Abstract
Dendritic cells (DCs) are bone marrow-derived professional antigen-presenting cells. The in vitro generation of DCs from either bone marrow or blood is routine in mammals. Their distinct morphology and phenotype and their unique ability to stimulate naïve T cells are used to define DCs. In this study, chicken bone marrow cells were cultured in the presence of recombinant chicken granulocyte–macrophage colony-stimulating factor (GM-CSF) and recombinant chicken interleukin-4 (IL-4) for 7 days. The cultured population showed the typical morphology of DCs, with the surface phenotype of major histocompatibility complex (MHC) class II+ (high), CD11c+ (high), CD40+ (moderate), CD1·1+ (moderate), CD86+ (low), CD83− and DEC-205−. Upon maturation with lipopolysaccharide (LPS) or CD40L, surface expression of CD40, CD1·1, CD86, CD83 and DEC-205 was greatly increased. Endocytosis and phagocytosis were assessed by fluorescein isothiocyanate (FITC)-dextran uptake and fluorescent bead uptake, respectively, and both decreased after stimulation. Non-stimulated chicken bone marrow-derived DCs (chBM-DCs) stimulated both allogeneic and syngeneic peripheral blood lymphocytes (PBLs) to proliferate in a mixed lymphocyte reaction (MLR). LPS- or CD40L-stimulated chBM-DCs were more effective T-cell stimulators in MLR than non-stimulated chBM-DCs. Cultured chBM-DCs could be matured to a T helper type 1 (Th1)-promoting phenotype by LPS or CD40L stimulation, as determined by mRNA expression levels of Th1 and Th2 cytokines. We have therefore cultured functional chBM-DCs in a non-mammalian species for the first time.
Keywords: bone marrow, chicken, dendritic cells, granulocyte–macrophage colony-stimulating factor, interleukin-4
Introduction
In common with the vast majority of avian species, and other non-mammalian vertebrates, the chicken lacks lymph nodes. The chicken faces as wide a spectrum of pathogen challenge as mammals. Adaptive immune responses are readily measurable, and vaccination is highly effective. Antigen presentation therefore obviously occurs, either in the spleen or at local sites of diffuse structure. For the last two decades, studies have been carried out to demonstrate the existence of chicken dendritic cells (DCs) in different tissues.1–6 A recent report7 described cells in tissue sections that stained with an anti-chicken CD83 monoclonal antibody as having unique attributes akin to both DCs and follicular DCs. However, as yet there is no substantive evidence that these cells are DCs. Recently, progress has been made on the characterization of chicken epidermal DCs6 and DCs isolated from caecal tonsils.8 However, still little is known about the function and migration of chicken DCs.
DCs were first described as Langerhans cells in the skin in 1868. In 1973 Steinman and Cohn identified DCs from mouse spleen and named them for their typical morphology.9,10 DCs are professional antigen-presenting cells of the immune system with the unique capacity to initiate primary immune responses.10–12 DC progenitors are derived from haematopoietic stem cells in the bone marrow and seed in non-lymphoid tissues where they develop into immature DCs. Immature DCs can capture antigen by phagocytosis, macropinocytosis and endocytosis. These cells are specialized in antigen capture and processing but are poor T-cell stimulators. Upon activation by pathogen-associated molecular patterns, they migrate out of non-lymphoid tissues into T-cell regions of secondary lymphoid tissues where they complete their maturation. Mature DCs express up-regulated major histocompatibility complex (MHC) class II and costimulatory molecules.13,14 They are specialized in presenting collected antigens (Ags) to T cells to initiate immune responses.
Expansion of DCs for study in vitro is necessary because DCs are rare populations in all body tissues.15,16 In the 1990s, DCs were generated in vitro in sufficient numbers and purity for functional studies.17,18 In mammals, much of the initial characterization of DC function was on blood- or bone marrow-derived DCs, grown out in vitro under various conditions [in the presence of granulocyte–macrophage colony-stimulating factor (GM-CSF) alone, or GM-CSF plus interleukin (IL)-4, either with or without FMS-like tyrosine kinase 3 ligand (Flt3L) ligand, tumour necrosis factor (TNF)-α, interferon (IFN)-γ or other cytokines].18–24 From these initial studies, reagents and hypotheses were developed towards functional analyses of tissue DCs, both ex and in vivo.24–26 We have followed a similar approach to begin to characterize DCs in the chicken. Following our successful cloning and characterization of the chicken T helper type 2 (Th2) cytokine cluster, for the first time in a non-mammalian species, we produced recombinant chicken IL-4 and GM-CSF.27,28 Using these two cytokines, we have developed a method to culture chicken DCs from bone marrow cells in vitro and have characterized their phenotype and function.
Materials and methods
Chicken bone marrow preparation
Inbred line 72 and C.B12 birds were kept under specific pathogen-free (SPF) conditions in the Experimental Animal House at the Institute for Animal Health (IAH), Compton, UK. Femurs of 4–12-week-old birds were removed and isolated from the surrounding muscle tissue using sterile instruments. Femurs were then placed into a Universal container with enough sterile phosphate-buffered saline (PBS) to submerge the bone. Both ends of the bone were cut with scissors and the marrow flushed with phosphate-buffered saline (PBS) using a 10-ml syringe with a 0·45-mm-diameter needle (21 G). Clusters within the marrow suspension were disaggregated by vigorous pipetting. After one wash in PBS, the cells were suspended in PBS and loaded onto an equal volume of Histopaque-1119 (1·119 g/ml at 25°; Sigma-Aldrich, Poole, UK) and centrifuged at 1200 g for 30 min. Cells at the interface were collected and washed twice with PBS. Cells were re-suspended in PBS and mixed 1 : 1 with trypan blue solution. Trypan blue negative cells were counted as viable under the microscope in a haemocytometer chamber. Usually 8 × 107 cells were obtained from one bird.
Generation and maturation of chicken bone marrow-derived DCs (chBM-DCs)
Cells obtained from femurs were cultured at a final concentration of 1 × 106 cells/ml in six-well plates in pre-warmed RPMI-1640 (Invitrogen, Paisley, UK) complete medium containing 10% chicken serum (Invitrogen), 1% non-essential amino acids, 1% L-glutamine, 1 U/ml penicillin and 1 μg/ml streptomycin, for 7 days at 41°, 5% CO2. Recombinant chicken GM-CSF and IL-4 were added to the culture medium.
Recombinant chicken GM-CSF and IL-4 were produced from COS-7 cells transfected with pCIneo (Promega, Southampton, UK) expressing the relevant cytokine. Different dilutions (1/50, 1/100, 1/250, 1/500 and 1/1000) of COS cell culture supernatant containing recombinant GM-CSF or IL-4 were used to optimize the culture conditions. Three-quarters of the medium was replaced with fresh, pre-warmed complete medium every 2 days, and non-adherent cells (such as granulocytes and dead cells) were therefore removed at day 2 and day 4. To induce maturation, cultured cells were stimulated with lipopolysaccharide (LPS; 200 ng/ml; Sigma-Aldrich) or CD40L (3 μg/ml)29 from day 6 for 24 hr. At day 7 of culture, cells were harvested by gentle pipetting using Pasteur pipettes for characterization.
