Abstract
Endodontic infections are polymicrobial infections resulting in bone destruction and tooth loss. The host response to these infections is complex, including both innate and adaptive mechanisms. Osteopontin (OPN), a secreted, integrin-binding protein, functions in the regulation of immune responses and enhancement of leucocyte migration. We have assessed the role of OPN in the host response to endodontic infection using a well-characterized mouse model. Periapical bone loss associated with endodontic infection was significantly more severe in OPN-deficient mice compared with wild-type 3 weeks after infection, and was associated with increased areas of inflammation. Expression of cytokines associated with bone loss, interleukin-1α (IL-1α) and RANKL, was increased 3 days after infection. There was little effect of OPN deficiency on the adaptive immune response to these infections, as there was no effect of genotype on the ratio of bacteria-specific immunoglobulin G1 and G2a in the serum of infected mice. Furthermore, there was no difference in the expression of cytokines associated with T helper type 1/type2 balance: IL-12, IL-10 and interferon-γ. In infected tissues, neutrophil infiltration into the lesion area was slightly increased in OPN-deficient animals 3 days after infection: this was confirmed by a significant increase in expression of neutrophil elastase in OPN-deficient samples at this time-point. We conclude that OPN has a protective effect on polymicrobial infection, at least partially because of alterations in phagocyte recruitment and/or persistence at the sites of infection, and that this molecule has a potential therapeutic role in polymicrobial infections.
Keywords: bone loss, cytokine, migration, neutrophil, osteopontin, peri-apical
Introduction
Endodontic infections are typically polymicrobial infections of the dental root canal system.1,2 Bacterial species gain access to this space through defects in the tooth structure, often advanced caries or stress-related cracks and fissures. The associated inflammatory response at the apex of the root results in loss of the surrounding peri-apical bone. These infections, together with periodontitis, are unusual in combining bone resorption with a polymicrobial infection. The inflammatory response to these infections has been best characterized in the mouse system, and involves a robust activation of the innate immune system. The resultant bone loss is much more severe in animals with impaired neutrophil3,4 or macrophage5 function. The role of the adaptive immune system in these infections is less clear – mice lacking the classic T helper type 1 (Th1) cytokines interleukin-12 (IL-12) and interferon-γ (IFN-γ) have comparable susceptibility to endodontic infections to wild-type mice,6 whereas IL-10-deficient mice are significantly more susceptible to infection-associated bone loss.7
Osteopontin (OPN) is a secreted phosphoprotein with various roles in the immune responses. It is made by T cells and macrophages, and binds to a series of integrins, as an intact protein or as proteolytically cleaved fragments.8 Its activities associated with immune/inflammatory responses include regulation of Th1/Th2 balance,9 enhancement of dendritic cell function10 and regulation of IL-17 production.11 It is also important in the regulation of the innate immune response, enhancing the accumulation of neutrophils and macrophages at sites of injury.12–14 Osteopontin has been shown to down-regulate IL-1015 so we predicted that it might enhance bone destruction associated with endodontic infections.
Here we have assessed the host response to endodontic infections in OPN-deficient mice. Unexpectedly, we found that in the absence of OPN, the inflammatory response and resultant bone loss associated with these infections was much more severe than in wild-type (WT) mice. We present data suggesting that this observation may be related to the role of OPN in the innate immune system.
Materials and methods
Mice
Wild-type and OPN−/− mice were maintained on a 129 (S1,S7) mixed background16 as separate colonies under specific pathogen-free conditions. Colonies were maintained to minimize inbreeding, and WT and OPN−/− colonies were interbred every 2 years. All procedures were approved by the Forsyth Institutional Animal Care and Use Committee.
Periapical infection
Periapical infections were performed using an established protocol.2,6,7 Briefly, mice, 6–12 weeks of age, were anaesthetized with ketamine/xylazine and immobilized and mounted on a jaw retraction board. Molar pulps were exposed by using a #1/4 round bur under a surgical microscope. Ten microlitres of bacterial suspension at 1010 cells/ml in 2% carboxymethyl cellulose was inoculated into the exposed root canal. Mice were allowed to recover and were maintained under standard conditions until they were sacrificed. On death, mandibles were dissected and fixed in 4% paraformaldehyde before analysis by micro-computed tomography (microCT) or histology. For RNA preparation, bone blocks containing the first molars were dissected, cleaned of soft tissue and snap frozen in liquid nitrogen. Trizol reagent (Invitrogen, Carlsbad, CA) was used to prepare total RNA from crushed bone blocks.
Bacterial preparations
Common human endodontic pathogens Prevotella intermedia ATCC 25611, Streptococcus intermedius ATCC 27335, Fusobacterium nucleatum ATCC 25586 and Peptostreptococcus micros ATCC 33270 were grown on tryptic soy broth with yeast agar plates, and subsequently in mycoplasma liquid medium under anaerobic conditions (80% N2, 10% H2 and 10% CO2). The cells were harvested by centrifugation at 7000 g for 15 min and resuspended in phosphate-buffered saline (PBS) and quantified spectrophotometrically. For pulp infection, a mixture of the four species was diluted into 2% carboxymethyl cellulose in PBS at 2·5 × 109 each species/ml.
MicroCT was performed on isolated, fixed mandibles using a Skyscan-1172 or a Shimadzu SMX-225CT cone-beam type tomograph. Areas of bone loss were determined as described in Leshem et al.17 Briefly, acquired stacks were re-sliced using ImageJ software (Wayne Rasband, National Institutes of Health, Bethesda, MD) to obtain the ‘pivot’ section, which included the mesial and distal roots of the mandibular first molar and which exhibited a patent distal root canal apex. The area of bone loss (radiolucency) in this section was measured using Photoshop (Adobe, San Jose, CA) and ImageJ, and expressed in mm2. This analysis was performed by an investigator blinded to the genotype of the samples.
