Abstract
Cyclooxygenase (Cox) inhibitors are among the most widely used and commonly prescribed medications. Relatively little is understood about their influence on human immune responses. Herein, we discovered a novel and important mechanism whereby non-steroidal anti-inflammatory drugs (NSAIDs) blunt human B-cell antibody production. We demonstrate that the Cox-2 selective small molecule inhibitors SC-58125 and NS-398 attenuate the production of human antibody isotypes including immunoglobulin M (IgM), IgG1, IgG2, IgG3 and IgG4. In addition, inhibition of Cox-2 significantly reduced the generation of CD38+ IgM+ and CD38+ IgG+ antibody-secreting cells. Interestingly, we discovered that inhibition of Cox-2 activity in normal human B cells severely reduced the messenger RNA and protein levels of the essential plasma cell transcription factor, Blimp-1. These observations were mirrored in Cox-2-deficient mice, which had reduced CD138+ plasma cells and a near loss of Blimp-1 expression. These new findings demonstrate a critical role for Cox-2 in the terminal differentiation of human B lymphocytes to antibody-secreting plasma cells. The use of NSAIDs may adversely influence the efficacy of vaccines, especially in the immunocompromised, elderly and when vaccines are weakly immunogenic.
Keywords: antibody responses, B cells, CpG DNA, transcription factors, vaccines
Introduction
Generation of antibody is a goal of vaccination and is essential for effective immune responses against pathogens. Transcription factors, including Blimp-1 and Xbp-1, regulate the terminal differentiation of B lymphocytes to plasma cells, which are responsible for antibody production. Blimp-1, a transcriptional repressor, is necessary for plasma cell differentiation, as well as for maintenance of the plasma cell phenotype.1–3 Mice deficient in Blimp-1 fail to produce antibodies against both T-independent and T-dependent antigens, indicating that Blimp-1 is required for antibody production.3–5 Blimp-1 represses genes such as Pax5, c-myc and Bcl-6 that are important for the function of mature B cells.2,6 Expression of Blimp-1 is necessary for the expression of Xbp-1, a transcriptional activator that prepares a plasma cell to become an antibody-secreting factory.2,7 Xbp-1 controls the expression of proteins that are responsible for increased cell volume, protein synthesis, protein folding and enlarged endoplasmic reticulum, all important for plasma cell function.7,8
Cyclooxygenases are enzymes that regulate inflammation, at least in part, through the production of lipid mediators called eicosanoids. The constitutively expressed isoform cyclooxygenase-1 (Cox-1) maintains homeostatic levels of eicosanoids, while the inducible isoform Cox-2 is responsible for elevated mediator production, so controlling inflammation. It was previously thought that only tissue structural cells expressed Cox-2. However, Cox-2 can be expressed by immune cells including T cells, macrophages and B cells.9,10 Human B cells express Cox-2 after exposure to provoking agents such as CpG DNA, CD40 ligand and B-cell receptor (BCR) engagement.11,12 This was further confirmed by Hanten et al.,13 who demonstrated that activation of human B cells with ligands of Toll-like receptors 7 and 9 increased Cox-2 transcript levels. Cox-2 activity in B cells is important for optimal antibody production.12,14 We previously demonstrated that Cox-2-deficient mice have impaired antibody responses to human papillomavirus-16 virus-like particles.15 Cox-2 inhibitor-treated mice also showed reduced B-cell responses to T-dependent antigens, including tetanus and diphtheria toxin.16
The purpose of the present study was to determine whether the reduction in total immunoglobulin G (IgG) levels caused by Cox-2 inhibition influenced all human IgG isotypes and whether or not CD38+ antibody-secreting cells were influenced. Finally, we wanted to test the hypothesis that Cox-2 inhibition attenuated the expression of key transcription factors important for B-cell differentiation to plasma cells. Collectively, our findings support the concept that the use of Cox inhibitors can counteract the goal of vaccines, by inhibiting the generation of plasma cells which produce antibodies, important for fighting infections.
Materials and methods
Reagents and culture conditions
Human B lymphocytes isolated from peripheral blood mononuclear cells (PBMC) were cultured in RPMI-1640 (GIBCO/Invitrogen, North Andover, MA) supplemented with 10% fetal bovine serum, 2 mm l-glutamine, 5 × 105 m 2-mercaptoethanol, 10 mm HEPES and 50 μg/ml gentamicin. CpG oligodeoxynucleotide (ODN) 2395 5′-TCGTCGTTTTCGGCGCGCGCCG-3′ was purchased from the Coley Pharmaceutical Group (Wellesley, MA) and used to stimulate B cells at a concentration of 1 μg/ml. Stimulation of BCR was performed using a rabbit anti-human F(ab′)2 anti-IgM antibody fragment (Jackson ImmunoResearch Laboratories, West Grove, PA) at 2 μg/ml. Arachidonic acid (Nu-Chek Prep, Elysian, MN) dissolved in ethanol was supplemented in culture at a concentration of 10 μm. Mitomycin C (Sigma-Aldrich, St Louis, MO) was added to cell cultures to prevent cell division, acting as a control for carboxyfluorescein succinimidyl ester (CFSE) analysis. SC-58125 and NS-398, (Cayman Chemical, Ann Arbor, MI) small molecule Cox-2 selective inhibitors, were dissolved in dimethyl sulphoxide (DMSO), and used at concentrations of 5, 10 and 20 μm. Cox-2 inhibitors were added on days 0, 3 and 5 of culture unless otherwise stated.
