Introduction
The production of bone by neoplastic cells that have an osteoblastic phenotype is the common trait of all bone forming neoplasms. The tumor bone can be lamellar or woven in architecture and may mimic cortical or cancellous elements. In benign tumors, the neoplastic bone usually consists of relatively well-formed trabeculae of woven bone, whereas in malignant bone forming tumors (osteosarcoma), it is usually deposited in a coarse lace-like pattern. Lamellar cortical-type neoplastic bone is generally only present in osteoma (and enostosis), however, it can rarely be a component of well-differentiated osteosarcoma.
Osteoma
Osteoma is a benign, slow growing bone forming tumor that consists primarily of well-differentiated mature, compact or cancellous bone. They usually arise on the surfaces of the cranial vault (outer table—exostotic and inner table—enostotic), jaw, paranasal sinuses, and orbit [17, 22, 42]. Cranial osteomas are named according to the bone from which they arise, whereas osteomas of the paranasal sinuses are designated in relation to the sinus which they invade [4]. In the paranasal sinuses, osteomas most frequently involve the frontal sinus, followed in descending order by the ethmoid sinus, maxillary antrum, and sphenoid sinus [11, 12, 30, 41].
Although rare in children, osteomas affect all age groups but are most commonly diagnosed in the fourth or fifth decades of life [12, 41].
Osteomas are often asymptomatic and are frequently an incidental finding on imaging studies performed for unrelated conditions. Osteomas when large or situated in a strategic location can cause a variety of signs and symptoms including painless swelling, facial asymmetry and symptoms secondary to nasal or paranasal sinus obstruction such as sinusitis, nasal discharge and mucocele formation [12, 13, 16, 45]. Orbital osteomas and paranasal sinus osteomas that protrude into the orbit can cause a variety of ocular abnormalities such as exophthalmos, proptosis, ptosis, diplopia, lid edema or swelling and amaurosis fugax [16, 46]. In extraordinary cases the tumor may grow intracranially and cause neurological complications [1].
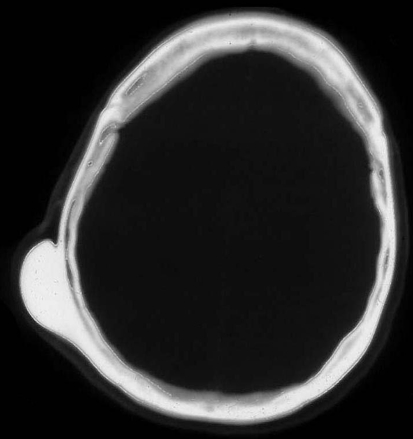
Classically, osteomas manifest as a round well-circumscribed homogeneous radiodensity on radiographs; however, several different patterns of mineralization may be seen (Fig. 1) [11].
Fig. 1.
Axial CT of skull showing round well defined osteoma attached to the outer table
Grossly, osteomas are covered externally by a thin layer of fibrous periosteum. They are round or oval, hard, tan-white, bosselated, well circumscribed and attached to the underlying bone by a broad base or occasionally by a small stalk. On bisection they are dense or sclerotic with narrow (compact type) or prominent (spongiotic) intertrabecular spaces [38].
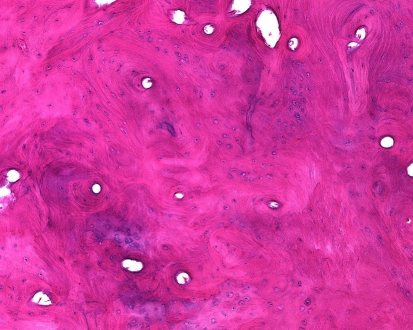
Histologically, compact osteomas are composed of sheets of predominantly lamellar bone with haversian-like systems of variable size and shape that often blends imperceptibly with the underlying normal cortex (Fig. 2). Foci of woven bone and fibrous tissue, at times reminiscent of a fibro-osseous lesion may be present. Spongy osteomas are constructed of cancellous bone with intertrabecular hematopoietic bone marrow or fat. Regardless of the type of bone, the osseous surfaces show minimal osteoblastic or osteoclastic activity and the osteocytes are small and inconspicuous.
Fig. 2.
Osteoma composed of disorganized appearing cortical-like bone having a predominately lamellar pattern
Patients with Gardner’s syndrome, an autosomal dominant disorder characterized by colonic polyps and soft tissue tumors, may have multiple osteomas. Osteomas of the skull may be the initial finding in these patients [41].
Only patients with symptomatic osteomas should be treated, generally by simple excision. Recurrences are very rare, even in incompletely excised lesions. Malignant transformation has not been reported.