Histology and immunohistochemistry
Fixed mandibles were decalcified in 5% formic acid/10% citrate, and embedded in paraffin. The entire mandible was sectioned at 6 μm/section, and every fifth section was stained with haematoxylin & eosin to identify the lesion area. Images of the stained sections were obtained with a dissecting microscope and imported into IQBase software (Mediacybernetics, Bethesda, MD). Bone loss in appropriate sections was estimated by measuring the distance from the first molar proximal root surface to the closest bone edge at the bottom of the root and on both sides using the measurement functions in IQBase. These numbers were obtained for all stained sections spanning the base of the root (four to six sections), and averaged. Neutrophils were identified with antibody 7/418 (AbD Serotec, Raleigh, NC) at 0·22 μg/ml, and macrophages with F4/8019 (Harlan) at 1 : 10; both were detected with biotinylated goat anti-rat antibody and the Vector ELITE ABC kit (Vector Laboratories, Burlingame, CA). Osteoclasts were identified using a rabbit antiserum to cathepsin K, as previously described.20 No primary controls were included in each experiment, and there was no reactivity of the secondary antibodies alone. Semi-quantitative estimates of phagocyte accumulation in tissue sections were obtained by measuring the area of intense staining using ImageJ or IQBase: in 3-day samples, the root canal of infected mice stained strongly for neutrophils, and the neutrophil accumulation was estimated by measurement of the length of the pulp chamber occupied by neutrophils.
Quantitative reverse transcription–polymerase chain reaction (qPCR)
One to two micrograms RNA prepared from bone blocks (approx. 5 mm3, containing the infected molar and associated bone, from which gingival tissue was removed) was reverse transcribed using standard techniques; for each sample a control reaction was performed without reverse transcriptase. Complementary DNA (cDNA) was subjected to qPCR using primers at 200–300 μm and Sybr green technology in a total volume of 20 μl. Master mix was either purchased from BioRad (Hercules, CA) or was home-made21 using standard Taq polymerase (NE Biolabs, Ipswich, MA). For each assay, standards were prepared by amplifying a DNA fragment encompassing the qPCR primer sites: this fragment was purified, quantified and used for absolute quantification. Results, in molecules/μl were divided by the geometric mean of results from two control genes: glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and EF1a1,22 to give relative expression. Primers (Invitrogen) for IL-1α, IL-1β, IFN-γ, IL-10 and IL-12p40 were described in Akilesh et al.23 Receptor activator of nuclear factor κB ligand (RANKL) primers are described elsewhere.20 Other primers used were GAPDH: left: 5′-CGAAGGTGGAAGAGTGGGAG-3′; right: 5′-TGAAGCAGGCATCTGAGGG-3′; EF1a1: left: 5′-GGAAA TTCGAGACCAGCAAA-3′; right: 5′-ACACCAGC AGCAACAATCAG-3′; neutrophil elastase: left: 5′-TGTGAACGGCCTAAATTTCC-3′; right: 5′-GGTCAAAG CCATTCTCGAAG-3′.
Enzyme-linked immunsorbent assay
Antibody subtypes were determined as previously described.24 In brief, 96-well microtitre plates were coated with fixed F. nucleatum (optical density 580 nm = 0·3) and blocked with 1% bovine serum albumin. Sera from infected mice collected on killing were serially diluted in PBS as indicated and 100 μl was added to each well. After incubation and washing, specific immunoglobulin G (IgG) subclasses were detected with biotinylated rabbit anti-mouse IgG1 or IgG2a (BD Biosciences PharMingen, San Diego, CA). Wells were then incubated with streptavidin-conjugated horseradish peroxidase (Invitrogen), after which substrate and chromogen were added, and absorbance was read on an enzyme-linked immunsorbent assay (ELISA) plate reader (Dynatech, Chantilly, VT).
Statistical analyses
Significance of differences was calculated by two–way analysis of variance with Bonferroni post-test (bone loss determinations), or by two-tailed t-test. Graph-Pad Prism (Graph Pad Software, LaJolla, CA) software was used for statistical calculations.
Results
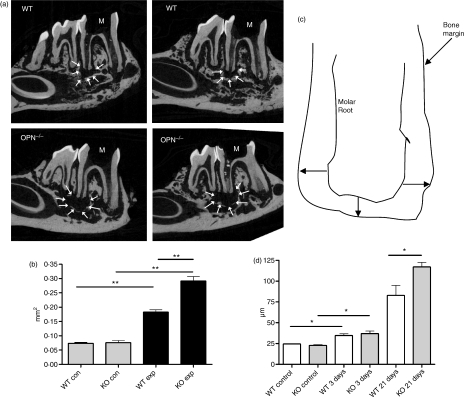
Wild-type and OPN-deficient mice (both males and females at 5–12 weeks of age) on a 129 (S1, S7) mixed background were subjected to dental pulp exposure, and infected with a mixture of four human endodontic pathogens including P. intermedia, S. intermedius, F. nucleatum and P. micros. Three weeks after infection, mice were killed, and the infected mandibles were removed, fixed and analysed by microCT as described.7Figure 1 shows that bone loss associated with these endodontic infections was significantly higher in OPN−/− mice than in WT animals. The area of radiolucency in unexposed mice was minimal (average 0·07 mm2); it was not different between WT and OPN−/− mice – this radiolucent area represents the normal periodontal ligament that anchors teeth to the underlying bony structure. Following pulp exposure and infection, the area of bone loss averaged 0·18 mm2 in WT mice, but was 55% higher in OPN−/− animals (0·28 mm2, Fig. 1b). When corrected for the radiolucent area observed in unexposed areas, the extent of bone loss in OPN−/− mice was more than twice that seen in WT mice. This result was confirmed in an independent experiment (data not shown).
