Abstract
Deficiency of protease-activated receptor-2 (PAR2) modulates inflammation in several models of inflammatory and autoimmune disease, although the underlying mechanism(s) are not understood. PAR2 is expressed on endothelial and immune cells, and is implicated in dendritic cell (DC) differentiation. We investigated in vivo the impact of PAR2 activation on DCs and T cells in PAR2 wild-type (WT) and knockout (KO) mice using a specific PAR2 agonist peptide (AP2). PAR2 activation significantly increased the frequency of mature CD11chigh DCs in draining lymph nodes 24 hr after AP2 administration. Furthermore, these DCs exhibited increased expression of major histocompatibility complex (MHC) class II and CD86. A significant increase in activated (CD44+ CD62−) CD4+ and CD8+ T-cell frequencies was also observed in draining lymph nodes 48 hr after AP2 injection. No detectable change in DC or T-cell activation profiles was observed in the spleen. The influence of PAR2 signalling on antigen transport to draining lymph nodes was assessed in the context of delayed-type hypersensitivity. PAR2 WT mice that were sensitized by skin-painting with fluorescein isothiocyanate (FITC) to induce delayed-type hypersensitivity possessed elevated proportion of FITC+ DCs in draining lymph nodes 24 hr after FITC painting when compared with PAR2 KO mice (0·95% versus 0·47% of total lymph node cells). Collectively, these results demonstrate that PAR2 signalling promotes DC trafficking to the lymph nodes and subsequent T-cell activation, and thus provides an explanation for the pro-inflammatory effect of PAR2 in animal models of inflammation.
Keywords: AP2, dendritic cells (DCs), protease-activated receptor-2 (PAR2), T cells
Introduction
The protease-activated receptor-2 (PAR2) has been implicated in the pathogenesis of several inflammatory and autoimmune disorders,1–10 and is expressed in a wide variety of human tissues and cells.11–15 PAR2 knockout (KO) mice are relatively resistant to inflammation16 and PAR2 has been shown to affect multiple aspects of the tissue response to injury and inflammation.17 The underlying cellular mechanisms remain unclear.
PAR2 belongs to a family of seven transmembrane domain receptor proteins that are activated by proteolysis. Enzymatic digestion exposes an N-terminus ligand sequence that binds intramolecularly to the activation site on the extracellular loop II,18 initiating a G-protein-mediated cell-signalling cascade19,20 and nuclear factor-kappa B (NF-κB)-regulated gene transcription.21–23 Serine proteases such as thrombin, trypsin, neutrophil proteinase-3 (PR3) and mast cell tryptase, as well as coagulation factors VIIa and Xa, have been shown to activate PAR2.24–28
Physiopathologically, PAR2 can be activated during injury, inflammation or atopy.27 Experimentally, PAR2 can also be activated using a synthetic agonist hexapeptide, SLIGRL (AP2), which emulates the tethered ligand without the need for proteolysis.28–31 In this study we used an AP2 peptide modified by replacement of the N-terminal serine with a 2-furoyl group and a C-terminal ornithine (2-furoyl-LIGRLO), as this peptide represents the most potent, selective and metabolically stable (longest half-life) PAR2 agonist peptide available.32,33
PAR2 expression on leucocytes has been reported but not well characterized.9,10 In a recent human study, immature monocyte-derived DCs (MoDCs) were shown to differentiate to a mature phenotype in the presence of PR3. Furthermore, by blocking the protease activity of PR3 with a serine protease inhibitor, dendritic cell (DC) maturation was inhibited.34 DCs are critical orchestrators of the immune system and provide a link between innate and adaptive immunity. Acting as professional antigen-presenting cells (APCs), DCs sample and process antigen in peripheral tissues and transport it to secondary lymphoid organs, mainly the lymph node, where they activate T cells and modulate the acquired immune response.35 Danger signals act on DC innate immune receptors to induce acquired immunity. The best known of these receptors are the toll-like receptors (TLRs) that can activate DCs and stimulate their differentiation from haematopoietic progenitors.36 Moreover, TLR4 and PAR2 have been recently reported to cooperate and exert synergistic pro-inflammatory effects.37
In this study, we investigated the in vivo effects of PAR2 activation on DCs and T cells using AP2 as a PAR2 ligand and compared the effects observed in PAR2 KO and wild-type (WT) mice. Our results showed that PAR2 signalling leads to an enhanced state of DC and T-cell activation that could account for the observed contribution of PAR2 in immune-mediated inflammatory disorders.
Materials and methods
Animals
PAR2 KO (B6 background) mice were a gift of Dr S. Coughlin.16 PAR2 KO and WT littermates, 8–12 weeks of age, were used in the experiments. Mice were anaesthetized with inhaled isoflurane and then killed by cervical dislocation. Institutional approval was obtained for all animal experiments.
