Abstract
Objectives Immunohistochemistry (IHC) can be helpful in the diagnosis of minor salivary gland neoplasms including those that have been incisionally biopsied or fragmented during surgery that do not contain key diagnostic features on hematoxylin and eosin sections. IHC has been used as an adjunct to distinguish among many salivary gland neoplasms using both qualitative and quantitative methods. The objective of this study was to determine whether a distinctive immunoreactivity staining pattern to GFAP can be consistently observed among three selected minor salivary gland neoplasms and thus serve as a diagnostic adjunctive procedure. Study Design Glial fibrillary acidic protein (GFAP) reactivity was examined among 78 minor salivary gland neoplasms: 27 canalicular adenomas (CAA), 21 pleomorphic adenomas (PA) and 30 polymorphous low grade adenocarcinomas (PLGA). Each case was evaluated by two oral and maxillofacial pathologists (OMP) blinded to the diagnosis. Consensus was reached on the pattern of GFAP reactivity among the neoplastic cells and on the similarities and differences among the cases. Results Ninety-six percent (96%) of CAAs demonstrated a distinctive linear immunoreactive pattern among cells in proximity to connective tissue interface. All (100%) PAs demonstrated diffuse immunopositivity within tumor cells. All (100%) PLGAs showed little or no intralesional reactivity and no peripheral linear immunoreactivity. Additional challenge cases were examined by outside OMPs to demonstrate the utility of these findings. Conclusions This study demonstrates that the pattern of GFAP immunoreactivity may be an adjunct to diagnosis among PA, CAA and PLGA. The pattern of distinctly linear GFAP immunoreactivity at the tumor/connective tissue interface in CAA has not been reported previously. This distinctive feature may permit the pathologist to differentiate among CAA, PA and PLGA when an incisional biopsy and/or fragmentation cause key diagnostic features to be absent. Because each of these neoplasms requires a different treatment approach, this can be of major significance.
Keywords: Salivary gland neoplasms, Glial fibrillary acidic protein, Immunohistochemistry, Canalicular adenoma, Polymorphous low-grade adenocarcinoma, Pleomorphic adenoma
Introduction
Diagnosis of minor salivary gland neoplasms relies upon the presence of distinct morphologic features. Preservation of tumor architecture in adequately excised samples provides the pathologist with the ideal opportunity to render a definitive diagnosis on routine hematoxylin and eosin stained sections; however, fragmented or incisionally biopsied minor salivary gland neoplasms can become a diagnostic challenge when key morphologic features have become distorted. This challenge is encountered regularly with minor salivary gland neoplasms of the hard palate that often are incompletely excised or fragmented.
The adjunctive use of immunohistochemistry (IHC) has been researched extensively in diagnosis of minor salivary gland tumors. Most studies have developed quantitative and qualitative methods to distinguish among tumors. For example, previous investigators including Gnepp and colleagues, Castle and colleagues and Curran and colleagues have demonstrated lack of glial fibrillary acid protein (GFAP) reactivity is an adjunct in differentiating between pleomorphic adenoma (PA) and polymorphous low grade adenocarcinoma (PLGA) [1–3]. This lack of reactivity has been attributed to the loss of ability of malignant cells to express GFAP [4–7]. Clinical application of these methods is useful when fragmented neoplasms with equivocal features are encountered.
A similar diagnostic challenge may be encountered when canalicular adenomas (CAA) are incisionally biopsied or fragmented during surgery. While most CAA have classic monotonous morphologic features that contain pseudocystic spaces, they also often contain hypercellular areas that may be indistinguishable from cellular areas seen in PA or PLGA [8, 9].
This study examines whether pattern of GFAP reactivity can be a reliable adjunct in the diagnosis of CAA, PLGA and PA when they are fragmented or present with equivocal diagnostic features. The objective of this study was to determine whether a specific immunoreactivity pattern to GFAP can be consistently observed within each of these three minor salivary gland tumors and thereby serve as an adjunct in their diagnosis.
Methods and Materials
After obtaining Institutional Review Board approval for use of archived tissue samples, 27 CAAs, 21 PAs and 30 PLGAs were reviewed by three oral and maxillofacial pathologists (OMPs) for consensus on diagnoses. In addition, three cases of basal cell adenoma (BCA) were retrieved and reviewed.
Paraffin embedded tissue was cut at 4 μ and placed on positively charged slides. All cases were immunostained with rabbit polyclonal antibodies to GFAP (Dako Carpinteria, CA, catalog number M0761, mouse monoclonal, clone 6F2) using standard IHC techniques, including use of antigen retrieval methods. Slides were then placed on a Dako Autostainer immunostaining system. Primary antibody was diluted 1:500. Slides were then counterstained in hematoxylin. Sections of brain served as positive GFAP controls for each batch.
