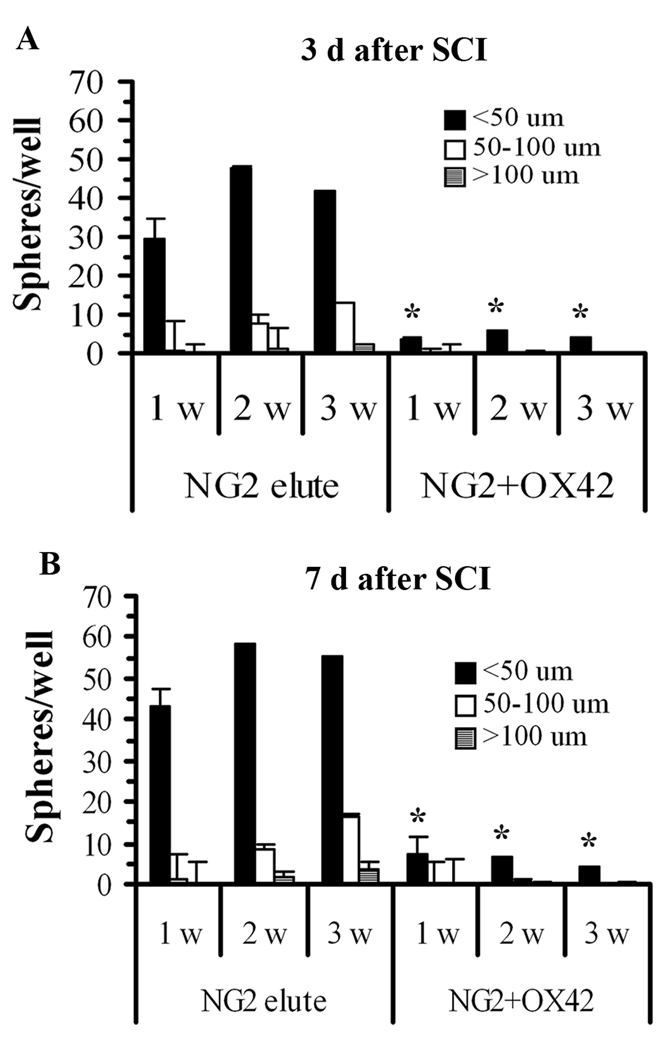
NG2+ Sphere Growth was Inhibited by OX42+ Cells or Conditioned Medium
To investigate the potential interaction of NG2+ and OX42+ cells in the injured spinal cord, we examined the effect of adding purified OX42+ cells or medium conditioned by them on sphere formation and growth of purified NG2+ cells. The overall yield of cells from the 3 day injured spinal cord was about 3:1 OX42+ cells to NG2+ cells, but because immunohistochemistry (Fig. 1) indicated that in the lesion zone the ratio appeared even higher, we plated co-cultures at a ratio of 5 OX42+ cells to 1 NG2+ cell. The co-cultures were grown under conditions that supported proliferation of both types of cells with defined media supplemented with FGF2 and GGF2 as well as 1% serum. NG2+ sphere growth was significantly reduced in the co-cultures by 1 week after plating (Fig. 6). Only about 5 small-sized spheres per well appeared at maximum over 3 weeks in culture, suggesting that activated macrophages from both 3 day (Fig. 6A) and 7 day (Fig. 6B) SCI tissue interfere with NG2+ cell proliferation and sphere formation in vitro.
Figure 6.
OX42+ cells inhibit sphere formation of purified NG2+ cells. A: The sphere-forming ability of NG2+ cells purified from 3 day SCI tissue is inhibited by the presence of microglia/macrophages purified from the same tissue. The numbers and sizes of spheres are significantly reduced in co-culture (5 OX42+:1 NG2+) at 1, 2 and 3 weeks in vitro. B: There is a similar pattern of sphere formation of cells derived from spinal cords at 7 days SCI. N = 5. In some cases the error bars are too small to be discerned. *Significantly different than equal number of NG2+ elute cells in the absence of OX42+ cells.