Agonist peptide
The synthetic agonist peptide 2-furoyl-LIGRLO-amide (AP2) (Bachem AG, Bubendorf, Switzerland) was suspended in sterile phosphate-buffered saline (PBS). For in vivo studies, AP2 was administrated as an intraperitoneal (i.p.) injection at 1 μm/kg.38
Cell preparation
Single-cell suspensions from harvested spleen and lymph nodes were prepared by incubating organs for 25 min at 25° in 1 mg/ml of collagenase D (Roche, Penzberg, Germany) + 105 μg/ml of DNAse I (Roche) in RPMI medium (Invitrogen, Basel, Switzerland) supplemented with 50 U/ml of penicillin, 50 μg/ml of streptomycin and 5% fetal calf serum (FCS) (Sigma-Aldrich, Buchs, Switzerland) and then filtered through a 40-μm nylon cell strainer. Cells were subsequently washed twice with supplemented RPMI medium. Viable cell counts were performed using Trypan Blue.
Fluorescein isothiocyanate painting
Mice were shaved 1 day before fluorescein isothiocyanate (FITC) painting. FITC powder (Sigma-Aldrich) was dissolved in a 50 : 50 (v/v) mixture of acetone and dibutylphthalate to obtain a 0·8% FITC solution. FITC solution (20 μl) was applied onto the abdominal skin of mice, and 24 hr later antigen-draining inguinal lymph nodes were harvested and digested with collagenase solution, as described above, before analysis was performed.
Flow cytometry
Cells were suspended in fluorescence-activated cell sorter (FACS) buffer (3% FCS, 5 mm EDTA in PBS). Cells were incubated with conjugated monoclonal antibodies (mAbs) in the presence of Fc blockers. All data acquisition was performed on a FACScanto™ flow cytometer (Becton-Dickinson, San Jose, CA). The mAbs used (Becton-Dickinson) were: CD11c-phycoerythrin Cy7 (PECy7), CD11c-phycoerythrin (PE), major histocompatibility complex (MHC) class II-PE, MHC class II-FITC, B220-PECy7, B220-allophycocyanin Cy7 (APCCy7), B220-FITC, CD11b-PE, CD11b-allophycocyanin, CD80-phycoerythrin Cy5 (PECy5), CD40-allophycocyanin, CD86-allophycocyanin, CD8α-phycoerythrin Cy5.5 (PECy5.5), CD8α-PECy7, CD8α-FITC, CD4-FITC, CD4-APCCy7, CD24-FITC, CD3-PECy5.5, CD44-allophycocyanin and CD62L-FITC.
The DC subpopulation analysed included plasmocytoid (CD11c+, CD11b−, CD8α+/−, B220+) and conventional (CD11c+, CD11b+, CD8α−, B220− and CD11c+, CD11b−, CD8α+, B220−) DCs, as well as CD11chigh total DCs. MHC class II, CD40, CD80 and CD86 expression served as indicators for DC maturation. T cells were indentified as CD3+ and either CD4+, CD8− (for CD4 T cells) or CD4−, CD8+ (for CD8+ T cells). CD44 and CD62L expression were used to assess the T-cell activation status.
Statistical analysis
All values were expressed as the mean ± standard error of the mean (SEM). Variation between data sets was evaluated using the Student’s t-test of unpaired data for AP2 experiments and the paired t-test for the FITC painting experiments, with a 95% confidence interval. A P value of < 0·05 was considered statistically significant. Data were analysed using graphpad prism software (GraphpPad Software, La Jolla, CA).
Results
We initially analysed different DC subset frequencies in lymph nodes of naive PAR2 WT and KO mice to determine the contribution of PAR2 to DC development and trafficking under steady-state conditions. DC proportions were comparable between naive PAR2 WT and KO mice (Table 1), indicating that the absence of PAR2 does not grossly impact on DC development and homing to secondary lymphoid organs under non-inflammatory conditions. To confirm that PAR2 deficiency has no influence on DC development, we next investigated whether there are differences in bone marrow-derived dendritic cell (BMDC) formation capacity between PAR2 KO and WT bone marrow precursors in vitro. When cultured in the presence of granulocyte–monocyte colony-stimulating factor (GM-CSF), no significant differences were observed in the generation of BMDC from PAR2 WT and KO bone marrow-derived progenitors (data not shown).
Table 1.
Inguinal lymph node dendritic cell (DC) subset frequencies present in naive protease-activated receptor-2 (PAR2) wild-type (WT) and knockout (KO) mice
| WT | KO | |
|---|---|---|
| CD11c+ B220− (cDC, % of total cells) | 1·365 ± 0·04455 | 1·235 ± 0·06941 |
| CD11c+ B220− CD11b+ CD8a− (% of cDC) | 66·00 ± 1·900 | 63·05 ± 6·450 |
| CD11c+ B220− CD11b− CD8a+ (% of cDC) | 15·03 ± 1·021 | 16·88 ± 2·581 |
| CD11c+ B220+ (pDC, % of total cells) | 0·4200 ± 0·1348 | 0·5400 ± 0·1991 |
Values represent mean values ± standard error of the mean (SEM). For all groups, n = 6.
cDC, conventional DC; pDC, plasmoctoid DC.