Two OMPs, who were blinded to the diagnosis, viewed the slides independently for patterns of GFAP reactivity.
Results
Among the three tumor types, three distinct patterns emerged: (1) distinct intracytoplasmic reactivity within a single row of tumor cells at the tumor/connective tissue interface, (2) intracytoplasmic immunoreactivity of diffuse intralesional cells and (3) weak luminal or no reactivity. Reactive cases then were classified as strong linear, weak linear, strong diffuse or weak diffuse patterns. The two OMPs then concurred on the staining pattern classification of each case. The single row of distinctly immunoreactive cells at the tumor/connective tissue interface was assessed at least 2 mm from the border of the specimen to avoid false positive interpretation of what is commonly referred to as “edge-effect” of immunoreactants.
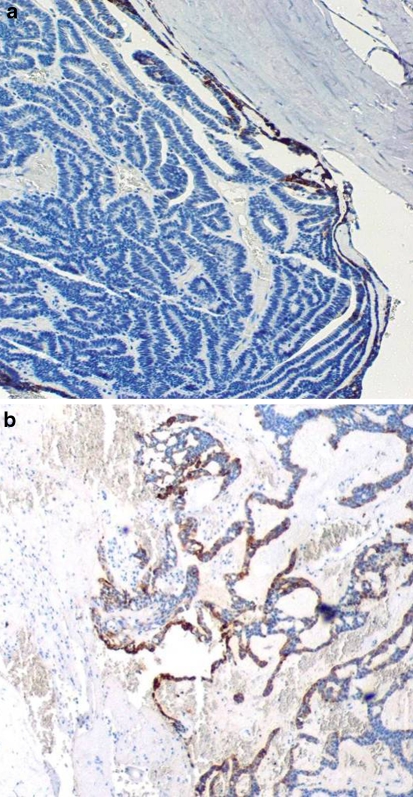
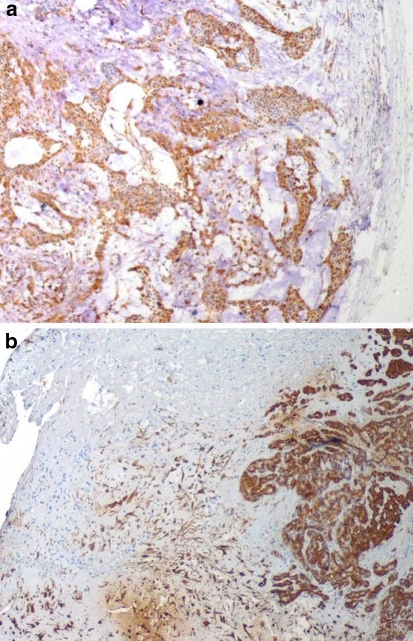
Twenty-six (96%) CAAs demonstrated either strong or weak intracytoplasmic reactivity that was confined to a row of cells at the tumor/connective tissue interface (Fig. 1). One CAA demonstrated weak diffuse positivity of tumor cells but also demonstrated a row of strong immunoreactive cells at the tumor/connective tissue interface. One CAA was not reactive. All 21 PAs demonstrated either strong or weak diffuse intracytoplasmic positivity within tumor cells (Fig. 2). In addition to strong intralesional positivity, two cases demonstrated a weakly linear row of immunoreactive cells at the tumor/connective tissue interface.
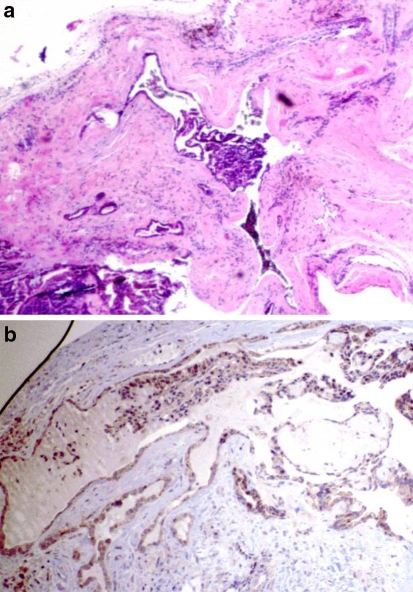
Fig. 1.