Observation of morphology
Effects of different concentrations of recombinant chicken GM-CSF and IL-4 on cell differentiation were recorded by observing cell morphology, clustering and cell growth. The cell cultures were photographed after 7 days of culture with a digital camera on an inverted microscope.
Phenotypic analysis by flow cytometry
Immunofluorescence labelling was carried out using allophycocyanin (APC)-labelled mouse anti-chicken MHC II [2G11, immunoglobulin G1 (IgG1)]30 in combination with putative mouse anti-chicken CD11c (8F2, IgG2a), CD1·1 (IgG1),31,32 CD40 (AV79, IgG2a),33 CD86 (IAH F853 AG2, IgG1) or DEC-205 (IgG1) monoclonal antibodies (mAbs) (Table 1) or polyclonal anti-chicken CD83.8 Fluorescein isothiocyanate (FITC)-labelled F(ab′)2 fragments of polyclonal rabbit anti-goat/sheep immunoglobulins (DAKO, Ely, UK) were used as secondary antibodies for anti-chicken CD83 and FITC-labelled F(ab′)2 fragments of polyclonal goat anti-mouse immunoglobulins (DAKO) were used to detect the other antibodies. For intracellular marker labelling, cells were fixed in 1% paraformaldehyde for 10 min and then permeabilized in PBS/0·1% saponin/1% bovine serum albumin (BSA) for 15 min. For all the labelling steps, cells (0·5–1·0 × 106 cells/ml) were incubated for 10 min at room temperature with appropriate dilutions of the primary or secondary mAbs in U-bottomed 96-well microtitre plates with two washes between each step. PBS containing 1·0% BSA and 0·1% sodium azide was used as dilution buffer and washing buffer for surface marker staining and PBS/0·1% saponin/1% BSA was used for intracellular marker staining. After the final wash, cells were analysed on a FACSCalibur (BD Bioscience, Cowley, UK).
Table 1.
Monoclonal antibodies used in flow cytometry analysis and imaging
| Monoclonal antibody | Antigen specificity | Source/ reference |
|---|---|---|
| 2G11 | MHC class II | 30 |
| 8F2 | Putative CD11c | Bernd Kaspers (University of Munich), unpublished |
| CB3 | Chicken CD1·1 | 31,32 |
| AV79 | CD40 | 33 |
| Anti-chicken CD86 | CD86 | John Young (IAH), unpublished1 |
| Anti-chicken DEC-205 | DEC-205 | Colin Butter and Karen Staines (IAH), unpublished2 |
| Polyclonal anti-chicken CD83 | CD83 | 7 |
Immunization and screening with extracellular domain of chicken CD86 (accession number AM050135).
Immunization and screening with fusion protein expressing mouse CD8 signal sequence and chicken DEC-205 C-type lectin domains 4, 5 and 6, fused to human immunoglobulin heavy chain C2 and C3 domains. Specificity demonstrated to chicken DEC-205.
IAH, Institute for Animal Health; MHC, major histocompatibility complex.
Endocytosis of FITC-dextran by chBM-DCs
Non-stimulated chBM-DCs (1 × 106 cells per sample) were harvested on day 7 and incubated with FITC-dextran (40 000 MW; Sigma-Aldrich) at 1 mg/ml in a 41° incubator (surface binding and endocytosis) or on ice (surface binding but no endocytosis) for different times (from 20 min to 2 hr) to determine a suitable time-point for subsequent assays. Cells were washed four times with ice-cold PBS, re-suspended in 200 μl of PBS, and analysed by flow cytometry (BD Bioscience). All subsequent assays were carried out with an incubation time of 20 min.
Phagocytosis assay
Phagocytosis was assessed using 0·5-μm carboxylate-modified fluorescent red latex beads (Sigma-Aldrich) and 0·5-μm Fluoresbrite® YG Carboxylate Microspheres (Polysciences, Northampton, UK). Briefly, chBM-DCs were harvested at day 6, washed and then cultured with chicken serum-opsonized latex beads (red or yellow-green) in RPMI-1640 medium at 108 particles/ml at 41°. After 1 hr of incubation, cells were washed three times with cold PBS and then cultured with RPMI-1640 medium containing 200 ng/ml LPS on fetal calf serum (FCS)-coated 13-mm coverslips. After 24 hr, cells were washed and cultured with the other colour latex beads for 1 hr. Cells cultured with both red and yellow-green latex beads prior to LPS stimulation were used as a control. Cells cultured with yellow-green beads or red beads at day 6 for 24 hr and then cultured with the opposite colour beads, without stimulation, were used as another control. Cells were washed three times with cold PBS, fixed with 4% paraformaldehyde and then stained with 4′,6-Diamidino-2-phenylindole dihydrochloride (DAPI) solution (Sigma-Aldrich UK) for 10 min. Cells were washed three times with PBS, mounted with Vectashield® mounting medium (Vector Laboratories, Peterborough, UK) on slides and visualized using a confocal microscope.
T-cell proliferation assays [mixed lymphocyte reaction (MLR)]
The primary T-cell stimulatory capacity of chBM-DCs was tested in MLR. Non-stimulated, LPS-stimulated or CD40L-stimulated chBM-DCs were used as stimulator cells. These cells were washed, counted and irradiated at 2000 rad (Gammacell 1000 Elite; Nordion International Inc., Fleurus, Belgium). Syngenic and allogeneic peripheral blood lymphocytes (PBLs) were isolated from blood of 4–8-week-old inbred line 72 or C.B12 chickens. Briefly, whole blood with ethylenediaminetetraacetic acid (EDTA) as an anti-coagulant was diluted with an equal volume of PBS and layered on 1·077 histopaque (10771; Sigma-Aldrich). Following centrifugation for 30 min at 1200 g, the cells at the interface were collected, washed, counted and used as responder cells. Graded numbers of stimulator cells were added to 96-well round-bottomed cell culture plates and 105 responder cells were added, giving stimulator:responder ratios of 1 : 2, 1 : 6, 1 : 18, 1 : 54, 1 : 162 and 1 : 486 in a culture volume of 200 μl. Control cultures contained responder or stimulator cells only. All experiments were performed in at least in triplicate. After 3 days of culture in 5% CO2 at 37°, each well received 1 μCi of [3H]thymidine, after which the culture was continued for 16 hr. Incorporation of [3H]thymidine [corrected counts per minute (c.c.p.m.)] was measured in a scintillation β-counter (PerkinElmer, Beaconsfield, UK).
