Abstract
Human alveolar epithelial cells actively contribute to the innate immune response in the lung and play an important role in mycobacterial dissemination during primary infection, by undergoing cell death and by releasing mycobacteria. In the present study, we report that natural lysophospholipids, such as lysophosphatidic acid or sphingosine 1-phosphate, reduce Mycobacterium tuberculosis-induced cytotoxicity and enhance anti-mycobacterial activity in the A549 cell line, used as a model of type II alveolar epithelial cells. Intracellular mycobacterial killing was strictly dependent on phagolysosome maturation, which in turn was promoted by the activation of a Ca2+dependent phospholipase D. Finally, the restriction of mycobacteria in highly microbiocidal compartments was associated, in vitro, with a significant decrease in mycobacterial dissemination to macrophages. Taken as whole, these results suggest that the pulmonary lysophospholipid microenvironment may play a protective role during the early phases of host–pathogen interaction by enhancing anti-mycobacterial activity in type II alveolar epithelial cells.
Keywords: alveolar epithelial cells, dissemination, lysophospholipid, primary infection, tuberculosis
Introduction
Tuberculosis is a globally occurring disease that causes in excess of 2 million deaths and affects more than 8 million people annually.1 Primary infection occurs when aerosol-droplet nuclei containing a small number of bacilli are deposited in the alveoli. Once deposited, they can be phagocytosed by alveolar macrophages2 or invade and replicate within alveolar epithelial cells.3 During this early phase, the pulmonary microenvironment and its interplay with the local innate immune response influence the outcome of the primary infection, which, in the vast majority of cases, results in the infection being contained.4 Despite this containment, dissemination from the site of primary infection occurs early on, leading to the distribution of Mycobacterium tuberculosis (MTB) to other sites, such as the apical portions of the pulmonary parenchyma, where the bacillus can remain in a latent state for decades. In this context, during latency MTB can be harboured in a variety of cells, including alveolar and interstitial macrophages, type II alveolar epithelial cells, endothelial cells and fibroblasts.5
Epidemiological data provide evidence that human protective immunity against MTB exists and may involve both innate and adaptive immune responses.6,7 The local pulmonary innate immune response involves several cell types (chiefly macrophages, neutrophils and epithelial cells) and several host antimicrobial factors, present in airway epithelial lining fluid, which are able to directly kill invading microbes.8,9 The role played by host lipids in the activation of the anti-mycobacterial immune response is emerging and recently reported results drew attention to the possible contribution of some lipids (e.g. sphingomyelin, ceramide, phosphatidylinositol) to the enhancement of anti-mycobacterial activity in mouse macrophages.10,11 Also, lysophosphatidic acid (LPA) and sphingosine 1-phosphate (S1P), two lysophospholipids known to be involved in a wide range of biological processes such as cell survival, differentiation, migration and morphogenesis,12 have recently been described to induce anti-mycobacterial activity in human macrophages through a mechanism that involves phospholipase D (PLD) activation and phagolysosome maturation.13,14
Based on this knowledge, we wondered whether LPA and/or S1P may serve to control mycobacterial infection in type II alveolar epithelial cells. Our results showed that both lysophospholipids (i) are cytoprotective in MTB-infected type II alveolar epithelial cells, (ii) induce Ca2+-dependent PLD activation, which, in turn, promotes phagolysosome maturation, (iii) induce phagolysosome maturation dependent intracellular mycobacterial killing and (iv) inhibit mycobacterial dissemination to macrophages in vitro.
Materials and methods
Bacteria
Pathogenic MTB H37Rv was grown in Middlebrook 7H9 broth supplemented with albumin dextrose catalase. Mycobacteria were then harvested, suspended in sterile phosphate-buffered saline (PBS), pH 7·2, aliquoted and stored at −80° until use. Before infection, aliquots of mycobacteria were grown on 7H10 plates in order to titre the bacteria after thawing.
Cell cultures
The human lung adenocarcinoma epithelial cell line, A549 (ATCC, Rockville, MD), was used as model of human type II alveolar epithelial cells. Cells were grown in complete medium consisting of RPMI-1640 supplemented with 10% fetal bovine serum (FBS), 2 mm l-glutamine and 5 μg/ml of gentamicine, and split when a confluent cell monolayer was attained. In several preliminary experiments, cell viability was then monitored by lactate dehydrogenase release (CytoTox96 kit; Promega, Milan, Italy), 2 and 4 days after the cells became confluent.