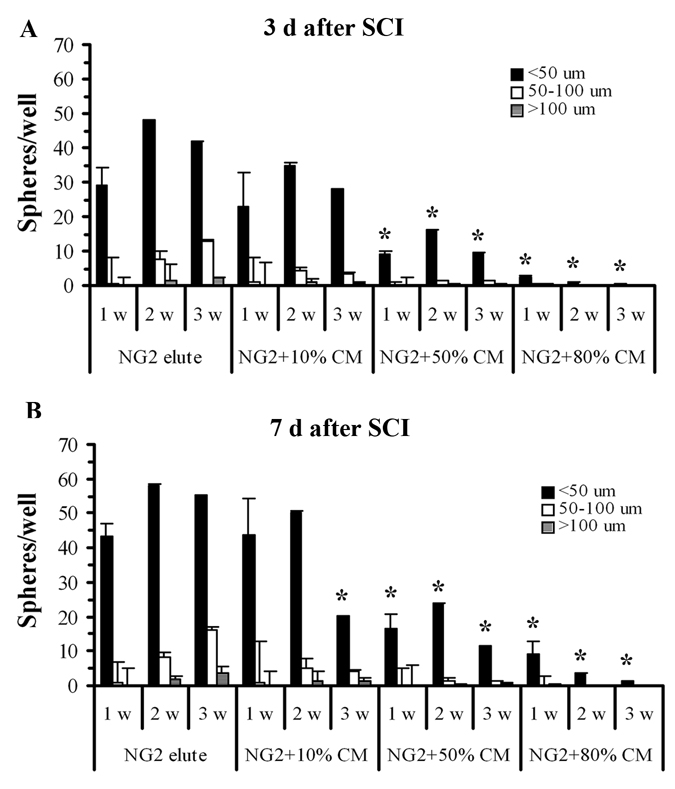
To investigate whether inhibition by microglia/macrophages of NG2+ cell growth was mediated by secreted factors, CM from cultured OX42+ cells purified from injured spinal cord tissue were applied in different concentrations to purified NG2+ cell cultures. We found that the numbers and sizes of the spheres were significantly inhibited when the cells were co-cultured with 50% or 80% CM. However, a low concentration of CM did not significantly affect sphere formation by NG2+ cells (Fig. 7A). Sphere formation by NG2+ cells purified from 7 day SCI tissue was also significantly reduced by conditioned medium in a dose-related manner (Fig. 7B), suggesting that factor(s) secreted by OX42+ cells during much of the first week after SCI could limit survival and proliferation of NG2+ cells.
Figure 7.
OX42+ cell-CM reduces the numbers and size of spheres from purified NG2+ cells. The numbers and sizes of the spheres are significantly inhibited when the purified NG2+ cells from 3 d SCI (A) or 7d SCI (B) tissue are co-cultured with medium conditioned by microglia/macrophages isolated from 3 or 7d injured spinal cord. N = 5. *Significantly different from the NG2+ elute cells in the absence of conditioned medium.
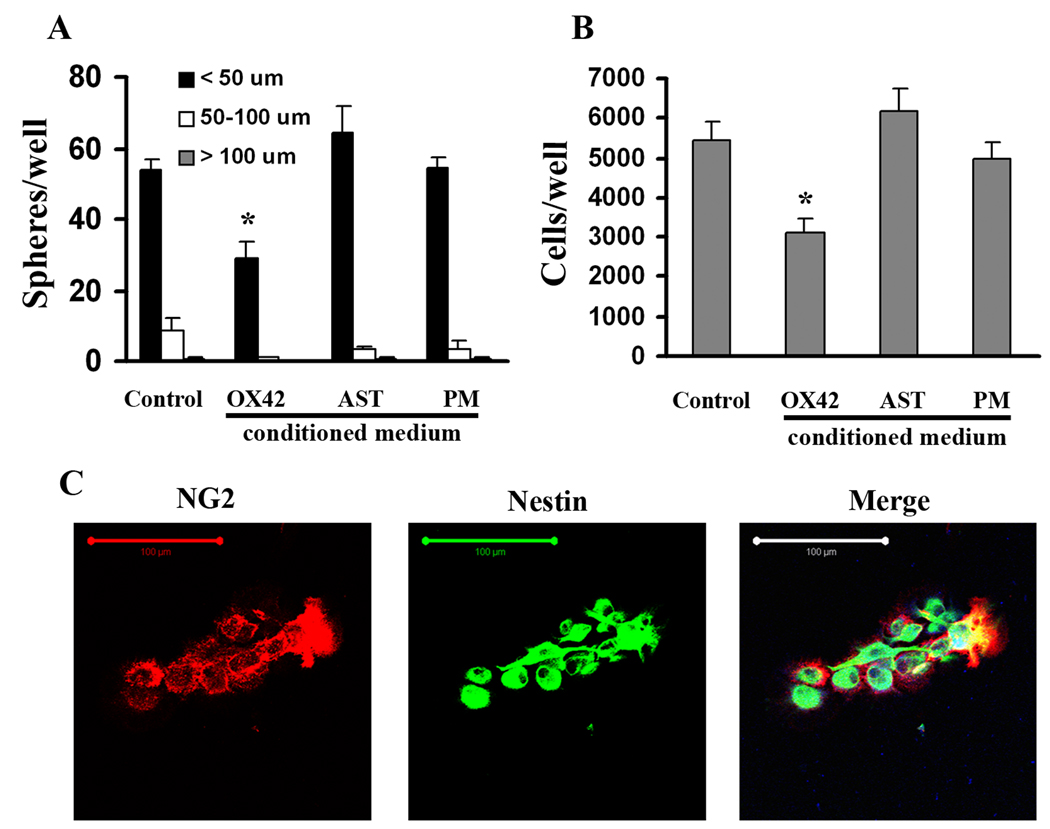
NG2+ Cell Growth is Not Inhibited by CM from Normal Peritoneal Macrophages or Astrocytes
To investigate the specificity of the inhibitory effects of OX42+ cells from the injured spinal cord on sphere formation of NG2+ cells, we prepared and tested CM from normal peritoneal macrophages and from astrocyte cultures. NG2+ cells purified from the injured spinal cord after 3 days SCI were cultured in uncoated 96-well plates at a density of 2,000 cells/well in DMEM/F12/1×B27 supplemented with FGF2 and GGF2. The CMs were diluted 1:1 with the growth medium to make 50% CM and applied to purified NG2+ cell cultures. We found that the numbers and sizes of the spheres in cultures with CM from normal peritoneal macrophages or astrocytes were similar to the control group (NG2+ cells without CM) after 2 weeks culture (Fig. 8A). However, there was significant reduction of sphere formation when NG2+ cells were cultured with the OX42+ cell-CM, indicating a specific inhibitory effect of activated microglia/ macrophages from the injured spinal cord. It also ruled out the possibility that sphere formation is compromised due to dilution of fresh nutrients in the medium by the addition of the CM.