Chicken cytokine and chemokine mRNA expression by chBM-DCs assessed by real-time quantitative reverse transcription–polymerase chain reaction (qRT-PCR)
Total RNA from control chBM-DCs and from chBM-DCs incubated with either LPS or CD40L was isolated using an RNeasy Mini kit (Qiagen, Crawley, UK). Cytokine and chemokine mRNA levels were quantified using TaqMan qRT-PCR, a well-described method.34–37 Primers and probes for cytokine, chemokine and 28S RNA-specific amplification have been described previously but for clarity are given in Table 2. The qRT-PCR was performed using the TaqMan fast universal PCR master mix and one-step RT-PCR master mix reagents (Applied Biosystems, Cheshire, UK). Amplification and detection of specific products were performed using the Applied Biosystems 7500 Fast Real-Time PCR System with the following cycle profile: one cycle of 48° for 30 min, one cycle of 95°C for 20 seconds, 40 cycles of 95° for 3 seconds, and 40 cycles of 60° for 30 seconds. Quantification was based on the increased fluorescence detected by the 7500 Fast Sequence Detection System as a result of hydrolysis of the target-specific probes by the 5′ nuclease activity of the rTth DNA polymerase during PCR amplification. The passive reference dye 6-carboxy-c-rhodamine, which is not involved in amplification, was used for normalization of the reporter signal. Results are expressed in terms of the threshold cycle value (Ct), the cycle at which the change in the reporter dye passes a significance threshold (ΔRn).
Table 2.
Real-time quantitative reverse transcriptase–polymerase chain reaction (RT-PCR) probes and primers
| RNA target | Probe/primer sequence (5′-3′) | Accession number |
|---|---|---|
| 28S | Probe (FAM)-AGGACCGCTACGGACCTCCACCA-(TAMRA) | X59733 |
| F GGCGAAGCCAGAGGAAACT | ||
| R GACGACCGATTTGCACGTC | ||
| IFN-γ | Probe (FAM)-TGGCCAAGCTCCCGATGAACGA-(TAMRA) | Y07922 |
| F GTGAAGAAGGTGAAAGATATCATGGA | ||
| R GCTTTGCGCTGGATTCTCA | ||
| IL-1β | Probe (FAM)-CCACACTGCAGCTGGAGGAAGCC-(TAMRA) | AJ245728 |
| F GCTCTACATGTCGTGTGTGATGAG | ||
| R TGTCGATGTCCCGCATGA | ||
| IL-2 | Probe (FAM)-ACTGAGACCCAGGAGTGCACCCAGC-(TAMRA) | AJ224516 |
| F TTGGAAAATATCAAGAACAAGATTCATC | ||
| R TCCCAGGTAACACTGCAGAGTTT | ||
| IL-4 | Probe (FAM)-AGCAGCACCTCCCTCAAGGCACC-(TAMRA) | AJ621735 |
| F AACATGCGTCAGCTCCTGAAT | ||
| R TCTGCTAGGAACTTCTCCATTGAA | ||
| IL-6 | Probe (FAM)-AGGAGAAATGCCTGACGAAGCTCTCCA-(TAMRA) | AJ309540 |
| F GCTCGCCGGCTTCGA | ||
| R GGTAGGTCTGAAAGGCGAACAG | ||
| IL-12α | Probe (FAM)-CCAGCGTCCTCTGCTTCTGCACCTT-(TAMRA) | AY262751 |
| F TGGCCGCTGCAAACG | ||
| R ACCTCTTCAAGGGTGCACTCA | ||
| IL-13 | Probe (FAM)-CATTGCAAGGGACCTGCACTCCTCTG-(TAMRA) | AJ621735 |
| F CACCCAGGGCATCCAGAA | ||
| R TCCGATCCTTGAAAGCCACTT | ||
| CXCLi1 | Probe (FAM)-TCGCTGAACGTGCTTGAGCCATACCTT-(TAMRA) | Y14971 |
| F TGGCTCTTCTCCTGATCTCAATG | ||
| R GCACTGGCATCGGAGTTCA | ||
| CXCLi2 | Probe (FAM)-TCTTTACCAGCGTCCTACCTTGCGACA-(TAMRA) | AJ009800 |
| F GCCCTCCTCCTGGTTTCAG | ||
| R TGGCACCGCAGCTCATT |
F, forward primer; IFN, interferon; IL, interleukin; R, reverse primer.
To account for variation in sampling and RNA preparation, the Ct values for cytokine- or chemokine-specific product for each sample were normalized using the Ct value of the 28S rRNA product for the same sample. Standard plots of Ct against log10(RNA) were obtained for all genes including the 28S RNA. Normalized Ct values were calculated using the formula 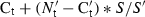 , where
, where 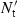 is the mean Ct for 28S RNA among all samples,
is the mean Ct for 28S RNA among all samples, 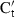 is the mean Ct for 28S RNA in the sample and S and S′ are the slopes of the regressions of the standard plots for the cytokine mRNA and the 28S RNA, respectively. This effectively achieves interpolations on the standard plots to obtain the cytokine Ct values that would have been obtained had all samples had the same (mean) amount of 28S RNA. Results are expressed either as 40-Ct, using the normalized value, or as fold-difference from levels in control chBM-DCs. Non-stimulated chBM-DCs harvested at the same time-points as LPS- or CD40L-stimulated chBM-DCs were compared for expression levels of the different cytokines and chemokines. There were no statistically significant differences in expression for any cytokines or chemokines in non-stimulated chBM-DCs at any time-point. Therefore, expression values in the non-stimulated samples at time zero (0 hr) were used as the reference.
is the mean Ct for 28S RNA in the sample and S and S′ are the slopes of the regressions of the standard plots for the cytokine mRNA and the 28S RNA, respectively. This effectively achieves interpolations on the standard plots to obtain the cytokine Ct values that would have been obtained had all samples had the same (mean) amount of 28S RNA. Results are expressed either as 40-Ct, using the normalized value, or as fold-difference from levels in control chBM-DCs. Non-stimulated chBM-DCs harvested at the same time-points as LPS- or CD40L-stimulated chBM-DCs were compared for expression levels of the different cytokines and chemokines. There were no statistically significant differences in expression for any cytokines or chemokines in non-stimulated chBM-DCs at any time-point. Therefore, expression values in the non-stimulated samples at time zero (0 hr) were used as the reference.
Results
Cells generated by culturing chicken bone marrow cells with recombinant chicken GM-CSF and IL-4 have the morphology of typical DCs
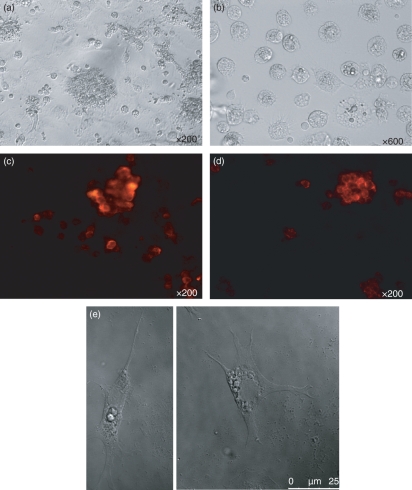
Chicken bone marrow cells were cultured at 1 × 106 cells/ml in the presence of recombinant chicken GM-CSF and IL-4. There are no internationally agreed units of activity for avian cytokines. COS cell culture supernatants which contained chicken GM-CSF or IL-4 were each used at a dilution of 1 : 250 to maximize the number of cell aggregates, which were evident on the culture surface by day 4, as seen under an inverted light microscope (Fig. 1a). These aggregates grew and became floating or loosely adherent at day 7, at which point they were easily dislodged by swirling and pipetting. Higher magnification revealed that many individual cells and the peripheral cells of the aggregates displayed a veiled or dendritic appearance (Fig. 1b). All the cell aggregates were positive when stained with anti-chicken MHC class II (2G11) and mAb 8F2 (putative anti-chicken CD11c) (Fig. 1c,d). When the cell aggregates were stimulated with LPS for 24 hr, many cells had large veils as a sign of maturation (Fig 1e). Yields of non-stimulated cells were 25–50% of starting bone marrow cell numbers. Stimulation with LPS or CD40L for 24 hr reduced the number of cultured cells, presumably as a result of the death of some mature DCs after overstimulation. When chicken bone marrow cells were cultured under the same conditions as above, but without adding GM-CSF and IL-4, no cell aggregates were observed and only a few live cells were left in the plates by day 7.
