Abstract
Exposure of the developing brain to a wide variety of drugs of abuse (eg., stimulants, opioids, ethanol, etc.) can induce life-long changes in behavior and neural circuitry. However, the long-term effects of exposure to therapeutic, psychotropic drugs have only recently begun to be appreciated. Antipsychotic drugs are little studied in this regard. Here we quantitatively analyzed dendritic architecture in adult mice treated with paradigmatic typical- (haloperidol) or atypical (olanzapine) antipsychotic drugs at developmental stages corresponding to fetal or fetal plus early childhood stages in humans. In layer 3 pyramidal cells of the medial and orbital prefrontal cortices and the parietal cortex and in spiny neurons of the core of the nucleus accumbens, both drugs induced significant changes (predominantly reductions) in the amount and complexity of dendritic arbor and the density of dendritic spines. The drug-induced plasticity of dendritic architecture suggests changes in patterns of neuronal connectivity in multiple brain regions that are likely to be functionally significant.
Keywords: Antipsychotic, dendrite, development, cortex, nucleus accumbens, plasticity
INTRODUCTION
It has long been recognized that exposure of the developing brain to a wide variety of drugs of abuse (eg., stimulants, opioids, ethanol, etc.) can induce life-long changes in behavior and neural circuitry. The frank, teratogenic effects of early exposure to some therapeutic, psychotropic drugs (eg., some mood stabilizers and anticonvulsants) has long been recognized. However, the potential, more subtle, long-term functional and structural consequences of exposure of the developing brain to these and other therapeutic agents have come to be studied and recognized only relatively recently. Such effects should be expected: these therapeutic drugs, like drugs of abuse, modulate the functions of a broad spectrum of neurotransmitter systems and ion channels (Andersen and Navalta, 2004; Baldessarini, 2001a; Baldessarini, 2001b), which can alter the development of neural circuitry either directly (Frost and Cadet, 2000; Henschel et al., 2008), or by their impact on the level and pattern of neuroelectric activity, which modulates neuronal development by additional pathways (Cowan et al., 1984; Fink and Gothert, 2007; Hensch, 2005; Katz and Shatz, 1996).
Schizophrenia and depression/bipolar disease have high incidences (1% and 15–20% respectively) and women are at an elevated risk of these diseases in their child bearing years (Kessler et al., 1993; Weissman, 1987). Many patients need to remain on their medications during pregnancy in order for them to manage their lives successfully. However, numerous drugs, haloperidol and olanzapine for example, pass efficiently from the maternal to the placental circulation (Newport et al., 2007). Furthermore, prescription of antidepressants to children and adolescents has exploded see ((Andersen and Navalta, 2004) for a review) and antipsychotics are becoming increasingly used for the treatment of pediatric and adolescent bipolar disease (Chang, 2008), autism (McDougle et al., 2008) and other developmental diseases. Thus, a better understanding of the risks and benefits of the drugs used to treat these diseases is of the utmost importance.
Multiple technical, logistical and ethical obstacles hinder the study of the long-term effects of maternal psychotropic drug therapy in humans. Many of these difficulties can be mitigated in controlled studies using animals. In the cerebral cortex, the schedule of neuronal proliferation, migration and formation of connections suggests that neurodevelopmental events that take place in humans in the second and third trimesters occur, respectively, during the third week of gestation and the first 10–14 days of life in rodents (Bayer et al., 1993; Rakic, 1975; Sidman and Rakic, 1973). Thus, treatment of pregnant or neonatal rodents with psychotropic drugs has been used as a model to study the long-term effects of human fetal exposure to these agents.
Data on the consequences of early exposure to antipsychotic drugs (APDs) are scanty: Several of the most commonly used antipsychotics, including olanzapine, haloperidol and risperidone, efficiently cross the placental barrier, resulting in placental plasma concentrations 49–72% of those in the maternal circulation (Newport et al., 2007). Administration of haloperidol to pregnant rat dams induces in their offspring increased open field activity, symptoms of anxiety and stereotypic rearing, and reduced scratching and licking/washing. (Singh and Singh, 2002; Singh et al., 1997) Haloperidol treatment of pregnant dams reduces dopamine D2 receptor binding in progeny, whereas haloperidol treatment of dams during nursing causes an increase. These changes persist weeks (and maybe more) following the termination of drug administration and are paralleled, respectively, by decreases and increases in apomorphine-induced stereotopy when the progeny are 5 weeks old (Rosengarten and Friedhoff, 1979). Haloperidol exposure during the first two postnatal weeks reduces the number of spontaneously active midbrain dopaminergic neurons (Zhang et al., 1996). There are also age-dependent effects of haloperidol on dopamine metabolism in the prefrontal cortex of postnatal rats (Teicher et al., 1993). Treatment of pregnant dams with haloperidol, risperidone or quetiapine also causes deficits in spatial task learning in progeny; haloperidol and risperidone also interfere with task retention (Rosengarten and Quartermain, 2002). Fetal risperidone exposure also increases open field activity, whereas fetal sulpiride exposure induces defects in Morris water maze performance (Zuo et al., 2008).
Dendritic branching patterns and spine density determine which populations of afferent axons terminate upon a population of neurons and the relative weighting of each type of input. The vast majority of synaptic inputs onto neurons are on dendrites or dendritic spines, and the amount of synaptic input cells receive varies with the amount of dendritic surface available (Harris and Kater, 1994). Over 90% of excitatory synapses are on dendritic spines, and synaptogenesis, associated with experiences like learning or environmental complexity, is reflected by changes in the number of dendritic spines (Greenough et al., 1990; Kolb et al., 1998; Rampon et al., 2000; Woolley, 1999). Thus, changes in dendritic architecture are reliably associated with functional alterations induced by neuroendocrine changes (Li et al., 2004; Rudick and Woolley, 2001; Woolley, 2000; Yankova et al., 2001), drugs of abuse (Kolb et al., 2004; Robinson and Kolb, 2004; Williams et al., 2004), pharmacotherapy (Diaz Heijtz et al., 2003), learning (Chang and Greenough, 1982; Leuner et al., 2003; Moser et al., 1994; Stewart and Rusakov, 1995), living in isolated versus complex environments (Greenough et al., 1990; Kolb et al., 2003; van Praag et al., 2000) and recovery of function after brain damage (Biernaskie and Corbett, 2001; Jones et al., 1996; Kolb and Gibb, 1991; Kolb et al., 2004) (see also (Kolb and Whishaw, 1998) for a review). Throughout the life span, the external environment and internal body conditions can modify dendritic form and other aspects of neural circuitry (Kolb et al., 1998).