Infection and evaluation of intracellular mycobacterial growth
A confluent A549 cell monolayer, composed of about 106 cells, was exposed for 3 hr to MTB at a multiplicity of infection (MOI) of 10 bacilli per cell in six-well plates. After removal of extracellular bacilli, cells were stimulated for 2 days with either 0·5 μm LPA (Calbiochem, San Diego, CA) or 0·5 μm S1P (Calbiochem). As short-chain primary alcohols, such as ethanol and 1-buthanol, inhibit PLD-dependent phosphatidic acid (PA) generation and induce the formation of the metabolically inactive phosphatidylethanol (PetOH), primary alcohols were used to investigate the role of PLD activity in LPA-induced or S1P-induced intracellular mycobacterial killing. In this context, MTB-infected A549 cells were incubated with 0·5 μm of either LPA or S1P in the presence or absence of 0·3% ethanol, 0·3% 1-buthanol, or 0·3% 2-buthanol (used as a negative control), as described previously.15 In order to ascertain whether phagolysosome maturation was responsible for intracellular mycobacterial killing, 10 μm chloroquine or 20 mm NH4Cl was added to MTB-infected cells together with LPA or S1P. Colony-forming unit (CFU) assays were performed on day 2 after infection, as previously described.13 Any modification, in terms of cell viability, was not detected in any of the experimental conditions employed (data not shown).
Cell viability assay
A confluent A549 cell monolayer was infected for 3 hr with MTB at an MOI of 10 in 96-well plates. After removal of extracellular bacilli, cells were stimulated with either LPA or S1P at 0·05, 0·5 or 5 μm, for 2 days. Cytotoxicity was measured using the MTT Cell Proliferation Assay Kit (Molecular Probes, Milan, Italy). The MTT assay is based on the cleavage of the yellow tetrazolium salt, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), to purple formazan crystal in metabolically active cells. The formazan is then solubilized, and the concentration determined by measuring the optical density at 540 nm. The assay is sensitive with the colorimetric signal proportional to the viable cell number.
Intracellular Ca2+ measurement and analysis of PLD activity
Intracellular Ca2+ was measured after labelling cells with the fluorescent intracellular Ca2+ indicator Fluo-3/AM (Molecular Probes), as described previously,15 followed by a 5-min incubation at 37° with 0·5 μm of either LPA or S1P. Fluo-3 fluorescence was followed by the use of a Perkin Elmer LS50B luminescence spectrometer (Perkin Elmer LS50B luminescence spectrometer, Waltham, MA). Analysis of PLD activity was performed on A549 cells stimulated for 18 hr with 0·5 μm of either LPA or S1P. Cells were harvested and tested for PLD activity using the Amplex Red Phospholipase D assay kit according to the manufacturer’s instructions (Molecular Probes). In several experiments, 2 mm EDTA or 3 mm EGTA (Calbiochem) were added 15 min before the addition of LPA or S1P, whereas 20 μm 1,2-bis(2-aminophenoxy)ethane-N,N,N1,N1-tetraacetic acid tetraicis (acetoxymethyl ester) (BAPTA-AM) (Sigma-Aldrich, Milan, Italy) was added 30 min before stimulation with LPA or S1P.
Confocal microscopy
The degree of maturation of MTB-containing endosomes was assessed in A549 cells stimulated for 18 hr with 0·5 μm of either LPA or S1P by analyzing the co-localization of bacilli with lysosomes after staining the mycobacteria with auramine and the lysosomes with either (i) the acidophilic dye Lysotracker Red (Molecular Probes), or (ii) Alexa Fluor 647 monoclonal anti-LAMP-3 [LAMP refers lysosomal associated membrane protein] (IgG1, clone MX-49.129.5; Santa Cruz Biotechnology, Inc., Heidelberg, Germany), or monoclonal anti-LAMP-1 (IgG2b, clone 25; Transduction Laboratories, Becton Dickinson, MD) followed by incubation with Alexa Fluor 568 goat anti-mouse IgG monoclonal antibody (Molecular Probes), as previously described.15 The samples were analyzed by confocal laser scanning microscopy using a Leica TCS-SP2 operating system, Wetzlar, Germany.
Analysis of in vitrodissemination to human macrophages
A confluent A549 cell monolayer was exposed for 3 hr to MTB at an MOI of 10 in six-well plates. After removal of extracellular bacilli, cells were stimulated for 2 days with either 0·5 μm LPA or 0·5 μm S1P. Thereafter, supernatant was collected and centrifuged at 18 000 g for 5 min. The pellet containing mycobacteria was then resuspended in 1 ml of culture medium and administered to the human monocyte cell line (THP-1) cells previously induced to differentiate by 3 days of incubation with 20 ng/ml of phorbol 12-myristate 13-acetate (PMA). Finally, a CFU assay was performed 4 days after exposure to A549-derived mycobacteria, as previously described.13
Statistical analysis
Statistical analysis was carried out using the Graphpad Prism 3.0 software package (GraphPad Software Inc., La Jolla, CA). The Student’s t-test was used to compare differences between means. A P-value of < 0·05 was considered statistically significant.