Figure 1.
Morphology of chicken bone marrow cells cultured for 7 days in the presence of recombinant chicken granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4. (a) Cell aggregates at day 7; magnification ×200. (b) Single cells at day 7 without any stimulation; magnification ×600. (c, d) Major histocompatibility complex class II (MHC II) (2G11) and putative CD11c (8F2) staining; magnification ×200. (e) Single cells after stimulation with lipopolysaccharide (LPS) for 24 hr. The data are representative of at least five independent experiments in which dendritic cells (DCs) from each inbred line were cultured.
Non-stimulated cells have the phenotype of immature DCs and LPS- or CD40L-stimulated cells have the phenotype of mature DCs
Flow cytometry was used to evaluate the cell surface expression of markers typically expressed on DCs. In mammalian species, these markers include MHC class II, CD11c, CD40, CD80, CD83, CD86 and DEC-205.10 In mammals, immature bone marrow-derived DCs show only moderate surface expression of MHC class II molecules and no or low levels of costimulatory molecules.15,38 In contrast, mature bone marrow-derived DCs express increased levels of MHC class II, CD40 and costimulatory molecules such as CD80 or CD86.15,38 CD83 is one of the most characteristic cell surface markers for fully matured DCs39,40 and human DCs express surface CD83 upon activation.41 DEC-205 is abundant in intracellular granules of immature mouse bone marrow-derived DCs,42–44 whereas mature but not immature DCs express high levels of surface DEC-205 in humans.45
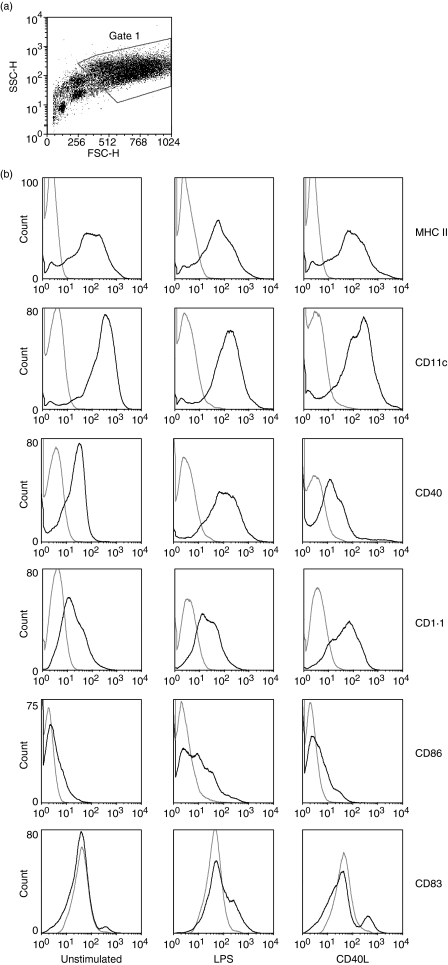
The results of single-colour flow cytometry analysis of the cultured cells harvested on day 7 of culture are shown in Fig. 2. The cultured cells were a heterogenous population, as shown in the side scatter/forward scatter (SSC/FSC) plot (Fig. 2a). The flow cytometry data were therefore gated to exclude cell debris and smaller cells, according to cell granularity and size (SSC/FSC parameters). The gated population included about 70% of all events.
Figure 2.
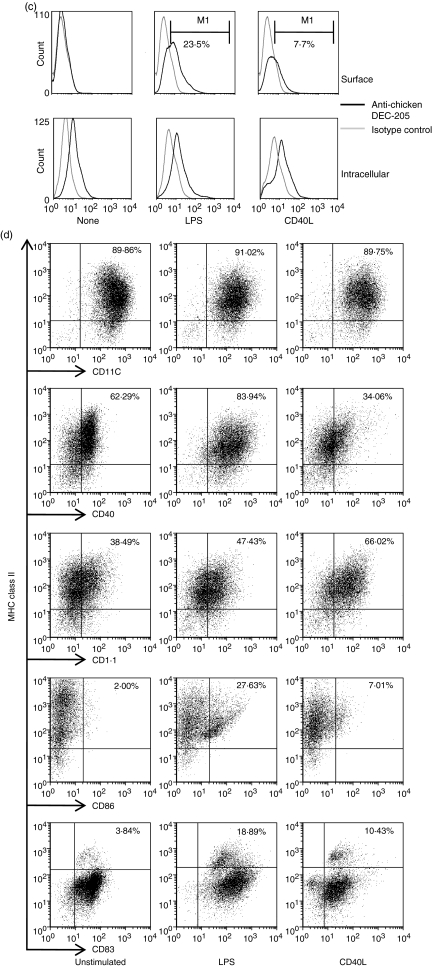
Comparative flow cytometric analysis of surface antigens on cells cultured for 7 days in granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 with or without stimulation with lipopolysaccharide (LPS) or CD40L. (a) Cells were gated based on side scatter/forward scatter (SSC/FSC) parameters. (b) Expression of surface antigens on non-stimulated cells (None), LPS-stimulated cells (LPS) or CD40L-stimulated cells (CD40L). Black lines, anti-chicken cell surface marker monoclonal antibodies (mAbs); grey lines, isotype control mAbs. (c) DEC-205 expression on live (Surface) or saponin-permeabilized (Intracellular) chicken bone marrow-derived dendritic cells (chBM-DCs). (d) Proportions of double-positive cells are shown in dot plots. The data shown are representative of three independent experiments.
Surface expression of cell surface molecules is shown in Fig. 2b. Phenotypic analyses of non-stimulated cells showed high expression of MHC class II and 8F2 antigen (putative CD11c), moderate expression of CD1·1 and CD40, low expression of CD86 and no CD83 or DEC-205 expression, suggesting that these cells are relatively immature. Compared with non-stimulated cells, significantly increased surface expression of CD1·1, CD83, CD86 and DEC-205 was observed on both LPS-stimulated and CD40L-stimulated cells and significantly increased expression of CD40 was observed on LPS-stimulated cells.