In the present study, we investigated the long-term effects of early exposure to haloperidol and olanzapine, prototypic “typical” and “atypical” APDs, respectively, on layer 3 pyramidal neurons in the medial prefrontal cortex, orbital prefrontal cortex, parietal cortex and core of the nucleus accumbens. We chose these regions for study because they express dopaminergic and/or serotonergic receptors on which the drugs might be expected to act, because they have been extensively studied with respect to the actions of other drugs and because (except in the parietal cortex), these structures are affected by diseases for which APDs are administered. We found that chronic drug administration to neonatal female mice induced, in all 4 brain regions, numerous, long lasting changes - generally reductions - in the amount and complexity of dendritic arbor and in dendritic spine density. These data suggest additional changes in the function of these brain regions and the behaviors to which they contribute.
METHODS
Subjects
All subjects were C57Bl/6 female mice bred, born and raised in our colony in an AALAC-accredited animal care facility at the University of Maryland School of Medicine. Founder mice were obtained from Jackson Laboratories. The diurnal light cycle was 14h light, 10h dark. All litters were culled to 8 pups on the day of birth to avoid effects due to differences in litter size and consequent potential variation in maternal care.
Experimental treatments
All our experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committees of the University of Maryland, Baltimore and the University of Lethbridge.
In preliminary studies, we examined the plasma concentration of haloperidol and olanzapine as a function of drug dose. We treated mice with haloperidol at 6- or 12 mg/kg, IP, 2/d, or olanzapine at 1 mg/kg, IP, 2/d, (5ml/kg body weight for both drugs) from postnatal day 3 (P3; first 24h of life = P0) until euthanasia on P21. The two daily injections were spaced 6–12h apart. 6- or 2 hours after the morning injection on P21, the mice were decapitated and trunk blood was obtained for measurement of plasma concentrations of haloperidol or olanzapine, respectively. A minimum of 6 independent samples (each prepared from blood pooled from multiple, similarly treated mouse pups) was used to measure the plasma drug concentration at each dose. The resultant plasma concentrations of haloperidol were: 14.9 +/− 3.1 ng/ml (mean +/− standard error; n=12) and 21.2 +/− 4.0 ng/ml (n=6) at the 6- and 12 mg/kg doses respectively. For olanzapine the plasma concentration was 7.22 +/− 1.15 ng/ml (n=6; dose = 1 mg/kg). The half life of these drugs in rodents is on the order of about 2–4h (Kapur et al., 2003); thus the mice were never actually in steady state and the average drug concentrations over a whole day were lower than the values we measured. Although we did not conduct formal tests, activity levels of the mice were normal. In addition, APD-treated mice gained weight over the course of drug treatment at the same rate as vehicle injected (0.9% saline, 5ml/kg), littermate controls and at the age of euthanasia, the weights of APD-treated and saline treated mice did not differ significantly (data not shown).
For our experimental treatments we used 12 mg/kg for the haloperidol injections and 1 mg/kg for olanzapine. Control animals were littermates injected with vehicle. For each of the 3 treatments, mice were treated either on P3-10 or P3-20. Within each litter, at least 2 mice were injected, respectively, with 0.9% NaCl, haloperidol or olanzapine. Mice for each of the 6 treatment-duration groups were obtained from at least 3 litters to avoid effects due to differences in maternal care.
Histological processing
When the mice were adults (6 months of age), they were administered an overdose of sodium pentobarbital and perfused with 0.9% saline. The brains were removed, processed with the Golgi-Cox technique, sectioned on a vibratome at 200 µm and mounted onto slides, as previously described (Gibb and Kolb, 1998)
Quantitative Golgi analysis
We analyzed the dendritic architecture of layer 3 pyramidal cells of the orbital prefrontal cortex (OPC; area AID of (Zilles, 1985), medial prefrontal cortex (MPC; area Cg3 (Zilles, 1985) and parietal cortex (area Par of (Zilles, 1985), and spiny neurons in the core of the nucleus accumbens (NAc). In each region of interest, neurons were traced using a 100X oil immersion objective and a camera lucida. In order to be included in the data analysis, neuronal dendritic trees (a) had to be well impregnated and not obscured by blood vessels, astrocytes, or heavy clusters of dendrites from other neurons and (b) the apical and basal arborizations had to be largely intact. For each cortical neuron, separate measures were made on apical and basal dendrites; neurons in the NAc were considered to have only basal dendrites because their dendritic fields are approximately radially symmetric.
We measured total dendritic length by counting the number of intersections of dendrites with a series of concentric spheres at 25 µm intervals from the center of the soma and multiplying by 25 µm (Sholl, 1965). To measure the complexity of the dendritic arbor we counted the total number of dendritic segments. The majority of excitatory inputs to the neurons we studied are on dendritic spines. Thus, we measured the spine density on a minimum length of 50 µm of one distal, terminal branch of the apical dendrite and on one third-order basilar dendritic branch for each neuron studied. All measurements were made by an investigator who was blind to treatment group.
For each hemisphere, measures were obtained for 5 neurons in each region of interest. Within each region, the value of each measure was taken to be the average of the values obtained from the 5 neurons studied. In our statistical analyses, n = 6 hemispheres/treatment group-duration. We made separate statistical analyses of each measure of dendritic form within each of the 4 regions of interest. In each analysis, we first determined the statistical significance of the effects of treatment, treatment duration and their interaction were analyzed using 2-way ANOVA. At each treatment duration, we then compared the effects of treatment by 1-way ANOVA followed by Fisher’s PLSD test.