The most important entity in the differential diagnosis is juxtacortical well-differentiated (parosteal) osteosarcoma. Unlike osteomas that frequently arise in the skull bones, parosteal osteosarcomas are extremely rare in this location. Although bone formation can be extensive in parosteal osteosarcoma, the neoplastic trabeculae of woven bone are separated by a cellular fibrous stroma that contains occasional mitotic figures, and these features are not seen in osteoma.
Osteoid Osteoma
Osteoid osteoma is a benign bone forming tumor characterized by its small size, limited growth potential, and classic pattern of pain. It accounts for approximately 12% of benign bone tumors, and similar to osteoblastoma predominantly affects children and young adults, particularly males [5, 16, 21, 39]. By definition, osteoid osteoma is 1–2 cm in diameter; morphologically similar lesions larger than 2 cm are classified as osteoblastomas. Most osteoid osteomas of the head and neck affect the posterior elements of the cervical vertebrae [21, 23, 35]. Osteoid osteomas of the craniofacial and jaw bones are exceptionally uncommon where they usually arise in the mandible [20]. Between 5 and 10% of osteoid osteomas arise within the vertebral column and approximately 25% of these affect the cervical vertebrae. Patients typically complain of severe localized pain that is often worse at night and relieved by aspirin or other non-steroidal anti-inflammatory medications [5, 15, 16, 21, 39]. Additionally, the majority of patients with cervical osteoid osteoma exhibit torticollis and often scoliosis [23, 35].
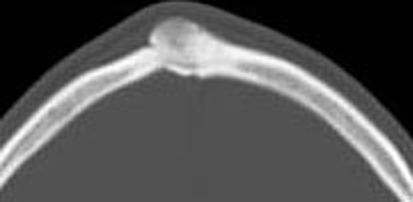
Radiographically, osteoid osteomas exhibit a central lucency (the nidus) with patchy mineralization located centrally. The nidus is surrounded by a zone of sclerosis (the reactive zone) that can be so extensive as to obscure the underlying lesion (Fig. 3) [15, 16].
Fig. 3.
Axial CT of frontal bone containing an osteoid osteoma. The tumor is small, round, well demarcated and surrounded by sclerotic reactive bone
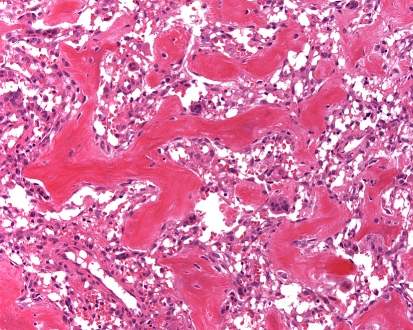
Grossly, the nidus is usually tan-white gritty and dark red representing the partially mineralized woven bone and rich capillary vascular supply, respectively. Histologically, the nidus is composed of haphazardly intersecting trabeculae of woven bone that are prominently rimmed by osteoblasts (Fig. 4). The bone matrix is supported by a richly vascularized loose connective tissue that contains fibroblasts and delicate collagen fibers. All of the various patterns of bone matrix production including solid sheets, focal lace-like arrangements and trabeculae that may be seen in osteoblastoma may also be present within osteoid osteoma. In effect, the microscopic appearance of the nidus of an osteoid osteoma and osteoblastoma are indistinguishable [21, 26]. However, they differ in that the periphery of osteoid osteoma has a greater degree of reactive sclerosis. Additionally immunohistochemical stains for neurofilament and S-100 protein readily identify nerve fibers within the reactive zone and nidus of osteoid osteomas, whilst osteoblastomas lack these structures [34].
Fig. 4.
Osteoid osteoma composed of haphazardly interconnecting trabeculae of woven bone lined by prominent osteoblasts
In the past osteoid osteoma used to be treated with curettage or en bloc resection, however, in recent years percutaneous radiofrequency ablation has become the treatment of choice. This less invasive procedure appears to be as successful in treating these patients as the traditional operative approach [37].
The differential diagnosis of osteoid osteoma includes osteoblastoma, osteosarcoma and osteomyelitis. Osteoblastoma, although microscopically indistinguishable from osteoid osteoma differs by virtue of its larger size, lack of marked reactive sclerosis, and the absence of detectable nerve fibers. Additionally, patients with osteoblastomas usually do not exhibit the characteristic pattern of nocturnal localized pain that is relieved by aspirin. Osteosarcoma is larger than osteoid osteoma, exhibits a poorly defined margin radiographically, and microscopically demonstrates greater cytologic atypia and mitoses. Localized chronic osteomyelitis (Brodie’s abscess) may clinically and radiographically simulate osteoid osteoma, however, microscopically it is associated with acute and chronic inflammation, granulation tissue and bone necrosis. The peripheral sclerotic rim surrounding a Brodie’s abscess may be indistinguishable from the reactive zone of an osteoid osteoma. Microscopic evaluation of the central lucency (the nidus) is required to definitely separate these two lesions.