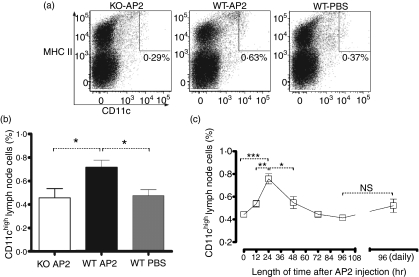
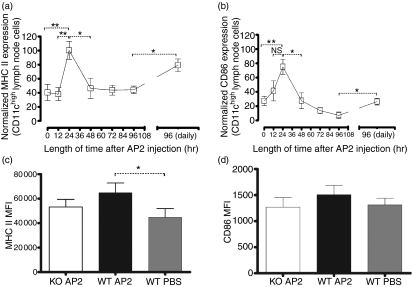
In order to determine the contribution of PAR2 activation to DC maturation and trafficking to secondary lymphoid organs, we utilized a PAR2 agonist peptide (AP2) to activate PAR2 in vivo. A single dose of AP2 was administered to PAR2 WT and KO mice and, 24 hr later, the DC frequencies in draining lymph nodes were assessed. Our analyses revealed an increased frequency of CD11chigh cells in PAR2 WT mice 24 hr after AP2 injection when compared with similarly treated PAR2 KO or PBS-injected WT mice (Fig. 1a,b). To understand in more detail the impact of PAR2 activation on DC maturation kinetics, a time-course experiment was set up. Draining inguinal lymph node CD11chigh DCs were analysed from PAR2 WT mice at 12, 24, 48, 72 and 96 hr following a single AP2 injection. Mice injected with PBS only served as negative controls for DC analysis. CD11chigh DC numbers peaked in inguinal lymph nodes 24 hr after administration of AP2 before gradually returning to baseline levels (Fig. 1c). To assess the influence of AP2 on DC activation and differentiation, the expression of MHC class II and of CD86 were characterized on lymph node CD11chigh DCs at the various time-points. In AP2-treated mice, DCs significantly up-regulated MHC class II (Fig. 2a) and CD86 (Fig. 2b) on their cell surface, with peak expression observed 24 hr after AP2 administration. After 24 hr, expression of these markers returned to basal levels. Daily administration of AP2 did not significantly increase DC numbers (Fig. 1c) at the 96 hr time-point, but they expressed higher levels of MHC class II and CD86 (Fig. 2a,b respectively). No consistent differences were observed in the spleen (data not shown).
Figure 1.
(a) Inguinal lymph node MHC+ CD11chigh dendritic cell (DC) numbers from protease-activated receptor-2 (PAR2) wild-type (WT) and knockout (KO) mice 24 hr following intraperitoneal (i.p.) injection with 1 μm/kg of AP2 or with phosphate-buffered saline (PBS). Plots are representative of four mice per group. MHC II, major histocompatibility complex class II. (b) Inguinal lymph node CD11chigh DC numbers from PAR2 WT and KO mice 24 hr following i.p. injection with 1 μm/kg of AP2 or with PBS. For all groups, n = 4. Error bars represent mean values ± standard error of the mean (SEM). For P values: *P < 0·05. (c) Kinetics of CD11chigh DCs in draining inguinal lymph nodes after a single AP2 dose (left) and 96 hr after daily i.p. injections with 1 μm/kg of AP2 (right). Data are expressed as the percentage of total lymph node cells. Error bars represent mean values ± SEM. For the 0-, 24- and 48-hr groups, n = 7; for the 12- and 72-hr groups, n = 4; and for the 96-hr and daily injection (for 96 hr) groups, n = 3. Data were pooled from two independent experiments. For P values: *P < 0·05, **P < 0·01, ***P < 0·001 and NS, not statistically significant.
Figure 2.
(a) Kinetics of major histocompatibility complex (MHC) class II expression on dendritic cells (DCs) from inguinal lymph nodes after a single 1 μm/kg dose of AP2 (left) and at 96 hr after daily intraperitoneal (i.p.) injections of 1 μm/kg of AP2 (right). Data were pooled from two independent experiments and are expressed as a percentage of the highest geometric mean of MHC class II fluorescence. Error bars represent the mean values ± standard error of the mean (SEM). For the 0-, 24- and 48-hr groups, n = 7; for the 12- and 72-hr groups, n = 4; and for the 96-hr and daily injection (for 96 hr) groups, n = 3.. For P values: *P < 0·05, **P < 0·01. (b) Kinetics of CD86 expression on DCs from inguinal lymph nodes after a single 1 μm/kg AP2 dose (left) and at 96 hr after daily i.p. injections with 1 μm/kg of AP2 (right). Data were pooled from two independent experiments and are expressed as a percentage of the highest geometric mean of CD86 fluorescence. Error bars represent mean values ± standard error of the mean (SEM). For the 0-, 24- and 48-hr groups, n = 7; for the 12- and 72-hr groups, n = 4; and for the 96-hr and daily injection (for 96 hr) groups, n = 3. For P values: *P < 0·05, **P < 0·01 and NS, not statistically significant. (c) MHC class II expression (geometric mean of fluorescence) on protease-activated receptor-2 (PAR2) wild-type (WT) and knockout (KO) DCs from inguinal lymph nodes 24 hr after a single i.p. injection with 1 μm/kg of AP2 or phosphate-buffered saline (PBS). Plots are representative of four mice per group. Error bars represent the mean values ± SEM. For P values: *P < 0·05. (d) CD86 expression (geometric mean of fluorescence) on PAR2 WT and KO DCs from inguinal lymph nodes 24 hr after a single i.p. injection with 1 μm/kg of AP2 or PBS. Plots are representative of four mice per group. Error bars represent mean values ± SEM.