(a) Intact CAA demonstrating intense linear GFAP immunostaining (40×). (b) Fragmented CAA with linear GFAP immunoreactivity at tumor/connective tissue interface (40×)
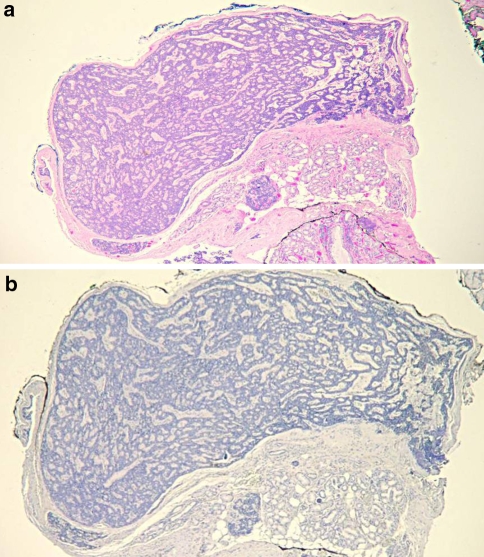
Fig. 2.
(a) Intact PA demonstrating diffuse intralesional GFAP immunoreactivity (40×). (b) Fragmented PA with diffuse intralesional GFAP immunoreactivity and no reactivity at tumor/connective tissue interface (40×)
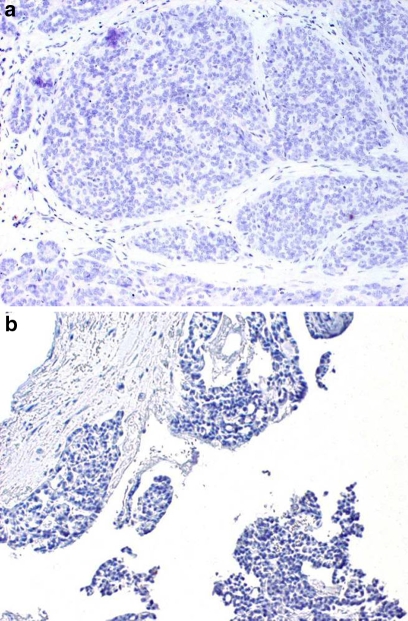
All 30 PLGAs showed little or no immunoreactivity to GFAP. There was almost universal negativity among the tumor cells, with the exception of occasional faint positivity in luminal cells. (Fig. 3) One case demonstrated a weak linear immunoreactivity adjacent to areas of perineural invasion but not at the tumor/connective tissue interface.
Fig. 3.
(a) PLGA demonstrating negative GFAP immunoreactivity of tumor cells with no reactivity at tumor/connective tissue interface (40×). (b) Fragmented PLGA demonstrating negative GFAP immunoreactivity of tumor cells with no reactivity at tumor/connective tissue interface (40×)
Between-lesion differences in GFAP immunoreactivity reactivity were analyzed using multiple Fisher exact tests with Bonferroni adjustment. There were statistically significant differences (P < 0.001) between groups (Table 1).
Table 1.
Fisher’s exact test with Bonferroni adjustment
| Neoplasm | Diffuse intralesional pattern | Linear pattern at tumor/CT interface | No reactivity pattern |
|---|---|---|---|
| PA (n = 21) | 21* | 2a | 0 |
| CAA (n = 27) | 0 | 26* | 1 |
| PLGA (n = 30) | 1a | 0 | 30* |
*P < 0.001
aCases with secondary patterns that did not alter statistical significance
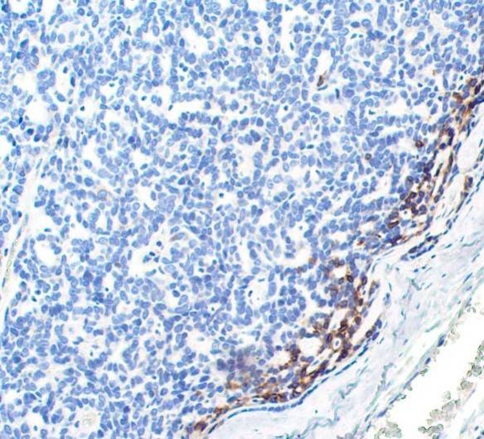
Three basal cell adenomas (BCA) were used to compare reactivity pattern to CAA because both tumors have distinctly monotonous features and in the past had often been misclassified or grouped together as so-called “monomorphic adenoma”. Here, they each demonstrated strong intracytoplasmic reactivity in a single row of cells at the tumor/connective tissue interface (Fig. 4), similar to CAA. These cases were not included in statistical analysis due to low numbers. Also, BCA rarely occurs in minor salivary glands and rarely is included in a minor salivary gland differential diagnosis.
Fig. 4.