Chicken DEC-205 was detected in permeabilized non-stimulated cells and LPS- or CD40L-stimulated cells, but only expressed on the surface of LPS-stimulated (23·5%) and CD40L-stimulated (7·7%) cells (Fig. 2c). DEC-205 was detected intracellularly in both non-stimulated and LPS- or CD40L-stimulated cells, but was only expressed on the surface of the stimulated cells (Fig. 2c).
To characterize the cell population in more detail, double-colour flow cytometric analyses were performed using MHC class II as a reference molecule. The results of double-colour analysis are shown in Fig. 2d, which also illustrates the effects of 24 hr of treatment with LPS or CD40L on cultured cells. The proportions of MHC II+/CD40+, MHC II+/CD1·1+, MHC II+/CD86+ and MHC II+/CD83+ cells were elevated after LPS or CD40L stimulation. Therefore the stimulated cells showed the typical phenotype of mature DCs and henceforth will be described as such.
LPS or CD40L stimulation results in decreased endocytosis and phagocytosis by chBM-DCs
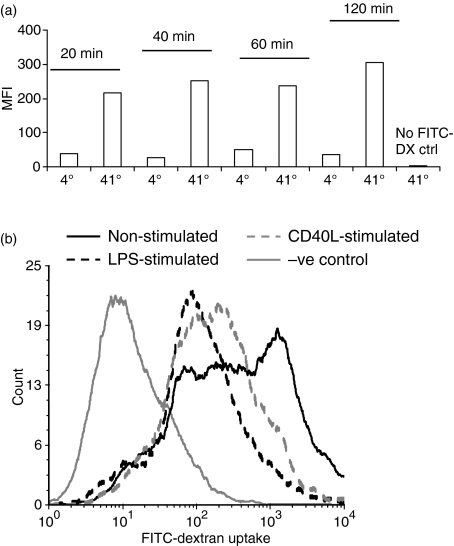
Receptor-mediated endocytic activity was assayed by uptake of FITC-dextran. ChBM-DCs showed a clear temperature-dependent endocytic activity for FITC-dextran (Fig. 3). From the time–course assay (Fig. 3a), non-stimulated chBM-DCs had the capability to take up FITC-dextran with low background binding from the first time-point we measured. No significant increase of mean fluorescence intensity (MFI) was detected after incubation for a further 40 min, and therefore a 20-min incubation was used in subsequent assays.
Figure 3.
Uptake of fluorescein isothiocyanate (FITC)-dextran (1 mg/ml) by chicken bone marrow-derived dendritic cells (chBM-DCs) after 7 days of culture in granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4. (a) Time–course of endocytosis. (b) FITC-dextran uptake by non-stimulated chBM-DCs (solid black line), lipopolysaccharide (LPS)-stimulated chBM-DCs (dashed black line), CD40L-stimulated chBM-DCs (dashed grey line) and 4° negative control cells (solid grey line). The data shown are representative of three independent experiments.
Different preparations of chBM-DCs (non-stimulated, LPS-stimulated or CD40L-stimulated) were compared for their ability to take up FITC-dextran (Fig. 3b). Non-stimulated chBM-DCs showed a high level of endocytic ability. In contrast, LPS- or CD40L-induced maturation resulted in significantly reduced endocytic activity of the highly endocytically active cells.
To investigate whether these cells possess phagocytic capacity, non-stimulated chBM-DCs were co-cultured with fluorescent micro-latex beads. The non-stimulated chBM-DCs phagocytosed both red and green beads (Fig. 4a) when presented with a mixture of the two. Non-stimulated chBM-DCs that phagocytosed yellow-green beads at day 6 did not phagocytose red beads after subsequent LPS stimulation (Fig. 4b). However, non-stimulated chBM-DCs that phagocytosed yellow-green beads at day 6 still phagocytosed red beads after 24 hr of incubation in the absence of LPS stimulation (Fig. 4c). Approximately 90% of the population of cultured cells phagocytosed beads.
Figure 4.
The capacity of chicken bone marrow-derived dendritic cells (chBM-DCs) to phagocytose two different coloured latex beads, with images taken through a confocal microscope. (a) ChBM-DCs following incubation with a mixture of red and yellow-green latex beads. (b) ChBM-DCs following incubation with yellow-green beads before lipopolysaccharide (LPS) stimulation and red beads afterwards. (c) chBM-DCs following incubation with yellow-green beads at day 6 for 24 hr without LPS stimulation followed by addition of red beads.
ChBM-DCs stimulate proliferation of naïve T cells in MLRs
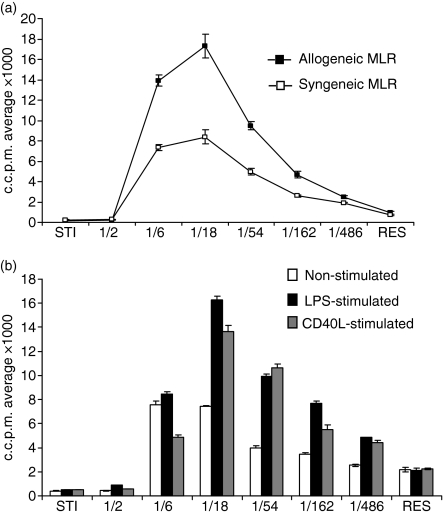
Non-stimulated chBM-DCs effectively stimulated both allogeneic and syngeneic MLRs, compared with controls of responder cells only (Fig. 5a). Maximal stimulation was higher in the allogeneic than the syngeneic MLR, although it was achieved at the same effector:target ratio for both. After LPS or CD40L stimulation, chBM-DCs enhanced their allostimulatory capacity in that maximal stimulation was increased, a characteristic of DCs in mammals (Fig. 5b). With all stimulators, the maximal response was obtained at a 1 : 18 chBM-DC:PBL ratio.
Figure 5.
Mixed lymphocyte reaction (MLR) with chicken bone marrow-derived dendritic cells (chBM-DCs). (a) MLR with non-stimulated chBM-DCs. (b) Comparative analysis of non-stimulated (white bars), lipopolysaccharide (LPS)-stimulated (black bars) or CD40L-stimulated (grey bars) chBM-DCs. The data are representative of three independent experiments for each inbred line. c.c.p.m., corrected counts per minute. STI = stimulator cells (chBM-DCs); RES = responder cells (PBLs).