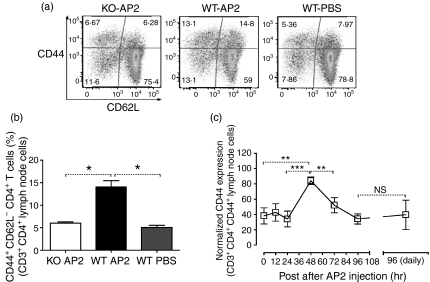
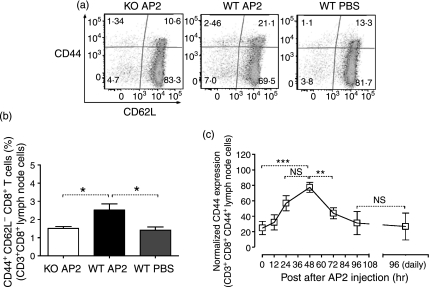
Lymph node CD4+ and CD8+ T cells were also characterized for expression of activation markers following single and repeated injection of AP2, as described above. Both CD4+ and CD8+ T cells expressed peak levels of CD44, a marker of mature activated T cells, 48 hr after a single AP2 injection (Figs 3c and 4c), followed by a return to basal levels thereafter. After repeated daily administration of AP2, the expression of CD44 on T cells did not increase above basal levels (Figs 3c and 4c). No consistent differences were detected in the spleen (data not shown).
Figure 3.
(a) CD4+ T-cell activation in the inguinal lymph nodes of protease-activated receptor-2 (PAR2) wild-type (WT) and knockout (KO) mice 48 hr after a single intraperitoneal (i.p.) injection with 1 μm/kg of AP2 or phosphate-buffered saline (PBS). CD3+ CD4+ CD8− gated cells were analysed for CD44 (y axis) and CD62 (x axis) expression. Plots are representative of three mice per group. (b) Activated PAR2 WT and KO CD4+ T cells (CD3+ CD4+ CD8−CD44+ CD62L−) in inguinal lymph nodes 48 hr after a single i.p. injection with 1 μm/kg of AP2 or PBS. For all groups, n = 3. Error bars represent mean values ± standard error of the mean (SEM). For P values: *P < 0·05. (c) Kinetics of CD44 expression in CD4+ T cells from inguinal lymph nodes after a single 1 μm/kg AP2 dose (left) and 96 hr after daily i.p. injections with 1 μm/kg of AP2 (right). Data were pooled from two independent experiments and are expressed as a percentage of the highest geometric mean of CD44 fluorescence. Error bars represent the mean values ± SEM. For the 0-, 24- and 48-hr groups, n = 7; for the 12- and 72-hr groups, n = 4; and for the 96-hr and daily injection (for 96 hr) groups, n = 3. For P values: **P < 0·01, ***P < 0·001 and NS, not statistically significant.
Figure 4.
(a) CD8+ T-cell activation in the inguinal lymph nodes of protease-activated receptor-2 (PAR2) wild-type (WT) and knockout (KO) mice 48 hr after a single intraperitoneal (i.p.) injection with 1 μm/kg of AP2 or phosphate-buffered saline (PBS). CD3+ CD8+ CD4− gated cells were analysed for CD44 (y axis) and CD62 (x axis) expression. Plots are representative of three mice per group. (b) Activated PAR2 WT and KO CD8+ T cells (CD3+ CD8+ CD4−CD44+ CD62L−) in inguinal lymph nodes 48 hr after a single i.p. injection with 1 μm/kg of AP2 or PBS. For all groups, n = 3. Error bars represent mean values ± standard error of the mean (SEM). For P values: *P < 0·05. (c) Kinetics of CD44 expression in CD8+ T cells from inguinal lymph nodes after a single 1 μm/kg AP2 dose (left) and at 96 hr after daily i.p. injection with 1 μm/kg of AP2 (right). Data were pooled from two independent experiments and are expressed as a percentage of the highest geometric mean of CD44 fluorescence. Error bars represent mean values ± SEM. For the 0-, 24- and 48-hr groups, n = 7; for the 12- and 72-hr groups, n = 4; and for the 96-hr and daily injection (for 96 hr) groups, n = 3. For P values: **P < 0·01, ***P < 0·001 and NS, not statistically significant.
In order to show that the AP2-mediated effects on DCs and T cells were PAR2 specific, we proceeded to compare the impact of AP2-treated PAR2 WT mice (designated WT AP2), PAR2 WT mice receiving PBS alone (WT PBS) and PAR2 KO mice injected with AP2 (KO AP2). WT AP2 DCs expressed significantly higher levels of MHC class II compared with WT PBS DCs. CD86 expression on WT AP2 was also higher compared with controls, but did not reach significance (Fig. 2c,d). As for T cells, WT AP2 showed a twofold increase of CD44high and CD44+ CD62L−, CD4+ and CD8+ T cells compared with both controls (Figs 3a and 4a). CD4+ T cells displaying an activated phenotype (CD3+ CD4+ CD44high CD62L−) were more prominent in the draining lymph node of the WT AP2 group when compared with control groups (Fig. 3b). A similar trend was also observed for activated CD8+ T cells (CD3+ CD8+ CD44high CD62L−) in the WT AP2 group (Fig. 4b). These results show that PAR2 activation promotes increased numbers of DCs in the draining lymph nodes as well as the expression of DC maturation markers. At 24 hr after the DC peak, we found evidence of lymph node T-cell activation after a single injection of AP2.