Figure 1.
(a) Micro-computed tomography images of periapical bone loss in wild type (WT) and osteopontin-deficient (OPN−/−) mice 21 days after infection. A single section through each mandible is shown, and the area of bone loss is indicated by arrows. Each panel represents a different mouse. (b) Quantification of bone loss in WT and OPN−/− (KO). Con, unexposed; exp, exposed, infected mice. **P < 0·01; n = 3 (control) or n = 5 (infected). Experiment shown is one of two experiments. (c) Diagram of measurements used for quantification of bone loss in histological sections. Lengths of the lines indicated by arrows were measured and averaged to estimate bone loss. (d) Quantification of bone loss in histological sections. Bone loss was determined from haematoxylin & eosin-stained sections from control, uninfected animals (n = 2 or 3), infected animals 3 days after infection (n = 4 or 5) or infected animals 21 days after infection (n = 4 or 5). *P< 0·05.
Bone loss was also estimated in histological sections as described in Materials and methods. These measurements confirmed the bone loss observed by microCT 21 days after infection, and the significantly increased bone loss occurring in the OPN-deficient mice (Fig. 1c). At 3 days after infection, there was a significant amount of bone loss adjacent to the infected pulp chamber, with many osteoclasts apparent (data not shown). However, the extent of bone loss at this time-point was not different between WT and OPN-deficient animals.
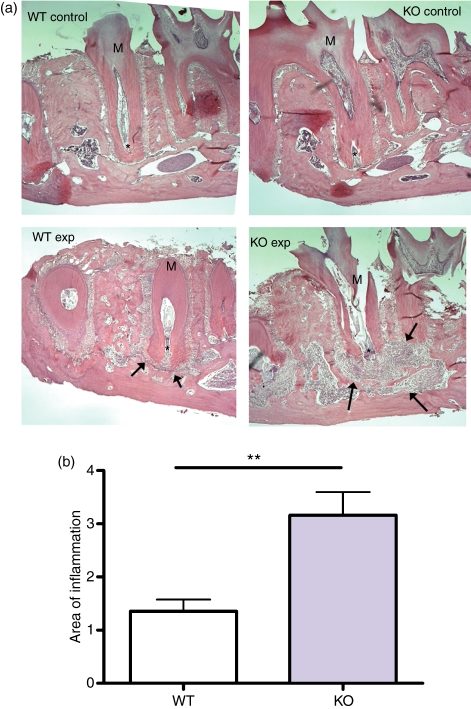
The bone loss in infected animals was secondary to the inflammatory infiltration occurring in response to bacterial infection. This inflammatory response was quantified in haematoxylin & eosin-stained decalcified sections of infected mandibles at 21 days after infection (Fig. 2). One section from each mandible was selected that had the greatest amount of inflammation and the area of inflammation was measured using ImageJ software. Maximal inflammation was more than twice as extensive in the OPN-deficient mandibles as in the WT tissues.
Figure 2.
(a) Histological sections (haematoxylin & eosin-stained) of wild-type (WT) and osteopontin-deficient (OPN−/−) control (Con) and exposed, infected mice (Exp) 21 days after infection. Asterisk indicates the apex of the root. Arrows indicate the area of inflammation. Objective magnification: 10 ×. (b) Quantification of areas of inflammation (in arbitrary units) in WT and OPN−/− (KO) mice 21 days after infection. **P = 0·01; n = 4 or 5.
The pro-inflammatory molecules known as IL-1 (comprising both IL-1α and IL-1β) are responsible for much of the pathology in these periapical infections25 and can mediate osteoclast activation and function.26 We used qPCR to evaluate the effect of OPN deficiency on IL-1 expression in the periapical lesions. Interleukin-1α, but not IL-1β, was significantly increased in lesions from OPN-deficient mice compared with WT mice at early times after infection (Fig. 3a). Consistent with the increased bone loss seen in these animals, RANKL expression was also increased in OPN-deficient mice. By 21 days, however, there were no significant differences in the expression of these cytokines between the two genotypes (Fig. 3b). The number of osteoclasts was greatly elevated in the periapical region of infected mice at 3 days after infection, as compared with control, unexposed animals. However, the number of osteoclasts in these areas was not different between WT and OPN-deficient animals (Fig. 3c). This is consistent with the similar extent of bone loss in the WT and OPN-deficient mice at this time-point. Together these results suggest that OPN acts to enhance the bone loss seen at later times, which reflects the increased bone resorption between 3 and 21 days after infection.
Figure 3.
Expression of osteoclast-activating cytokines in periapical lesions. (a) Interleukin-1α (IL-1α; n = 11), IL-1β (n = 8), and receptor activator of nuclear factor-κB ligand (RANKL; n = 11) expression was measured by quantitative polymerase chain reaction in extracts of infected tissues 3 days after infection, and normalized to expression of control genes. (b) Similar analysis was performed in RNA from infected tissues 21 days after infection. *P < 0·05; n = 5 or 6.