B-lymphocyte isolation
Units of peripheral blood were obtained from healthy donors [not taking any non-steroidal anti-inflammatory drugs (NSAIDs) or other medications] under ethical permission provided by the Research Subjects Review Board at the University of Rochester. B cells were isolated as described previously.11,12 Briefly, PBMC were isolated using Ficoll–Paque (Amersham Biosciences, Piscataway, NJ) gradient centrifugation. The B cells were labelled with CD19 Dynabeads (Invitrogen) and CD19 Dynabead-cell rosettes were disrupted using CD19 Detachabead (Invitrogen). Cells obtained by this method of isolation were > 98% CD19+. B cells were purified from Cox-2-deficient mice (B6.129P2-Ptgs2tm1Unc) and wild-type control splenocytes (Taconic Farms Inc., Hudson, NY) using a CD19 magnetic antibody cell sorter (MACS) separation protocol (Miltenyi Biotec, Auburn, CA). Purified CD19+ B cells were cultured with lipopolysaccharide (LPS; 10 μg/ml) for 72 hr.
Antibody production assessment
Positively isolated CD19+ human B cells (5 × 105 cells/ml) were cultured in 96-well round-bottom plates for 7 days in the presence of CpG ODN 2395, anti-IgM and arachidonic acid (10 μm). Vehicle control or Cox-2 selective inhibitors, SC-58125 or NS-398, were added at onset of culture and on days 3 and 5. Levels of IgM and IgG in the supernatants were assessed by enzyme-linked immunosorbent assay (ELISA; Bethyl Laboratories, Montgomery, TX) on day 7 as described previously.11,12 The immunoglobulins IgG1, IgG2, IgG3 and IgG4 were measured using the human IgG subclass isotyping kit (Millipore, Billerica, MA) and analysed on the Luminex 100 system (Upstate/Millipore).
Prostaglandin E2 enzyme immunoassay
Human PBMCs (2 × 105/well) were left untreated or stimulated with CpG plus anti-IgM for 24 hr in the presence of SC-58125 or NS-398. Supernatants were collected and analysed for prostaglandin E2 (PGE2) levels by enzyme immunoassay (Cayman Chemical).
Flow cytometric analysis
Purified human B-cell viability was assessed by 7-aminoactinomycin D (7-AAD) staining using BD Bioscience’s Cell Viability Solution. Cells were surface stained for allophycocyanin-conjugated CD19 and phycoerythrin-conjugated CD38 (CD38-PE; BD Biosciences, San Jose, CA). Proliferation was assessed by CFSE (Molecular Probes/Invitrogen, Carlsbad, CA) labelling of cells before agonist/drug treatment. Cells were incubated with 5 μm CFSE for 5 min at room temperature and washed three times before stimulation in culture for 7 days. For intracellular staining, CD19+ purified human B cells were fixed and permeabilized using the Caltag fix and perm kit (Caltag Laboratories/Invitrogen, Burlingame, CA) and stained for intracellular fluorescein isothiocyanate-conjugated IgM (IgM-FITC) or IgG-FITC (BD Biosciences). Freshly isolated wild-type and Cox-2-deficient mouse splenocytes were stained for CD19-PE (BD Biosciences), CD21-FITC (eBioscience, San Diego, CA) and CD23-biotin (BD Biosciences) to assess marginal zone B-cell populations. Secondary labelling was performed with streptavidin-allophycocyanin (Caltag Laboratories/Invitrogen). Wild-type and Cox-2-deficient B cells were stained for surface CD138-PE (BD Biosciences) expression after 72 hr of culture. Fluorescently labelled cells were analysed on a FACSCalibur flow cytometer (BD Biosciences) and results were analysed using FlowJo software (Tree Star Inc., Ashland, OR).
Real-time reverse transcription–polymerase chain reaction
Following 24, 48, 72 and 96 hr culture of human B cells (3 × 106 cells/ml), total RNA was isolated using a Qiagen RNAeasy mini kit. RT Superscript III and random primers (Invitrogen, Carlsbad, CA) were used to reverse transcribe isolated RNA to complementary DNA. Steady-state levels of Blimp-1, Xbp-1, Pax5 and 7S (housekeeping control) messenger RNA (mRNA) were assessed by real-time polymerase chain reaction (PCR). Primers used included Blimp-1 sense 5′-GTGTCAGAACGGGATGAAC-3′ and antisense 5′-TGTTAGAACGGTAGAGGTCC-3′, Xbp-1 sense 5′-TGGCGGTATTGACTCTTCAG-3′ and antisense 5′-ACGAGGTCATCTTCTACAGG-3′, Pax5 sense 5′-TTGCTCATCAAGGTGTCAGG-3′ and antisense 5′-TAGGCACGGTGTCATTGTC-3′ and 7S sense 5′-ACCACCA GGTTGCCTAAGGA-3′ and antisense 5′-CACGGGAGT TTTGACCTGCT-3′. As previously described, iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) was used to quantify amplified products and results were analysed with the Bio-Rad Icycler software.11,12 Blimp-1, Xbp-1 and Pax5 mRNA steady-state levels were normalized to 7S expression. Fold mRNA decrease was determined by comparing mRNA steady-state levels from vehicle-treated peripheral human B cells with SC-58125-treated B cells.
Western blotting
Purified normal human B lymphocytes were lysed in ELB buffer: 50 mm HEPES (pH 7.0), 0.1% nonidet P-40, 250 mm NaCl, 10 mm NaF, 5 mm ethylenediamine tetraacetic acid, 0.1 mm Na3VO4, 5 μm ZnCl2 to obtain whole cell lysate. Protein was quantified using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL). Gradient sodium dodecyl sulphate –polyacrylamide gel electrophoresis gels (Pierce/Thermo Fisher Scientific, Rockford, IL) were loaded with 10 μg of protein and transferred to polyvinylidene fluoride membranes (Millipore). Western blots were probed with mouse anti-human Blimp-1 (Novus, Littleton, CO), mouse anti-human AID (Cell Signaling Technology, Beverly, MA), rabbit anti-human Xbp-1 (Novus), rabbit anti-human Pax5 (Millipore, Billerica, MA), mouse anti-human actin control (Calbiochem/EMD Chemicals, Gibbstown, NJ) and anti-GAPDH control (Calbiochem). Secondary antibody labelling was performed using horseradish peroxidase-conjugated goat anti-mouse or goat anti-rabbit antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) after washing. Western blots were visualized by autoradiography after incubation with enhanced chemiluminescence (Perkin Elmer Life Sciences Inc., Boston, MA).
