Abstract
The regulatory role of tumour necrosis factor-α (TNF-α) on the expression of suppressor of cytokine signalling 3 (SOCS-3) in response to lipopolysaccharide (LPS) was examined using peritoneal macrophages from TNF-α-deficient mice. The LPS-induced SOCS-3 expression was markedly augmented in macrophages from wild-type mice whereas such augmentation was not seen in the cells from TNF-α-deficient mice. However, there was no significant difference in the level of SOCS-3 messenger RNA expression between macrophages from wild-type mice and those from TNF-α-deficient mice. The addition of exogenous TNF-α augmented the LPS-induced SOCS-3 expression in macrophages from TNF-α-deficient mice. The pulse chase analysis suggested augmented degradation of LPS-induced SOCS-3 protein in macrophages from TNF-α-deficient mice. Moreover, MG 132, a 26S proteasome inhibitor, sustained the LPS-induced SOCS-3 expression in those cells. The tyrosine phosphorylation of SOCS-3 was definitely induced in LPS-stimulated macrophages from TNF-α-deficient mice but not wild-type mice. A tyrosine phosphatase inhibitor enhanced the tyrosine phosphorylation of SOCS-3 in wild-type mice and accelerated the degradation. Therefore, it was suggested that TNF-α prevented the degradation of SOCS-3 protein via inhibition of the tyrosine phosphorylation in LPS-stimulated macrophages.
Keywords: lipopolysaccharide, suppressor of cytokine signalling-3, tumour necrosis factor-α-deficient mice, tumour necrosis factor-α, tyrosine phosphorylation
Introduction
Suppressor of cytokine signalling (SOCS) proteins are a family of intracellular proteins that are involved in a wide range of biological processes, especially regulation of responses of immune cells to cytokines.1–4 All of these proteins contain a central Src homology 2 (SH2) domain and a C-terminal SOCS box that may target signalling intermediates to proteasomal degradation.5–8 They act as feedback inhibitors induced by cytokines and bacterial products, such as lipopolysaccharide (LPS) and CpG DNA. Of these, SOCS-3 is known to play an important role in the regulation of macrophage function. Several pro-inflammatory stimuli such as tumour necrosis factor-α (TNF-α), LPS and CpG DNA, or interleukin-10 (IL-10) induce the expression of SOCS-3 not only in human and rat liver macrophages but also in a macrophage cell line.9–12 However, the regulatory mechanism of the SOCS-3 expression induced by pro-inflammatory stimuli has not been fully understood.
The SH2 domain of SOCS-3 contains a 35-residue unstructured PEST (proline-, glutamicacid-, serine- and threonine-rich) -motif insertion that increases SOCS-3 turnover and affects the protein stability.13 One lysine residue, a potential ubiquitination site, is found at position 6 (Lys-6) in the N-terminal region of SOCS-3 and the Lys-6 is a key residue for regulating SOCS-3 stability through the SOCS-box.14 The SOCS box interacts with elongin C and the complex prevents proteasome-mediated degradation of the SOCS protein.15–17 SOCS-3 is strongly phosphorylated by Janus-activated kinase (JAK) in response to many growth factors, including IL-2, IL-6, erythropoietin, epidermal growth factor and platelet-derived growth factor.18–20 Recently, it has been reported that the tyrosine phosphorylation of SOCS-3 disrupts the complex of the SOCS box and elongin C and enhances proteasome-mediated degradation of SOCS-3.21 In the present study we studied a regulatory role of TNF-α in LPS-induced SOCS-3 expression by using peritoneal macrophages from TNF-α-deficient mice. Here we report that TNF-α may prevent the degradation of SOCS-3 protein on LPS stimulation by inhibiting the tyrosine phosphorylation.
Materials and methods
Mice
B10.D2 male mice, 6 weeks of age, were supplied by Japan SLC (Hamamatsu, Japan). The TNF-α-deficient (TNF-α−/−) B10.D2 mice were provided by K. Sekikawa (National Institute of Animal Health, Tsukuba, Japan) and K. Saito (Gifu University). All animal experiments were approved by the Animal Care Committee of Aichi Medical University and carried out under the guide for care and use of laboratory animals.
Reagents
Lipopolysaccharide from Escherichia coli O55 was obtained from Sigma-Aldrich (St Louis, MO). Recombinant TNF-α was purchased from Roche Applied Science (Indianapolis, IN). Mouse antibodies to TNF-α and interferon-γ (IFN-γ) were obtained from R&D Systems (Minneapolis, MN). Anti-phosphotyrosine antibody (4G10) and anti-SOCS-3 rabbit polyclonal antibody (C005) were obtained from Upstate Biotechnology (Hamburg, Germany) and IBL (Hamburg, Germany), respectively.
Cell culture
Mice were injected intraperitoneally with 1 ml sterile 10% thioglycollate (Remel, Kansas City, MO). Three days later, thioglycollate-elicited peritoneal cells were obtained by washing out the peritoneal cavity with RPMI-1640 medium (Gibco-BRL, Gaithersburg, MD) containing 5% fetal calf serum (FCS). The cells were suspended in RPMI-1640 medium containing 5% FCS without antibiotics and were incubated in a plastic dish for 5 hr. The culture medium was removed and the adherent cells, as peritoneal macrophages, were washed twice with RPMI-1640 medium and then incubated in RPMI-1640 medium containing 5% FCS and antibiotic-antimycotic (Gibco, Invitrogen, Carlsbad, CA) at 37° in a humidified 5% CO2 incubator for 24 hr.