RESULTS
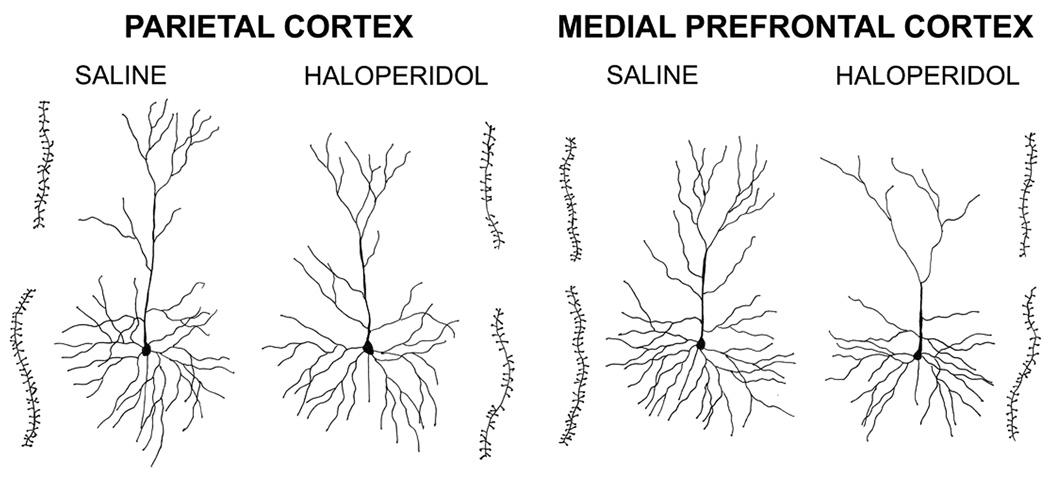
Qualitative inspection of layer 3 pyramidal cells in OPC, MPC and Par, and spiny stellate cells in the NAc, reveals that neonatal exposure to haloperidol or olanzapine, for both treatment durations, generally appears to reduce the total amount of dendritic arbor, dendritic branching complexity and dendritic spine density (Fig. 1). Apical and basal dendrites both appear to be affected.
Fig. 1.
Camera lucida drawings of typical pyramidal cells in the parietal and medial prefrontal cortices of mice treated with saline or haloperidol on P3-10. The insets immediately adjacent to each cell are drawings of terminal apical (upper) or third order basal (lower) dendritic segments that were used to calculate spine densities. The cells shown were selected because they were close to the group averages for our 3 measures of dendritic form.
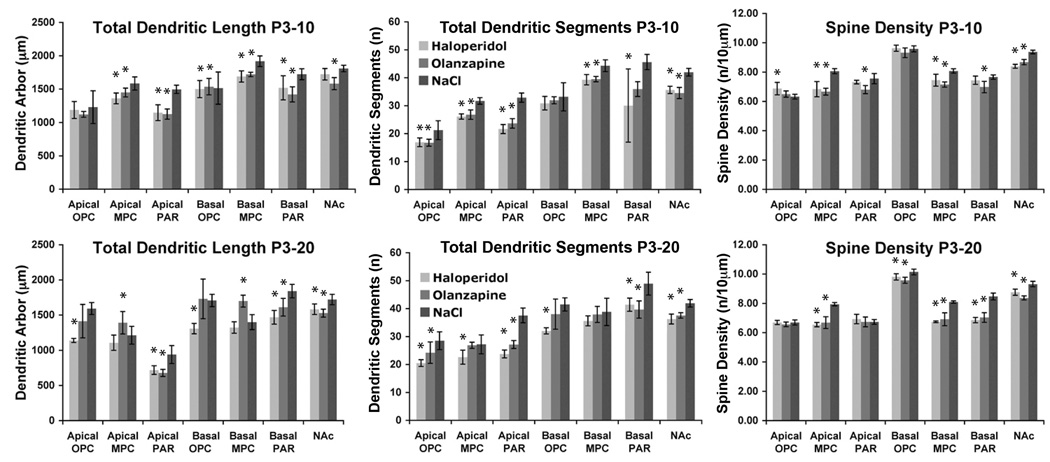
Our quantitative analysis confirms these impressions. Fig. 2 shows the means and standard deviations for the 3 measures of dendritic form, for each combination of treatment and treatment duration within the 4 regions of interest. The results of the 2-way ANOVA are summarized in Table I. Treatment had a significant effect on 21/21 (=100%) of the measures of dendritic form in all 4 regions of interest (Table II). The duration of treatment had a significant effect in 16/21 (=76%) of the measures of dendritic form and there was a significant interaction of treatment and duration for 12/21 (=57%) of the measures.
Fig. 2.
Total dendritic arbor, number of dendritic segments and dendritic spine density in adult, female mice treated from P3-10 or P3-20 with haloperidol, olanzapine or (as a control) saline. Data from apical and basal dendritic arbors are calculated separately for each cortical area. 5 neurons were measured in each region of interest in each hemisphere. n =6 hemispheres/treatment group-duration. Asterisks indicate measures in APD treated mice that differ significantly (p<0.05) from those obtained in saline treated mice. OPC, MPC, Par and NAc indicate the orbital and medial prefrontal cortices, parietal cortex and nucleus accumbens core, respectively.
Table I. 2-way Statistical Analysis.
Summary of 2-way ANOVA evaluations of the significance of APD treatment, treatment duration and their interaction for the 21 independent parameters measured in this study. S=significant effects; N=non-significant effects.
| PARAMETER | TREATMENT | DURATION | TREATMENT X DURATION |
|---|---|---|---|
| AID Apical Dendritic Segments | S | S | N |
| AID Apical Spines | S | N | S |
| AID Apical Total Dendritic Arbor | S | S | S |
| AID Basal Dendritic Segments | S | S | S |
| AID Basal Spines | S | S | N |
| AID Basal Total Dendritic Arbor | S | N | S |
| Cg3 Apical Dendritic Segments | S | S | S |
| Cg3 Apical Spines | S | N | N |
| Cg3 Apical Total Dendritic Arbor | S | S | S |
| Cg3 Basal Dendritic Segments | S | S | N |
| Cg3 Basal Spines | S | S | S |
| Cg3 Basal Total Dendritic Arbor | S | S | S |
| NAc Core Dendritic Segments | S | S | S |
| NAcCoreSpines | S | N | S |
| NAc Core Total Dendritic Arbor | S | S | N |
| Par Apical Dendritic Segments | S | S | N |
| Par Apical Spines | S | S | S |
| Par Apical Total Dendritic Arbor | S | S | N |
| Par Basal Dendritic Segments | S | S | N |
| Par Basal Spines | S | N | S |
| Par Basal Total Dendritic Arbor | S | S | N |
| % Significant | 100 | 76 | 57 |
Table II. Summary of Results.