Figure 8.
NG2+ cell growth is not inhibited by CM from normal peritoneal macrophages (PM) or astrocytes (AST). The numbers and sizes of the spheres (A) as well as total cell numbers (B) in the presence of the media conditioned by normal PM or AST were similar to the control group (NG2+ cells alone) after 2 weeks culture. There was reduction of sphere formation and total cell numbers when NG2+ cells were cultured with the OX42+ cell-CM. N = 3. *Significantly different from the NG2+ cells in the absence of conditioned medium. C: Representative cells from sphere culture with OX42+ cell-CM. All cells show co-expression of NG2 and nestin. Scale bar = 100 µm.
In addition, after 2 weeks free-floating culture, the spheres in each well were collected, enzymatically dissociated, and counted by hemocytometer. As shown in Figure 8B, the OX42+ cell-conditioned medium significantly reduced total cell numbers, while the conditioned medium from normal peritoneal macrophages or astrocytes did not exert a similar effect on NG2+ cell growth.
To examine the phenotype of cells in the spheres in the control and the OX42+ CM groups, we transferred individual spheres onto PDL-coated glass coverslips for 2 h, followed by fixation and immunocytochemistry. We analyzed 78 consecutive cells in the control group and 98 in the conditioned medium-treated group, and found that all were positive for both NG2 and nestin. Figure 8C shows representative cells from a culture grown with OX42+ CM.
Potential Inhibitory Factors Expressed by Microglia/macrophages from the Injured Spinal Cord
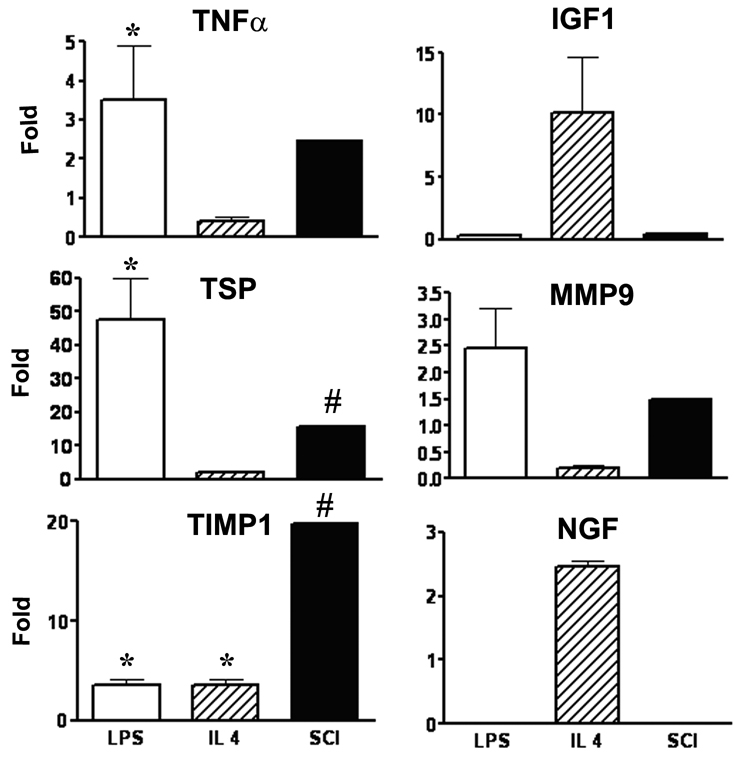
To identify factors that could be involved in the inhibitory effect of microglia/macrophages from the injured cord on NG2+ cells’ response, real-time quantitative RT-PCR was used to measure the relative expression of candidate genes for secreted factors that might be expected to impact growth of the NG2+ cell spheres in vitro and oligodendrogenesis in vivo. Since the cell yield of OX42 cells from normal, naïve spinal cord is extremely low, we used normal peritoneal macrophages as a control. As the expression pattern of activated microglia/macrophages from the injured spinal cord has not previously studied, we used two types of activated peritoneal macrophages as controls. LPS treatment was used to model the classical activation pathway and IL-4 treatment to induce a major alternative activation pathway (Gordon 2003). As shown in Figure 9, compared to normal peritoneal macrophages, the OX42+ cells purified from the injured spinal cord appeared to have dramatically increased expression of TSP, and TIMP1, that was beyond the 95% confidence limits for these genes in the control group. This pattern was different from that of IL-4 activated macrophages. The increase in TSP was similar to that of LPS-stimulated macrophages but these cells did not show the increased expression of TIMP1 demonstrated by the purified OX42+ cells. TNFα expression was significantly increased in the LPS-activated peritoneal macrophages and also increased beyond the range seen in the control group in the cells from the injured spinal cord but did not reach the 95% confidence limit. There was no evidence of NGF mRNA expression in the microglia/macrophages purified from the injured spinal cord.