Reverse transcription-polymerase chain reaction (RT-PCR) and real-time PCR
The total RNA was isolated from cells using an RNeasy minikit (Qiagen Sciences, Gaithersburg, MD) in accordance with the manufacturer’s protocols. Total RNA was reverse-transcribed to complementary DNA using a RT system with random hexamers (Toyobo, Tokyo, Japan). Expression of messenger RNA (mRNA) was analysed with StepOne real-time PCR, according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA). The reaction mixture consisted of SYBR green PCR master mix (Toyobo) and sequence-specific primers: β-actin sense, 5′-ATGACCCAGATCATGTTTGA-3′, antisense, 5′-TACGACCAGAGGCATACAG-3′; SOCS-3 sense, 5′-GCC AATGTCTTCCCAGTGTT-3′, antisense, 5′-ATTCACCCA GGTGGCTACAG-3′.
Immunoblotting and immunoprecipitation
Cells (2 × 107) were lysed in 550 μl lysis buffer containing 150 mm NaCl, 50 mm Tris–HCl (pH 7·6), 0·125% Nonidet P-40, 2 mm ethylenediaminetetraacetic acid, 1 mm Na3VO4, 1 mm NaF and protease inhibitor cocktail (Sigma, St Louis, MO) for 30 min on ice. Insoluble material was removed by centrifugation, and pre-cleared with TrueBlot anti-rabbit immunoglobulin IP beads (eBioscience, San Diego, CA) on ice for 30 min. The protein concentration was determined by the bicinchoninic acid protein assay reagent (Pierce, Rockford, IL) and the lysates were immunoprecipitated with appropriately diluted anti-SOCS-3 antibody at 4° overnight. The precipitated proteins were analysed with sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE). The immune complexes were detected with a 1 : 1000 dilution of horseradish peroxidase-conjugated anti-rabbit and anti-mouse immunoglobulin G (IgG) antibody (eBioscience) and the bands were visualized with a chemiluminescent reagent (Pierce). The chemiluminescence was analysed by a light capture system (AE6955; Atto Corp., Tokyo, Japan) with cool saver analyser.
Pulse chase analysis
The NEG772 EasyTag express protein labelling mix [35S] was purchased from PerkinElmer Life and Analytical Sciences (Albany, Boston, MA). Peritoneal macrophages (1·5 × 106/well) were washed twice in phosphate-buffered saline and incubated in methionine/cystine-free Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen) for 30 min at 37°. The cells were incubated with LPS (0·1 μg/ml) and [35S]methionine (100 μCi/well) for 30 min, followed by the addition of DMEM containing cold methionine and cystine, and 10% FCS. The cells were harvested 0 and 2 hr after the addition and the cellular lysates were immunoprecipitated with anti-SOCS-3 antibody and analysed using SDS–PAGE. After drying the gel, it was scanned with a Fuji BAS 5000 machine (Tokyo, Japan).
Statistical analysis
Statistical analysis was performed using Student’s t-test, with P< 0·01 considered to indicate a significant difference. Experimental results are expressed as the mean value of triplicates with SD in at least three independent experiments.
Results
Attenuated SOCS-3 expression in LPS-stimulated peritoneal macrophages from TNF-α-deficient mice
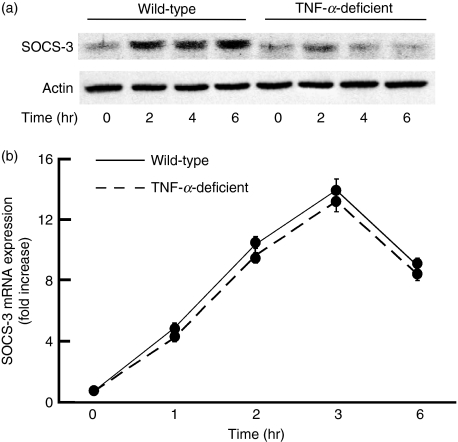
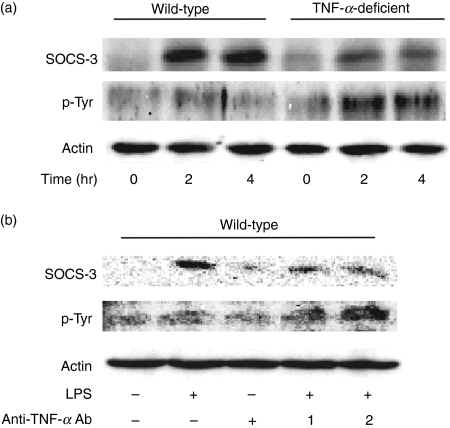
First, peritoneal macrophages from TNF-α-deficient mice were confirmed to produce no TNF-α in response to LPS (data not shown). To clarify the involvement of TNF-α, the level of SOCS-3 protein expression was compared in response to LPS between macrophages from wild-type and TNF-α-deficient mice (Fig. 1). Macrophages were stimulated with 0·1 μg/ml LPS for 2, 4 and 6 hr, and the level of SOCS-3 expression was analysed by immunoblotting with an anti-SOCS-3 antibody (Fig. 1a). The level of SOCS-3 protein expression in wild-type mice increased 2 hr after LPS treatment and the augmented SOCS-3 expression continued up to 6 hr. On the other hand, no such augmentation was seen in LPS-stimulated macrophages from TNF-α-deficient mice. Next, the level of SOCS-3 mRNA expression was examined with semi-quantitative PCR analysis (Fig. 1b). There was no significant difference in LPS-induced SOCS-3 mRNA expression between wild-type and TNF-α-deficient mice, excluding the involvement of the SOCS-3 mRNA stability in the difference of the SOCS-3 expression.