Summary of APD-induced changes in dendritic architecture in adult, female mice treated from P3-10 or P3-20 with haloperidol, olanzapine or (as a control) saline. Measures from apical and basal dendritic arbors are calculated separately for each cortical area. H/S and O/S indicate, respectively, the ratio of the mean measures in haloperidol- and olanzapine treated mice to corresponding measures in control, saline treated mice. For measures that were significantly different from control values (p<0.05), the ratios are in bold type.
| P3-10 Apical |
P3-10 Basal |
P3-20 Apical |
P3-20 Basal |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Dendritic Segments |
Spine Density |
Total Dendritic Length |
Total Dendritic Segments |
Spine Density |
Total Dendritic Length |
Total Dendritic Segments |
Spine Density |
Total Dendritic Length |
Total Dendritic Segments |
Spine Density |
Total Dendritic Length |
|
| OPC | ||||||||||||
| 0.80 | 1.09 | 0.97 | 0.93 | 1.01 | 0.99 | H/S | 0.72 | 1.00 | 0.71 | 0.77 | 0.97 | 0.77 |
| 0.79 | 1.03 | 0.91 | 0.96 | 0.97 | 1.01 | 0/S | 0.85 | 0.98 | 0.89 | 0.92 | 0.94 | 1.01 |
| MPC | ||||||||||||
| 0.82 | 0.85 | 0.86 | 0.89 | 0.92 | 0.88 | H/S | 0.83 | 0.82 | 0.91 | 0.92 | 0.83 | 0.95 |
| 0.85 | 0.83 | 0.91 | 0.89 | 0.89 | 0.90 | 0/S | 0.99 | 0.84 | 1.15 | 0.98 | 0.85 | 1.21 |
| Par | ||||||||||||
| 0.66 | 0.97 | 0.77 | 0.66 | 0.97 | 0.88 | H/S | 0.77 | 1.03 | 0.74 | 0.85 | 0.81 | 0.80 |
| 0.72 | 0.90 | 0.75 | 0.79 | 0.91 | 0.82 | 0/S | 0.72 | 1.00 | 0.77 | 0.81 | 0.83 | 0.88 |
| NAc | ||||||||||||
| 0.85 | 0.90 | 0.95 | H/S | 0.87 | 0.94 | 0.92 | ||||||
| 0.82 | 0.93 | 0.87 | 0/S | 0.90 | 0.90 | 0.89 | ||||||
Table. 2 shows the mean value of each measure obtained in haloperidol- or olanzapine treated mice as a fraction of the mean value obtained in control, saline treated mice (statistically significant [p<0.05], drug-induced changes are shown in bold type). Of the total of 84 measures obtained in APD-treated mice (Table I), 59/84 (=70%) differed significantly from control values. 56/59 (95%) of these changes were decreases. The magnitude of the decreases ranges from 6–34%, whereas the increases are 9–21%.
Fig. 2 and Table II also show that there are no clear trends for statistically significant, APD induced changes in any of the 3 measures to be more pronounced in a particular region of interest, for apical vs basal dendrites, for haloperidol vs olanzapine or for one or the other of the two treatment durations.
DISCUSSION
Our data show that exposure of the rodent brain to haloperidol or olanzapine during developmental stages corresponding approximately to the human third trimester (P3-10), or to the third trimester plus early childhood (P3-20), causes numerous, significant, long-term changes in the amount and complexity of dendritic arbor and in dendritic spine density in the OPC, MPC, Par and NAc. Dendritic architecture is a key determinant of the amount, spectrum and weighting of inputs to each neuronal population. Thus, the pervasive, APD-induced dendritic alterations are indicative of changes in neural circuits that are likely to alter the function of the regions in which they occur. We plan to test this hypothesis in behavioral studies of animals exposed to APDs early in life, guided by previous data from adult animals on the behavioral effects of brain lesions and changes in neuronal activity during the performance of various behavioral tasks.
Our data show that even low levels of APDs can cause significant, long lasting abnormalities of dendritic form. In this study, the mean plasma concentrations of haloperidol and olanzapine were 21- and 7 ng/ml, 6 and 2 hours, respectively, after the last of a series of twice daily injections from P3-21. Although the plasma half lives of these drugs in humans are 12–36h for haloperidol and 20–54h for olanzapine (Baldessarini, 2001b), they are in the 2–4h range in adult rats and mice (Kapur et al., 2003). Thus, twice daily injection of haloperidol or olanzapine at the doses used in this study are likely to produce brief spikes in tissue concentrations of the drugs and the mean plasma levels of these APDs would be comparable to or lower than those commonly used therapeutically in humans - 5–20 ng/ml (Baldessarini, 2001b) and 9–208 ng/ml (Bergemann et al., 2004; Gex-Fabry et al., 2003; Olesen and Linnet, 1999; Perry et al., 2001; Perry et al., 1997), respectively. To our knowledge, the rates of APD metabolism in immature rodents and human children have not been determined and compared to those for adults of the same species. However, for many other hepatically metabolized, drugs, the metabolic rates are significantly higher in immature rodents than in adults. Thus, the effective doses of APDs in the neonatal mice of this study are likely to be even lower than one might calculate based on drug half life in adults. If APD metabolic rate is similarly elevated in human fetuses, nursing infants or children, then similar oscillations of plasma APD concentration may also occur in immature humans.
Although the exact spectrum of dendritic measures altered by early APD treatment varied somewhat across treatment conditions, the effect of the drugs on all 4 neuronal populations studied was overwhelmingly a reduction in the total amount of dendritic arbor, the complexity of dendritic branching and dendritic spine density, for both apical and basal dendrites (Table II). Both drugs are potent D2 receptor antagonists and olanzapine is also a potent serotonin type 2A (5HT2A) receptor antagonist. Thus, differences in the spectrum of effects of the two drugs are likely to arise from differences in their effects on 5HT2A receptor signaling or differences in their spectra of action on other receptors, eg., the 5HT1A, nicotinic acetylcholine, α-adrenergic and histamine receptors. Similarly, differences in the spectrum of APD treatment effects in different neuronal populations may reflect variations among those populations with respect to the intensity, localization or weighting of signaling by various receptor types in the ontogeny or maintenance of the dendritic parameters we studied.
There are no studies of the effects on entire dendritic arbors of APD administration to adult animals, with which our results on the effects of APD administration to neonates can be directly compared. Electron microscopic studies of neuropil demonstrate that in the corpus striatum of adult rats, 6 months of haloperidol treatment decreases the density of asymmetric synapses (Roberts et al., 1995) and dendritic spines (Kelley et al., 1997), whereas 6 mo of olanzapine treatment has no effect on dendritic spine density (Roberts, 2001). In layer VI of MPC, 4- and 12 months of haloperidol treatment reduce the density of dendritic spines and of asymmetric axon terminal synapses on dendritic spines (Benes et al., 1985; Vincent et al., 1991). Consistent with these changes, 12 months of haloperidol treatment decreases spinophilin levels in the orbital and dorsolateral prefrontal cortices of adult monkeys, homologues of OPC and MPC, respectively, in the rat (Lidow et al., 2001). Altered cortical spinophilin expression appears to be specific to regions that receive a heavy dopaminergic input. (Law et al., 2004; Lidow et al., 2001). The density of asymmetric synaptic contacts on dendritic spines in layer 6 of MPC is also reduced after 1 year of treatment with clozapine (Benes et al., 1985), that like olanzapine, is an atypical antipsychotic that antagonizes 5HT2A receptors. Shorter (3–4 week) haloperidol treatments appear to have opposite effects: They increase dendritic spine density (Kerns et al., 1992) and the density of synapses on dendritic spines and dendritic shafts (Uranova et al., 1991) in the corpus striatum and increase the density of synapses on dendritic shafts in layer 6 of area MPC (Klinzova et al., 1990). In the rat prefrontal cortex, 26 days of systemic, low dose haloperidol or clozapine treatment increases the expression of genes coding glycolytic proteins, protein kinases and presynaptic proteins and decrease the expression of genes coding protein phosphatases (MacDonald et al., 2005). These changes are consistent with the synaptic plasticity induced in the adult prefrontal cortex by short-term treatment with haloperidol.