Results
Cox-2 inhibitors attenuate human antibody production regardless of isotype
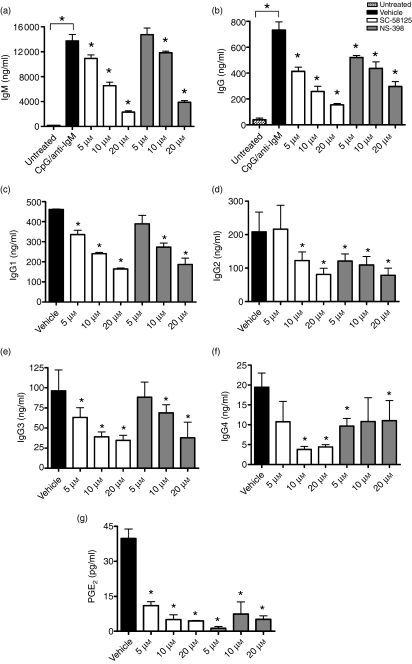
Human peripheral blood B cells express Cox-2 upon activation and Cox-2 activity is necessary for optimal production of IgM and IgG.11,12 To determine if Cox-2 selective inhibitors preferentially influence the production of certain human antibody isotypes, we assessed production of IgM, total IgG, IgG1, IgG2, IgG3 and IgG4 following a 7-day stimulation with CpG plus anti-IgM. Peripheral blood human B cells were treated with either SC-58125 or NS-398, both small molecule Cox-2 selective inhibitors. Production of IgM and total IgG was measured by ELISA (Fig. 1a,b), while IgG1, IgG2, IgG3 and IgG4 (Fig. 1c–f) isotypes were measured using Luminex technology. We observed a significant decrease in IgM, total IgG, IgG1, IgG2, IgG3 and IgG4 following treatment of B cells with SC-58125. NS-398 also significantly inhibited the production of IgM, total IgG, IgG1, IgG2 and IgG3. Treatment with NS-398 also reduced IgG4 production, although, not in a dose-dependent manner. Based on PGE2 production from isolated PBMC the concentrations of Cox-2 selective inhibitors used to attenuate antibody production are sufficient to significantly inhibit Cox-2 activity (Fig. 1g). These new data demonstrate that both SC-58125 and NS-398 significantly attenuated production of all isotypes, indicating that Cox-2 inhibitors do not selectively inhibit antibody isotype production.
Figure 1.
Cyclooxygenase-2 (Cox-2) inhibitors attenuate immunoglobulin M (IgM) and IgG production. Purified human B cells were left untreated or stimulated with oligodeoxynucleotide 2395 plus anti-IgM and cultured for 7 days in the presence of vehicle control (black), 5 μm, 10 μm or 20 μm SC-58125 (white) or NS-398 (grey). (a) IgM and (b) total IgG were measured in media supernatants by enzyme-linked immunosorbent assay. IgG1 (c), IgG2 (d), IgG3 (e) and IgG4 (f) were assessed using a Luminex multiplex assay. Peripheral blood mononuclear cells were cultured for 24 hr in the presence of SC-58125 or NS-398 following stimulation with CpG plus anti-IgM. (g) Prostaglandin (PG) E2 was measured in supernatants, demonstrating that concentrations of selective inhibitors used abrogated Cox-2 activity. Data are represented as mean ± SD compared with vehicle alone, *P < 0·05.
Cox-2 selective inhibitors do not significantly influence normal B-cell viability or proliferation
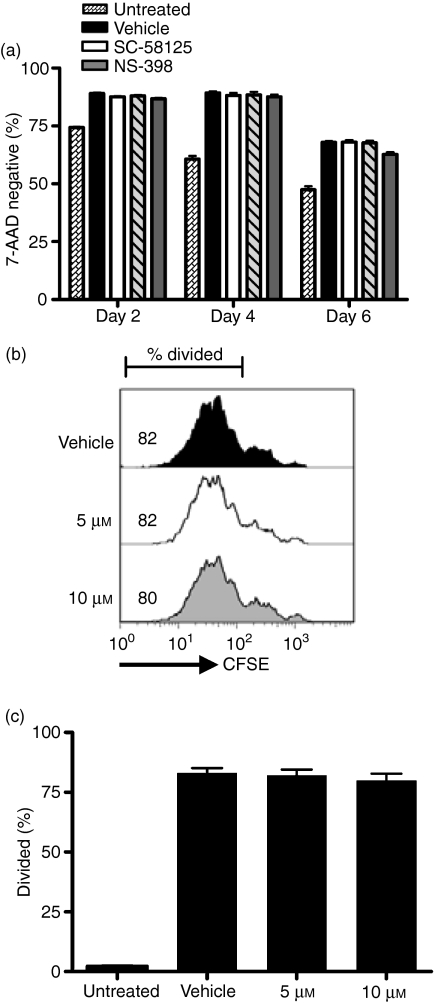
The global decrease in antibody production induced by Cox-2 inhibition could be a result of reduced B-cell viability or proliferation. Therefore, we measured the percentage of cells that excluded 7-AAD on days 2, 4 and 6 of culture. Neither SC-58125 (Fig. 2a) nor NS-398 (data not shown) significantly affected the viability of activated human B cells measured on any of these days. To demonstrate whether or not attenuated antibody production was a result of decreased proliferation, purified blood B cells were labelled with CFSE before stimulation with CpG plus anti-IgM. After 7 days of culture, little difference was observed in CFSE profiles and per cent divided cells in SC-58125-treated B-cell cultures (Fig. 2b). Similar results were observed for B cells treated with NS-398, a different Cox-2 selective inhibitor (data not shown). The percentages of divided B cells following treatment with SC-58125 were averaged from three different donors (Fig. 2c). No significant change in the per cent divided B cells following Cox-2 inhibitor treatment was detected, indicating that a decrease in proliferation does not account for the attenuation of antibody production.