Figure 9.
Comparison of alterations in gene expression in microglia/macrophages from the injured spinal cord (SCI) to normal peritoneal macrophages (PM) and peritoneal macrophages activated by lypopolysaccharide (LPS) or interleukin-4 (IL-4). Results are expressed as fold change from the average of control, normal peritoneal macrophages for 3 separate preparations of LPS and IL-4 activated macrophages and the combined RNA from 5 separate preparations of MACS-purified OX42+ cells from the spinal cord at 3 days after injury. *Significantly different from the normal peritoneal macrophages (ANOVA); #beyond 95% confidence limit of the control values.
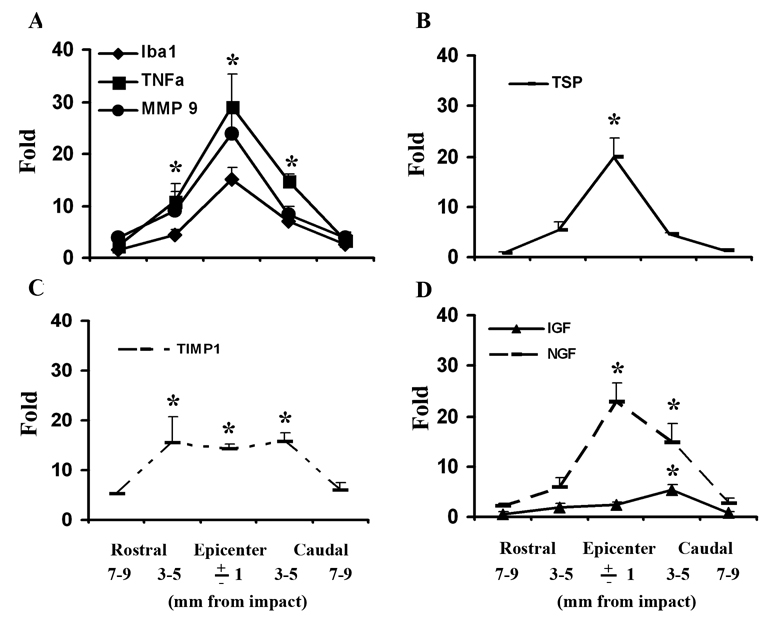
We also studied the pattern of expression of these genes, as well as for Iba1 (an expression marker for microglia/macrophages) in sequential 2 mm thick coronal tissue segments from a 2 cm length of cord centered on the injury epicenter at 3 days after SCI, and from uninjured controls (n = 5 per group). We found dramatically increased expression of Iba1, TNFα, and MMP9 at the injured epicenter and significant increases at 3–5 mm rostral and caudal it that correlated with the expression of Iba1 (Fig. 10A). Expression of TSP was only significantly higher at the epicenter (Fig. 10B). Expression of TIMP1, an inhibitor for MMP9 was extremely high at the epicenter, but also similarly increased at 3–5 mm rostral and caudal to it (Fig. 10C). Because of the different patterns of increased expression the ratio of MMP9/TIMP1 expression was much higher at the injury epicenter than it at the border areas rostral or caudal to it. The pattern of expression of IGF and of NGF did not appear to correlate with that of Iba1 (Fig. 10D).
Figure 10.
Patterns of gene expression in 2 mm thick coronal tissue segments from a 2 cm length of cord centered on the injury epicenter at 3 days after SCI. (A) There was significantly higher expression of Iba1, TNFα, MMP9 at the epicenter as well as at 3–5 mm rostral and caudal to it, compared with the uninjured tissue. (B) TSP was only significantly higher than control at the epicenter. (C) TIMP1 was more broadly up-regulated with similar high expression at the epicenter and 3–5 mm rostral and caudal to it. (D) The expression of IGF was only significantly altered at 3–5 mm caudal to epicenter, while NGF mRNA was found significantly greater at the epicenter as well as 3–5 mm caudal to it. N = 5. *Significantly different from the uninjured spinal cord.
DISCUSSION
We demonstrate that purified NG2-expressing cells from the injured adult spinal cord in the first week after SCI have the capacity to form spherical masses in culture, whose growth is stimulated by the combination of FGF2 and GGF2 and inhibited by purified endogenous microglia/macrophages from the injured spinal cord or medium conditioned by them. The inhibition of NG2+ cell growth by the CM from microglia/macrophages from the injured spinal cord appears specific, as similar effects are not seen with CM from normal peritoneal macrophages or cultured astrocytes. It is associated with increased expression of genes reported to affect OPC survival and/or differentiations that we detected in freshly purified OX42+ cells from the injured cord and also in segments of injured spinal cord tissue demonstrating high expression of a microglia/macrophage marker gene (Iba1) in vivo. Thus, our results suggest that products of microglia/macrophages may impede oligodendrogenesis in microglia/macrophage rich-lesion areas (niches) chronically after SCI.