Figure 1.
Effect of lipopolysaccharide (LPS) on the expression of suppressor of cytokine signalling 3 (SOCS-3) in macrophages from tumour necrosis factor-α (TNF-α)-deficient mice. Macrophages from wild-type and TNF-α-deficient mice were incubated with LPS (0·1 μg/ml) for various hours. The expression of SOCS-3 protein (a) and messenger RNA (b) was analysed by immunoblotting and real-time polymerase chain reaction, respectively. (a) A typical experiment of three independent experiments is shown. (b) The experimental result was normalized using the housekeeping gene β-actin. The fold increase was calculated based on the value at 0 hr.
Involvement of TNF-α in LPS-induced SOCS-3 expression in macrophages
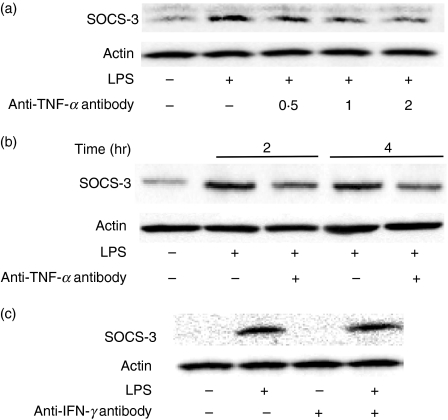
In the preceding section, LPS-induced SOCS-3 expression in macrophages from TNF-α-deficient mice was much less than that in the cells from wild-type mice, suggesting that TNF-α is involved in LPS-induced SOCS-3 expression. The effect of anti-TNF-α neutralizing antibody on LPS-induced SOCS-3 expression in macrophages from wild-type mice was examined (Fig. 2). Macrophages were pre-treated with anti-TNF-α antibody (0·5, 1 and 2 μg/ml) for 1 hr and then stimulated with LPS (0·1 μg/ml) for 4 hr. The LPS-induced SOCS-3 expression was analysed by immunoblotting. The LPS-induced SOCS-3 expression remarkably increased in the absence of the antibody. However, it was significantly inhibited by the addition of anti-TNF-α antibody (Fig. 2a). Subsequently, the effect of the neutralizing antibody on the kinetics of the LPS-induced SOCS-3 expression was examined. Macrophages from wild-type mice were pre-treated with anti-TNF-α antibody (2 μg/ml) for 1 hr and then incubated with LPS (0·1 μg/ml) for 2 or 4 hr. The pre-treatment with anti-TNF-α antibody significantly reduced the LPS-induced SOCS-3 expression 2 and 4 hr after LPS treatment (Fig. 2b). There was no significant difference in LPS-induced SOCS-3 mRNA expression between pre-treatment with or without anti-TNF-α antibody (data not shown). Furthermore, the effect of anti-IFN-γ-neutralizing antibody on the LPS-induced SOCS-3 expression was examined. Macrophages from wild-type mice were pre-treated with anti-IFN-γ antibody (2 μg/ml) for 1 hr and then stimulated with LPS (0·1 μg/ml) for 4 hr (Fig. 2c). There was no alteration in the LPS-induced SOCS-3 expression between pre-treatment with and without anti-IFN-γ antibody, suggesting that TNF-α might specifically inhibit it.
Figure 2.
Effect of anti-tumour necrosis factor-α (TNF-α) antibody on the lipopolysaccharide (LPS)-induced suppressor of cytokine signalling 3 (SOCS-3) expression in macrophages from wild-type mice. (a) Macrophages from wild-type mice were pre-treated with anti-TNF-α antibody (0·5, 1 or 2 μg/ml) for 1 hr and then incubated with LPS (0·1 μg/ml) for 4 hr. (b) Macrophages were pre-treated with anti-TNF-α antibody (2 μg/ml) for 1 hr and then incubated with LPS (0·1 μg/ml) for 2 and 4 hr. (c) Macrophages were pre-treated with anti-interferon-γ antibody (2 μg/ml) for 1 hr and then stimulated with LPS (0·1 μg/ml) for 4 hr. The expression of SOCS-3 protein was analysed by immunoblotting. A typical experiment of three independent experiments is shown.
Augmentation by exogenous TNF-α of the SOCS-3 expression in LPS-stimulated macrophages from TNF-α-deficient mice
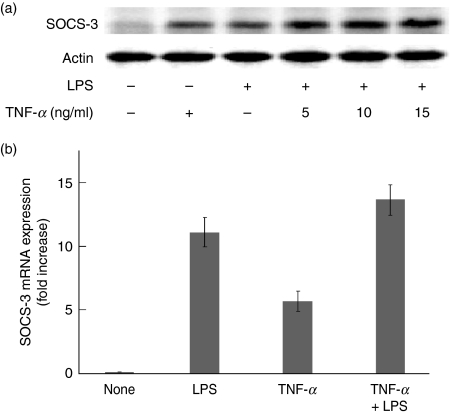
The effect of exogenous TNF-α on the SOCS-3 expression in LPS-stimulated macrophages from TNF-α-deficient mice was examined. Macrophages from TNF-α-deficient mice were stimulated with mouse recombinant TNF-α (5, 10 or 15 ng/ml) in the presence or absence of LPS (0·1 μg/ml) for 4 hr and the SOCS-3 expression was analysed by immunoblotting. Addition of exogenous TNF-α definitely induced the expression of SOCS-3 and it augmented LPS-induced SOCS-3 expression in macrophages from TNF-α-deficient mice (Fig. 3a).