ChBM-DCs can be matured to a Th1-promoting phenotype by LPS and CD40L stimulation, as assessed by cytokine expression
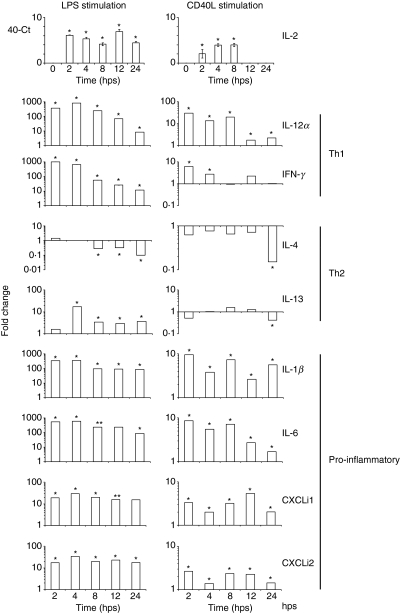
The cytokine environment is critical to the differentiation of naïve T helper cells into subsets 1 and 2 (Th1 and Th2) during immune responses. As the major antigen-presenting cells, DCs have the unique function to prime T-cell responses and polarize Th1/Th2 differentiation by cytokine secretion.46,47 In humans, LPS and CD40L stimulate immature DCs to induce Th1 differentiation.48 In this study, expression levels of mRNA for Th1 (IL-2, IL-12α and IFN-γ) and Th2 cytokines (IL-4 and IL-13) were assessed by real-time qRT-PCR in chBM-DCs (Fig. 6). No IL-2 mRNA was detected in non-stimulated chBM-DCs, whereas LPS- and CD40L-stimulated chBM-DCs expressed high levels of IL-2 mRNA, although for CD40L-stimulated chBM-DCs high expression was only seen at early time-points. Both LPS and CD40L activated chBM-DCs to up-regulate expression levels of IL-12α and IFN-γ mRNA, with unchanged or decreased levels of IL-4 mRNA at the same time-points. Non-stimulated chBM-DCs expressed IL-13 mRNA at low levels, but these were up-regulated after LPS stimulation and down-regulated after CD40L stimulation. Based on these results, we conclude that LPS and CD40L activated chMB-DCs to a Th1-promoting phenotype.
Figure 6.
Quantification of mRNA levels of interleukin (IL)-2, T helper type 1 (Th1) [IL-12α and interferon (IFN)-γ], Th2 (IL-4 and IL-13) and pro-inflammatory cytokines (IL-1β and IL-6) and chemokines (CXCLi1 and CXCLi2) in non-stimulated, lipopolysaccharide (LPS)- or CD40L-stimulated chicken bone marrow-derived dendritic cells (chBM-DCs), at various times (hr) post-stimulation (hps). Data are expressed as fold change in cytokine mRNA levels compared with those in non-stimulated chBM-DCs, except for IL-2 (data expressed as 40-Ct, as IL-2 mRNA was not expressed in non-stimulated chBM-DCs). *, levels statistically significantly different from those in non-stimulated chBM-DCs; P < 0·05.
LPS and CD40L also induced up-regulation of mRNA levels of pro-inflammatory cytokines (IL-1β and IL-6) and chemokines (the IL-8-like molecules CXCLi1 and CXCLi2).
Discussion
When generated in vitro from bone marrow haematopoietic precursors or peripheral blood monocytes with different cytokine or cytokine combinations, immature DCs and mature DCs are defined in mammals18,23 by their distinct morphology, phenotype and function, which mirror those found in vivo.
In this study, we describe the efficient generation of DCs from chicken bone marrow precursors. We followed a method similar to that used to generate large numbers of DCs in mammals.23,49 Chicken bone marrow cells were cultured in the presence of recombinant chicken GM-CSF and IL-4 for 7 days. The cultured cells had the morphology of typical mammalian DCs.15,18 The majority of non-stimulated cultured cells showed high surface expression of MHC class II+ and putative CD11c, moderate or low levels of costimulatory molecules, and no surface expression of CD83 or DEC-205. They actively endocytosed FITC-dextran and phagocytosed fluorescent latex beads but were poor stimulators of PBLs in MLRs. By these criteria, we define them as immature chBM-DCs.
Immature DCs can be induced to mature in vitro with many different stimuli, and LPS and CD40L are strong inducers of DC maturation in mammals.49,50 We used LPS and chicken CD40L to stimulate chBM-DCs to investigate whether they drive the maturation of immature chBM-DCs.
LPS- and CD40L-stimulated chBM-DCs had elevated surface expression levels of costimulatory molecules (CD40 and CD86), CD83 and DEC-205, compared with those seen on non-stimulated chBM-DCs. In mammalian species these criteria are used to characterize mature DCs.12 Our results therefore suggest that LPS and CD40L drive chBM-DCs to become phenotypically mature.
Receptor-mediated endocytosis of dextran is dependent upon expression of the mannose receptor, which in mammals is expressed at high levels on immature DCs and is low or absent on mature DCs.51,52 In our study, LPS- or CD40L-stimulated mature chBM-DCs showed a significant decrease in their capability to take up FITC-dextran compared with that of immature non-stimulated chBM-DCs. LPS- or CD40L-stimulated mature chBM-DCs showed no phagocytic capacity, as measured by uptake of fluorescent microbeads. These results suggest that, upon maturation, chBMDCs lose the ability to phagocytose but maintain a reduced capacity for endocytosis.
In vitro, DCs can induce an MLR.12,53 The most striking difference between DCs and macrophages is that only DCs are effective stimulators in syngeneic MLRs.54 Non-stimulated chBM-DCs can stimulate both allogeneic and syngeneic MLRs. After LPS or CD40L stimulation, chBM-DCs dramatically enhanced their allostimulatory capacity. This demonstrates that the chBM-DCs took up and processed antigen for presentation to T cells in vitro, and ultimately makes the strongest case that immunostimulatory chBM-DCs were indeed produced by this method. LPS and CD40L were therefore shown to be strong inducers of functional maturation of chBM-DCs.
Recently we demonstrated that polarized Th1 and Th2 reactions occur in the chicken, in response to challenge with intracellular and extracellular pathogens, respectively.27,55 We matured chBM-DCs with LPS or CD40L to assess mRNA expression levels of Th1 and Th2 cytokines. The chBM-DCs can be matured to a Th1-promoting phenotype by LPS and CD40L stimulation, as demonstrated by increased levels of expression of IL-12α and IFN-γ mRNA and decreased levels of IL-4 mRNA.
It was recently observed that, in mammals, DCs but not macrophages transiently produce IL-2 following bacterial encounter. IL-2 mRNA was up-regulated at early time-points after Gram-negative bacterial stimulation, probably associated with the initiation of adaptive immune responses.56,57 No IL-2 mRNA was detected in unstimulated chBM-DCs but it was transiently produced at early time-points post-stimulation with LPS or CD40L.
In conclusion, we describe an efficient method to culture chBM-DCs which have been characterized in terms of their morphology, phenotypic expression of DC markers and function (phagocytosis, endocytosis and ability to present antigen as characterized by MLR). LPS and CD40L are inducers of chBM-DC maturation, as evident by down-regulation of phagocytic and endocytic capacity, up-regulation of costimulatory molecules CD83 and DEC-205, and up-regulation of the capacity to stimulate proliferation of naïve T cells in vitro. In addition, chBM-DCs expressed high levels of IL-12α mRNA and could be matured to a Th1-promoting phenotype, at least in terms of the cytokines they expressed, by LPS and CD40L stimulation. This is the first report of cultured DCs in a non-mammalian species. Our ability to generate chBM-DCs in vitro, the development of more DC-specific reagents and the further understanding of DC biology will enable us to isolate and further investigate ex vivo chicken DCs, with the ultimate aim of understanding the migration of DCs and the site of antigen presentation in a species that lacks lymph nodes.