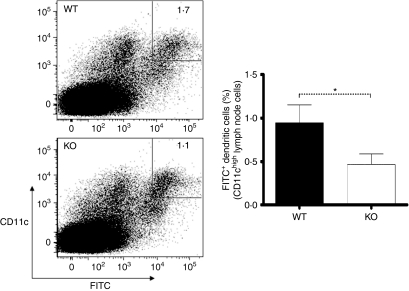
We next wanted to assess whether PAR2 signalling could enhance antigen uptake and transport by DCs, from the periphery to the draining lymph nodes, in an inflammatory context. For this purpose, FITC painting experiments were performed on PAR2 WT and KO littermate mice, and FITC transport to the draining lymph nodes by DCs was compared. FITC has been shown to be a contact sensitizer that induces Type IV hypersensitivity39 and allows visualization of DC migration and antigen take-up.40 At 24 hr after FITC painting, WT mice had a higher proportion of FITC+ DCs in draining lymph nodes compared with PAR2 KO mice. The mean percentage of FITC+ DCs in the draining lymph nodes 24 hr after FITC painting on the skin was 0·95% for WT mice versus 0·47% for their KO littermate counterparts (Fig. 5). Most FITC-bearing DCs found in the lymph nodes had a CD11b+ CD8α− phenotype (data not shown).
Figure 5.
Fluorescein isothiocyanate (FITC) uptake and transport by CD11chigh dendritic cells (DCs) to inguinal lymph nodes 24 hr after FITC painting of the abdominal skin. FITC+ CD11chigh DCs were gated (left) and their frequency was compared between protease-activated receptor-2 (PAR2) knockout (KO) and wild-type (WT) littermates (right). For all groups, n = 7. Error bars represent mean values ± standard error of the mean (SEM). For P values: *P < 0·05. The data are representative of three independent experiments.
Discussion
Although an immunoregulatory role of PAR2 in inflammation is widely acknowledged, the mechanism(s) that account for its influence are not well understood. With their central role in adaptive and innate immunity, DCs are strong candidates to explain how PAR2 modulates the immune response during inflammation. Previous reports have shown that PAR2 activation has an effect on BMDC development and maturation in vitro, and boosts acquired immune responses during allergy.41,42 In this study, we demonstrated that PAR2 activation, in the absence of adjuvant or other immune stimuli, leads to increased numbers of DCs bearing strengthened antigen-presentation and costimulatory potential in draining lymph nodes. This is followed by an increase in CD4+ and CD8+ T lymphocytes that bear markers of an effector-memory phenotype. We also showed that DC capture and transport of antigen from the periphery to draining lymph nodes following an acute inflammatory insult can be enhanced by PAR2 activation.
The effect of a single systemic AP2 dose on draining lymph node cell populations was followed for 72 hr on PAR2 WT mice and was compared with WT PBS and KO AP2 littermate control groups. The use of the highly selective 2-furoyl-LIGRLO-NH2 agonist peptide allowed us to dissect PAR2-specific regulation of the immune system. The mobilization of DCs to draining lymph nodes on day 1 and the activation of T cells within the same lymph nodes on day 2 were the most striking PAR2-induced effects observed. A cause–effect relationship between both observations is suggested, but the cross-talk between DC migration and T-cell activation was not documented. The events observed might be part of a cascade initiated by an undetected cell type, or might be the result of a synergistic sum of effects. Potential AP2 targets could be, for example, the stromal cells of the lymph node that produce CCL19 and CCL21 chemokines to attract DC,43 or the high endothelial venules (HEV) that participate in the regulation of DC trafficking to the lymph nodes.44 It is very likely that DCs, rather than AP2, directly activate T cells, as Fiorucci et al.45 demonstrated that direct stimulation of CD4 T cells with AP2 in vitro abrogates induced CD44 up-regulation. Nevertheless, these results demonstrate that PAR2-specific signalling promotes DC migration to draining lymph nodes and subsequent T-cell activation. In further support of these findings are the functional observations that AP2 peptide co-administration intranasally with ovalbumin (OVA) promotes OVA-mediated airways inflammation, which is associated with an observed increase in the proliferation of T helper 2 (Th2) OVA-specific T cells upon OVA restimulation ex vivo41 and that the absence of PAR2 signalling suppresses myelin oligodendrocyte glycoprotein (MOG) induced-experimental autoimmune encephalomyelitis (EAE) severity in mice, which is linked with decreased MOG-specific T-cell reactivity and infiltration.7
Enhanced DC migration to draining lymph nodes is relevant if this migration is accompanied by antigen capture in peripheral tissues and antigen transport to the lymph nodes for presentation to T cells. Ebeling et al.41 previously reported that artificial activation of PAR2 led to enhanced antigen transport to the spleen in the context of sensitization to inhaled allergen. Our results, from FITC painting, showed that in the absence of PAR2, there is reduced trafficking of antigen to draining lymph nodes. These results imply that PAR2 activation by endogenous mechanisms during acute skin inflammation can enhance antigen transport from the skin to the draining lymph nodes. Topical FITC was administered in the presence of irritants known to induce a type IV hypersensitivity reaction, but this skin irritant solution contained no proteases. It is known that local inflammation liberates proteases such as mast cell tryptase and PR3, enzymes that can activate PAR2 and can account for the observed increase of antigen-bearing DC trafficking to draining lymph nodes. Expression of CCR7 by PAR2-activated DCs may have led to increased chemotaxis to draining lymph nodes. Although induction of CCR7 by PAR2 has not been documented, CCR7 is known to be up-regulated during DC maturation. Furthermore, these DCs had migrated to draining lymph nodes, leading to the assumption that these DCs were already equipped to follow the CCL19 and CCL21 chemotactic gradient. Overall, these results show that PAR2 signalling can promote DC antigen transport to lymph nodes draining sites of inflammation.