We previously reported that MACS-purified NG2+ cells from the 3-day injured spinal cord subjected to clonal analysis can differentiate into oligodendrocytes or astrocytes (Yoo and Wrathall 2007). The current study demonstrates that NG2+ cells purified from injured adult rat spinal cord exhibit increased proliferative ability compared to NG2+ cells purified from the normal adult spinal cord. Robust sphere formation and growth also occurs in cultures of cells from the final elute, from which NG2+ cells had been removed. This provides evidence for the presence of other stem/progenitor cells in the injured spinal cord as has been postulated from BrdU labeling studies in vivo (Horky et al. 2006). Further studies in vitro will be needed to characterize these other stem/progenitor cells in terms of their origin, sensitivity to growth factors, differentiation potential, etc.
Neural stem/progenitor cells can be induced to proliferate in vitro in response to FGF2 (Shihabuddin et al. 1997), and a continuous supply of FGF2 is necessary to repress differentiation and to maintain a population of rapidly dividing stem/progenitor cells (Liu et al. 2005; Ostenfeld and Svendsen 2004). In our study, NG2+ cells proliferated in vitro and formed spherical masses in media supplemented with FGF2 at a concentration found effective previously (Liu et al. 2005; Shihabuddin et al. 1997), and we found no beneficial effect of added EGF. However, the addition of GGF2 in combination with FGF2 greatly enhanced the number and size of spheres from NG2+ cells purified from the injured spinal cord. In contrast, there was no additional effect for cultures from uninjured spinal cord. This apparent difference in sensitivity of NG2+ cells from the injured cord to GGF2 is interesting in the context of the up-regulation of FGF2 and GGF2 in spinal cord tissue after injury and the temporal-spatial correlation of the pattern of up-regulation of these growth factors and proliferation of NG2+ cells after injury (Zai and Wrathall 2005).
The proliferation of NG2+ cells after SCI is associated with the replacement of oligodendrocytes and astrocytes (and adult NG2+ cells) after SCI. Many NG2+ cells can be labeled with BrdU in the first week after SCI (Horky et al. 2006; Zai and Wrathall 2005), and many cells labeled in the first week express antigenic markers of mature CNS glia by 6 weeks after injury (Rosenberg et al. 2005; Zai and Wrathall 2005). Further, quantitative analysis of cells in the tissue surrounding the chronic lesion indicates that the glial cells lost acutely after injury have been replaced (Horky et al. 2006). However, the lesion itself remains largely devoid of astrocytes or oligodendrocytes, but contains many macrophages even chronically at 2 months or more after injury in the rat (Wrathall et al. 1998). In addition, by then axons myelinated by Schwann cells are present in the central lesion area (Beattie et al. 1997; Wrathall et al. 1998). These are believed to be derived from the p75+/NG2+ cells described in the lesion site in earlier weeks after injury and characterized as invading Schwann cells (McTigue et al. 2006). In this context, our finding inhibition of proliferation of cultured NG2+ cells isolated at 3 days after SCI by purified endogenous microglia/macrophages, or media conditioned by them, is intriguing. At this early time after injury NG2+/P75+ Schwann cells precursors are rare. They represent less than 2% of the dissociated cells from the 3 day injured spinal cord (unpublished results). Based on our previous studies, the NG2+ cells in the first week after SCI are primarily OPCs (Lytle et al. 2009) and bipotential glial progenitors (Yoo and Wrathall 2007). Collectively, our results suggest that the absence of CNS macroglial replacement in the central lesion after a clinically-relevant contusion injury may be due, at least in part, to factors produced and secreted by the predominant cell type in this location, activated microglia and macrophages, during the first week after injury. Of course, there are important caveats to studies of cells in culture, and such a hypothesis must be tested in vivo in the future.