The reductions in dendritic spine density that we observe in mice neonatally treated for 8–18 days with haloperidol or olanzapine more nearly resemble the reduction in spine density induced by 4–12 months of haloperidol treatment in adult rodents than they do the increases induced by 3–4 weeks of treatment in adults. The reasons for this are unknown, but could reflect differences between the immature and mature brains with respect to their profiles of gene expression (Stead et al., 2006) and/or the functionality of diverse intracellular and intercellular signaling systems including the dopaminergic (Andersen and Navalta, 2004; Andersen et al., 2000; Foote and Morrison, 1987; Frost and Cadet, 2000; Teicher et al., 1995) (also Andersen, personal communication) and serotonergic (Basura and Walker, 2000; Foote and Morrison, 1987; Frost and Cadet, 2000; Lidov and Molliver, 1982) systems.
The changes in dendritic architecture induced by neonatal APD treatment are clearly long lasting, as we observed them in 6 month old animals. This contrasts with data on the duration of effects induced by APD treatment in adults, where the decrease in asymmetric synaptic density in the corpus striatum induced by 6 months of haloperidol treatment is partly reversed 4 weeks after drug withdrawal (Roberts et al., 1995). Spine density also shows a trend toward recovery (Roberts et al., 1995) and both measures might come closer to control levels with increased time off drug. (Haloperidol treatment of adult rats also induces reversible changes in the number of tyrosine hydroxylase positive neurons in the substantia nigra (Levinson et al., 1998)). One reason for this crucial difference between the effects of APDs on the developing and mature brains may be that early perturbations of the brain can engender a cascade of subsequent alterations in the development of neural circuitry, a dimension of complexity that is relatively less important in the mature brain (Frost and Cadet, 2000). This may be why some effects of early exposure to antidepressants (Ansorge et al., 2008; Vogel et al., 1990) and anxiolytics (Depino et al., 2008) are delayed. Another important potential difference between immature and adult brains may be that that their functional responses to the drugs are opposite. This is the case for stimulants used to treat attention deficit hyperactivity disorder in children and adolescents (Andersen and Navalta, 2004), anxiolytic drugs (Depino et al., 2008; Henschel et al., 2008) and antidepressants (Ansorge et al., 2008; Vogel et al., 1990), inter alia. Whereas this issue remains to be investigated for APDs at both the structural and functional levels, the increase in dendritic spine density in the neuropil induced by 24 days of haloperidol treatment in adult rats (Kerns et al., 1992) supports this possibility.
Our data indicate no clear differences in the magnitude or spectrum of long-term effects on dendritic form between mice treated with APDs on P3-10 and those treated on P3-20. This suggests that drug treatment on P3-10 is responsible for most of the effects of early APD exposure and that continued treatment only marginally modifies those effects. The long-term consequences of early exposure to nicotine and tactile stimulation (B. Kolb and R. Gibb, unpublished observations) are similarly affected by treatment duration. Additional experiments would be required to determine if this is due to a rapid saturation of the effects of early APD exposure or to a “critical period” during which APD treatment can induce long-term changes in dendritic form.
It is well established that prenatal maternal stress and neonatal stress of progeny can induce long lasting functional and structural abnormalities: Dysfunction of the maternal HPA axis during pregnancy can induce long-term behavioral- (Rice et al., 2007; Weinstock, 2001) and HPA axis dysfunction (Weinstock, 2001; Welberg and Seckl, 2001) and neuroanatomical abnormalities (Alves et al., 1997; Weinstock, 2001) in offspring. These effects appear to be mediated by maternal adrenal hormone secretion (Zagron and Weinstock, 2006). However, the effects of early APD exposure reported here cannot be due solely to stress of dams or progeny by the twice daily drug injections: 1) The dendritic architecture of mature mice subjected to repeated APD treatment differed significantly from that of littermate control mice injected with drug-free vehicle, who were subjected to the same levels of stress and maternal care. 2) A recent study of the effects of daily injections of antidepressants during the first 3 postnatal weeks did not find significant behavioral differences between control mice subjected to daily vehicle injections and mice that were handled but not injected over the same interval (Ansorge et al., 2008). Although stress induced in dams or pups by the injection procedure may modulate the effects of early APD exposure on dendritic form, this may render the present experiments more naturalistic because maternal schizophrenia (Walker et al., 2008) and bipolar disease (Daban et al., 2005; McEwen, 2003) are accompanied by physiological stress that can interact with APDs in producing their effects on fetuses. Neonatal humans and rats may also be stressed postnatally by the impact of maternal disease or stress on mother-progeny interactions.
CONCLUSION
Exposure of the rodent brain to typical (haloperidol) and atypical (olanzapine) APDs at stages of development corresponding to fetal stages in humans induces abnormalities of dendritic form in multiple brain regions. The functional significance and mechanisms of these changes are important avenues of future study. In order to guide physicians and patients in the use of these drugs during pregnancy, it will also be necessary to determine how the presence of endophenotypes of human psychiatric disease (eg., maternal stress) and APD treatment interact in producing their long term effects on offspring.
Acknowledgment
Grants: Supported by grants 1R21 DA13628 and 5R01 MH074083 from the National Institutes of Health, a Research Enhancement Award Program from the Research Service of the US Department of Veterans Affairs and Intramural grant 05-003 from the Women’s Health Research Group of the University of Maryland School of Medicine (DOF) and the Canadian Institutes of Health Research (BK). We thank Dr. Rosy Roberts for critical comments on the manuscript.