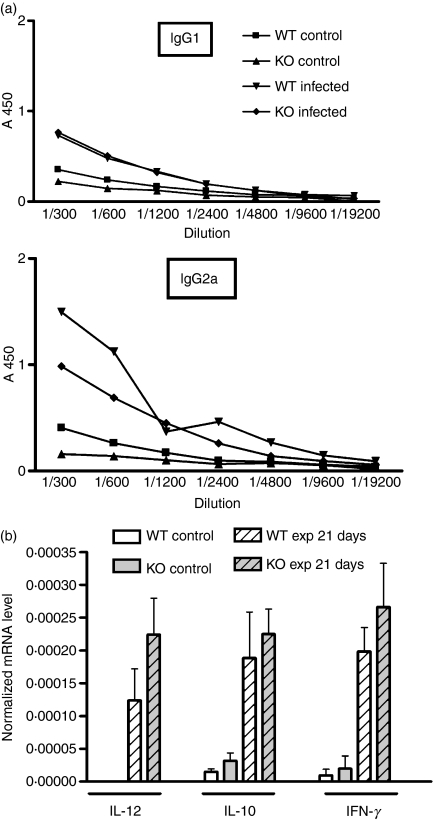
Osteopontin has been associated with the Th1 response, which is known to exacerbate inflammation-associated bone loss in our endodontic infection model.27 It can also suppress the expression of IL-10,9 which has an anti-inflammatory role in these infections.28 To assess the effect of OPN on the Th1/Th2 response in these infections, the serological response of infected animals to bacterial infection was determined 3 weeks after infection. Levels of IgG1 and IgG2a, were determined in sera from infected mice by ELISA using F. nucleatum as antigen: this species has been shown previously to elicit a strong immune response.7 The ratio of the expression of these isoforms reflects the Th1/Th2 balance, such that IGg2a ≥ IgG1 indicates a Th1 bias, whereas lower IgG2a suggests a Th2 polarization.24,29 In WT mice, the humoral immune response to this species included both IgG1 and IgG2a, although the titre of IgG2a was somewhat higher, perhaps reflecting a Th1 bias. There were no significant changes in either IgG1 or IgG2a levels in the absence of OPN (Fig. 4a), suggesting that there is no alteration in the Th1/Th2 polarization in these lesions in the absence of OPN. This idea is supported by analysis of messenger RNA (mRNA) levels for a series of cytokines in the periapical lesions at 21 days after infection. While OPN has been reported to enhance IL-12 expression and suppress IL-10,9 IL-12, IL-10 and IFN-γ mRNA levels were similar in both WT and OPN-deficient mice (Fig. 4b). Expression of IFN-γ and IL-12 was undetectable at 3 days after infection (data not shown). Together, these data suggest that the effect of OPN on the inflammatory response in this system is not through effects on the adaptive immune response.
Figure 4.
(a) Levels of immunoglobulin G1 (IgG1) and IgG2 isotypes reactive against Fusobacterium nucleatum in sera from wild-type (WT) and osteopontin-deficient (OPN−/−) mice at 21 days after infection. Serial dilutions of mouse sera were reacted with immobilized F. nucleatum, and detected with isotype-specific secondary antibodies. Each point represents the average of results from three serum samples from individual mice. There is no significant difference between the WT and OPN−/− samples at any dilution. (b) Cytokine expression in periapical tissue was measured by quantitative polymerase chain reaction 21 days after infection as described in Fig. 3. There are no significant differences between genotypes for any of the cytokines.
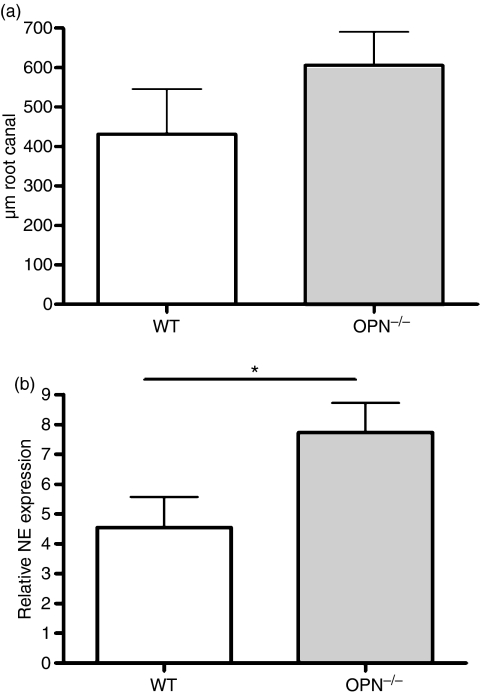
To evaluate the effects of OPN on the innate immune response, we examined the accumulation of neutrophils and macrophages in the areas of periapical infection. Neutrophil accumulation was examined by immunohistochemistry using a neutrophil-specific antibody (7/4)18 at 3 days after infection to examine the early response to bacterial infection: at this time-point there was a slight but non-significant trend to higher neutrophil accumulation in the root canals of infected OPN−/− mice, as compared with WT (Fig. 5a). At all time-points, however, neutrophil infiltration was extensive and was difficult to quantify accurately by histological analysis. To more accurately quantify neutrophil accumulation and function in 3-day samples, therefore, neutrophil elastase was measured by qPCR in cDNA samples prepared from periapical tissues. This analysis demonstrated significantly increased neutrophil accumulation and/or function in the absence of OPN (Fig. 5b). Together, these results suggest that OPN regulates both neutrophil infiltration and persistence at sites of infection. Macrophage numbers were assessed by immunohistochemistry with the macrophage-specific antibody F4/8019, and were similar to controls in the peri-apical region 3 days after infection. By 21 days after infection, macrophage numbers were greatly increased in infected animals compared with controls, but semi-quantitative analysis of staining in the peri-apical area did not show any difference in macrophage numbers at this time-point between the two genotypes (data not shown).
Figure 5.
(a) Neutrophil accumulation within the pulp chamber of infected animals was assessed by immunohistochemistry. Sections of mandibles 3 days after infection were reacted with rat anti-neutrophil antibody 7/4, and the length of the pulp chamber with neutrophil accumulation was measured; n = 4 or 5. (b) Neutrophil elastase (NE) was measured by quantitative polymerase chain reaction in RNA from infected mandibles 3 days after infection, and normalized to control genes. *P < 0·05, n = 6 or 7.