BCA demonstrating intense GFAP immunoreactivity at tumor/connective tissue interface (100×)
Application of Findings
A set of 12 challenge cases stained with hematoxylin and eosin was reviewed by three OMPs blinded to the objectives of the study. The OMPs did not reach consensus on six cases. Review of GFAP reactivity of those six cases demonstrated that four showed reactivity patterns seen in the study cases consistent with our findings in CAA. In only two cases did the GFAP fail to contribute to the final diagnosis.
An addition to the challenge cases, two subsequent cases provided an opportunity to apply our findings. Figure 5a shows a case submitted to The Ohio State University Oral and Maxillofacial Pathology Laboratory showing a fragmented neoplasm of the posterior hard palate with the differential diagnosis of PA vs. PLGA. Figure 5b shows GFAP reactivity of the neoplasm to be an adjunct in establishing the diagnosis of canalicular adenoma.
Fig. 5.
(a) Fragmented neoplasm of hard palate with equivocal features (20×). (b) GFAP reactivity shows linear pattern c/w CAA (40×)
Another case involved a 60 year-old Caucasian female who presented in 1997 with a tumor of the left upper buccal mucosa with features of a canalicular adenoma but with multifocal areas suggestive of infiltration. At that time, the tumor was signed out by general surgical pathologists as “low grade adenocarcinoma with canalicular adenoma”. In 2005, the patient returned with a mass of the upper lip, 5 cm from the original site. The histopathology was identical to the first neoplasm (Fig. 6a). The tumor formed numerous well-circumscribed tumor nodules that closely resembled canalicular adenoma. Occasional foci were less circumscribed and extended into surrounding muscle. There was significant discussion concerning whether this tumor represented recurrent carcinoma (Due to the distance from the original surgical site, the surgeon strongly felt this was a new focus and not a recurrence from the 1997 tumor), multifocal CAA or an adenocarcinoma. GFAP demonstrated no reactivity and none of the tumor lobules revealed a band-like staining of the tumor/connective tissue interface (Fig. 6b). Due to the GFAP findings, the second neoplasm was signed out as “low-grade adenocarcinoma, NOS”.
Fig. 6.
(a) Neoplasm of upper lip with multiple foci (20×). (b) GFAP is negative with no peripheral linear reactivity (20×)
Discussion
The objective of this study was to determine if a differential pattern of GFAP immunoreactivity can assist the pathologist who is confronted with a fragmented minor salivary gland neoplasm that demonstrates equivocal histopathologic features. GFAP reactivity previously has been reported in a number of studies of salivary gland neoplasms. Nishimura showed that a quantitative difference in GFAP reactive cells can differentiate PA from BCA [10]. We, along with Gnepp, showed that GFAP can differentiate PLGA from PA based on quantity of reactive cells [1, 3]. Direct comparison of GFAP immunoreactivity among PA, PLGA and CAA, however, has not been reported.
This study emphasizes that a pattern of reactivity to GFAP, rather than quality or quantity of reactive cells, can be useful in differentiating CAA, PA and PLGA, and suggests similar findings in BCA. Quantification of GFAP-positive tumor cells that is useful in differentiating PA from PLGA was not shown to be useful in the present study when differentiating PLGA from CAA because GFAP positive cells within PLGA were scant or negative in both neoplasms. However, we have demonstrated that CAA reacts to GFAP antibodies with an intense distinct row of immunoreactive cells at the tumor/connective tissue interface (Fig. 1) not seen in PA or PLGA. GFAP also helped differentiate between CAA and PA because any weak linear reactivity at the tumor/ connective tissue interface of PAs was accompanied by strong diffuse positivity among tumor cells.
Multifocal CAA often presents as multiple discrete clinical nodules but may include microscopic foci of tumor cells suggesting a malignancy [11]. Our case seen in Fig. 6a, b demonstrates the utility of GFAP in the final diagnosis of such a case.
A possible explanation for the pattern of GFAP reactivity is the fact that in normal ducts, GFAP-positive myoepithelial cells are found at the periphery only. CAA and BCA are believed to be derived mainly from basal and ductal epithelium with myoepithelial and myoepithelial-derived cells making up only a small portion of the tumor [7]. In CAA, proliferation of ductal and/or basal cells may be at the expense of the myoepithelial cells that remain in their ‘normal’ orientation during tumor development, causing this peripheral row of distinctly GFAP-immunoreactive cells at the connective tissue interface. PA, on the other hand, is derived from variable numbers of ductal and myoepithelial cells that form variable patterns as they proliferate. This leads to greater numbers of the GFAP positive myoepithelial cells within the tumor and thus to a more diffuse generalized pattern of GFAP reactivity. The lack of any reactivity pattern in PLGA is due to altered or absent ability of malignant cells to express GFAP [12].