However, if activated microglia/macrophages in the lesion inhibit oligodendrogenesis after SCI in vivo, what mechanisms may be involved? Our gene expression study of MACS purified OX42+ cells from the injured spinal cord indicates several possible factors. One gene expressed by microglia/macrophages from the injured spinal cord, TNFα, has been shown to induce apoptosis in an oligodendrocyte precursor cell line (Hamanoue et al. 2004) and is particularly toxic in the absence of sufficient IGF (Ye and D'Ercole 1999), a gene whose expression we found to be relatively low in the microglia/macrophages from the injured spinal cord and in the spinal cord tissue from the injury epicenter. The up-regulation of TSP1 seen in the purified cells and in epicenter tissue could also be important in inhibiting the growth of the NG2+ cells as, among other functions, TSP1 is known to bind to and sequester FGF2 (Margosio et al. 2003). FGF2 is important for the growth of the NG2+ neurospheres in culture as shown in the present study and is reported to play a significant role in remyelination in vivo (Frost et al. 2003; Mocchetti et al. 1996). MMP9 is also up-regulated in the cells from the injured spinal cord and is known to play a negative role in outcome after SCI, as demonstrated in MMP9 null mice (Noble et al. 2002). However, other studies have shown that MMP9 is essential for oligodendrocytes process outgrowth and its expression is correlated with myelination of the corpus callosum (Oh et al. 1999). Further, MMP9 knock out mice have impaired remyelination (Larsen et al. 2003) and the tissue inactivator of MMP9, TIMP1, can decrease oligodendrocytes process outgrowth in culture (Uhm et al. 1998). We find up-regulation of both MMP9 and TIMP1 expression in activated microglia/macrophages from the injured spinal cord and high expression of both in injured tissue from the epicenter. However, the regional pattern of TIMP1 up-regulation after SCI strongly suggests that cells other than microglia/macrophages must be considered as TIMP1 is similarly increased in regions with both very high and also those with much more moderate expression of Iba1. Most intriguingly, if the expression profiles reflect protein levels, the ratio of MMP9 to TIMP1 differs markedly at the injury epicenter compared to adjacent border regions. This possibility and its potential effect on oligodendrogenesis after SCI in these different regions deserve attention in future studies.
Despite the recent report concluding that production of proNGF by microglia is an important mediator of oligodendrocyte apoptosis after SCI (Yune et al. 2007), we did not detect mRNA for NGF in the microglia/macrophages from the injured spinal cord, and NGF mRNA was also very low in the LPS and IL-4 activated peritoneal macrophages.
The interaction between OPCs and inflammatory cells has been extensively studied in the context of Multiple Sclerosis [for review, see (Franklin and Kotter 2008)]. Removal of myelin debris by phagocytic macrophages is necessary for OPC differentiation after demylination. Activation of microglia by injection of LPS into intact spinal cord appears to stimulate NG2+ cell proliferation (Schonberg et al. 2007). Similarly, although there are many microglia/macrophages in the residual spinal cord white matter after SCI, NG2+ cells proliferate in these regions in vivo, and mature oligodendrocytes lost after SCI are replaced by relatively successful oligodendrogenesis (Zai and Wrathall 2005). However, in the lesion per se, oligodendrocytes are lost, but are not replaced even chronically after injury. Our results suggest that the difference is that the very high concentration of endogenous microglia/macrophages and factors secreted by them may contribute to an inhibitory microenvironment in the lesion per se that interferes with the proliferation of endogenous NG2+ oligodendorcyte progenitor cells. We postulate that this inhibition may play a role in creating the typical chronic central lesion seen in clinically-relevant contusion injury in which astrocyte and oligodendrocyte replacement does not occur.
Supplementary Material
Loss of oligodendrocyte lineage cells from the lesion area chronically after SCI in the CNP-EGFP mouse. CNP-EGFP cells (green) are distributed in both grey and white matter (WM) in uninjured control tissue. The white dotted line delineates the border of grey matter (GM). By 1 day at the injury epicenter, EGFP cells are visibly lost dorso-centrally. The loss in the central lesion continues at 7 days, and these cells are not replaced chronically at 42 days post-injury. Fluorescent photomicrographs of 10 µm coronal sections from the spinal cord at T8 of CNP-EGFP transgenic mice that were part of a previous study (Lytle et. al., 2009). Scale bar = 250 µm.
Acknowledgments
This research was supported by funds from the National Institutes of Health (NINDS RO1 NS NS035647) and the Christopher Reeve and Sam Schmidt Foundations. We thank Acorda Therapeutics for the gift of the human recombinant GGF2.
REFERENCES
- Beattie MS, Bresnahan JC, Komon J, Tovar CA, Van Meter M, Anderson DK, Faden AI, Hsu CY, Noble LJ, Salzman S, et al. Endogenous repair after spinal cord contusion injuries in the rat. Exp Neurol. 1997;148(2):453–463. doi: 10.1006/exnr.1997.6695. [DOI] [PubMed] [Google Scholar]