Discussion
Osteopontin has been shown to be important in resistance to viral and microbial infection: frequently this resistance has been associated with its role in regulating the Th1 response. For instance, OPN-deficient mice are more susceptible than WT mice to several human pathogens, including Listeria monocytogenes,9Plasmodium chabaudi chabaudi30 and Mycobacterium bovis bacillus Calmette–Guérin.31 Here, we demonstrate for the first time that OPN is an important aspect of the host response to polymicrobial infections, showing that these infections are much more severe in mice that lack OPN.
Our results suggest that while OPN plays a major role in the host response to these polymicrobial infections, this role seems not to be related to its role in the adaptive immune response. There was no change in the immunoglobulin subtype response to F. nucleatum in the absence of OPN, nor did we detect a significant change in expression of Th1/Th2 cytokines in infected tissues in the presence or absence of OPN. The role of OPN in regulation of IL-10 has been clearly shown, particularly via the dendritic cell response to viral infections.9,32 We assessed IL-10 expression at the RNA level in peri-apical tissues: whether stimulated T cells from lymph nodes draining these infections would express lower levels of IL-10 is unclear. However, IL-10-deficient mice have more severe bone loss than WT mice in our periapical lesion model,7 suggesting that if OPN is acting to regulate IL-10 expression then OPN-deficient mice would be protected from bone loss, rather than the increased susceptibility we observed. Together, these considerations suggest that OPN function in these periapical lesions is independent of its effects on IL-10 expression, and most likely related to its function in regulating the innate immune system.
Osteopontin has multiple effects on cells of the myeloid lineage.8 It is chemotactic for neutrophils,33,34 although its effects on these cells are still not well understood. Osteopontin is also chemotactic for macrophages, and enhances migration of this cell type14,35–38 in response to some, but not all, chemoattractants. The OPN-deficient macrophages are defective in killing tumour cells39 and bacterial cells,31 and defective phagocytosis has also been reported.40 Our results are consistent with these reports, suggesting that OPN deficiency results in increased neutrophil persistence in vivo in response to bacterial infection. So, increased neutrophil elastase levels in OPN-deficient mice may be a reflection of a defect in neutrophil killing or clearance mediated by macrophages or may reflect an alteration in neutrophil function in the absence of OPN. An alternative explanation, that OPN deficiency results in increased recruitment of neutrophils to the site of infection, is also possible, although this would be unexpected, based on the known effects of OPN on cell migration. Analysis of these lesions at different times of infection is required to understand the detailed mechanism of this effect.
Defects in macrophage function or accumulation have been previously shown to result in increased bone loss in these endodontic infections.5 In the absence of the macrophage chemoattractant MCP-1, monocyte recruitment to the site of infection is impaired, and the resulting bone loss is significantly increased. A similar mechanism may be occurring in the absence of OPN. However, neutrophil defects are strongly associated with the tissue damage in both human and experimental endodontic infections (reviewed in ref. 2), so we cannot rule out an effect of OPN on this cell type as well.
The effects of OPN on phagocytes are probably mediated through its ability to bind to the integrins important in myeloid cells: the αvβ3, and the α4β1 and α9β1 integrins.41–43 The innate immune response to infection includes a rapid accumulation of neutrophils at the site of infection: these cells make a variety of toxic products that can kill invading bacteria, but also cause tissue damage.44 Neutrophils and other leucocytes arrive at sites of infection from the bloodstream through a co-ordinated response including arrest at the site of the inflamed endothelium, extravasation through the endothelial layer, and migration to the site of infection. Leucocyte arrest on endothelial cells is mediated by selectin binding to endothelial lectins, resulting in slow rolling, followed by integrin-mediated arrest.45 Chemokine expression on the surface of endothelial cells triggers changes in leucocyte integrin affinity, resulting in rapid binding of β2-containing integrins to endothelial intercellular adhesion molecule-1 and α4-containing integrins to vascular cell adhesion molecule-1. Following arrest, there is rapid release of these integrin contacts allowing leucocytes to move to endothelial cell junctions, and migrate through these junctions. Finally, phagocytes migrate through the tissue to bacterially infected areas. OPN or its fragments bind to the α4β1 and α9β1 integrins through the SLAYGLR sequence: these integrins are important in all these steps of infiltration;46,47 hence OPN may be important in any of these aspects of phagocyte extravasation. The exact mechanism remains obscure, however, and further work is required to elucidate the molecular interactions. Important questions include whether OPN regulates the function of these cell types, or if its effect is mostly related to cell migration.
The role of leucocyte extravasation in the development of mouse periapical lesions was explored using P/E selectin double-deficient mice.48 These animals developed extensive bone loss similar to the OPN-deficient mice. There were also extensive systemic effects, including splenomegaly, which was not observed in the OPN-deficient mice (data not shown) and a 50% decrease in neutrophil accumulation in the inflammatory site. Hence, the effect of OPN deficiency on neutrophil accumulation is not as severe as that of selectin deficiency, perhaps reflecting the redundancy of the integrin ligands available for extravasation. These integrins undergo rapid changes in affinity for their ligands during inflammation, and it is not known how these changes affect the binding of OPN.45,49,50 CD44 isoforms are also implicated in the effects of OPN,51 and additionally there is evidence that an intracellular form of OPN may have physiological importance.36,52
At early times after infection, we observed increased expression of IL-1α and RANKL in infected tissues from OPN-deficient mice: both these cytokines are associated with inflammation-associated bone resorption.26,53 Hence, the mechanism of the increased bone resorption in these mice is probably related to the increased expression of IL-1α and RANKL: further work is needed to determine the cell types expressing these factors in endodontic infections and the role of OPN in their regulation. OPN has been shown to be required for bone resorption in mice in response to ovariectomy or hind-limb suspension,54,55 and this effect is probably the result of a defect in osteoclast function in the absence of OPN.36,56,57 Consequently, it was unexpected to see increased bone loss in the OPN-deficient mice. The effect of OPN on osteoclasts suggests that the bone loss secondary to endodontic infection that we observed in OPN-deficient mice might be restricted by the osteoclast defect, and could be more severe in the absence of this defect. Alternatively, factors may be produced during the course of the response to infection that can override the osteoclast defect, as has been suggested in bone loss associated with metastatic tumour growth in the bone.20,58
Mice infected with M. bovis develop granulomas, and the number and size of these granulomas are higher in mice deficient for OPN expression.31 This effect was shown to be unrelated to the adaptive immune response; rather there was a defect in bacterial killing by OPN-deficient macrophages. Hence, the effect of OPN in our model of endodontic infection seems to resemble the host response to M. bovis. It is not clear if the mechanism of host response is the same in both these models, but this similarity illustrates the generality of the OPN dependency of aspects of the innate immune response.