Figure 2.
Cyclooxygenase-2 (Cox-2) inhibitors do not significantly influence human B-cell viability or proliferation. Viability of human B cells was assessed by 7-aminoactinomycin D (7-AAD) exclusion. (a) Per cent viable cells analysed on days 2, 4 and 6 are represented as bar graphs for SC-58125 treatments. B cells were stained with carboxyfluorescein succinimidyl ester before agonist stimulation and SC-58125 treatment to analyse possible changes in proliferation by flow cytometry after 7 days of culture in two individual donors. Per cent divided cell gates were drawn based on undivided mitomycin-C-treated B cells. Vehicle-treated and SC-58125-treated B-cell CFSE profiles and per cent cells divided are shown for one representative donor (b) and per cent cells divided for multiple donors (n= 3) (c). Data are represented as mean ± SD.
Inhibition of Cox-2 prevents differentiation of human B cells to CD38+ antibody-secreting cells
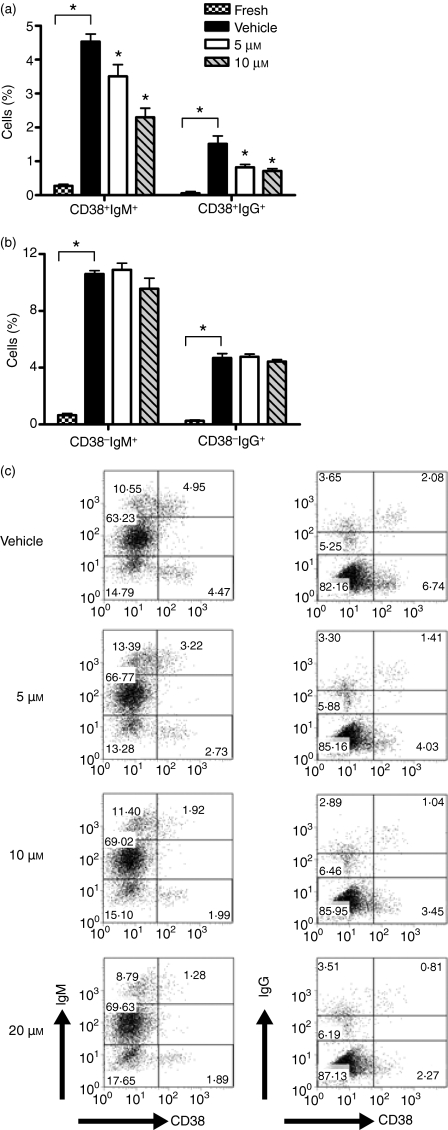
We next investigated whether attenuated antibody production was caused by a reduction in the differentiation of human B cells to antibody-secreting cells. Human plasma cell precursors, defined by multiple investigators as CD38+ antibody-secreting cells,17–19 can be generated in vitro. On day 7 of culture, B cells were stained for surface expression of CD38 and CD19, as well as for intracellular IgM or IgG. Intracellular antibody gates were determined based upon unpermeabilized stained controls. Multiple blood donors were assessed via this method with similar results. Freshly isolated B cells express a relatively low frequency of CD38+ antibody-secreting cells, which is significantly increased following 7 days of stimulation with CpG plus anti-IgM (Fig. 3a). A significant reduction in the frequency of CD38+ antibody-secreting cells was observed following treatment with SC-58125 (Fig. 3a,c). In contrast there was no change in the frequency of CD38− Ig+-secreting cells (Fig. 3b). Generation of IgM-secreting, CD38+ B cells was significantly attenuated in a dose-dependent manner (Fig. 3c). These results mirrored the decrease in antibody production measured by ELISA (Fig. 1). Similarly, CD38+ IgG-secreting cells were also significantly decreased following treatment with the Cox-2 inhibitor (Fig. 3c). These new data demonstrate that the Cox-2 selective inhibitor, SC-58125 attenuated the ability of B cells to differentiate to CD38+ antibody-secreting plasma cell precursors.
Figure 3.
Inhibition of cyclooxygenase-2 (Cox-2) prevents the differentiation of B cells to antibody-secreting cells. CD19+ B cells cultured for 7 days in the presence of oligodeoxynucleotide 2395 plus anti-immunoglobulin M (IgM) and treated with dimethylsulphoxide vehicle, 5, 10 or 20 μm SC-58125 were stained for surface expression of CD38-phycoerythrin and intracellular IgM- or IgG-fluorescein isothiocyanate. Gates for intracellular staining were drawn based on unpermeabilized cells. Bar graphs depict changes in CD38+ (a) CD38− (b) antibody-secreting cell populations in freshly isolated B cells or following activation and treatment with SC-58125. (c) Flow cytometry dot plots show changes in CD38+ IgM+ and CD38+ IgG+ in the presence of SC-58125 in another donor. Data shown are representative of two donors; however, multiple donors showed similar decreases in CD38+ Ig+ cells. Data are represented as mean ± SD, *P < 0·05.
B-cell differentiation to plasma cells and expression of the essential transcription factor, Blimp-1, is Cox-2 dependent
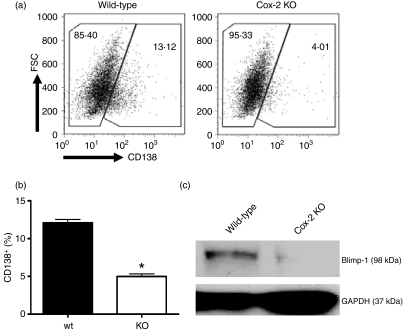
Cox-2 knockout mice were next used to study the vital role of Cox-2 in B-cell differentiation to plasma cells. CD19+ B cells were isolated from the spleens of wild-type and Cox-2-deficient mice. Analysis of wild-type and Cox-2-deficient splenocytes revealed no significant differences in overall CD19+ cells or marginal zone B cells (CD19+ CD21+ CD23−), indicating that B-cell populations are similar. Following a 72-hr stimulation with LPS, Cox-2-deficient mice had a 60% reduction in the number of CD138+ plasma cells compared with wild-type controls (Fig. 4a,b). This indicates impairment in the differentiation of B cells to plasma cells in mice lacking Cox-2.