- Butt AM, Kiff J, Hubbard P, Berry M. Synantocytes: new functions for novel NG2 expressing glia. J Neurocytol. 2002;31(6–7):551–565. doi: 10.1023/a:1025751900356. [DOI] [PubMed] [Google Scholar]
- Cao Q, Xu XM, Devries WH, Enzmann GU, Ping P, Tsoulfas P, Wood PM, Bunge MB, Whittemore SR. Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. J Neurosci. 2005;25(30):6947–6957. doi: 10.1523/JNEUROSCI.1065-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CA, Mott RT, Sharpe H, Xu Q, Van Nostrand WE, Vitek MP. Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. J Neuroinflammation. 2006;3:27. doi: 10.1186/1742-2094-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, Gage FH, Anderson AJ. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci U S A. 2005;102(39):14069–14074. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJ, Kotter MR. The biology of CNS remyelination: the key to therapeutic advances. J Neurol. 2008;255 Suppl 1:19–25. doi: 10.1007/s00415-008-1004-6. [DOI] [PubMed] [Google Scholar]
- Frost EE, Nielsen JA, Le TQ, Armstrong RC. PDGF and FGF2 regulate oligodendrocyte progenitor responses to demyelination. J Neurobiol. 2003;54(3):457–472. doi: 10.1002/neu.10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Grossman SD, Rosenberg LJ, Wrathall JR. Temporal-spatial pattern of acute neuronal and glial loss after spinal cord contusion. Exp Neurol. 2001;168(2):273–282. doi: 10.1006/exnr.2001.7628. [DOI] [PubMed] [Google Scholar]
- Hagg T, Oudega M. Degenerative and spontaneous regenerative processes after spinal cord injury. J Neurotrauma. 2006;23(3–4):264–280. doi: 10.1089/neu.2006.23.263. [DOI] [PubMed] [Google Scholar]
- Hamanoue M, Yoshioka A, Ohashi T, Eto Y, Takamatsu K. NF-kappaB prevents TNF-alpha-induced apoptosis in an oligodendrocyte cell line. Neurochem Res. 2004;29(8):1571–1576. doi: 10.1023/b:nere.0000029571.39497.56. [DOI] [PubMed] [Google Scholar]
- Horky LL, Galimi F, Gage FH, Horner PJ. Fate of endogenous stem/progenitor cells following spinal cord injury. J Comp Neurol. 2006;498(4):525–538. doi: 10.1002/cne.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner PJ, Power AE, Kempermann G, Kuhn HG, Palmer TD, Winkler J, Thal LJ, Gage FH. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20(6):2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovcevski I, Wu J, Karl N, Leshchyns'ka I, Sytnyk V, Chen J, Irintchev A, Schachner M. Glial scar expression of CHL1, the close homolog of the adhesion molecule L1, limits recovery after spinal cord injury. J Neurosci. 2007;27(27):7222–7233. doi: 10.1523/JNEUROSCI.0739-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PH, Wells JE, Stallcup WB, Opdenakker G, Yong VW. Matrix metalloproteinase-9 facilitates remyelination in part by processing the inhibitory NG2 proteoglycan. J Neurosci. 2003;23(35):11127–11135. doi: 10.1523/JNEUROSCI.23-35-11127.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ford BD, Mann MA, Fischbach GD. Neuregulin-1 increases the proliferation of neuronal progenitors from embryonic neural stem cells. Dev Biol. 2005;283(2):437–445. doi: 10.1016/j.ydbio.2005.04.038. [DOI] [PubMed] [Google Scholar]
- Lu FG, Wong CS. Time-dependent neurosphere-forming ability of adult rat spinal cord after irradiation. Radiat Res. 2007;168(4):453–461. doi: 10.1667/RR0591.1. [DOI] [PubMed] [Google Scholar]
- Lytle JM, Chittajallu R, Wrathall JR, Gallo V. NG2 cell response in the CNP-EGFP mouse after contusive spinal cord injury. Glia. 2009;57(3):270–285. doi: 10.1002/glia.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytle JM, Wrathall JR. Glial cell loss, proliferation and replacement in the contused murine spinal cord. Eur J Neurosci. 2007;25(6):1711–1724. doi: 10.1111/j.1460-9568.2007.05390.x. [DOI] [PubMed] [Google Scholar]
- Margosio B, Marchetti D, Vergani V, Giavazzi R, Rusnati M, Presta M, Taraboletti G. Thrombospondin 1 as a scavenger for matrix-associated fibroblast growth factor 2. Blood. 2003;102(13):4399–4406. doi: 10.1182/blood-2003-03-0893. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Liu XZ, Qu Y, Liu S, Mickey SK, Turetsky D, Gottlieb DI, Choi DW. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med. 1999;5(12):1410–1412. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Tripathi R, Wei P. NG2 colocalizes with axons and is expressed by a mixed cell population in spinal cord lesions. J Neuropathol Exp Neurol. 2006;65(4):406–420. doi: 10.1097/01.jnen.0000218447.32320.52. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Wei P, Stokes BT. Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J Neurosci. 2001;21(10):3392–3400. doi: 10.1523/JNEUROSCI.21-10-03392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocchetti I, Rabin SJ, Colangelo AM, Whittemore SR, Wrathall JR. Increased basic fibroblast growth factor expression following contusive spinal cord injury. Exp Neurol. 1996;141(1):154–164. doi: 10.1006/exnr.1996.0149. [DOI] [PubMed] [Google Scholar]
- Nishiyama A. Polydendrocytes: NG2 cells with many roles in development and repair of the CNS. Neuroscientist. 2007;13(1):62–76. doi: 10.1177/1073858406295586. [DOI] [PubMed] [Google Scholar]