REFERENCES
- Alves SE, Akbari HM, Anderson GM, Azmitia EC, McEwen BC, Strand FL. Neonatal ACTH administration elicits long-term changes in forebrain monoamine innervation. Subsequent disruptions in hypothalamic-pituitary-adrenal and gonadal function. Ann N Y Acad Sci. 1997;814:226–251. doi: 10.1111/j.1749-6632.1997.tb46160.x. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Navalta CP. Altering the course of neurodevelopment: a framework for understanding the enduring effects of psychotropic drugs. Int J Dev Neurosci. 2004;22(5–6):423–440. doi: 10.1016/j.ijdevneu.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37(2):167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Ansorge MS, Morelli E, Gingrich JA. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J Neurosci. 2008;28(1):199–207. doi: 10.1523/JNEUROSCI.3973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldessarini RJ. Drugs and the treatment of psychiatric disorders: Depression and anxiety disorders. In: Hardman JG, Limbird LE, Gilman AG, editors. Goodman & Gilman's The pharmacological basis of therapeutics. 10 ed. New York: McGraw-Hil; 2001a. pp. 447–484. [Google Scholar]
- Baldessarini RJ. Drugs and the treatment of psychiatric disorders: Psychosis and mania. In: Hardman JG, Limbird LE, Gilman AG, editors. Goodman & Gilman's The pharmacological basis of therapeutics. 10 ed. New York: McGraw Hill; 2001b. pp. 485–520. [Google Scholar]
- Basura GJ, Walker PD. Serotonin 2A and 2C receptor biosynthesis in the rodent striatum during postnatal development: mRNA expression and functional linkage to neuropeptide gene regulation. Synapse. 2000;38(2):216–225. doi: 10.1002/1098-2396(200011)38:2<216::AID-SYN12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14(1):83–144. [PubMed] [Google Scholar]
- Benes FM, Paskevich PA, Davidson J, Domesick VB. Synaptic rearrangements in medial prefrontal cortex of haloperidol-treated rats. Brain Res. 1985;348(1):15–20. doi: 10.1016/0006-8993(85)90353-1. [DOI] [PubMed] [Google Scholar]
- Bergemann N, Frick A, Parzer P, Kopitz J. Olanzapine plasma concentration, average daily dose, and interaction with co-medication in schizophrenic patients. Pharmacopsychiatry. 2004;37(2):63–68. doi: 10.1055/s-2004-815527. [DOI] [PubMed] [Google Scholar]
- Biernaskie J, Corbett D. Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury. J Neurosci. 2001;21(14):5272–5280. doi: 10.1523/JNEUROSCI.21-14-05272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F-LF, Greenough WT. Lateralized effects of monocular training on dendritic branching in adult split-brain rats. BrRes. 1982;232:283–292. doi: 10.1016/0006-8993(82)90274-8. [DOI] [PubMed] [Google Scholar]
- Chang KD. The use of atypical antipsychotics in pediatric bipolar disorder. J Clin Psychiatry. 2008;69 Suppl 4:4–8. [PubMed] [Google Scholar]
- Cowan WM, Fawcett JW, O'Leary DDM, Stanfield BB. Regressive events in neurogenesis. Science. 1984;225:1258–1265. doi: 10.1126/science.6474175. [DOI] [PubMed] [Google Scholar]
- Daban C, Vieta E, Mackin P, Young AH. Hypothalamic-pituitary-adrenal axis and bipolar disorder. The Psychiatric clinics of North America. 2005;28(2):469–480. doi: 10.1016/j.psc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Depino AM, Tsetsenis T, Gross C. GABA homeostasis contributes to the developmental programming of anxiety-related behavior. Brain Res. 2008;1210:189–199. doi: 10.1016/j.brainres.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Diaz Heijtz R, Kolb B, Forssberg H. Can a therapeutic dose of amphetamine during pre-adolescence modify the pattern of synaptic organization in the brain? Eur J Neurosci. 2003;18(12):3394–3399. doi: 10.1046/j.0953-816x.2003.03067.x. [DOI] [PubMed] [Google Scholar]
- Fink KB, Gothert M. 5-HT receptor regulation of neurotransmitter release. Pharmacol Rev. 2007;59(4):360–417. doi: 10.1124/pr.107.07103. [DOI] [PubMed] [Google Scholar]
- Foote SL, Morrison JH. Development of the noradrenergic, serotonergic, and dopaminergic innervation of neocortex. Current Topics in DevBiol. 1987;21:391–423. doi: 10.1016/s0070-2153(08)60145-3. [DOI] [PubMed] [Google Scholar]
- Frost DO, Cadet JL. Effects of drug-induced neurotoxicity on development of neural circuitry: A hypothesis. BrResRev. 2000;34:103–118. doi: 10.1016/s0165-0173(00)00042-4. [DOI] [PubMed] [Google Scholar]
- Gex-Fabry M, Balant-Gorgia AE, Balant LP. Therapeutic drug monitoring of olanzapine: the combined effect of age, gender, smoking, and comedication. Therapeutic drug monitoring. 2003;25(1):46–53. doi: 10.1097/00007691-200302000-00007. [DOI] [PubMed] [Google Scholar]
- Gibb R, Kolb B. A method for vibratome sectioning of Golgi-Cox stained whole rat brain. J Neurosci Methods. 1998;79(1):1–4. doi: 10.1016/s0165-0270(97)00163-5. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Withers GS, Wallace CS. Morphological changes in the nervous system arising from behavioral experience: what is the evidence that they are involved in learning and memory? In: Squire LR, Lindenlaub E, editors. The Biology of Memory. New York: F. K. Schattauder Verlag; 1990. pp. 159–185. [Google Scholar]
- Harris KM, Kater SB. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu Rev Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6(11):877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Henschel O, Gipson KE, Bordey A. GABAA receptors, anesthetics and anticonvulsants in brain development. CNS & neurological disorders drug targets. 2008;7(2):211–224. doi: 10.2174/187152708784083812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Kleim JA, Greenough WT. Synaptogenesis and dendritic growth in the cortex opposite unilateral sensorimotor cortex damage in adult rats: a quantitative electron microscopic examination. Brain Res. 1996;733(1):142–148. doi: 10.1016/0006-8993(96)00792-5. [DOI] [PubMed] [Google Scholar]
- Kapur S, VanderSpek SC, Brownlee BA, Nobrega JN. Antipsychotic dosing in preclinical models is often unrepresentative of the clinical condition: a suggested solution based on in vivo occupancy. J Pharmacol Exp Ther. 2003;305(2):625–631. doi: 10.1124/jpet.102.046987. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kelley JJ, Gao XM, Tamminga CA, Roberts RC. The effect of chronic haloperidol treatment on dendritic spines in the rat striatum. Exp Neurol. 1997;146(2):471–478. doi: 10.1006/exnr.1997.6552. [DOI] [PubMed] [Google Scholar]