Figure 4.
B-cell differentiation to plasma cells and expression of the essential transcription factor, Blimp-1, is cyclooxygenase-2 (Cox-2) dependent. Purified CD19+ B cells from spleens of wild-type and Cox-2 deficient mice were cultured with 10 μg/ml lipopolysaccharide (LPS) for 72 hr. Cells were analysed by flow cytometry for CD138 surface expression. (a) Dot plots show events based on forward scatter and CD138 expression. (b) Representative bar graph showing the percentage of CD138+ cells after LPS stimulation. (c) Western blot probed for Blimp-1 and GAPDH control following 72 hr of culture with 10 μg/ml LPS. Data are represented as mean ± SD, *P < 0·05.
Next, we tested whether expression of the essential plasma cell transcriptional regulator, Blimp-1, was regulated by Cox-2. Following 72 hr of activation with LPS, Western blots were probed for expression of Blimp-1 in both Cox-2 knockout and wild-type B-cell protein extracts (Fig. 4c). Interestingly, Cox-2-deficient mice had an approximately 25-fold lower Blimp-1 protein expression compared with wild-type controls (Fig. 4c). This further demonstrates that B-cell differentiation is Cox-2-dependent.
Cox-2 inhibition attenuates the expression of transcriptional regulators essential for human plasma cell differentiation
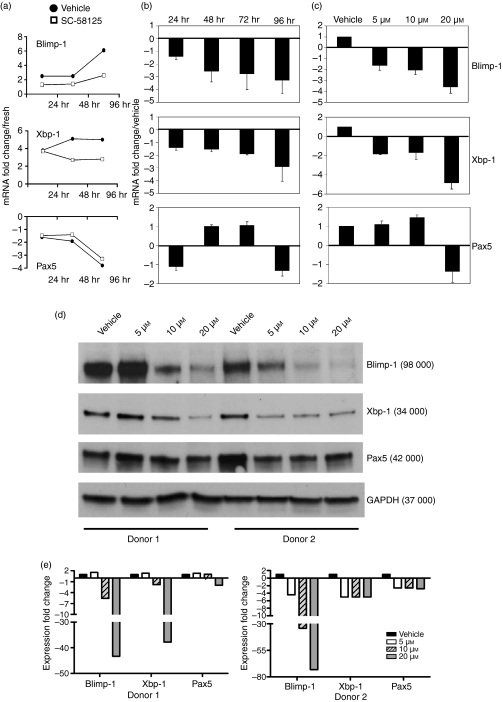
To determine if the reduced generation of CD38+ antibody-secreting cells was a result of impaired differentiation of human B cells, we investigated whether the expression of plasma cell transcriptional regulators was influenced. We assessed both mRNA steady-state levels and protein expression of Blimp-1 and Xbp-1, which are essential transcription factors necessary for plasma cell differentiation. Pax5, a transcription factor important for initiating and maintaining the B-cell phenotype, was also investigated. Purified human B cells from three different donors activated for 24, 48, 72 or 96 hr were treated with either DMSO (vehicle) or the Cox-2 selective inhibitor SC-58125. RNA was extracted at each time-point, reverse transcribed, and subjected to real-time PCR analysis for Blimp-1, Xbp-1 and Pax5 expression. Messenger RNA steady-state levels of each transcription factor were normalized to 7S control mRNA steady-state levels. Comparing levels of Blimp-1, Xbp-1 and Pax5 with freshly isolated B-cell mRNA demonstrated that Pax5 mRNA steady-state levels decreased following stimulation with CpG plus anti-IgM, while Blimp-1 and Xbp-1 expression was enhanced (Fig. 5a). The mRNA fold-expression decrease after Cox-2 inhibitor treatment was determined by dividing the normalized mRNA expression values of the vehicle-treated cells by the normalized values of the SC-58125-treated cells (Fig. 5b,c). Following treatment of three different human donors with SC-58125, Blimp-1 mRNA expression was decreased 2·6 ± 0·8-fold by 24 hr, 2·8 ± 1·2-fold by 72 hr and 3·3 ± 1·1-fold by 96 hr (Fig. 5b). At the 20-μm dose Blimp-1 levels were reduced by 3·6 ± 0·5-fold after 72 hr of incubation (Fig. 5c). Over the time–course, Xbp-1 mRNA expression was decreased (1·9 ± 0·1-fold) in the presence of SC-58125 at 72 hr (Fig. 5b). By 96 hr after Cox-2 inhibitor treatment we observed a 2·9 ± 1·2-fold decrease. Treatment of B cells with 20 μm SC-58125 for 72 hr resulted in a 4·9 ± 0·6-fold decrease in Xbp-1 mRNA expression (Fig. 5c). In contrast, Pax5 mRNA expression was relatively unchanged following inhibition of Cox-2 (Fig. 5b,c). These new data indicate that inhibition of Cox-2 reduced mRNA transcript levels of the transcription factors, Blimp-1 and Xbp-1, which are essential for the differentiation of B cells to plasma cells.
Figure 5.