Results
LPA and S1P are cytoprotective and reduce intracellular mycobacterial viability in MTB-infected type II alveolar epithelial cells
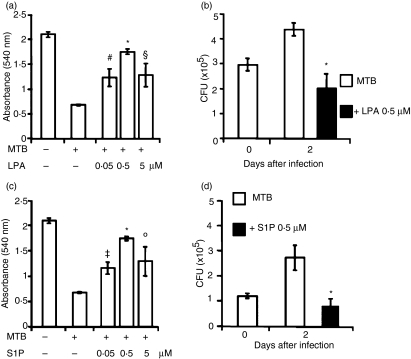
MTB has been shown to be cytopathic for type II alveolar epithelial cells16 and cell death may favour mycobacterial dissemination by releasing bacteria into the interstitial spaces or into the blood vessels.17 On these grounds, we assessed the role of lysophospholipids on cell viability following in vitro MTB infection. As the cell monolayer was infected18 and in order to minimize the basal level of cell death in confluent cells, in a preliminary investigation we monitored the cell viability of the monolayer 2 and 4 days after the culture became confluent. The results showed 95 ± 0·7% cell viability on day 2, which decreased to 59 ± 5% on day 4, after the monolayer reached confluence. On the basis of these results, we decided to analyze mycobacterial growth on day 2 after infection. The results showed that treatment with either LPA (Fig. 1a) or S1P (Fig. 1c), at concentrations from 0·05 to 5 μm, significantly rescued A549 cells from MTB-induced cytotoxicity and that 0·5 μm appeared to be the optimal dose to achieve this effect. Lysophospholipids are known to play a cytoprotective role in different cell types18 and to activate the mycobacteriocidal response in macrophages.13,14 To investigate whether the enhancement of cell viability is associated with an increased capability of lysophospholipid-activated A549 cells to kill intracellular mycobacteria, we analysed intracellular mycobacterial growth in S1P- or LPA-stimulated MTB-infected A549 cells. The results showed that treatment with LPA (Fig. 1b) or with S1P (Fig. 1d) significantly reduces intracellular mycobacterial viability.
Figure 1.
Lysophosphatidic acid (LPA) and sphingosine 1-phosphate (S1P) are cytoprotective and induce anti-mycobacterial activity in Mycobacterium tuberculosis (MTB)-infected type II alveolar epithelial cells. (a, c) A549 cells were infected with MTB and then stimulated with 0·05, 0·5, or 5 μm LPA (a) or with 0·05, 0·5, or 5 μm S1P (c) for 2 days. The MTT assay was performed to evaluate cell viability. Data are reported as mean ± standard deviation (SD) of triplicate values and are representative of three different experiments. (b, d) A549 cells were infected with MTB and then stimulated with 0·5 μm of either LPA (b) or S1P (d) for 2 days. Colony-forming unit (CFU) counts are expressed as means ± SD of the triplicate values and are representative of three separate experiments. *P ≤ 0·001, #P = 0·002, §P = 0·005, ‡P = 0·001, °P = 0·01 in comparison with MTB-infected cells (data were analyzed using the unpaired Student’s t-test).
LPA and S1P induce Ca2+-dependent PLD activation
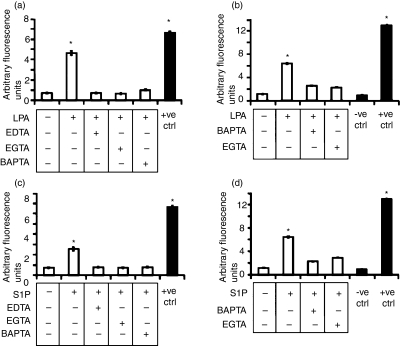
Intracellular calcium elevation is required for many different signal transduction pathways, including activation of the anti-mycobacterial response.15,19 On these grounds, we analyzed the cytosolic Ca2+ influx following stimulation with lysophospholipids in type II alveolar epithelial cells. Our results showed a significant increase in the concentration of cytosolic Ca2+ following stimulation with 0·5 μm LPA (Fig. 2a) or with 0·5 μm S1P (Fig. 2c). In addition, treatment with 2 mm EDTA or 3 mm EGTA, chelating extracellular Ca2+, and treatment with 20 μm BAPTA-AM, chelating intracellular Ca2+, almost completely inhibited the LPA- or S1P-induced increase of cytosolic Ca2+.