Acknowledgments
This research was undertaken with the financial support of Intervet and the BBSRC. The authors would like to thank Professors Max Cooper and Chen-lo Chen for the kind gift of CB3 monoclonal antibody, Professor Bernd Kaspers (Munich, Germany) for kindly providing the putative anti-chicken CD11c mAb (8F2) and Professor Ian McConnell (Cambridge, UK) for polyclonal sheep anti-chicken CD83.
Disclosures
None.
References
- 1.Perez Torres A, Millan Aldaco DA. Ia antigens are expressed on ATPase-positive dendritic cells in chicken epidermis. J Anat. 1994;184:591–6. [PMC free article] [PubMed] [Google Scholar]
- 2.Gallego M, Olah I, Del Cacho E, Glick B. Anti-S-100 antibody recognizes ellipsoid-associated cells and other dendritic cells in the chicken spleen. Dev Comp Immunol. 1993;17:77–83. doi: 10.1016/0145-305x(93)90017-k. [DOI] [PubMed] [Google Scholar]
- 3.Gallego M, del Cacho E, Lopez-Bernad F, Bascuas JA. Identification of avian dendritic cells in the spleen using a monoclonal antibody specific for chicken follicular dendritic cells. Anat Rec. 1997;249:81–5. doi: 10.1002/(SICI)1097-0185(199709)249:1<81::AID-AR10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 4.Gallego M, Del Cacho E, Felices C, Varas A, Bascuas JA. Distribution of bursal secretory dendritic cells in the chicken. Anat Rec. 1996;246:372–6. doi: 10.1002/(SICI)1097-0185(199611)246:3<372::AID-AR8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 5.Gallego M, Del Cacho E, Zapata A, Bascuas JA. Ultrastructural identification of the splenic follicular dendritic cells in the chicken. Anat Rec. 1995;242:220–4. doi: 10.1002/ar.1092420211. [DOI] [PubMed] [Google Scholar]
- 6.Igyarto BZ, Lacko E, Olah I, Magyar A. Characterization of chicken epidermal dendritic cells. Immunology. 2006;119:278–88. doi: 10.1111/j.1365-2567.2006.02432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansell C, Zhu XW, Brooks H, Sheppard M, Withanage S, Maskell D, McConnell I. Unique features and distribution of the chicken CD83+ cell. J Immunol. 2007;179:5117–25. doi: 10.4049/jimmunol.179.8.5117. [DOI] [PubMed] [Google Scholar]
- 8.Del Cacho E, Gallego M, Lopez-Bernard F, Sanchez-Acedo C, Lillehoj HS. Isolation of chicken follicular dendritic cells. J Immunol Methods. 2008;334:59–69. doi: 10.1016/j.jim.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–62. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 11.Austyn JM. New insights into the mobilization and phagocytic activity of dendritic cells. J Exp Med. 1996;183:1287–92. doi: 10.1084/jem.183.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 13.Granucci F, Ferrero E, Foti M, Aggujaro D, Vettoretto K, Ricciardi-Castagnoli P. Early events in dendritic cell maturation induced by LPS. Microbes Infect. 1999;1:1079–84. doi: 10.1016/s1286-4579(99)00209-9. [DOI] [PubMed] [Google Scholar]
- 14.Winzler C, Rovere P, Rescigno M, et al. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J Exp Med. 1997;185:317–28. doi: 10.1084/jem.185.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 16.Vremec D, Zorbas M, Scollay R, Saunders DJ, Ardavin CF, Wu L, Shortman K. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J Exp Med. 1992;176:47–58. doi: 10.1084/jem.176.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258–61. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 18.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lardon F, Snoeck HW, Berneman ZN, et al. Generation of dendritic cells from bone marrow progenitors using GM-CSF, TNF-alpha, and additional cytokines: antagonistic effects of IL-4 and IFN-gamma and selective involvement of TNF-alpha receptor-1. Immunology. 1997;91:553–9. doi: 10.1046/j.1365-2567.1997.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pulendran B, Smith JL, Caspary G, Brasel K, Pettit D, Maraskovsky E, Maliszewski CR. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci USA. 1999;96:1036–41. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romani N, Gruner S, Brang D, et al. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheicher C, Mehlig M, Zecher R, Reske K. Dendritic cells from mouse bone marrow: in vitro differentiation using low doses of recombinant granulocyte-macrophage colony-stimulating factor. J Immunol Methods. 1992;154:253–64. doi: 10.1016/0022-1759(92)90199-4. [DOI] [PubMed] [Google Scholar]
- 23.Talmor M, Mirza A, Turley S, Mellman I, Hoffman LA, Steinman RM. Generation or large numbers of immature and mature dendritic cells from rat bone marrow cultures. Eur J Immunol. 1998;28:811–7. doi: 10.1002/(SICI)1521-4141(199803)28:03<811::AID-IMMU811>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 24.Maraskovsky E, Daro E, Roux E, et al. In vivo generation of human dendritic cell subsets by Flt3 ligand. Blood. 2000;96:878–84. [PubMed] [Google Scholar]
- 25.Hanada K, Tsunoda R, Hamada H. GM-CSF-induced in vivo expansion of splenic dendritic cells and their strong costimulation activity. J Leukoc Biol. 1996;60:181–90. doi: 10.1002/jlb.60.2.181. [DOI] [PubMed] [Google Scholar]
- 26.Garrigan K, Moroni-Rawson P, McMurray C, Hermans I, Abernethy N, Watson J, Ronchese F. Functional comparison of spleen dendritic cells and dendritic cells cultured in vitro from bone marrow precursors. Blood. 1996;88:3508–12. [PubMed] [Google Scholar]
- 27.Avery S, Rothwell L, Degen WD, Schijns VE, Young J, Kaufman J, Kaiser P. Characterization of the first nonmammalian T2 cytokine gene cluster: the cluster contains functional single-copy genes for IL-3, IL-4, IL-13, and GM-CSF, a gene for IL-5 that appears to be a pseudogene, and a gene encoding another cytokinelike transcript, KK34. J Interferon Cytokine Res. 2004;24:600–10. doi: 10.1089/jir.2004.24.600. [DOI] [PubMed] [Google Scholar]
- 28.Kaiser P, Poh TY, Rothwell L, et al. A genomic analysis of chicken cytokines and chemokines. J Interferon Cytokine Res. 2005;25:467–84. doi: 10.1089/jir.2005.25.467. [DOI] [PubMed] [Google Scholar]
- 29.Tregaskes CA, Glansbeek HL, Gill AC, Hunt LG, Burnside J, Young JR. Conservation of biological properties of the CD40 ligand, CD154 in a non-mammalian vertebrate. Dev Comp Immunol. 2005;29:361–74. doi: 10.1016/j.dci.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Kaufman J, Skjoedt K, Salomonsen J, Simonsen M, Du Pasquier L, Parisot R, Riegert P. MHC-like molecules in some nonmammalian vertebrates can be detected by some cross-reactive xenoantisera. J Immunol. 1990;144:2258–72. [PubMed] [Google Scholar]