Inhibition of cyclooxygenase-2 (Cox-2) attenuates the expression of transcription factors essential for plasma cell differentiation. RNA was isolated from freshly purified human peripheral blood B cells and cells stimulated with oligodeoxynucleotide (ODN) 2395 plus anti-immunoglobulin M (IgM) and treated with dimethylsulphoxide vehicle or the Cox-2 selective inhibitor SC-58125. Blimp-1, Xbp-1 and Pax5 messenger RNA (mRNA) steady-state levels were first normalized to 7S mRNA steady-state levels. (a) Blimp-1, Xbp-1 and Pax5 mRNA steady-state level fold change compared with freshly isolated B cells from one representative donor. Fold decrease of Blimp-1, Xbp-1 and Pax5 levels were determined by comparing vehicle-treated B cells with those treated with SC-58125. The mRNA fold decrease was averaged for four donors. (b) The mRNA fold decrease in the presence of 10 μm SC-58125 shown over a time–course of 24, 48, 72 and 96 hr; (c) mRNA fold decrease shown at 72 hr with 5, 10 or 20 μm doses of SC-58125; (d) ODN 2395 plus anti-IgM stimulated B-cell protein lysates (10 μg) collected at 72 hr from two separate donors were run using sodium dodecyl sulphate–polyacrylamide gel electrophoresis. The Western blot was probed for Blimp-1, Xbp-1, Pax5 and GAPDH control. (e) Densitometry results were normalized to GAPDH expression. Data are represented as mean ± SEM.
To further demonstrate that the decrease in Blimp-1 and Xbp-1 mRNA was seen at the translational level, protein was extracted from activated human B cells treated with vehicle or SC-58125. A Western blot containing these samples from two different donors was probed for the expression of Blimp-1, Xbp-1, Pax5 and GAPDH as a loading control (Fig. 5d). Blimp-1 protein expression was strongly decreased in the presence of Cox-2 inhibitors (Fig. 5d). Densitometry analysis indicated that SC-58125 reduced B cell Blimp-1 expression sixfold (10 μm) and 43-fold (20 μm) in one donor and fourfold (5 μm), 34-fold (10 μm) and 73-fold (20 μm) in the second donor (Fig. 5d,e). Xbp-1 protein levels were modestly decreased following incubation with the Cox-2 inhibitor. Densitometry analysis indicated that SC-58125 reduced B-cell Xbp-1 expression twofold (10 μm) and 38-fold (20 μm) in one donor and fivefold (5 μm) for all doses in the second donor (Fig. 5d). Blimp-1 and Xbp-1 protein levels in freshly isolated or untreated B cells were not detectable by Western blot (data not shown). Pax5 protein levels appear relatively unchanged, although donor 2 had slightly lower levels compared with vehicle control (2·5-fold decrease). These data demonstrate that inhibition of Cox-2 strongly decreases Blimp-1 steady-state mRNA and protein levels, which indicates that Cox-2 activity is essential for optimal generation of antibody-secreting plasma cells.
Discussion
The NSAIDs, including Cox-2 selective inhibitors, are commonly used in the treatment of acute inflammation, chronic pain and arthritis. More recent benefits have been investigated, including the use of these drugs to delay the onset of Alzheimer’s disease and as supplements for cancer chemotherapy.14,20,21 Although interest in using NSAIDs for new therapies is expanding, relatively little is known about how these drugs influence the human immune system. We provide new evidence that NSAIDs, through the inhibition of Cox-2, blunt B-cell antibody production. Our results reveal a novel mechanism for attenuated antibody production whereby Cox-2 activity is essential for the terminal differentiation of B lymphocytes. These findings implicate that the use of NSAIDs that inhibit Cox-2 dampen humoral immune responses.
Human B-cell production of total IgM and IgG is attenuated in the presence of NSAIDs.11,12 Herein, we further demonstrated that the Cox-2 selective inhibitors, SC-58125 and NS-398, blunted the production of IgM and all IgG isotypes (IgG1, IgG2, IgG3 and IgG4). Therefore, Cox-2 plays an essential role in the optimal production of antibody in general and is not biased towards any particular human antibody isotype. This indicates that Cox-2 plays a broad role in the differentiation of human B cells to antibody-secreting plasma cells.
Cox-2 selective inhibitors can affect cell growth and survival.22,23 Therefore, viability of normal activated B cells treated with Cox-2 inhibitors was evaluated to rule out their role in the attenuation of antibody production. Mongini et al.24 showed that Cox-2 inhibitors decreased the viability of human B2 cells undergoing cell division. However, the doses used in that study were approximately 10-fold higher than those used herein. Under our lower-dose conditions, neither SC-58125 nor NS-398 influenced the overall viability of human B cells. B-cell differentiation is linked to cellular proliferation, with the probability of generating antibody-secreting cells increasing upon each successive division.25 However, human B-cell proliferation, as assessed by CFSE labelling, was not significantly influenced in the presence of Cox-2 selective inhibitors, and so does not contribute to attenuated antibody production.
It is difficult to generate CD138+ human plasma cells in vitro. Therefore, we investigated changes in plasma cell precursor populations, a commonly used approach.17–19 Plasma cell precursors have been defined by numerous investigators as CD38+ antibody-secreting cells.17–19 Arce et al.17 demonstrated that CD38− IgG-secreting cells generated from blood-derived B cells gave rise to CD38+ antibody-secreting plasma cell precursors. We observed no change in the frequency of CD38− antibody-secreting cells after treatment with Cox-2 inhibitors. In contrast, inhibition of Cox-2 significantly impaired the generation of CD38+ antibody-secreting cells, supporting the reduced levels of IgM and IgG observed in culture. This new finding suggests that Cox-2 controls the progression of CD38− antibody-secreting cells to CD38+ antibody-secreting plasma cell precursors. Inhibiting the terminal differentiation of B cells would result in a lack of plasma cells available to produce antibodies in response to vaccination or infection. Preventing memory B cells from differentiating into long-lived plasma cells would also severely attenuate responses to secondary infections. Our results, therefore, implicate an essential role for Cox-2 in optimal humoral immunity to infection and vaccination.