In conclusion, our results suggest that OPN has a protective effect in endodontic infections at least partially through an effect on neutrophil persistence. A possible mechanism for these observations is that OPN deficiency may affect macrophage recruitment or function, such that macrophage-dependent neutrophil clearance is impaired. Understanding the mechanism of action of OPN in these infections may lead to new therapeutic approaches to treat polymicrobial infections.
Acknowledgments
The authors thank Martha O’Hara for help with immunohistochemistry, and Justine Dobeck for expert tissue sectioning. This work was supported by grant DK067685 from the NIDDK/NIH (SRR) and by the High-Tech Research Center Program at Private Universities from the Japanese Ministry of Education, Culture, Sports, Science, and Technology.
Disclosures
The authors report no conflicts of interest.
References
- 1.Nair PNR. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit Rev Oral Biol Med. 2004;15:348–81. doi: 10.1177/154411130401500604. [DOI] [PubMed] [Google Scholar]
- 2.Stashenko P, Teles R, D’Souza R. Periapical inflammatory responses and their modulation. Crit Rev Oral Biol Med. 1998;9:498–521. doi: 10.1177/10454411980090040701. [DOI] [PubMed] [Google Scholar]
- 3.Niederman R, Kelderman H, Socransky S, et al. Enhanced neutrophil emigration and Porphyromonas gingivalis reduction following PGG-glucan treatment of mice. Arch Oral Biol. 2002;47:613–8. doi: 10.1016/s0003-9969(02)00042-0. [DOI] [PubMed] [Google Scholar]
- 4.Stashenko P, Wang CY, Riley E, Wu Y, Ostroff G, Niederman R. Reduction of infection-stimulated periapical bone resorption by the biological response modifier PGG glucan. J Dent Res. 1995;74:323–30. doi: 10.1177/00220345950740010701. [DOI] [PubMed] [Google Scholar]
- 5.Chae P, Im M, Gibson F, Jiang Y, Graves DT. Mice lacking monocyte chemoattractant protein 1 have enhanced susceptibility to an interstitial polymicrobial infection due to impaired monocyte recruitment. Infect Immun. 2002;70:3164–9. doi: 10.1128/IAI.70.6.3164-3169.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sasaki H, Balto K, Kawashima N, et al. Gamma interferon (IFN-gamma) and IFN-gamma-inducing cytokines interleukin-12 (IL-12) and IL-18 do not augment infection-stimulated bone resorption in vivo. Clin Diagn Lab Immunol. 2004;11:106–10. doi: 10.1128/CDLI.11.1.106-110.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sasaki H, Hou L, Belani A, et al. IL-10, but not IL-4, suppresses infection-stimulated bone resorption in vivo. J Immunol. 2000;165:3626–30. doi: 10.4049/jimmunol.165.7.3626. [DOI] [PubMed] [Google Scholar]
- 8.Ramaiah SK, Rittling S. Pathophysiological role of osteopontin in hepatic inflammation, toxicity and cancer. Toxicol Sci. 2007;103:4–13. doi: 10.1093/toxsci/kfm246. [DOI] [PubMed] [Google Scholar]
- 9.Ashkar S, Weber GF, Panoutsakopoulou V, et al. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–4. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 10.Renkl AC, Wussler J, Ahrens T, et al. Osteopontin functionally activates dendritic cells and induces their differentiation toward a Th1-polarizing phenotype. Blood. 2005;106:946–55. doi: 10.1182/blood-2004-08-3228. [DOI] [PubMed] [Google Scholar]
- 11.Shinohara ML, Kim JH, Garcia VA, Cantor H. Engagement of the Type I interferon receptor on dendritic cells inhibits T helper 17 cell development: role of intracellular osteopontin. Immunity. 2008;29:68–78. doi: 10.1016/j.immuni.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banerjee A, Apte UM, Smith R, Ramaiah SK. Higher neutrophil infiltration mediated by osteopontin is a likely contributing factor to the increased susceptibility of females to alcoholic liver disease. J Pathol. 2006;208:473–85. doi: 10.1002/path.1917. [DOI] [PubMed] [Google Scholar]
- 13.Diao H, Kon S, Iwabuchi K, et al. Osteopontin as a mediator of NKT cell function in T cell-mediated liver diseases. Immunity. 2004;21:539–50. doi: 10.1016/j.immuni.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Giachelli CM, Lombardi D, Johnson RJ, Murry CE, Almeida M. Evidence for a role of osteopontin in macrophage infiltration in response to pathological stimuli in vivo. Am J Pathol. 1998;152:353–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Shinohara ML, Jansson M, Hwang ES, Werneck MB, Glimcher LH, Cantor H. T-bet-dependent expression of osteopontin contributes to T cell polarization. Proc Natl Acad Sci USA. 2005;102:17101–6. doi: 10.1073/pnas.0508666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Rittling SR. Novel murine mammary epithelial cell lines that form osteolytic bone metastases: effect of strain background on tumor homing. Clin Exp Metastasis. 2003;20:111–20. doi: 10.1023/a:1022675031185. [DOI] [PubMed] [Google Scholar]
- 17.Leshem O, Kashino SS, Goncalves RB, et al. Th1 biased response to a novel Porphyromonas gingivalis protein aggravates bone resorption caused by this oral pathogen. Microbes Infect. 2008;10:664–72. doi: 10.1016/j.micinf.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsch S, Gordon S. Polymorphic expression of a neutrophil differentiation antigen revealed by monoclonal antibody 7/4. Immunogenetics. 1983;18:229–39. doi: 10.1007/BF00952962. [DOI] [PubMed] [Google Scholar]