We failed to find a direct role for PAR2 in the differentiation of BMDC (data not shown), unlike the results reported by Fields et al.,46 who found that PAR2-deficient bone marrow did not spontaneously develop BMDCs. Although it is possible that the addition of interleukin-4 (IL-4) may explain the discrepancies between these results, we believe that the absence of PAR2 has only a minor effect on the differentiation of bone marrow progenitors into DCs. This hypothesis is supported by our findings that in naive PAR2 WT and KO mice, the DC frequencies in secondary lymphoid organs are equivalent. Although PAR2 KO mice exhibit a delayed onset of inflammation in most animal models, they are immunocompetent and are able to survive in a non-specific pathogen-free (SPF) environment, making a fundamental DC development defect unlikely.
Altogether, we demonstrate that PAR2 activation promotes DC maturation, antigen transport to draining lymph nodes and enhances lymph node T-cell activation, thus enhancing immune activation and explaining the phenotype observed in animal models. A better comprehension of this mechanism of PAR2 immunomodulation may prove particularly useful for vaccine development, as well as in allergy, autoimmunity and inflammatory disease.
Disclosures
None of the authors of this paper have conflicts of interest to disclose.
References
- 1.Busso N, Frasnelli M, Feifel R, Cenni B, Steinhoff M, Hamilton J, So A. Evaluation of protease-activated receptor 2 in murine models of arthritis. Arthritis Rheum. 2007;56:101–7. doi: 10.1002/art.22312. [DOI] [PubMed] [Google Scholar]
- 2.Ferrell WR, Lockhart JC, Kelso EB, et al. Essential role for proteinase-activated receptor-2 in arthritis. J Clin Invest. 2003;111:35–41. doi: 10.1172/JCI16913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansen KK, Sherman PM, Cellars L, et al. A major role for proteolytic activity and proteinase-activated receptor-2 in the pathogenesis of infectious colitis. Proc Natl Acad Sci USA. 2005;102:8363–8. doi: 10.1073/pnas.0409535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawagoe J, Takizawa T, Matsumoto J, et al. Effect of protease-activated receptor-2 deficiency on allergic dermatitis in the mouse ear. Jpn J Pharmacol. 2002;88:77–84. doi: 10.1254/jjp.88.77. [DOI] [PubMed] [Google Scholar]
- 5.Nakano S, Mishiro T, Takahara S, et al. Distinct expression of mast cell tryptase and protease activated receptor-2 in synovia of rheumatoid arthritis and osteoarthritis. Clin Rheumatol. 2007;26:1284–92. doi: 10.1007/s10067-006-0495-8. [DOI] [PubMed] [Google Scholar]
- 6.Napoli C, de Nigris F, Wallace JL, Hollenberg MD, Tajana G, De Rosa G, Sica V, Cirino G. Evidence that protease activated receptor 2 expression is enhanced in human coronary atherosclerotic lesions. J Clin Pathol. 2004;57:513–6. doi: 10.1136/jcp.2003.015156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noorbakhsh F, Tsutsui S, Vergnolle N, et al. Proteinase-activated receptor 2 modulates neuroinflammation in experimental autoimmune encephalomyelitis and multiple sclerosis. J Exp Med. 2006;203:425–35. doi: 10.1084/jem.20052148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seeliger S, Derian CK, Vergnolle N, et al. Proinflammatory role of proteinase-activated receptor-2 in humans and mice during cutaneous inflammation in vivo. FASEB J. 2003;17:1871–85. doi: 10.1096/fj.02-1112com. [DOI] [PubMed] [Google Scholar]
- 9.Su X, Camerer E, Hamilton JR, Coughlin SR, Matthay MA. Protease-activated receptor-2 activation induces acute lung inflammation by neuropeptide-dependent mechanisms. J Immunol. 2005;175:2598–605. doi: 10.4049/jimmunol.175.4.2598. [DOI] [PubMed] [Google Scholar]
- 10.Takizawa T, Tamiya M, Hara T, Matsumoto J, Saito N, Kanke T, Kawagoe J, Hattori Y. Abrogation of bronchial eosinophilic inflammation and attenuated eotaxin content in protease-activated receptor 2-deficient mice. J Pharmacol Sci. 2005;98:99–102. doi: 10.1254/jphs.scz050138. [DOI] [PubMed] [Google Scholar]
- 11.D’Andrea MR, Derian CK, Leturcq D, et al. Characterization of protease-activated receptor-2 immunoreactivity in normal human tissues. J Histochem Cytochem. 1998;46:157–64. doi: 10.1177/002215549804600204. [DOI] [PubMed] [Google Scholar]