Transcriptional regulators, such as Blimp-1 and Xbp-1 are indispensible for the differentiation of B lymphocytes to plasma cells.3,26 Shapiro-Shelef et al.27 demonstrated that, in mice, antigen-specific antibodies in serum were lost when Blimp-1 was deleted from resident bone marrow plasma cells, indicating that Blimp-1 expression is essential for maintenance and survival of plasma cells. Blimp-1 targets and represses transcription of Pax5 and other factors that are important for maintaining the B-cell phenotype. Targeting Pax5 permits expression of Xbp-1 and paves the way for differentiating B cells to become antibody-producing factories.2,6,28 Human B-cell expression of Blimp-1 and Xbp-1 protein was attenuated in the presence of a Cox-2 selective inhibitor (see Fig. 5d). We also observed decreased Blimp-1 mRNA levels 24–48 hr after treatment with Cox-2 inhibitors and decreased Xbp-1 mRNA expression approximately 96 hr after treatment. This is consistent with the control hierarchy over Xbp-1, as Blimp-1 expression is necessary to induce Xbp-1 transcription. No significant changes in Pax5 expression occurred in B cells treated with Cox-2 inhibitors. However, inhibition of Pax5 function has been shown to occur before its repression by Blimp-1, indicating that although Pax5 expression does not change, its function may be inhibited.29
Interestingly, attenuated CD138+ plasma cell generation and Blimp-1 protein expression were discovered in Cox-2-deficient mouse B cells. This provides further evidence that B-cell terminal differentiation is Cox-2-dependent. Although both Blimp-1 and CD138 expression are attenuated in mouse B-cell cultures, this does not demonstrate that Blimp-1 is directly responsible for the decrease in the frequency of CD138+ cells. However, in the human B-cell cultures, Blimp-1 decreases early after Cox-2 inhibition and precedes CD38+ plasma cell precursor formation, suggesting that reduced Blimp-1 levels are responsible for decreased generation of human plasma cells. Blimp-1 is considered a master regulator of the plasma cell phenotype. Mice deficient in either Blimp-1 or Xbp-1 fail to generate significant numbers of plasma cells and produce relatively little serum antibody.3,26,30 We previously demonstrated that Cox-2-deficient mice immunized with HPV-16 virus-like particles displayed reduced neutralizing antibody titres and B-cell memory responses.15 Lupu et al.16 provide evidence that Cox-2 selective inhibitors impaired IgG production against T-dependent antigens, namely tetanus and diphtheria toxins. Autoimmune antibody production was also attenuated following treatment with Cox-2 selective inhibitors.31 These observations and our new in vitro results suggest that impaired in vivo antibody production is a result of decreased B-cell differentiation to antibody-secreting plasma cells. Likewise, our results show reduced human Blimp-1 and Xbp-1 expression in the presence of Cox-2 inhibitors, which is important in the decreased generation of plasma cell precursors (CD38+ antibody-secreting cells) and overall reduced antibody levels.
Our results reveal that Cox-2 is essential for the differentiation B cells to antibody-secreting cells, providing a mechanism for the involvement of Cox-2 in attenuated antibody production. Use of Cox-2 inhibitors during vaccination or infection could, therefore, impair the generation of plasma cells, which are important regulators of immunity. Without effective generation of plasma cells, patients may be more vulnerable to infections that rely on antibody-mediated immune responses, particularly elderly patients, who often take Cox-2 selective inhibitors and NSAIDs. Ultimately, our findings indicate that taking Cox-2 selective inhibitors or other NSAIDs that inhibit Cox-2 may reduce the efficacy of vaccines, as well as blunt immune responses to invading pathogens.
Acknowledgments
The authors would like to thank Dr Ignacio Sanz and Tam Quach for providing T-cell-depleted human tonsil cells. This work was funded by the National Institutes of Health Grants DE011390, AI071064, ES01247 and the Training Program in Oral Sciences T32-DE007202.
Disclosures
The authors have no conflict of interest.
References
- 1.Martins G, Calame K. Regulation and functions of Blimp-1 in T and B lymphocytes. Annu Rev Immunol. 2008;26:133–69. doi: 10.1146/annurev.immunol.26.021607.090241. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nat Rev Immunol. 2005;5:230–42. doi: 10.1038/nri1572. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro-Shelef M, Lin KI, McHeyzer-Williams LJ, Liao J, McHeyzer-Williams MG, Calame K. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity. 2003;19:607–20. doi: 10.1016/s1074-7613(03)00267-x. [DOI] [PubMed] [Google Scholar]
- 4.Lindroth K, Fernandez C. The role of Blimp-1 in the GC reaction: differential expression of Blimp-1 upon immunization with TD and TI antigens. Immunol Lett. 2007;113:70–5. doi: 10.1016/j.imlet.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 5.Soro PG, Morales AP, Martinez MJ, Morales AS, Copin SG, Marcos MA, Gaspar ML. Differential involvement of the transcription factor Blimp-1 in T cell-independent and -dependent B cell differentiation to plasma cells. J Immunol. 1999;163:611–7. [PubMed] [Google Scholar]
- 6.Kallies A, Nutt SL. Terminal differentiation of lymphocytes depends on Blimp-1. Curr Opin Immunol. 2007;19:156–62. doi: 10.1016/j.coi.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Brewer JW, Hendershot LM. Building an antibody factory: a job for the unfolded protein response. Nat Immunol. 2005;6:23–9. doi: 10.1038/ni1149. [DOI] [PubMed] [Google Scholar]