- Kerns JM, Sierens DK, Kao LC, Klawans HL, Carvey PM. Synaptic plasticity in the rat striatum following chronic haloperidol treatment. Clinical neuropharmacology. 1992;15(6):488–500. doi: 10.1097/00002826-199212000-00006. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29(2–3):85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Klinzova AJ, Uranova NA, Haselhorst U, Schenk H. Synaptic plasticity in rat medial prefrontal cortex under chronic haloperidol treatment produced behavioral sensitization. J Hirnforsch. 1990;31(2):175–179. [PubMed] [Google Scholar]
- Kolb B, Forgie M, Gibb R, Gorny G, Rowntree S. Age, experience and the changing brain. Neurosci Biobehav Rev. 1998;22(2):143–159. doi: 10.1016/s0149-7634(97)00008-0. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gibb R. Environmental enrichment and cortical injury: behavioral and anatomical consequences of frontal cortex lesions. Cereb Cortex. 1991;1(2):189–198. doi: 10.1093/cercor/1.2.189. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gibb R, Gorny G. Experience-dependent changes in dendritic arbor and spine density in neocortex vary qualitatively with age and sex. Neurobiol Learn Mem. 2003;79(1):1–10. doi: 10.1016/s1074-7427(02)00021-7. [DOI] [PubMed] [Google Scholar]
- Kolb B, Pellis S, Robinson TE. Plasticity and functions of the orbital frontal cortex. Brain Cogn. 2004;55(1):104–115. doi: 10.1016/S0278-2626(03)00278-1. [DOI] [PubMed] [Google Scholar]
- Kolb B, Whishaw IQ. Brain plasticity and behavior. AnnRevPsychol. 1998;49:43–64. doi: 10.1146/annurev.psych.49.1.43. [DOI] [PubMed] [Google Scholar]
- Law AJ, Hutchinson LJ, Burnet PW, Harrison PJ. Antipsychotics increase microtubule-associated protein 2 mRNA but not spinophilin mRNA in rat hippocampus and cortex. J Neurosci Res. 2004;76(3):376–382. doi: 10.1002/jnr.20092. [DOI] [PubMed] [Google Scholar]
- Leuner B, Falduto J, Shors TJ. Associative memory formation increases the observation of dendritic spines in the hippocampus. J Neurosci. 2003;23(2):659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson AJ, Garside S, Rosebush PI, Mazurek MF. Haloperidol induces persistent downregulation of tyrosine hydroxylase immunoreactivity in substantia nigra but not ventral tegmental area in the rat. Neuroscience. 1998;84(1):201–211. doi: 10.1016/s0306-4522(97)00447-8. [DOI] [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, McEwen BS. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci U S A. 2004;101(7):2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidov HG, Molliver ME. An immunohistochemical study of serotonin neuron development in the rat: ascending pathways and terminal fields. Brain Res Bull. 1982;8(4):389–430. doi: 10.1016/0361-9230(82)90077-6. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Song ZM, Castner SA, Allen PB, Greengard P, Goldman-Rakic PS. Antipsychotic treatment induces alterations in dendrite- and spine-associated proteins in dopamine-rich areas of the primate cerebral cortex. Biol Psychiatry. 2001;49(1):1–12. doi: 10.1016/s0006-3223(00)01058-1. [DOI] [PubMed] [Google Scholar]
- MacDonald ML, Eaton ME, Dudman JT, Konradi C. Antipsychotic drugs elevate mRNA levels of presynaptic proteins in the frontal cortex of the rat. Biol Psychiatry. 2005;57(9):1041–1051. doi: 10.1016/j.biopsych.2005.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougle CJ, Stigler KA, Erickson CA, Posey DJ. Atypical antipsychotics in children and adolescents with autistic and other pervasive developmental disorders. J Clin Psychiatry. 2008;69 Suppl 4:15–20. [PubMed] [Google Scholar]
- McEwen BS. Mood disorders and allostatic load. Biol Psychiatry. 2003;54(3):200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- Moser MB, Trommald M, Andersen P. An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial learning in adult rats suggests the formation of new synapses. Proc Natl Acad Sci U S A. 1994;91(26):12673–12675. doi: 10.1073/pnas.91.26.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport DJ, Calamaras MR, DeVane CL, Donovan J, Beach AJ, Winn S, Knight BT, Gibson BB, Viguera AC, Owens MJ, Nemeroff CB, Stowe ZN. Atypical antipsychotic administration during late pregnancy: placental passage and obstetrical outcomes. Am J Psychiatry. 2007;164(8):1214–1220. doi: 10.1176/appi.ajp.2007.06111886. [DOI] [PubMed] [Google Scholar]
- Olesen OV, Linnet K. Olanzapine serum concentrations in psychiatric patients given standard doses: the influence of comedication. Therapeutic drug monitoring. 1999;21(1):87–90. doi: 10.1097/00007691-199902000-00013. [DOI] [PubMed] [Google Scholar]
- Perry PJ, Lund BC, Sanger T, Beasley C. Olanzapine plasma concentrations and clinical response: acute phase results of the North American Olanzapine Trial. J Clin Psychopharmacol. 2001;21(1):14–20. doi: 10.1097/00004714-200102000-00004. [DOI] [PubMed] [Google Scholar]
- Perry PJ, Sanger T, Beasley C. Olanzapine plasma concentrations and clinical response in acutely ill schizophrenic patients. J Clin Psychopharmacol. 1997;17(6):472–477. doi: 10.1097/00004714-199712000-00006. [DOI] [PubMed] [Google Scholar]
- Rakic P. Timing of major ontogenetic events in the visual cortex of the rhesus monkey. In: Buchwald NA, Brazier MAB, editors. Brain Mechanisms in Mental Retardation. New York: Academic Press; 1975. [DOI] [PubMed] [Google Scholar]
- Rampon C, Jiang CH, Dong H, Tang YP, Lockhart DJ, Schultz PG, Tsien JZ, Hu Y. Effects of environmental enrichment on gene expression in the brain. Proc Natl Acad Sci U S A. 2000;97(23):12880–12884. doi: 10.1073/pnas.97.23.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice F, Jones I, Thapar A. The impact of gestational stress and prenatal growth on emotional problems in offspring: a review. Acta Psychiatr Scand. 2007;115(3):171–183. doi: 10.1111/j.1600-0447.2006.00895.x. [DOI] [PubMed] [Google Scholar]