- 31.Pickel JM, Chen CL, Cooper MD. An avian B-lymphocyte protein associated with beta 2-microglobulin. Immunogenetics. 1990;32:1–7. doi: 10.1007/BF01787321. [DOI] [PubMed] [Google Scholar]
- 32.Salomonsen J, Sorensen MR, Marston DA, et al. Two CD1 genes map to the chicken MHC, indicating that CD1 genes are ancient and likely to have been present in the primordial MHC. Proc Natl Acad Sci USA. 2005;102:8668–73. doi: 10.1073/pnas.0409213102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kothlow S, Morgenroth I, Tregaskes CA, Kaspers B, Young JR. CD40 ligand supports the long-term maintenance and differentiation of chicken B cells in culture. Dev Comp Immunol. 2008;32:1015–26. doi: 10.1016/j.dci.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 34.Hughes S, Poh TY, Bumstead N, Kaiser P. Re-evaluation of the chicken MIP family of chemokines and their receptors suggests that CCL5 is the prototypic MIP family chemokine, and that different species have developed different repertoires of both the CC chemokines and their receptors. Dev Comp Immunol. 2007;31:72–86. doi: 10.1016/j.dci.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Harrison SM, Dove BK, Rothwell L, Kaiser P, Tarpey I, Brooks G, Hiscox JA. Down regulation of cyclin D1 by the avian coronavirus infectious bronchitis virus. FEBS Lett. 2007;581:1275–86. doi: 10.1016/j.febslet.2007.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrison SM, Tarpey I, Rothwell L, Kaiser P, Hiscox JA. Lithium chloride inhibits the coronavirus infectious bronchitis virus in cell culture. Avian Pathol. 2007;36:109–14. doi: 10.1080/03079450601156083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eldaghayes I, Rothwell L, Williams A, Withers D, Balu S, Davison F, Kaiser P. Infectious bursal disease virus: strains that differ in virulence differentially modulate the innate immune response to infection in the chicken bursa. Viral Immunol. 2006;19:83–91. doi: 10.1089/vim.2006.19.83. [DOI] [PubMed] [Google Scholar]
- 38.Lutz MB, Suri RM, Niimi M, Ogilvie AL, Kukutsch NA, Rossner S, Schuler G, Austyn JM. Immature dendritic cells generated with low doses of GM-CSF in the absence of IL-4 are maturation resistant and prolong allograft survival in vivo. Eur J Immunol. 2000;30:1813–22. doi: 10.1002/1521-4141(200007)30:7<1813::AID-IMMU1813>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 39.Prechtel AT, Steinkasserer A. CD83: an update on functions and prospects of the maturation marker of dendritic cells. Arch Dermatol Res. 2007;299:59–69. doi: 10.1007/s00403-007-0743-z. [DOI] [PubMed] [Google Scholar]
- 40.Zhou LJ, Tedder TF. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J Immunol. 1995;154:3821–35. [PubMed] [Google Scholar]
- 41.Cao W, Lee SH, Lu J. CD83 is preformed inside monocytes, macrophages and dendritic cells, but it is only stably expressed on activated dendritic cells. Biochem J. 2005;385:85–93. doi: 10.1042/BJ20040741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahnke K, Guo M, Lee S, Sepulveda H, Swain SL, Nussenzweig M, Steinman RM. The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II-positive lysosomal compartments. J Cell Biol. 2000;151:673–84. doi: 10.1083/jcb.151.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pierre P, Turley SJ, Gatti E, et al. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388:787–92. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- 44.Jiang W, Swiggard WJ, Heufler C, Peng M, Mirza A, Steinman RM, Nussenzweig MC. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature. 1995;375:151–5. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]
- 45.Guo M, Gong S, Maric S, Misulovin Z, Pack M, Mahnke K, Nussenzweig MC, Steinman RM. A monoclonal antibody to the DEC-205 endocytosis receptor on human dendritic cells. Hum Immunol. 2000;61:729–38. doi: 10.1016/s0198-8859(00)00144-0. [DOI] [PubMed] [Google Scholar]
- 46.Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 47.Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YJ. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–6. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 48.Liu YJ, Kanzler H, Soumelis V, Gilliet M. Dendritic cell lineage, plasticity and cross-regulation. Nat Immunol. 2001;2:585–9. doi: 10.1038/89726. [DOI] [PubMed] [Google Scholar]
- 49.Bai L, Feuerer M, Beckhove P, Umansky V, Schirrmacher V. Generation of dendritic cells from human bone marrow mononuclear cells: advantages for clinical application in comparison to peripheral blood monocyte derived cells. Int J Oncol. 2002;20:247–53. [PubMed] [Google Scholar]
- 50.Wurtzen PA, Nissen MH, Claesson MH. Maturation of dendritic cells by recombinant human CD40L-trimer leads to a homogeneous cell population with enhanced surface marker expression and increased cytokine production. Scand J Immunol. 2001;53:579–87. doi: 10.1046/j.1365-3083.2001.00910.x. [DOI] [PubMed] [Google Scholar]
- 51.Kato M, Neil TK, Fearnley DB, McLellan AD, Vuckovic S, Hart DN. Expression of multilectin receptors and comparative FITC-dextran uptake by human dendritic cells. Int Immunol. 2000;12:1511–9. doi: 10.1093/intimm/12.11.1511. [DOI] [PubMed] [Google Scholar]
- 52.Menges M, Baumeister T, Rossner S, Stoitzner P, Romani N, Gessner A, Lutz MB. IL-4 supports the generation of a dendritic cell subset from murine bone marrow with altered endocytosis capacity. J Leukoc Biol. 2005;77:535–43. doi: 10.1189/jlb.0804473. [DOI] [PubMed] [Google Scholar]
- 53.Austyn JM, Steinman RM, Weinstein DE, Granelli-Piperno A, Palladino MA. Dendritic cells initiate a two-stage mechanism for T lymphocyte proliferation. J Exp Med. 1983;157:1101–15. doi: 10.1084/jem.157.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guidos C, Wong M, Lee KC. A comparison of the stimulatory activities of lymphoid dendritic cells and macrophages in T proliferative responses to various antigens. J Immunol. 1984;133:1179–84. [PubMed] [Google Scholar]
- 55.Degen WG, Daal N, Rothwell L, Kaiser P, Schijns VE. Th1/Th2 polarization by viral and helminth infection in birds. Vet Microbiol. 2005;105:163–7. doi: 10.1016/j.vetmic.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 56.Granucci F, Vizzardelli C, Pavelka N, et al. Inducible IL-2 production by dendritic cells revealed by global gene expression analysis. Nat Immunol. 2001;2:882–8. doi: 10.1038/ni0901-882. [DOI] [PubMed] [Google Scholar]
- 57.Granucci F, Feau S, Angeli V, Trottein F, Ricciardi-Castagnoli P. Early IL-2 production by mouse dendritic cells is the result of microbial-induced priming. J Immunol. 2003;170:5075–81. doi: 10.4049/jimmunol.170.10.5075. [DOI] [PubMed] [Google Scholar]