- Noble LJ, Donovan F, Igarashi T, Goussev S, Werb Z. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J Neurosci. 2002;22(17):7526–7535. doi: 10.1523/JNEUROSCI.22-17-07526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh LY, Larsen PH, Krekoski CA, Edwards DR, Donovan F, Werb Z, Yong VW. Matrix metalloproteinase-9/gelatinase B is required for process outgrowth by oligodendrocytes. J Neurosci. 1999;19(19):8464–8475. doi: 10.1523/JNEUROSCI.19-19-08464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostenfeld T, Svendsen CN. Requirement for neurogenesis to proceed through the division of neuronal progenitors following differentiation of epidermal growth factor and fibroblast growth factor-2-responsive human neural stem cells. Stem Cells. 2004;22(5):798–811. doi: 10.1634/stemcells.22-5-798. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377(3):443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Rabchevsky AG, Sullivan PG, Scheff SW. Temporal-spatial dynamics in oligodendrocyte and glial progenitor cell numbers throughout ventrolateral white matter following contusion spinal cord injury. Glia. 2007;55(8):831–843. doi: 10.1002/glia.20508. [DOI] [PubMed] [Google Scholar]
- Rosenberg LJ, Teng YD, Wrathall JR. 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(f)quinoxaline reduces glial loss and acute white matter pathology after experimental spinal cord contusion. J Neurosci. 1999;19(1):464–475. doi: 10.1523/JNEUROSCI.19-01-00464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg LJ, Wrathall JR. Quantitative analysis of acute axonal pathology in experimental spinal cord contusion. J Neurotrauma. 1997;14(11):823–838. doi: 10.1089/neu.1997.14.823. [DOI] [PubMed] [Google Scholar]
- Rosenberg LJ, Zai LJ, Wrathall JR. Chronic alterations in the cellular composition of spinal cord white matter following contusion injury. Glia. 2005;49(1):107–120. doi: 10.1002/glia.20096. [DOI] [PubMed] [Google Scholar]
- Schonberg DL, Popovich PG, McTigue DM. Oligodendrocyte generation is differentially influenced by toll-like receptor (TLR) 2 and TLR4-mediated intraspinal macrophage activation. J Neuropathol Exp Neurol. 2007;66(12):1124–1135. doi: 10.1097/nen.0b013e31815c2530. [DOI] [PubMed] [Google Scholar]
- Shihabuddin LS, Ray J, Gage FH. FGF-2 is sufficient to isolate progenitors found in the adult mammalian spinal cord. Exp Neurol. 1997;148(2):577–586. doi: 10.1006/exnr.1997.6697. [DOI] [PubMed] [Google Scholar]
- Uhm JH, Dooley NP, Oh LY, Yong VW. Oligodendrocytes utilize a matrix metalloproteinase, MMP-9, to extend processes along an astrocyte extracellular matrix. Glia. 1998;22(1):53–63. doi: 10.1002/(sici)1098-1136(199801)22:1<53::aid-glia5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Wrathall JR, Li W, Hudson LD. Myelin gene expression after experimental contusive spinal cord injury. J Neurosci. 1998;18(21):8780–8793. doi: 10.1523/JNEUROSCI.18-21-08780.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrathall JR, Pettegrew RK, Harvey F. Spinal cord contusion in the rat: production of graded, reproducible, injury groups. Exp Neurol. 1985;88(1):108–122. doi: 10.1016/0014-4886(85)90117-7. [DOI] [PubMed] [Google Scholar]
- Ye P, D'Ercole AJ. Insulin-like growth factor I protects oligodendrocytes from tumor necrosis factor-alpha-induced injury. Endocrinology. 1999;140(7):3063–3072. doi: 10.1210/endo.140.7.6754. [DOI] [PubMed] [Google Scholar]
- Yoo S, Wrathall JR. Mixed primary culture and clonal analysis provide evidence that NG2 proteoglycan-expressing cells after spinal cord injury are glial progenitors. Dev Neurobiol. 2007;67(7):860–874. doi: 10.1002/dneu.20369. [DOI] [PubMed] [Google Scholar]
- Yuan X, Chittajallu R, Belachew S, Anderson S, McBain CJ, Gallo V. Expression of the green fluorescent protein in the oligodendrocyte lineage: a transgenic mouse for developmental and physiological studies. J Neurosci Res. 2002;70(4):529–545. doi: 10.1002/jnr.10368. [DOI] [PubMed] [Google Scholar]
- Yune TY, Lee JY, Jung GY, Kim SJ, Jiang MH, Kim YC, Oh YJ, Markelonis GJ, Oh TH. Minocycline alleviates death of oligodendrocytes by inhibiting pro-nerve growth factor production in microglia after spinal cord injury. J Neurosci. 2007;27(29):7751–7761. doi: 10.1523/JNEUROSCI.1661-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zai LJ, Wrathall JR. Cell proliferation and replacement following contusive spinal cord injury. Glia. 2005;50(3):247–257. doi: 10.1002/glia.20176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Loss of oligodendrocyte lineage cells from the lesion area chronically after SCI in the CNP-EGFP mouse. CNP-EGFP cells (green) are distributed in both grey and white matter (WM) in uninjured control tissue. The white dotted line delineates the border of grey matter (GM). By 1 day at the injury epicenter, EGFP cells are visibly lost dorso-centrally. The loss in the central lesion continues at 7 days, and these cells are not replaced chronically at 42 days post-injury. Fluorescent photomicrographs of 10 µm coronal sections from the spinal cord at T8 of CNP-EGFP transgenic mice that were part of a previous study (Lytle et. al., 2009). Scale bar = 250 µm.