Figure 2.
Lysophosphatidic acid (LPA) and sphingosine 1-phosphate (S1P) induce Ca2+ mobilization and phospholipase D (PLD) activation in type II alveolar epithelial cells. (a, c) A549 cells were labelled with 3 μm Fluo-3/AM, treated or not with EDTA, EGTA or BAPTA-AM, and further stimulated with 0·5 μm of either LPA (a) or S1P (c). The positive control consisted of A549 cells lysed with Triton X-100. Data are reported as mean ± standard deviation (SD) of triplicate values and are representative of five different experiments. (b, d) PLD activity was monitored using the Amplex Red Phospholipase D detection kit on A549 cells that were unstimulated or stimulated with 0·5 μm of either LPA (b) or S1P (d) in the presence or absence of EGTA and BAPTA-AM. The values are shown as means ± SD of triplicate values and are representative of three independent experiments. *P < 0·001 in comparison with untreated cells (data were analyzed using the unpaired Student’s t-test).
Human PLD is involved in molecular pathways leading to the activation of antimicrobial mechanisms.20 We therefore investigated the ability of LPA and S1P to increase PLD activity in type II alveolar epithelial cells. Our results showed a significant increase in enzymatic activity when either LPA (Fig. 2b) or S1P (Fig. 2d) was added to the cells. Moreover, the lysophospholipid-induced increase of PLD activity was found to be Ca2+ dependent, because enzymatic activity was completely abrogated by pretreatment with EGTA or BAPTA-AM. Finally, the specificity of the PLD assay was confirmed by incubating cells in the presence of 2,3-diphosphoglycerate (DPG), which is a direct competitive inhibitor of PLD. The results showed that the addition of 2,3-DPG almost completely inhibits LPA- or S1P-induced PLD activity, as assessed using the PLD assay kit, demonstrating the specificity of the PLD assay (Fig. 1, supplementary material).
LPA and S1P induce phagolysosome maturation by PLD activation
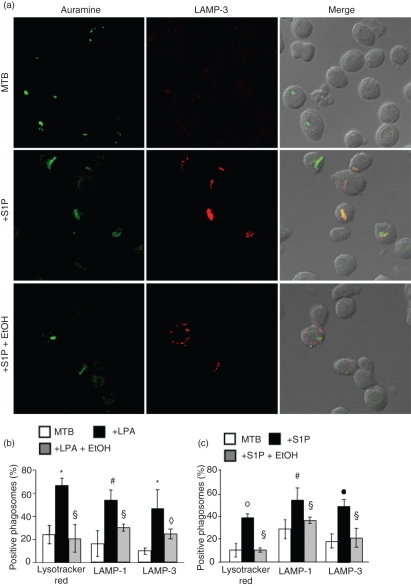
As macrophage PLD is involved in phagolysosome biogenesis in different experimental models,13–15,21 and MTB is known to reside in immature endosomes sequestered away from late endosomal compartments,2,4 the maturation of MTB-containing vacuoles in LPA- or S1P-stimulated cells was investigated by laser scanning confocal microscopy using two protein markers of lysosomes/late endosomes (LAMP-1 and LAMP-3) and the acidophilic dye, Lysotracker red. As expected, only a small percentage of MTB-containing phagosomes are acidic and express the lysosomal markers LAMP-1 and LAMP-3, consistent with an immature maturational state (Fig. 3b and c) and mycobacteria appeared green separated by the compartments stained with the different lysosomal markers used (Fig. 3a). By contrast, LPA or S1P stimulation induced a significant increase in the acidification and in the expression of LAMP-1 and LAMP-3 in MTB-containing phagosomes (Fig. 3b and c), which stained yellow (Fig. 3a). Finally, the addition of ethanol, in tandem with LPA or S1P, almost completely reversed this process.
Figure 3.
Lysophosphatidic acid (LPA) and sphingosine 1-phosphate (S1P) induce phagolysosome maturation in Mycobacterium tuberculosis (MTB)-infected type II alveolar epithelial cells. (a) Representative picture from three separate experiments shows the increase of green MTB residing in red LAMP-3-positive vacuoles [LAMP refers lysosomal associated membrane protein] after treatment with S1P and the reverse effect exerted by ethanol (EtOH). (b, c) Summary of the mean percentage ± standard deviation (SD) of MTB co-localizing in Lysotracker Red-, LAMP-1- and LAMP-3-positive vacuoles determined by counting ≥ 50 A549 cells per sample, after stimulation with LPA (b) or S1P (c). Four different experiments were assessed. *P = 0·01, •P = 0·006, ◊P = 0·003, °P = 0·001, #P = 0·03, §P = not significative (n.s.) in comparison with MTB-infected cells (data were analyzed using the unpaired Student’s t-test).