Figure 3.
Effect of exogenous tumour necrosis factor-α (TNF-α) on the lipopolysaccharide (LPS)-induced suppressor of cytokine signalling 3 (SOCS-3) expression in macrophages from TNF-α-deficient mice. (a) Macrophages were incubated with exogenous TNF-α (5, 10 or 15 ng/ml) and LPS (0·1 μg/ml) for 4 hr. (b) Macrophages were incubated with LPS (0·1 μg/ml), TNF-α (15 ng/ml), and TNF-α plus LPS for 2 hr. The expression of SOCS-3 protein and messenger RNA was analysed by immunoblotting (a) and real-time polymerase chain reaction (b), respectively. (a) A typical experiment of three independent experiments is shown. (b) The experimental result was normalized using the housekeeping gene β-actin. The fold increase was calculated based on the value of untreated control.
Next, the effect of TNF-α on the SOCS-3 mRNA expression was examined in macrophages from TNF-α-deficient mice with real-time PCR. There was no significant difference in the level of LPS-induced SOCS-3 mRNA expression between macrophages stimulated with or without exogenous TNF-α (Fig. 3b).
Augmented degradation of LPS-induced SOCS-3 protein in macrophages from TNF-α-deficient mice
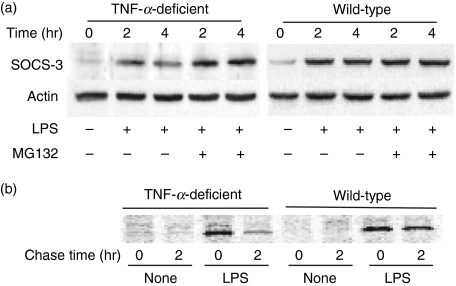
The expression of SOCS-3 protein is regulated by the proteasome-dependent pathway.6,14 To examine the involvement of proteasome in the attenuated SOCS-3 expression, macrophages were pre-treated with MG132 (10 μm), a 26S proteasome inhibitor, for 1 hr and stimulated with LPS (0·1 μg/ml) for 2 or 4 hr. Cellular lysates were analysed by immunoblotting using an anti-SOCS-3 antibody. As shown in Fig. 4(a), the SOCS-3 expression in macrophages from wild-type mice did not significantly alter 2 and 4 hr after LPS stimulation in the presence or absence of MG132. On the other hand, the SOCS-3 expression in macrophages from TNF-α-deficient mice significantly diminished at 4 hr in the absence of MG132. However, it did not diminish in the presence of MG132, suggesting that the reduced SOCS-3 expression in TNF-α-deficient mice might be responsible for proteasome-dependent degradation. Therefore, the SOCS-3 protein was labelled with [35S]methionine and chased for 0 and 2 hr to examine the augmented degradation (Fig. 4b). The LPS-induced SOCS-3 protein expression significantly diminished in macrophages from TNF-α-deficient mice 2 hr after chase whereas it did not diminish in those from wild-type mice.
Figure 4.
Augmented degradation of lipopolysaccharide (LPS)-induced suppressor of cytokine signalling 3 (SOCS-3) protein in macrophages from tumour necrosis factor-α (TNF-α) -deficient mice. (a) The effect of MG132, a 26S proteasome inhibitor, on the LPS-induced SOCS-3 expression in macrophages from TNF-α-deficient mice. Macrophages from wild-type or TNF-α-deficient mice were pre-treated with or without MG132 (10 μm) for 1 hr and then incubated with LPS (0·1 μg/ml) for 2 or 4 hr. The expression of SOCS-3 protein was analysed by immunoblotting. (b) Peritoneal macrophages were incubated with LPS (0·1 μg/ml) and [35S]methionine (100 μCi) for 30 min, followed by the addition of Dulbecco’s modified Eagle’s minimal medium containing cold methionine and cystine. The cell lysates were prepared 0 and 2 hr after the addition and analysed with immunoprecipitation and sodium dodecyl suphate–polyacrylamide gel electrophoresis. A typical experiment of three independent experiments is shown.
Induction of tyrosine phosphorylation of SOCS-3 in LPS-stimulated macrophages from TNF-α-deficient mice
The tyrosine residue of SOCS-3 is strongly phosphorylated by JAK in response to a number of cytokines and growth factors.12,20,21 First, the tyrosine phosphorylation of SOCS-3 was compared in LPS-stimulated macrophages between wild-type and TNF-α-deficient mice. Macrophages were treated with LPS (0·1 μg/ml) for 2 and 4 hr (Fig. 5a). The expression of SOCS-3 and tyrosine phosphorylation was analysed by using immunoprecipitation and immunoblotting. LPS caused the tyrosine phosphorylation of SOCS-3 in macrophages from TNF-α-deficient mice whereas it was not seen in the cells from wild-type mice. Subsequently, macrophages from wild-type mice were pre-treated with anti-TNF-α antibody (1 or 2 μg/ml) for 1 hr and then stimulated with LPS (0·1 μg/ml) for 4 hr. The tyrosine phosphorylation of SOCS-3 was strongly induced by the pre-treatment with anti-TNF-α antibody (Fig. 5b), suggesting that TNF-α might prevent LPS-induced SOCS-3 degradation through inhibiting the tyrosine phosphorylation.