- 19.Gordon S, Lawson L, Rabinowitz S, Crocker PR, Morris L, Perry VH. Antigen markers of macrophage differentiation in murine tissues. Curr Top Microbiol Immunol. 1992;181:1–37. doi: 10.1007/978-3-642-77377-8_1. [DOI] [PubMed] [Google Scholar]
- 20.Natasha T, Kuhn M, Kelly O, Rittling SR. Override of the osteoclast defect in osteopontin-deficient mice by metastatic tumor growth in the bone. Am J Pathol. 2006;168:551–61. doi: 10.2353/ajpath.2006.050480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pellissier F, Glogowski CM, Heinemann SF, Ballivet M, Ossipow V. Lab assembly of a low-cost, robust SYBR green buffer system for quantitative real-time polymerase chain reaction. Anal Biochem. 2006;350:310–2. doi: 10.1016/j.ab.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Vandesompele J, De PK, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akilesh S, Shaffer DJ, Roopenian D. Customized molecular phenotyping by quantitative gene expression and pattern recognition analysis. Genome Res. 2003;13:1719–27. doi: 10.1101/gr.533003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goncalves RB, Leshem O, Bernards K, Webb JR, Stashenko PP, Campos-Neto A. T-cell expression cloning of Porphyromonas gingivalis genes coding for T helper-biased immune responses during infection. Infect Immun. 2006;74:3958–66. doi: 10.1128/IAI.02029-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graves DT, Cochran D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J Periodontol. 2003;74:391–401. doi: 10.1902/jop.2003.74.3.391. [DOI] [PubMed] [Google Scholar]
- 26.Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest. 2005;115:282–90. doi: 10.1172/JCI23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stashenko P, Goncalves RB, Lipkin B, Ficarelli A, Sasaki H, Campos-Neto A. Th1 immune response promotes severe bone resorption caused by Porphyromonas gingivalis. Am J Pathol. 2007;170:203–13. doi: 10.2353/ajpath.2007.060597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasaki H, Suzuki N, Kent R, Jr, Kawashima N, Takeda J, Stashenko P. T cell response mediated by myeloid cell-derived IL-12 is responsible for Porphyromonas gingivalis-induced periodontitis in IL-10-deficient mice. J Immunol. 2008;180:6193–8. doi: 10.4049/jimmunol.180.9.6193. [DOI] [PubMed] [Google Scholar]
- 29.Faquim-Mauro EL, Coffman RL, Abrahamsohn IA, Macedo MS. Cutting edge: mouse IgG1 antibodies comprise two functionally distinct types that are differentially regulated by IL-4 and IL-12. J Immunol. 1999;163:3572–6. [PubMed] [Google Scholar]
- 30.Maeno Y, Nakazawa S, Yamamoto N, et al. Osteopontin participates in Th1-mediated host resistance against nonlethal malaria parasite Plasmodium chabaudi chabaudi infection in mice. Infect Immun. 2006;74:2423–7. doi: 10.1128/IAI.74.4.2423-2427.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nau GJ, Liaw L, Chupp GL, Berman JS, Hogan BL, Young RA. Attenuated host resistance against Mycobacterium bovis BCG infection in mice lacking osteopontin. Infect Immun. 1999;67:4223–30. doi: 10.1128/iai.67.8.4223-4230.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cantor H, Shinohara ML. Regulation of T-helper-cell lineage development by osteopontin: the inside story. Nat Rev Immunol. 2009;9:137–41. doi: 10.1038/nri2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koh A, da Silva AP, Bansal AK, et al. Role of osteopontin in neutrophil function. Immunology. 2007;122:466–75. doi: 10.1111/j.1365-2567.2007.02682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banerjee A, Lee JH, Ramaiah SK. Interaction of osteopontin with neutrophil α4β1 and α9β1 integrins in a rodent model of alcoholic liver disease. Toxicol Appl Pharmacol. 2008;233:238–46. doi: 10.1016/j.taap.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 35.Nystrom T, Duner P, Hultgardh-Nilsson A. A constitutive endogenous osteopontin production is important for macrophage function and differentiation. Exp Cell Res. 2007;313:1149–60. doi: 10.1016/j.yexcr.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 36.Zhu B, Suzuki K, Goldberg HA, et al. Osteopontin modulates CD44-dependent chemotaxis of peritoneal macrophages through G-protein-coupled receptors: evidence of a role for an intracellular form of osteopontin. J Cell Physiol. 2004;198:155–67. doi: 10.1002/jcp.10394. [DOI] [PubMed] [Google Scholar]