Lysophospholipid-induced inhibition of intracellular mycobacterial viability requires PLD activation and phagolysosome maturation
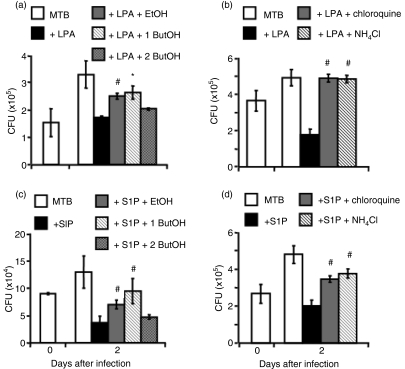
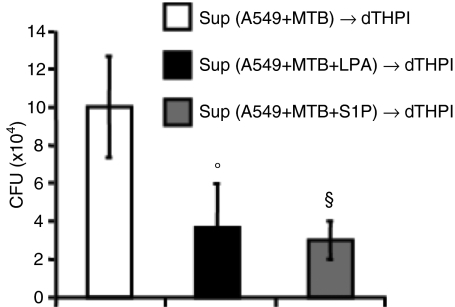
PLD involvement in lysophospholipid-induced anti-mycobacterial activity was investigated by mixing lysophospholipids with ethanol or 1-buthanol, which inhibit PLD-mediated PA production and promote the formation of the metabolically inactive PetOH.20 The results showed that lysophospholipid stimulation reduces intracellular mycobacterial viability and that the addition of ethanol or 1-butanol following stimulation with LPA (Fig. 4a) or S1P (Fig. 4c) significantly increases intracellular mycobacterial growth. Finally, lysophospholipid-induced anti-mycobacterial activity was mediated by phagolysosome maturation because the addition of the lysosomotropic agents chloroquine or NH4Cl significantly increased intracellular mycobacterial viability (Fig. 4b and d). Finally, in order to check whether the containment of bacilli in mycobacteriocidal compartments could prevent the release of mycobacteria and hence avoid infection of underlying macrophages,16 A549 cells were infected with MTB and stimulated or not with LPA or S1P. Two days later, the A549 cell supernatant was harvested, centrifuged and the mycobacteria collected were used to re-infect differentiated THP-1 cells. Intracellular mycobacterial growth was analysed in macrophages at 4 days after exposure to mycobacteria. The results showed that the activation of MTB-infected alveolar epithelial cells with lysophospholipids prevents mycobacterial release from A549 cells and infection of human macrophages (Fig. 5).
Figure 4.
Lysophosphatidic acid (LPA) and sphingosine 1-phosphate (S1P) stimulation reduces intracellular mycobacterial viability by a phospholipase D (PLD)-dependent pathway and a phagolysosome maturation-mediated mechanism. (a, c) A549 cells were infected with Mycobacterium tuberculosis (MTB) and incubated for 2 days with LPA (a) or S1P (c) plus ethanol (EtOH), 1-buthanol (1 ButOH) or 2-buthanol (2 ButOH) (as a control). Colony-forming unit (CFU) counts are expressed as means ± standard deviation (SD) of triplicate values and are representative of three separate experiments. (b, d) A549 cells were infected with MTB and incubated for 2 days with LPA (b) or S1P (d) plus NH4Cl or chloroquine. CFU counts are expressed as means ± SD of triplicate values and are representative of three separate experiments. *P = 0·001, #P < 0·001 in comparison with lysophospholipid-treated cells (data were analyzed using the unpaired Student's t-test).
Figure 5.
Lysophosphatidic acid (LPA) and sphingosine 1-phosphate (S1P) stimulation of alveolar epithelial cells reduces mycobacterial dissemination to macrophages. A549 cells were infected with Mycobacterium tuberculosis (MTB) and unstimulated or stimulated for 2 days with LPA or S1P. Thereafter, the supernatant (Sup) was centrifuged, and the mycobacterial pellet was resuspended in culture medium and then administrated to differentiated THP-1 (dTHP1) cells for 4 days when the colony-forming unit (CFU) assay was performed. CFU counts are expressed as means ± standard deviation (SD) of triplicate values and are representative of two separate experiments. °P = 0·01, §P = 0·006 in comparison with MTB-infected cells (data were analyzed using the unpaired Student’s t-test). dTHPI; SUP.