Figure 5.
Involvement of tumour necrosis factor-α (TNF-α) on the tyrosine phosphorylation of suppressor of cytokine signalling 3 (SOCS-3) in lipopolysaccharide (LPS)-stimulated macrophages. (a) Macrophages from wild-type and TNF-α-deficient mice were incubated with LPS (0·1 μg/ml) for 2 or 4 hr. (b) Macrophages from wild-type mice were pre-treated with anti-TNF-α antibody (1 and 2 μg/ml) for 1 hr and then incubated with LPS (0·1 μg/ml) for 4 hr. The SOCS-3 expression was analysed by immunoprecipitation with anti-SOCS-3 antibody and immunoblotting with anti-phosphotyrosine or SOCS-3 antibody. A typical experiment of three independent experiments is shown.
Effect of sodium pervanadate as a tyrosine phosphatase inhibitor on LPS-induced tyrosine phosphorylation in macrophages
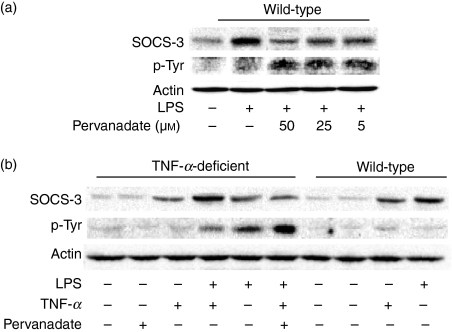
The effect of sodium pervanadate as a tyrosine phosphatase inhibitor on LPS-induced SOCS-3 tyrosine phosphorylation was examined. Macrophages from wild-type mice were pre-treated with sodium pervanadate for up to 50 μm for 1 hr and stimulated with LPS (0·1 μg/ml) for 4 hr. Cellular lysates were immunoprecipitated with anti-SOCS-3 antibody and then analysed by immunoblotting using an anti-phosphotyrosine antibody. The tyrosine phosphorylation of SOCS-3 was not detected in the LPS-stimulated macrophages (Fig. 6a). On the other hand, the tyrosine phosphorylation of SOCS-3 was clearly observed in the presence of sodium pervanadate. The LPS-induced SOCS-3 expression was reduced in the presence of sodium pervanadate.
Figure 6.
Effect of sodium pervanadate on the tyrosine phosphorylation of suppressor of cytokine signalling 3 (SOCS-3) in lipopolysaccharide (LPS)-stimulated macrophages from wild-type and tumour necrosis factor-α (TNF-α)-deficient mice. (a) Macrophages from wild-type mice were pre-treated with sodium pervanadate (5, 25 or 50 μm) for 1 hr and incubated with LPS (0·1 μg/ml) for 4 hr. (b) Macrophages from TNF-α-deficient mice were pre-treated with sodium pervanadate (50 μm) for 1 hr and incubated with LPS (0·1 μg/ml) or LPS plus TNF-α (15 ng/ml) for 4 hr. Macrophages from wild-type mice were treated with pervanadate, TNF-α or LPS. Cellular lysates were immunoprecipitated with anti-SOCS-3 antibody and the precipitates were analysed with immunoblotting with anti-phosphotyrosine or anti-SOCS-3 antibody. A typical experiment of three independent experiments is shown.
Next, macrophages from TNF-α-deficient mice were pre-treated with sodium pervanadate (50 μm) for 1 hr and stimulated with LPS (0·1 μg/ml) or the combination of LPS and TNF-α (15 ng/ml) for 4 hr (Fig. 6b). Exogenous TNF-α reduced LPS-induced tyrosine phosphorylation and augmented the SOCS-3 expression. Pervanadate significantly enhanced the tyrosine phosphorylation and reduced the SOCS-3 expression. In macrophages from wild-type mice neither TNF-α nor LPS induced the tyrosine phosphorylation of SOCS-3.
Discussion
In the present study we demonstrated that TNF-α augments LPS-induced SOCS-3 expression by preventing the degradation. Furthermore, TNF-α is suggested to prevent SOCS-3 protein degradation in LPS-stimulated peritoneal macrophages through inhibiting the tyrosine phosphorylation, which is essential for SOCS-3 protein stability. Several lines of evidence suggest that TNF-α is a critical regulator of SOCS-3 expression in LPS-stimulated macrophages: first, the expression of SOCS-3 protein was significantly impaired in response to LPS in macrophages from TNF-α-deficient mice; second, anti-TNF-α neutralizing antibody reduced LPS-induced SOCS-3 expression in macrophages from wild-type mice and exogenous TNF-α enhanced it in macrophages from TNF-α-deficient mice; third, the pulse chase analysis suggested the augmented degradation of LPS-induced SOCS-3 protein in macrophages from TNF-α-deficient mice; fourth, the LPS-induced SOCS-3 expression was sustained in macrophages from TNF-α-deficient mice in the presence of MG 132 as a 26S proteasome inhibitor; finally, the LPS-induced SOCS-3 expression was reduced by a tyrosine phosphatase inhibitor in macrophages from wild-type mice. Once again, TNF-α is suggested to regulate the SOCS-3 protein expression in LPS-stimulated macrophages via the tyrosine phosphorylation-dependent degradation. LPS-induced SOCS-3 expression can be regulated by activation of mitogen-activated protein (MAP) kinase, the production of endogenous IL-10, and signal transducer and activator of transcription 3 (STAT3) activation.10 The TNF-α-mediated degradation of SOCS-3 protein might be a mechanism for the regulation of LPS-induced SOCS-3 expression.