- 12.Damiano BP, Cheung WM, Santulli RJ, et al. Cardiovascular responses mediated by protease-activated receptor-2 (PAR-2) and thrombin receptor (PAR-1) are distinguished in mice deficient in PAR-2 or PAR-1. J Pharmacol Exp Ther. 1999;288:671–8. [PubMed] [Google Scholar]
- 13.Cocks TM, Fong B, Chow JM, et al. A protective role for protease-activated receptors in the airways. Nature. 1999;398:156–60. doi: 10.1038/18223. [DOI] [PubMed] [Google Scholar]
- 14.Santulli RJ, Derian CK, Darrow AL, Tomko KA, Eckardt AJ, Seiberg M, Scarborough RM, Andrade-Gordon P. Evidence for the presence of a protease-activated receptor distinct from the thrombin receptor in human keratinocytes. Proc Natl Acad Sci USA. 1995;92:9151–5. doi: 10.1073/pnas.92.20.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong W, McConalogue K, Khitin LM, Hollenberg MD, Payan DG, Bohm SK, Bunnett NW. Luminal trypsin may regulate enterocytes through proteinase-activated receptor 2. Proc Natl Acad Sci USA. 1997;94:8884–9. doi: 10.1073/pnas.94.16.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindner JR, Kahn ML, Coughlin SR, et al. Delayed onset of inflammation in protease-activated receptor-2-deficient mice. J Immunol. 2000;165:6504–10. doi: 10.4049/jimmunol.165.11.6504. [DOI] [PubMed] [Google Scholar]
- 17.Vergnolle N, Wallace JL, Bunnett NW, Hollenberg MD. Protease-activated receptors in inflammation, neuronal signaling and pain. Trends Pharmacol Sci. 2001;22:146–52. doi: 10.1016/s0165-6147(00)01634-5. [DOI] [PubMed] [Google Scholar]
- 18.Lerner DJ, Chen M, Tram T, Coughlin SR. Agonist recognition by proteinase-activated receptor 2 and thrombin receptor. Importance of extracellular loop interactions for receptor function. J Biol Chem. 1996;271:13943–7. [PubMed] [Google Scholar]
- 19.Bohm SK, Kong W, Bromme D, et al. Molecular cloning, expression and potential functions of the human proteinase-activated receptor-2. Biochem J. 1996;314:1009–16. doi: 10.1042/bj3141009. Pt 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molino M, Woolkalis MJ, Reavey-Cantwell J, Pratico D, Andrade-Gordon P, Barnathan ES, Brass LF. Endothelial cell thrombin receptors and PAR-2. Two protease-activated receptors located in a single cellular environment. J Biol Chem. 1997;272:11133–41. doi: 10.1074/jbc.272.17.11133. [DOI] [PubMed] [Google Scholar]
- 21.Kanke T, Macfarlane SR, Seatter MJ, Davenport E, Paul A, McKenzie RC, Plevin R. Proteinase-activated receptor-2-mediated activation of stress-activated protein kinases and inhibitory kappa B kinases in NCTC 2544 keratinocytes. J Biol Chem. 2001;276:31657–66. doi: 10.1074/jbc.M100377200. [DOI] [PubMed] [Google Scholar]
- 22.Belham CM, Tate RJ, Scott PH, Pemberton AD, Miller HR, Wadsworth RM, Gould GW, Plevin R. Trypsin stimulates proteinase-activated receptor-2-dependent and -independent activation of mitogen-activated protein kinases. Biochem J. 1996;320:939–46. doi: 10.1042/bj3200939. Pt 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelso EB, Lockhart JC, Hembrough T, et al. Therapeutic promise of proteinase-activated receptor-2 antagonism in joint inflammation. J Pharmacol Exp Ther. 2006;316:1017–24. doi: 10.1124/jpet.105.093807. [DOI] [PubMed] [Google Scholar]
- 24.Molino M, Barnathan ES, Numerof R, et al. Interactions of mast cell tryptase with thrombin receptors and PAR-2. J Biol Chem. 1997;272:4043–9. doi: 10.1074/jbc.272.7.4043. [DOI] [PubMed] [Google Scholar]
- 25.Camerer E, Huang W, Coughlin SR. Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc Natl Acad Sci USA. 2000;97:5255–60. doi: 10.1073/pnas.97.10.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belting M, Ahamed J, Ruf W. Signaling of the tissue factor coagulation pathway in angiogenesis and cancer. Arterioscler Thromb Vasc Biol. 2005;25:1545–50. doi: 10.1161/01.ATV.0000171155.05809.bf. [DOI] [PubMed] [Google Scholar]
- 27.Coughlin SR, Camerer E. Participation in inflammation. J Clin Invest. 2003;111:25–7. doi: 10.1172/JCI17564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- 29.Nystedt S, Emilsson K, Wahlestedt C, Sundelin J. Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci USA. 1994;91:9208–12. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nystedt S, Emilsson K, Larsson AK, Strombeck B, Sundelin J. Molecular cloning and functional expression of the gene encoding the human proteinase-activated receptor 2. Eur J Biochem. 1995;232:84–9. doi: 10.1111/j.1432-1033.1995.tb20784.x. [DOI] [PubMed] [Google Scholar]