Despite GFAP’s consistently distinct pattern of reactivity in this study, this may be of limited value when the biopsy specimen does not include portions of the tumor/connective tissue interface or when the interface has been disrupted by surgical procedures or processing effects. If, however, adequate zones of tumor/connective tissue interface are present within the biopsy, then the results of this study appear to be reliable and therefore may be useful. It is important to point out that this study does not evaluate other minor salivary gland neoplasms that may be included in the differential diagnosis of a given case. Analyzing GFAP patterns of immunoreactivity in additional tumor types will be the focus of future work.
In conclusion, the results of this study support the identification of the pattern of a single row of distinctly GFAP immunoreactive cells at the tumor/connective tissue interface as a reliable diagnostic criterion for differentiation among CAA, PA and PLGA in cases where the lack of classic histopathologic features does not permit definitive diagnosis.
Bibliography
- 1.Gnepp DR, el-Mofty S. Polymorphous low grade adenocarcinoma: glial fibrillary acidic protein staining in the differential diagnosis with cellular mixed tumors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:691–5. doi: 10.1016/S1079-2104(97)90321-8. [DOI] [PubMed] [Google Scholar]
- 2.Castle JT, Thompson LD, Frommett RA, Wenig BM, Kessler HP. Polymorphous low-grade adenocarcinoma: a clinicopathologic study of 164 cases. Cancer. 1999;86:207–19. doi: 10.1002/(SICI)1097-0142(19990715)86:2<207::AID-CNCR4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 3.Curran AE, White DK, Damm DD, Murrah VA. Polymorphous low-grade adenocarcinoma versus pleomorphic adenoma of minor salivary glands: resolution of a diagnostic dilemma by immunohistochemical analysis with glial fibrillary acidic protein. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:194–9. doi: 10.1067/moe.2001.111306. [DOI] [PubMed] [Google Scholar]
- 4.Gould VE, Koukoulis GK, Jansson DS, Franke WW, Moll R. Co-expression patterns of vimentin and glial fibrillary acidic protein in normal, hyperplastic and neoplastic breast. Am J Surg Pathol. 1990;137:1143–55. [PMC free article] [PubMed] [Google Scholar]
- 5.Liuder TM, Kros JM, Sillevis Smitt PA, Bent MJ, Vecht CJ. Glial fibrillary acidic protein and its fragments discriminate astrocytoma from oligodenroglioma. Electrophoresis. 1999;20:1087–91. doi: 10.1002/(SICI)1522-2683(19990101)20:4/5<1087::AID-ELPS1087>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 6.Murphy KG, Hatton JD. Role of glial fibrillary acidic protein expression in the biology of human glioblastoma U-373MG cells. J Neurosurg. 1998;89:997–1006. doi: 10.3171/jns.1998.89.6.0997. [DOI] [PubMed] [Google Scholar]
- 7.Zarbo RJ, Prasad AR, Regezi JA, Gown AM, Savera AT. Salivary gland basal cell and canalicular adenomas: immunohistochemical demonstration of myoepithelial cell participation and morphogenetic considerations. Arch Pathol Lab Med. 2000;124:401–5. doi: 10.5858/2000-124-0401-SGBCAC. [DOI] [PubMed] [Google Scholar]
- 8.Sharkey TE. Systematic evaluation of WHO classification of salivary gland tumors: a clinicopathologic study of 366 cases. Am J Clin Pathol. 1977;67:272–8. doi: 10.1093/ajcp/67.3.272. [DOI] [PubMed] [Google Scholar]
- 9.Ellis GL, Auclair PL. Tumors of the salivary glands. Third Series. Washington DC: Armed Forces Institute of Pathology; 1996. p. 100. [Google Scholar]
- 10.Nishimura T, Furukawa M, Kawahara E, Miwa A. Differential diagnosis of pleomorphic adenoma by immunohistochemical means. J Layngol Otol. 1991;105:1057–60. doi: 10.1017/s0022215100118183. [DOI] [PubMed] [Google Scholar]
- 11.Rousseau A, Mock D, Dover DG, Jordan RC. Multiple canalicular adenomas: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:346–50. doi: 10.1016/S1079-2104(99)70221-0. [DOI] [PubMed] [Google Scholar]
- 12.Viale G, Gambacorta M, Coggi G, Dell’Orto P, Milani M, Doglioni C. Glial fibrillary acidic protein immunoreactivity in normal and diseased human breast. Virchows Arch A Pathol Anat Histopathol. 1991;418:339–8. doi: 10.1007/BF01600164. [DOI] [PubMed] [Google Scholar]