There is no difference in the expression of SOCS-3 mRNA in response to LPS between macrophages from wild-type and TNF-α-deficient mice. Therefore, it is suggested that TNF-α exhibits no action on the LPS-induced SOCS-3 mRNA expression and stability. Recently, TNF-α is reported to activate MAPKAP kinase 2 (MK2) and enhance SOCS-3 mRNA stability in TNF-α-stimulated RAW 264.7 macrophage-like cells.22 Considering that MK2 is a downstream molecule of p38 MAP kinase and is activated by p38,23,24 the role of TNF-α is possibly to regulate the SOCS-3 mRNA stability via p38 MAP kinase activation. Lipopolysaccharide directly activates p38 MAP kinase via toll-like receptor 4 without the help of TNF-α,25 for this reason LPS itself may be able to enhance the SOCS-3 mRNA stability in the cells from TNF-α-deficient mice. This might be a reason why there is no significant difference in LPS-induced SOCS-3 mRNA expression between wild-type and TNF-α-deficient mice.
SOCS-3 is phosphorylated on two tyrosine residues within the C-terminal SOCS box region and the tyrosine phosphorylation accelerates the SOCS-3 protein degradation by suppressing the interaction between SOCS box and elongin C.21,26 It is consistent with our conclusion in the present study that TNF-α regulates the degradation of SOCS-3 protein through inhibiting the tyrosine phosphorylation. There are several reports on the inhibitory action of TNF-α on tyrosine phosphorylation. The TNF-α employs a protein-tyrosine phosphatase to inhibit phosphorylation and activation of c-met by hepatocyte growth factor.27 The TNF-α suppresses vascular endothelial cell growth factor-mediated signalling pathway and IL-6-dependent tyrosine phosphorylation of STAT3 by inhibiting tyrosine phosphorylation of cytoplasmic signalling molecules.28,29 Further, TNF-α suppresses the tyrosine phosphorylation of insulin receptor and its substrates.30 Therefore, TNF-α is able to inhibit the tyrosine phosphorylation of SOCS-3.
The SOCS family consists of eight members [SOCS-1 to SOCS-7 and cytokine-inducible Src homology 2 (SH2)-containing protein (CIS)] and shares a central SH2 domain and a C-terminal SOCS box.31 Expression of CIS, SOCS-1, SOCS-2 and SOCS-3 is induced by various cytokines,32 and the over-expression studies in various cell lines have demonstrated their inhibitory actions.5 The family members have been implicated in the negative regulation of several pathways, particularly the JAK/STAT pathway.33,34 Because this signalling pathway is responsible for the induction of the family members, they form part of a classical negative feedback circuit. The TNF-α is a potent pro-inflammatory cytokine that is produced in LPS-stimulated macrophages.35 It would be reasonable that TNF-α as a potent pro-inflammatory cytokine prevents the degradation of SOCS-3 protein, which exhibits the negative regulation of cytokine network. The regulation of SOCS-3 expression by TNF-α might play a critical role in the negative feedback mechanism of LPS-induced cytokine cascade.36 On the other hand, unlike TNF-α, IL-6 and epidermal growth factor produced by LPS induce SOCS-3 tyrosine phosphorylation via JAK19 and enhance the SOCS-3 degradation.21 The degradation of SOCS-3 might be differentially regulated by various cytokines in response to LPS.
In summary, LPS-induced SOCS-3 expression is attenuated in macrophages from TNF-α-deficient mice. It is suggested that TNF-α regulates LPS-induced SOCS-3 protein expression via tyrosine phosphorylation-dependent degradation. It might be a negative feedback mechanism so that excessive pro-inflammatory cytokines on LPS stimulation may not cause a harmful cytokine storm in the host.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan. We are grateful to K. Takahashi and A. Morikawa for their technical assistance.
Disclosures
The authors declare no conflict of interest.