- 37.Singh RP, Patarca R, Schwartz J, Singh P, Cantor H. Definition of a specific interaction between the early T lymphocyte activation 1 (Eta-1) protein and murine macrophages in vitro and its effect upon macrophages in vivo. J Exp Med. 1990;171:1931–42. doi: 10.1084/jem.171.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marcondes MC, Poling M, Watry DD, Hall D, Fox HS. In vivo osteopontin-induced macrophage accumulation is dependent on CD44 expression. Cell Immunol. 2008;254:56–62. doi: 10.1016/j.cellimm.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bourassa B, Monaghan S, Rittling SR. Impaired anti-tumor cytotoxicity of macrophages from osteopontin-deficient mice. Cell Immunol. 2004;227:1–11. doi: 10.1016/j.cellimm.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Heilmann K, Hoffmann U, Witte E, et al. Osteopontin as two-sided mediator of intestinal inflammation. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00428.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taooka Y, Chen J, Yednock T, Sheppard D. The integrin α9β1 mediates adhesion to activated endothelial cells and transendothelial neutrophil migration through interaction with vascular cell adhesion molecule-1. J Cell Biol. 1999;145:413–20. doi: 10.1083/jcb.145.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yokosaki Y, Matsuura N, Sasaki T, et al. The integrin α9β1 binds to a novel recognition sequence (SVVYGLR) in the thrombin-cleaved amino-terminal fragment of osteopontin. J Biol Chem. 1999;274:36328–34. doi: 10.1074/jbc.274.51.36328. [DOI] [PubMed] [Google Scholar]
- 43.Berton G, Lowell CA. Integrin signalling in neutrophils and macrophages. Cell Signal. 1999;11:621–35. doi: 10.1016/s0898-6568(99)00003-0. [DOI] [PubMed] [Google Scholar]
- 44.Dale DC, Boxer L, Liles WC. The phagocytes: neutrophils and monocytes. Blood. 2008;112:935–45. doi: 10.1182/blood-2007-12-077917. [DOI] [PubMed] [Google Scholar]
- 45.Alon R, Ley K. Cells on the run: shear-regulated integrin activation in leukocyte rolling and arrest on endothelial cells. Curr Opin Cell Biol. 2008;20:525–32. doi: 10.1016/j.ceb.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ulyanova T, Priestley GV, Banerjee ER, Papayannopoulou T. Unique and redundant roles of α4 and β2 integrins in kinetics of recruitment of lymphoid vs myeloid cell subsets to the inflamed peritoneum revealed by studies of genetically deficient mice. Exp Hematol. 2007;35:1256–65. doi: 10.1016/j.exphem.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Werr J, Xie X, Hedqvist P, Ruoslahti E, Lindbom L. β1 integrins are critically involved in neutrophil locomotion in extravascular tissue in vivo. J Exp Med. 1998;187:2091–6. doi: 10.1084/jem.187.12.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kawashima N, Niederman R, Hynes RO, Ullmann-Cullere M, Stashenko P. Infection-stimulated infraosseus inflammation and bone destruction is increased in P-/E-selectin knockout mice. Immunology. 1999;97:117–23. doi: 10.1046/j.1365-2567.1999.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng DQ, Woodard AS, Tallini G, Languino LR. Substrate specificity of αvβ3 integrin-mediated cell migration and phosphatidylinositol 3-kinase/AKT pathway activation. J Biol Chem. 2000;275:24565–74. doi: 10.1074/jbc.M002646200. [DOI] [PubMed] [Google Scholar]
- 50.Mahabeleshwar GH, Chen J, Feng W, Somanath PR, Razorenova OV, Byzova TV. Integrin affinity modulation in angiogenesis. Cell Cycle. 2008;7:335–47. doi: 10.4161/cc.7.3.5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weber GF, Zawaideh S, Hikita S, Kumar VA, Cantor H, Ashkar S. Phosphorylation-dependent interaction of osteopontin with its receptors regulates macrophage migration and activation. J Leukoc Biol. 2002;72:752–61. [PubMed] [Google Scholar]
- 52.Shinohara ML, Kim HJ, Kim JH, Garcia VA, Cantor H. Alternative translation of osteopontin generates intracellular and secreted isoforms that mediate distinct biological activities in dendritic cells. Proc Natl Acad Sci USA. 2008;105:7235–9. doi: 10.1073/pnas.0802301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xing L, Schwarz EM, Boyce BF. Osteoclast precursors, RANKL/RANK, and immunology. Immunol Rev. 2005;208:19–29. doi: 10.1111/j.0105-2896.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- 54.Ishijima M, Tsuji K, Rittling SR, et al. Resistance to unloading-induced three-dimensional bone loss in osteopontin-deficient mice. J Bone Miner Res. 2002;17:661–7. doi: 10.1359/jbmr.2002.17.4.661. [DOI] [PubMed] [Google Scholar]
- 55.Yoshitake H, Rittling SR, Denhardt DT, Noda M. Osteopontin-deficient mice are resistant to ovariectomy-induced bone resorption [published erratum appears in Proc Natl Acad Sci U S A 1999 Sep 14;96(19):10944] Proc Natl Acad Sci USA. 1999;96:8156–60. doi: 10.1073/pnas.96.14.8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chellaiah MA, Kizer N, Biswas R, et al. Osteopontin deficiency produces osteoclast dysfunction due to reduced CD44 surface expression. Mol Biol Cell. 2003;14:173–89. doi: 10.1091/mbc.E02-06-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tani-Ishii N, Tsunoda A, Umemoto T. Osteopontin antisense deoxyoligonucleotides inhibit bone resorption by mouse osteoclasts in vitro. J Periodontal Res. 1997;32:480–6. doi: 10.1111/j.1600-0765.1997.tb00563.x. [DOI] [PubMed] [Google Scholar]
- 58.Yagiz K, Rittling SR. Both cell-surface and secreted CSF-1 expressed by tumor cells metastatic to bone can contribute to osteoclast activation. Exp Cell Res. 2009;315:2442–52. doi: 10.1016/j.yexcr.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]