- 8.Iwakoshi NN, Lee AH, Vallabhajosyula P, Otipoby KL, Rajewsky K, Glimcher LH. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat Immunol. 2003;4:321–9. doi: 10.1038/ni907. [DOI] [PubMed] [Google Scholar]
- 9.Iniguez MA, Punzon C, Fresno M. Induction of cyclooxygenase-2 on activated T lymphocytes: regulation of T cell activation by cyclooxygenase-2 inhibitors. J Immunol. 1999;163:111–9. [PubMed] [Google Scholar]
- 10.Chen Y, Zhang J, Moore SA, Ballas ZK, Portanova JP, Krieg AM, Berg DJ. CpG DNA induces cyclooxygenase-2 expression and prostaglandin production. Int Immunol. 2001;13:1013–20. doi: 10.1093/intimm/13.8.1013. [DOI] [PubMed] [Google Scholar]
- 11.Bernard MP, Phipps RP. CpG oligodeoxynucleotides induce cyclooxygenase-2 in human B lymphocytes: implications for adjuvant activity and antibody production. Clin Immunol. 2007;125:138–48. doi: 10.1016/j.clim.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan EP, Pollock SJ, Murant TI, Bernstein SH, Felgar RE, Phipps RP. Activated human B lymphocytes express cyclooxygenase-2 and cyclooxygenase inhibitors attenuate antibody production. J Immunol. 2005;174:2619–26. doi: 10.4049/jimmunol.174.5.2619. [DOI] [PubMed] [Google Scholar]
- 13.Hanten JA, Vasilakos JP, Riter CL, Neys L, Lipson KE, Alkan SS, Birmachu W. Comparison of human B cell activation by TLR7 and TLR9 agonists. BMC Immunol. 2008;9:39. doi: 10.1186/1471-2172-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernard MP, Bancos S, Sime PJ, Phipps RP. Targeting cyclooxygenase-2 in hematological malignancies: rationale and promise. Curr Pharm Des. 2008;14:2051–60. doi: 10.2174/138161208785294654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryan EP, Malboeuf CM, Bernard M, Rose RC, Phipps RP. Cyclooxygenase-2 inhibition attenuates antibody responses against human papillomavirus-like particles. J Immunol. 2006;177:7811–9. doi: 10.4049/jimmunol.177.11.7811. [DOI] [PubMed] [Google Scholar]
- 16.Lupu AR, Cremer L, Durbaca S, Calugaru A, Herold A, Kerek F, Szegli G, Radu DL. COX-2 inhibitors can down-regulate in vivo antibody response against T-dependent antigens. Roum Arch Microbiol Immunol. 2006;65:59–65. [PubMed] [Google Scholar]
- 17.Arce S, Luger E, Muehlinghaus G, et al. CD38 low IgG-secreting cells are precursors of various CD38 high-expressing plasma cell populations. J Leukoc Biol. 2004;75:1022–8. doi: 10.1189/jlb.0603279. [DOI] [PubMed] [Google Scholar]
- 18.Avery DT, Ellyard JI, Mackay F, Corcoran LM, Hodgkin PD, Tangye SG. Increased expression of CD27 on activated human memory B cells correlates with their commitment to the plasma cell lineage. J Immunol. 2005;174:4034–42. doi: 10.4049/jimmunol.174.7.4034. [DOI] [PubMed] [Google Scholar]
- 19.Tarte K, De Vos J, Thykjaer T, et al. Generation of polyclonal plasmablasts from peripheral blood B cells: a normal counterpart of malignant plasmablasts. Blood. 2002;100:1113–22. [PubMed] [Google Scholar]
- 20.Nivsarkar M, Banerjee A, Padh H. Cyclooxygenase inhibitors: a novel direction for Alzheimer’s management. Pharmacol Rep. 2008;60:692–8. [PubMed] [Google Scholar]
- 21.de Groot DJ, de Vries EG, Groen HJ, de Jong S. Non-steroidal anti-inflammatory drugs to potentiate chemotherapy effects: from lab to clinic. Crit Rev Oncol Hematol. 2007;61:52–69. doi: 10.1016/j.critrevonc.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Grosch S, Maier TJ, Schiffmann S, Geisslinger G. Cyclooxygenase-2 (COX-2)-independent anticarcinogenic effects of selective COX-2 inhibitors. J Natl Cancer Inst. 2006;98:736–47. doi: 10.1093/jnci/djj206. [DOI] [PubMed] [Google Scholar]
- 23.Jana NR. NSAIDs and apoptosis. Cell Mol Life Sci. 2008;65:1295–301. doi: 10.1007/s00018-008-7511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mongini PK, Inman JK, Han H, Fattah RJ, Abramson SB, Attur M. APRIL and BAFF promote increased viability of replicating human B2 cells via mechanism involving cyclooxygenase 2. J Immunol. 2006;176:6736–51. doi: 10.4049/jimmunol.176.11.6736. [DOI] [PubMed] [Google Scholar]
- 25.Hasbold J, Corcoran LM, Tarlinton DM, Tangye SG, Hodgkin PD. Evidence from the generation of immunoglobulin G-secreting cells that stochastic mechanisms regulate lymphocyte differentiation. Nat Immunol. 2004;5:55–63. doi: 10.1038/ni1016. [DOI] [PubMed] [Google Scholar]
- 26.Reimold AM, Iwakoshi NN, Manis J, et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412:300–7. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro-Shelef M, Lin KI, Savitsky D, Liao J, Calame K. Blimp-1 is required for maintenance of long-lived plasma cells in the bone marrow. J Exp Med. 2005;202:1471–6. doi: 10.1084/jem.20051611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calame K. Activation-dependent induction of Blimp-1. Curr Opin Immunol. 2008;20:259–64. doi: 10.1016/j.coi.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Kallies A, Hasbold J, Fairfax K, et al. Initiation of plasma-cell differentiation is independent of the transcription factor Blimp-1. Immunity. 2007;26:555–66. doi: 10.1016/j.immuni.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Tirosh B, Iwakoshi NN, Glimcher LH, Ploegh HL. XBP-1 specifically promotes IgM synthesis and secretion, but is dispensable for degradation of glycoproteins in primary B cells. J Exp Med. 2005;202:505–16. doi: 10.1084/jem.20050575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turull A, Queralt J. Selective cyclooxygenase-2 (COX-2) inhibitors reduce anti-Mycobacterium antibodies in adjuvant arthritic rats. Immunopharmacology. 2000;46:71–7. doi: 10.1016/s0162-3109(99)00159-9. [DOI] [PubMed] [Google Scholar]