Discussion
The early phases of the interaction between the human host and MTB are crucial for the outcome of the infection. During this interaction, alveolar epithelial cells may influence the outcome of primary infection by promoting epithelial transcytosis and mycobacterial dissemination after binding with mycobacterial heparin-binding haemagglutinin22 or by releasing mycobacteria following cell death.16,17 In addition, alveolar epithelial cells can also participate in granuloma formation and mycobacterial containment during the effector phases of the adaptive immune response by presenting mycobacterial antigen to antigen-specific CD4+ T cells,23 and by limiting intracellular mycobacterial growth following stimulation with interferon-γ (IFN-γ).24 However, the possible role of alveolar epithelial cells in containing intracellular mycobacterial growth during the initial stages of infection, before the adaptive immune response takes place, has not been fully clarified. The results reported herein show that the lysophospholipid microenvironment protects type II alveolar epithelial cells from MTB-induced cytotoxicity and enhances the anti-mycobacterial response, leading to intracellular mycobacterial killing. These results, together with previously reported evidence showing that virulent MTB strains replicate more rapidly and are cytotoxic for alveolar epithelial cells,3,25 suggest that appropiate activation of these cells, in the context of the innate anti-mycobacterial immune response, may have important implications in the pathogenesis of tuberculosis.
PLD, and its metabolic product, PA, has been previously demonstrated to mediate phagolysosomal maturation in macrophages receiving different stimuli, including natural ligands, such as ATP,26 S1P and LPA,13,14 and microbial ligands, such as cytosine–phosphate–guanosine (CpG) oligodeoxynucleotides.15,20 The results reported herein, which show that phagolysosome maturation induced by lysophospholipids is mediated by the activation of host Ca2+-dependent PLD, strongly suggest that the activation of such a molecular pathway (i) is crucial for the activation of phagolysosome maturation-based antimicrobial response, (ii) is conserved among cell types and (iii) is at the intersection of different metabolic pathways, independent of the upstream stimulus received.
The most striking feature of pathogenic mycobacteria is their capability to arrest the normal process of phagosome maturation and to interfere with endosomal trafficking, thereby permitting their own survival in non-acidified vacuoles.2,4 Our results showed that acidification of endosomal compartments is required for lysophospholipid-induced intracellular mycobacterial killing. In fact, the treatment with the lysosomotropic agents chloroquine or NH4Cl, which increase the pH and can be considered as general lysosomal inhibitors, significantly enhances intracellular mycobacterial viability. The restriction of pathogenic mycobacteria inside intracellular mycobacteriocidal compartments of alveolar epithelial cells may play an important role in preventing dissemination and in controlling a primary infection before activated phagocyte recruitment at the site of infection occurs.
Dissemination of the tubercle bacilli from the site of primary infection constitutes an important step in the pathogenesis of tuberculosis. To allow the progression from infection to disease, the tubercle bacilli must gain access to the ‘vulnerable’ regions in the apex of the lungs.27 Thus, the results reported herein are coherent with a model in which the lysophospholipid extracellular microenvironment may protect alveolar epithelial cells from the MTB-induced cytopathic effect,16 presumably by enhancing their anti-mycobacterial activity, and hence may prevent mycobacterial release and dissemination to recently recruited tissue macrophages.
Approximately 30% of people exposed to MTB become infected, and 60–90% of those will have an effective immune response allowing successful containment of the infection.28 On these grounds, the natural consequence of the results reported herein is that the lipid composition of alveolar surfactant can play an important role, both during primary infection and latency, in ensuring the correct activation of the alveolar anti-mycobacterial response at the level of macrophages and alveolar epithelial cells. An altered surfactant phospholipid composition can be observed in a number of respiratory infections in children, where it is linked to disease and respiratory failure.29 Taken as a whole, these results show that the local lysophospholipid microenvironment may provide protective signals for both ‘conventional’ (e.g. macrophages) 13,14 as well as ‘unconventional’ (e.g. alveolar epithelial cells) immune cells, thereby limiting mycobacterial replication and dissemination, in particular, during the early phases of host–pathogen interaction, before any antigen-specific T helper-1 immune response takes place.
Acknowledgments
We would like to thank Dr Roberto Nisini (Italian Institute of Health), for his helpful suggestions and comments, and Dr Andrea Cabibbo and Dr Mark Livesey, for carefully reading the manuscript. The present study was supported financially by the Italian Ministry for Universities (COFIN 2006 and MIUR 60%).