References
- 1.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–65. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 2.Dalpke A, Heeg K, Bartz H, Baetz A. Regulation of innate immunity by suppressor of cytokine signaling (SOCS) proteins. Immunobiology. 2008;213:225–35. doi: 10.1016/j.imbio.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Wormald S, Hilton DJ. The negative regulatory roles of suppressor of cytokine signaling proteins in myeloid signaling pathways. Curr Opin Hematol. 2007;14:9–15. doi: 10.1097/00062752-200701000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Yoshimura A, Ohishi HM, Aki D, Hanada T. Regulation of TLR signaling and inflammation by SOCS family proteins. J Leukoc Biol. 2004;75:422–7. doi: 10.1189/jlb.0403194. [DOI] [PubMed] [Google Scholar]
- 5.Krebs DL, Hilton DJ. SOCS: physiological suppressors of cytokine signaling. J Cell Sci. 2000;113:2813–9. doi: 10.1242/jcs.113.16.2813. Pt 16. [DOI] [PubMed] [Google Scholar]
- 6.Zhang JG, Farley A, Nicholson SE, et al. The conserved SOCS box motif in suppressors of cytokine signaling binds to elongins B and C and may couple bound proteins to proteasomal degradation. Proc Natl Acad Sci USA. 1999;96:2071–6. doi: 10.1073/pnas.96.5.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babon JJ, Sabo JK, Soetopo A, Yao S, Bailey MF, Zhang JG, Nicola NA, Norton RS. The SOCS box domain of SOCS3: structure and interaction with the elonginBC-cullin5 ubiquitin ligase. J Mol Biol. 2008;381:928–40. doi: 10.1016/j.jmb.2008.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kile BT, Schulman BA, Alexander WS, Nicola NA, Martin HM, Hilton DJ. The SOCS box: a tale of destruction and degradation. Trends Biochem Sci. 2002;27:235–41. doi: 10.1016/s0968-0004(02)02085-6. [DOI] [PubMed] [Google Scholar]
- 9.Berlato C, Cassatella MA, Kinjyo I, Gatto L, Yoshimura A, Bazzoni F. Involvement of suppressor of cytokine signaling-3 as a mediator of the inhibitory effects of IL-10 on lipopolysaccharide-induced macrophage activation. J Immunol. 2002;168:6404–11. doi: 10.4049/jimmunol.168.12.6404. [DOI] [PubMed] [Google Scholar]
- 10.Qin H, Roberts KL, Niyongere SA, Cong Y, Elson CO, Benveniste EN. Molecular mechanism of lipopolysaccharide-induced SOCS-3 gene expression in macrophages and microglia. J Immunol. 2007;179:5966–76. doi: 10.4049/jimmunol.179.9.5966. [DOI] [PubMed] [Google Scholar]
- 11.Dalpke AH, Opper S, Zimmermann S, Heeg K. Suppressors of cytokine signaling (SOCS)-1 and SOCS-3 are induced by CpG-DNA and modulate cytokine responses in APCs. J Immunol. 2001;166:7082–9. doi: 10.4049/jimmunol.166.12.7082. [DOI] [PubMed] [Google Scholar]
- 12.Bode JG, Nimmesgern A, Schmitz J, et al. LPS and TNFalpha induce SOCS3 mRNA and inhibit IL-6-induced activation of STAT3 in macrophages. FEBS Lett. 1999;463:365–70. doi: 10.1016/s0014-5793(99)01662-2. [DOI] [PubMed] [Google Scholar]
- 13.Babon JJ, McManus EJ, Yao S, et al. The structure of SOCS3 reveals the basis of the extended SH2 domain function and identifies an unstructured insertion that regulates stability. Mol Cell. 2006;22:205–16. doi: 10.1016/j.molcel.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki A, Inagaki-Ohara K, Yoshida T, Yamanaka A, Sasaki M, Yasukawa H, Koromilas AE, Yoshimura A. The N-terminal truncated isoform of SOCS3 translated from an alternative initiation AUG codon under stress conditions is stable due to the lack of a major ubiquitination site, Lys-6. J Biol Chem. 2003;278:2432–6. doi: 10.1074/jbc.C200608200. [DOI] [PubMed] [Google Scholar]
- 15.Hanada T, Yoshida T, Kinjyo I, et al. A mutant form of JAB/SOCS1 augments the cytokine-induced JAK/STAT pathway by accelerating degradation of wild-type JAB/CIS family proteins through the SOCS-box. J Biol Chem. 2001;276:40746–54. doi: 10.1074/jbc.M106139200. [DOI] [PubMed] [Google Scholar]
- 16.Bullock AN, Rodriguez MC, Debreczeni JE, Songyang Z, Knapp S. Structure of the SOCS4-ElonginB/C complex reveals a distinct SOCS box interface and the molecular basis for SOCS-dependent EGFR degradation. Structure. 2007;15:1493–504. doi: 10.1016/j.str.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen XP, Losman JA, Cowan S, et al. Pim serine/threonine kinases regulate the stability of Socs-1 protein. Proc Natl Acad Sci USA. 2002;99:2175–80. doi: 10.1073/pnas.042035699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cacalano NA, Sanden D, Johnston JA. Tyrosine-phosphorylated SOCS-3 inhibits STAT activation but binds to p120 RasGAP and activates Ras. Nat Cell Biol. 2001;3:460–5. doi: 10.1038/35074525. [DOI] [PubMed] [Google Scholar]