- 31.Hollenberg MD, Saifeddine M, al-Ani B. Proteinase-activated receptor-2 in rat aorta: structural requirements for agonist activity of receptor-activating peptides. Mol Pharmacol. 1996;49:229–33. [PubMed] [Google Scholar]
- 32.Kanke T, Takizawa T, Kabeya M, Kawabata A. Physiology and pathophysiology of proteinase-activated receptors (PARs): PAR-2 as a potential therapeutic target. J Pharmacol Sci. 2005;97:38–42. doi: 10.1254/jphs.fmj04005x7. [DOI] [PubMed] [Google Scholar]
- 33.McGuire JJ, Saifeddine M, Triggle CR, Sun K, Hollenberg MD. 2-furoyl-LIGRLO-amide: a potent and selective proteinase-activated receptor 2 agonist. J Pharmacol Exp Ther. 2004;309:1124–31. doi: 10.1124/jpet.103.064584. [DOI] [PubMed] [Google Scholar]
- 34.Csernok E, Ai M, Gross WL, et al. Wegener autoantigen induces maturation of dendritic cells and licenses them for Th1 priming via the protease-activated receptor-2 pathway. Blood. 2006;107:4440–8. doi: 10.1182/blood-2005-05-1875. [DOI] [PubMed] [Google Scholar]
- 35.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–78. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 36.Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, Kincade PW. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–12. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rallabhandi P, Nhu QM, Toshchakov VY, Piao W, Medvedev AE, Hollenberg MD, Fasano A, Vogel SN. Analysis of proteinase-activated receptor 2 and TLR4 signal transduction: a novel paradigm for receptor cooperativity. J Biol Chem. 2008;283:24314–25. doi: 10.1074/jbc.M804800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawabata A, Kanke T, Yonezawa D, et al. Potent and metabolically stable agonists for protease-activated receptor-2: evaluation of activity in multiple assay systems in vitro and in vivo. J Pharmacol Exp Ther. 2004;309:1098–107. doi: 10.1124/jpet.103.061010. [DOI] [PubMed] [Google Scholar]
- 39.Thomas WR, Edwards AJ, Watkins MC, Asherson GL. Distribution of immunogenic cells after painting with the contact sensitizers fluorescein isothiocyanate and oxazolone. Different sensitizers form immunogenic complexes with different cell populations. Immunology. 1980;39:21–7. [PMC free article] [PubMed] [Google Scholar]
- 40.Macatonia SE, Edwards AJ, Knight SC. Dendritic cells and the initiation of contact sensitivity to fluorescein isothiocyanate. Immunology. 1986;59:509–14. [PMC free article] [PubMed] [Google Scholar]
- 41.Ebeling C, Lam T, Gordon JR, Hollenberg MD, Vliagoftis H. Proteinase-activated receptor-2 promotes allergic sensitization to an inhaled antigen through a TNF-mediated pathway. J Immunol. 2007;179:2910–7. doi: 10.4049/jimmunol.179.5.2910. [DOI] [PubMed] [Google Scholar]
- 42.Ebeling C, Forsythe P, Ng J, Gordon JR, Hollenberg M, Vliagoftis H. Proteinase-activated receptor 2 activation in the airways enhances antigen-mediated airway inflammation and airway hyperresponsiveness through different pathways. J Allergy Clin Immunol. 2005;115:623–30. doi: 10.1016/j.jaci.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 43.Marsland BJ, Battig P, Bauer M, et al. CCL19 and CCL21 induce a potent proinflammatory differentiation program in licensed dendritic cells. Immunity. 2005;22:493–505. doi: 10.1016/j.immuni.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 44.Yoneyama H, Matsuno K, Zhang Y, et al. Evidence for recruitment of plasmacytoid dendritic cell precursors to inflamed lymph nodes through high endothelial venules. Int Immunol. 2004;16:915–28. doi: 10.1093/intimm/dxh093. [DOI] [PubMed] [Google Scholar]
- 45.Fiorucci S, Mencarelli A, Palazzetti B, et al. Proteinase-activated receptor 2 is an anti-inflammatory signal for colonic lamina propria lymphocytes in a mouse model of colitis. Proc Natl Acad Sci USA. 2001;98:13936–41. doi: 10.1073/pnas.241377298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fields RC, Schoenecker JG, Hart JP, Hoffman MR, Pizzo SV, Lawson JH. Protease-activated receptor-2 signaling triggers dendritic cell development. Am J Pathol. 2003;162:1817–22. doi: 10.1016/S0002-9440(10)64316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]