Acknowledgments
The authors have no potential conflicts of interest.
Supporting Information
Additional Supporting information may be found in the online version of this article:
Figure S1. Analysis of phospholipase D (PLD) activity was performed on A549 cells stimulated for 18 hours with 0.5 μM of either LPA or S1P. Cells were harvested and tested for PLD activity using Amplex Red Phospholipase D assay kit according to the manufacturer's instructions (Molecular Probes, Italy). Finally, the specificity of PLD assay has been assessed by incubating the cells for 18 hours at 37 ° in the presence of 2 mM 2,3-diphosphoglycerate (DPG, Sigma) which is a direct competitive inhibitor of PLD (Kanaho Y., Nakai Y., Katoh M., Nozawa Y. The phosphatase inhibitor 2,3-Diphosphoglycerate interferes with phospholipase D activation in rabbit neutrophils. J. Biol. Chem. 1993: 268:12492 12497).
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.World Health Organization . Fact Sheet No. 104. Geneva: World Health Organization; 2006. [Google Scholar]
- 2.Flynn JL. Immunology of tuberculosis and implications in vaccine development. Tuberculosis (Edinb) 2004;84:93–101. doi: 10.1016/j.tube.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Bermudez LE, Goodman J. Mycobacterium tuberculosis invades and replicates within type II alveolar cells. Infect Immun. 1996;64:1400–6. doi: 10.1128/iai.64.4.1400-1406.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaufmann SH. How can Immunology contribute to the control of tuberculosis? Nat Rev Immunol. 2001;1:20–30. doi: 10.1038/35095558. [DOI] [PubMed] [Google Scholar]
- 5.Hernández-Pando R, Jeyanathan M, Mengistu G, Aguilar D, Orozco H, Harboe M, Rook GA, Bjune G. Persistence of DNA from Mycobacterium tuberculosis in superficially normal lung tissue during latent infection. Lancet. 2000;356:2133–8. doi: 10.1016/s0140-6736(00)03493-0. [DOI] [PubMed] [Google Scholar]
- 6.Ellner J, Hirsch C, Whalen C. Correlates of protective immunity to Mycobacterium tuberculosis in humans. Clin Infect Dis. 2000;30:S279–82. doi: 10.1086/313874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Crevel R, Ottenhof T, van der Meer J. Innate immunity to Mycobacterium tuberculosis. Adv Exp Med Biol. 2003;531:294–309. doi: 10.1007/978-1-4615-0059-9_20. [DOI] [PubMed] [Google Scholar]
- 8.Bals R, Hiemstra PS. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur Respir J. 2004;23:327–33. doi: 10.1183/09031936.03.00098803. [DOI] [PubMed] [Google Scholar]
- 9.Zhang P, Summer WR, Bagby GJ, Nelson S. Innate immunity and pulmonary host defense. Immunol Rev. 2000;173:39–51. doi: 10.1034/j.1600-065x.2000.917306.x. [DOI] [PubMed] [Google Scholar]
- 10.Anes E, Kuhnel MP, Bos E, Moniz-Pereira J, Habermann A, Griffiths G. Selected lipids activate phagosome actin assembly and maturation resulting in killing of pathogenic mycobacteria. Nat Cell Biol. 2003;5:793–802. doi: 10.1038/ncb1036. [DOI] [PubMed] [Google Scholar]
- 11.Gutierrez MG, Gonzalez AP, Anes E, Griffiths G. Role of lipids in killing mycobacteria by macrophages: evidence for NF-kB-dependent and -independent killing induced by different lipids. Cell Microbiol. 2009;11:406–20. doi: 10.1111/j.1462-5822.2008.01263.x. [DOI] [PubMed] [Google Scholar]
- 12.Ishii I, Fukushima N, Ye X, Chun J. Lysophospholipid receptors: signalling and biology. Annu Rev Biochem. 2004;73:321–54. doi: 10.1146/annurev.biochem.73.011303.073731. [DOI] [PubMed] [Google Scholar]
- 13.Garg SK, Volpe E, Palmieri G, et al. Sphingosine 1-phosphate induces antimicrobial activity both in vitro and in vivo. J Infect Dis. 2004;189:2129–38. doi: 10.1086/386286. [DOI] [PubMed] [Google Scholar]
- 14.Garg SK, Valente E, Greco E, et al. Lysophosphatidic acid enhances antimycobacterial activity both in vitro and ex vivo. Clin Immunol. 2006;121:23–8. doi: 10.1016/j.clim.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Greco E, De Spirito M, Papi M, Fossati M, Auricchio G, Fraziano M. CpG oligodeoxynucleotides induce Ca2+-dependent Phospholipase D leading to phagolysosome maturation and mycobacteriocidal activity in monocytes. Biochem Biophys Res Commun. 2006;347:963–9. doi: 10.1016/j.bbrc.2006.06.186. [DOI] [PubMed] [Google Scholar]