- 19.Sommer U, Schmid C, Sobota RM, et al. Mechanisms of SOCS3 phosphorylation upon interleukin-6 stimulation. Contributions of Src- and receptor-tyrosine kinases. J Biol Chem. 2005;280:31478–88. doi: 10.1074/jbc.M506008200. [DOI] [PubMed] [Google Scholar]
- 20.Peraldi P, Filloux C, Emanuelli B, Hilton DJ, Van Obberghen E. Insulin induces suppressor of cytokine signaling-3 tyrosine phosphorylation through janus-activated kinase. J Biol Chem. 2001;276:24614–20. doi: 10.1074/jbc.M102209200. [DOI] [PubMed] [Google Scholar]
- 21.Haan S, Ferguson P, Sommer U, Hiremath M, McVicar DW, Heinrich PC, Johnston JA, Cacalano NA. Tyrosine phosphorylation disrupts elongin interaction and accelerates SOCS3 degradation. J Biol Chem. 2003;278:31972–9. doi: 10.1074/jbc.M303170200. [DOI] [PubMed] [Google Scholar]
- 22.Ehlting C, Lai WS, Schaper F, et al. Regulation of suppressor of cytokine signaling 3 (SOCS3) mRNA stability by TNF-alpha involves activation of the MKK6/p38MAPK/MK2 cascade. J Immunol. 2007;178:2813–26. doi: 10.4049/jimmunol.178.5.2813. [DOI] [PubMed] [Google Scholar]
- 23.Ronkina N, Kotlyarov A, Gaestel M. MK2 and MK3 – a pair of isoenzymes? Front Biosci. 2008;13:5511–21. doi: 10.2741/3095. [DOI] [PubMed] [Google Scholar]
- 24.Kotlyarov A, Neininger A, Schubert C, Eckert R, Birchmeier C, Volk HD, Gaestel M. MAPKAP kinase 2 is essential for LPS-induced TNF-alpha biosynthesis. Nat Cell Biol. 1999;1:94–7. doi: 10.1038/10061. [DOI] [PubMed] [Google Scholar]
- 25.O’Neill LA, Dunne A, Edjeback M, Gray P, Jefferies C, Wietek C. Mal and MyD88: adapter proteins involved in signal transduction by Toll-like receptors. J Endotoxin Res. 2003;9:55–9. doi: 10.1179/096805103125001351. [DOI] [PubMed] [Google Scholar]
- 26.Cohney SJ, Sanden D, Cacalano NA, Yoshimura A, Mui A, Migone TS, Johnston JA. SOCS-3 is tyrosine phosphorylated in response to interleukin-2 and suppresses STAT5 phosphorylation and lymphocyte proliferation. Mol Cell Biol. 1999;19:4980–8. doi: 10.1128/mcb.19.7.4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugano M, Iwasaki Y, Abe M, Maeda T, Tsuchida K, Makino N. TNF-alpha employs a protein-tyrosine phosphatase to inhibit activation of hepatocyte growth factor receptor and hepatocyte growth factor-induced endothelial cell proliferation. Mol Cell Biochem. 2009;322:113–7. doi: 10.1007/s11010-008-9946-7. [DOI] [PubMed] [Google Scholar]
- 28.Guo DQ, Wu LW, Dunbar JD, et al. Tumor necrosis factor employs a protein-tyrosine phosphatase to inhibit activation of KDR and vascular endothelial cell growth factor-induced endothelial cell proliferation. J Biol Chem. 2000;275:11216–21. doi: 10.1074/jbc.275.15.11216. [DOI] [PubMed] [Google Scholar]
- 29.Bode JG, Schweigart J, Kehrmann J, Ehlting C, Schaper F, Heinrich PC, Haussinger D. TNF-alpha induces tyrosine phosphorylation and recruitment of the Src homology protein-tyrosine phosphatase 2 to the gp130 signal-transducing subunit of the IL-6 receptor complex. J Immunol. 2003;171:257–66. doi: 10.4049/jimmunol.171.1.257. [DOI] [PubMed] [Google Scholar]
- 30.Feinstein R, Kanety H, Papa MZ, Lunenfeld B, Karasik A. Tumor necrosis factor-alpha suppresses insulin-induced tyrosine phosphorylation of insulin receptor and its substrates. J Biol Chem. 1993;268:26055–8. [PubMed] [Google Scholar]
- 31.Kubo M, Hanada T, Yoshimura A. Suppressors of cytokine signaling and immunity. Nat Immunol. 2003;4:1169–76. doi: 10.1038/ni1012. [DOI] [PubMed] [Google Scholar]
- 32.Rane SG, Reddy EP. Janus kinases: components of multiple signaling pathways. Oncogene. 2000;19:5662–79. doi: 10.1038/sj.onc.1203925. [DOI] [PubMed] [Google Scholar]
- 33.Kamizono S, Hanada T, Yasukawa H, et al. The SOCS box of SOCS-1 accelerates ubiquitin-dependent proteolysis of TEL-JAK2. J Biol Chem. 2001;276:12530–8. doi: 10.1074/jbc.M010074200. [DOI] [PubMed] [Google Scholar]
- 34.Kimura A, Kinjyo I, Matsumura Y, et al. SOCS3 is a physiological negative regulator for granulopoiesis and granulocyte colony-stimulating factor receptor signaling. J Biol Chem. 2004;279:6905–10. doi: 10.1074/jbc.C300496200. [DOI] [PubMed] [Google Scholar]
- 35.Lee JY, Sullivan KE. Gamma interferon and lipopolysaccharide interact at the level of transcription to induce tumor necrosis factor alpha expression. Infect Immun. 2001;69:2847–52. doi: 10.1128/IAI.69.5.2847-2852.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larsen L, Ropke C. Suppressors of cytokine signalling: SOCS. APMIS. 2002;110:833–44. doi: 10.1034/j.1600-0463.2002.1101201.x. [DOI] [PubMed] [Google Scholar]