- Roberts RC. Effect of chronic olanzapine treatment on striatal synaptic organization. Synapse. 2001;39(1):8–15. doi: 10.1002/1098-2396(20010101)39:1<8::AID-SYN2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Roberts RC, Gaither LA, Gao XM, Kashyap SM, Tamminga CA. Ultrastructural correlates of haloperidol-induced oral dyskinesias in rat striatum. Synapse. 1995;20(3):234–243. doi: 10.1002/syn.890200307. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47 Suppl 1:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Rosengarten H, Friedhoff AJ. Enduring changes in dopamine receptor cells of pups from drug administration to pregnant and nursing rats. Science. 1979;203(4385):1133–1135. doi: 10.1126/science.570724. [DOI] [PubMed] [Google Scholar]
- Rosengarten H, Quartermain D. The effect of chronic treatment with typical and atypical antipsychotics on working memory and jaw movements in three- and eighteen-month-old rats. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(6):1047–1054. doi: 10.1016/s0278-5846(02)00221-x. [DOI] [PubMed] [Google Scholar]
- Rudick CN, Woolley CS. Estrogen regulates functional inhibition of hippocampal CA1 pyramidal cells in the adult female rat. J Neurosci. 2001;21(17):6532–6543. doi: 10.1523/JNEUROSCI.21-17-06532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholl DA. The organization of the cerebral cortex. New York: Hafner Publishing Co.; 1965. p. 0. [Google Scholar]
- Sidman RL, Rakic P. Neuronal migration, with special reference to developing human brain: a review. Brain Res. 1973;62(1):1–35. doi: 10.1016/0006-8993(73)90617-3. [DOI] [PubMed] [Google Scholar]
- Singh KP, Singh M. Effect of prenatal haloperidol exposure on behavioral alterations in rats. Neurotoxicol Teratol. 2002;24(4):497–502. doi: 10.1016/s0892-0362(02)00189-7. [DOI] [PubMed] [Google Scholar]
- Singh Y, Jaiswal AK, Singh M, Bhattacharya SK. Effect of prenatal haloperidol administration on anxiety patterns in rats. Indian journal of experimental biology. 1997;35(12):1284–1290. [PubMed] [Google Scholar]
- Stead JD, Neal C, Meng F, Wang Y, Evans S, Vazquez DM, Watson SJ, Akil H. Transcriptional profiling of the developing rat brain reveals that the most dramatic regional differentiation in gene expression occurs postpartum. J Neurosci. 2006;26(1):345–353. doi: 10.1523/JNEUROSCI.2755-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart MG, Rusakov DA. Morphological changes associated with stages of memory formation in the chick following passive avoidance training. Behav Brain Res. 1995;66(1–2):21–28. doi: 10.1016/0166-4328(94)00119-z. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Hostetter JC., Jr Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Res Dev Brain Res. 1995;89(2):167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Barber NI, Gelbard HA, Gallitano AL, Campbell A, Marsh E, Baldessarini RJ. Developmental differences in acute nigrostriatal and mesocorticolimbic system response to haloperidol. Neuropsychopharmacology. 1993;9(2):147–156. doi: 10.1038/npp.1993.53. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Orlovskaya DD, Apel K, Klintsova AJ, Haselhorst U, Schenk H. Morphometric study of synaptic patterns in the rat caudate nucleus and hippocampus under haloperidol treatment. Synapse. 1991;7(4):253–259. doi: 10.1002/syn.890070402. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1(3):191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Vincent SL, McSparren J, Wang RY, Benes FM. Evidence for ultrastructural changes in cortical axodendritic synapses following long-term treatment with haloperidol or clozapine. Neuropsychopharmacology. 1991;5(3):147–155. [PubMed] [Google Scholar]
- Vogel G, Neill D, Hagler M, Kors D. A new animal model of endogenous depression: a summary of present findings. Neurosci Biobehav Rev. 1990;14(1):85–91. doi: 10.1016/s0149-7634(05)80164-2. [DOI] [PubMed] [Google Scholar]
- Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annual review of clinical psychology. 2008;4:189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol. 2001;65(5):427–451. doi: 10.1016/s0301-0082(01)00018-1. [DOI] [PubMed] [Google Scholar]
- Weissman MM. Advances in psychiatric epidemiology: rates and risks for major depression. Am J Public Health. 1987;77(4):445–451. doi: 10.2105/ajph.77.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol. 2001;13(2):113–128. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- Williams MT, Brown RW, Vorhees CV. Neonatal methamphetamine administration induces region-specific long-term neuronal morphological changes in the rat hippocampus, nucleus accumbens and parietal cortex. Eur J Neurosci. 2004;19(12):3165–3170. doi: 10.1111/j.0953-816X.2004.03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS. Electrophysiological and cellular effects of estrogen on neuronal function. Critical reviews in neurobiology. 1999;13(1):1–20. doi: 10.1615/critrevneurobiol.v13.i1.10. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Effects of oestradiol on hippocampal circuitry. Novartis Found Symp. 2000;230:173–180. doi: 10.1002/0470870818.ch13. discussion 181-177. [DOI] [PubMed] [Google Scholar]
- Yankova M, Hart SA, Woolley CS. Estrogen increases synaptic connectivity between single presynaptic inputs and multiple postsynaptic CA1 pyramidal cells: a serial electron-microscopic study. Proc Natl Acad Sci U S A. 2001;98(6):3525–3530. doi: 10.1073/pnas.051624598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagron G, Weinstock M. Maternal adrenal hormone secretion mediates behavioural alterations induced by prenatal stress in male and female rats. Behav Brain Res. 2006;175(2):323–328. doi: 10.1016/j.bbr.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang L, Pitts DK. Prenatal haloperidol reduces the number of active midbrain dopamine neurons in rat offspring. Neurotoxicol Teratol. 1996;18(1):49–57. doi: 10.1016/0892-0362(95)02023-3. [DOI] [PubMed] [Google Scholar]
- Zilles K. The cortex of the rat: A stereotaxic atlas. Berlin: Springer-Verlag; 1985. [Google Scholar]
- Zuo J, Liu Z, Ouyang X, Liu H, Hao Y, Xu L, Lu XH. Distinct neurobehavioral consequences of prenatal exposure to sulpiride (SUL) and risperidone (RIS) in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(2):387–397. doi: 10.1016/j.pnpbp.2007.09.005. [DOI] [PubMed] [Google Scholar]