- 16.Danelishvili L, McGarvey J, Li YJ, Bermudez LE. Mycobacterium tuberculosis infection causes different levels of apoptosis and necrosis in human macrophages and alveolar epithelial cells. Cell Microbiol. 2003;5:649–60. doi: 10.1046/j.1462-5822.2003.00312.x. [DOI] [PubMed] [Google Scholar]
- 17.Russell DG. TB comes to a sticky beginning. Nat Med. 2001;7:894–5. doi: 10.1038/90926. [DOI] [PubMed] [Google Scholar]
- 18.Roy S, Sharma S, Sharma M, Aggarwal R, Bose M. Induction of nitric oxide release from the human alveolar epithelial cell line A549: an in vitro correlate of innate immune response to Mycobacterium tuberculosis. Immunology. 2004;112:471–80. doi: 10.1046/j.1365-2567.2004.01905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malik ZA, Denning GM, Kusner DJ. Inhibition of Ca(2+) signaling by Mycobacterium tuberculosis is associated with reduced phagosome-lysosome fusion and increased survival within human macrophages. J Exp Med. 2000;191:287–302. doi: 10.1084/jem.191.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Auricchio G, Garg SK, Martino A, et al. Role of macrophage phospholipase D in natural and CpG-induced antimycobacterial activity. Cell Microbiol. 2003;5:913–20. doi: 10.1046/j.1462-5822.2003.00330.x. [DOI] [PubMed] [Google Scholar]
- 21.Fairbairn IP, Stober CB, Dinakantha SK, Lammas DA. ATP-mediated killing of intracellular mycobacteria by macrophages is a P2X7-dependent process inducing bacterial death by phagosome-lysosome fusion. J Immunol. 2001;167:3300–7. doi: 10.4049/jimmunol.167.6.3300. [DOI] [PubMed] [Google Scholar]
- 22.Menozzi FD, Reddy VM, Cayet D, Raze D, Debrie AS. Dehouck MP. Mycobacterium tuberculosis heparin binding haemagglutinin adhesion (HBHA) triggers receptor mediated transcytosis without altering the integrity of tight junction. Microbes Infect. 2005;8:1–9. doi: 10.1016/j.micinf.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 23.Debbabi H, Ghosh S, Kamath AB, Alt J, deMello DE, Dunsmore S, Behar SM. Primary type II alveolar epithelial cells present microbial antigens to antigen specific CD4+ T cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:274–9. doi: 10.1152/ajplung.00004.2005. [DOI] [PubMed] [Google Scholar]
- 24.Radeff-Huang J, Seasholtz TM, Matteo RG, Brown JH. G protein mediated signaling pathways in lysophospholipid induced cell proliferation and survival. J Cell Biochem. 2004;92:949–66. doi: 10.1002/jcb.20094. [DOI] [PubMed] [Google Scholar]
- 25.McDonough KA, Kress Y. Cytotoxicity for lung epithelial cells is a virulence-associated phenotype of Mycobacterium tuberculosis. Infect Immun. 1995;63:4802–11. doi: 10.1128/iai.63.12.4802-4811.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kusner DJ, Adams J. ATP-induced killing of virulent Mycobacterium tuberculosis within human macrophages requires phospholipase D. J Immunol. 2000;164:379–88. doi: 10.4049/jimmunol.164.1.379. [DOI] [PubMed] [Google Scholar]
- 27.Balasubramanian V, Wiegeshaus EH, Taylor BT, Smith DW. Pathogenesis of tuberculosis: pathway to apical localization. Tuber Lung Dis. 1994;75:168–78. doi: 10.1016/0962-8479(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 28.Manabe C, Bishai WR. Latent Mycobacterium tuberculosis persistence, patience, and winning by waiting. Nat Med. 2000;6:1327–9. doi: 10.1038/82139. [DOI] [PubMed] [Google Scholar]
- 29.Mander A, Langton-Hewer S, Bernhard W, Warner JO, Postle AD. Altered phospholipid composition and aggregate structure of lung surfactant is associated with impaired lung function in young children with respiratory infections. Am J Respir Cell Mol Biol. 2002;27:714–21. doi: 10.1165/rcmb.4746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.