Osteoblastoma
Osteoblastoma is an uncommon bone forming neoplasm that accounts for approximately 1% of primary bone tumors [27, 28]. It develops most frequently in the posterior elements of the vertebrae and the metaphyses of long bones, however, approximately 10–15% arise within the bones of the craniofacial skeleton. The most common location is the mandible followed by the maxilla, although other bones may rarely be affected [8, 25, 27, 28, 31, 36, 39, 40, 43].
Osteoblastoma occurs predominantly in young adults and most are diagnosed in the 2nd through 4th decades of life. Males are affected twice as frequently as females [8, 27, 28, 39]. Patients present with a painful mass and craniofacial tumors may produce headache, tooth impaction and epistaxis. In a recent report of 24 cases of osteoblastomas of the maxilla and mandible there were 4 males and 20 females that ranged in age from 3 to 61 years. Approximately 80% of the cases were in the mandible and 20% in the maxilla [24].
Radiographically, osteoblastomas arise in the medullary cavity or on the surface of the bone. They appear as a mixed lytic and blastic mass; the intra-lesional mineralization is very variable and may be minimal or extensive, but is greatest centrally (Fig. 5). The tumors may expand the bone and are delineated by well-defined sclerotic margins. Some tumors have more poorly defined margins which can cause confusion with more aggressive tumors [8, 27, 28].
Fig. 5.
Axial CT of mandible showing large central osteoblastoma destroying the bone and extending into the soft tissues. The periphery of the tumor is surrounded by a thin layer of reactive bone
Grossly, most osteoblastomas measure between 2 and 5 cm and are solid, dark red, due to its rich vascularity, tan-white and gritty [8, 10, 27, 28, 39]. Cystic changes (aneurysmal bone cyst-like changes) are seen in approximately 10% of tumors. Microscopically, they are composed of haphazardly arranged trabeculae of neoplastic woven bone that are rimmed prominently by osteoblasts with scattered osteoclasts. The neoplastic osteoblasts are oval to round, have eosinophilic cytoplasm and eccentrically located uniform dark staining nuclei (Fig. 6). In some cases, the bone is deposited in sheets and in 5–10% of cases a cartilaginous matrix may be present [3, 8, 27]. The intertrabecular space is filled with richly vascular loose connective tissue that often contains foci of extravasated red blood cells. Mitoses are inconspicuous, and necrosis is usually not present unless there has been a prior pathologic fracture. In approximately 10–15% of tumors epithelioid osteoblasts may comprise the majority of the neoplastic cells [8, 10, 27]. These large cells contain abundant eosinophilic cytoplasm and nuclei with vesicular chromatin and prominent nucleoli. A paranuclear region of pallor is frequently present which corresponds ultrastructurally to a well-developed Golgi apparatus. Although osteoblastomas produce bone remodeling and expansion of the host bone, they do not demonstrate permeative growth. This latter feature is crucial in the distinction of these tumors from osteosarcoma. The pre-existing cortical and cancellous bone surrounding the neoplasm frequently demonstrates reactive changes including paratrabecular fibrosis, osteoclast activity and woven bone formation; however, permeative growth with entrapment of existing cancellous and/or cortical bone by tumor cells and matrix does not occur. The term “pseudomalignant osteoblastoma” has been applied to tumors that architecturally resemble conventional osteoblastoma but some of the neoplastic cells exhibit marked cytological atypia [6, 29]. The atypia is in the form of nuclear enlargement and intense hyperchromasia. It is not accompanied by increased mitotic activity or abnormal matrix production. The nuclear atypia is considered to be degenerative in nature. This finding is of no clinical significance except that it may be confused with osteosarcoma.
Fig. 6.
Large osteoblasts rimming neoplastic bone in osteoblastoma
The treatment of osteoblastoma is principally surgical excision. En bloc resection is usually curative; however, curettage results in a local recurrence rate of approximately 20%. Malignant transformation of osteoblastoma to osteosarcoma is exceptionally rare. In a review of 306 osteoblastomas from the Mayo Clinic, malignant degeneration occurred in only 2 cases [27].
The differential diagnosis of osteoblastoma includes osteoid osteoma, osteosarcoma and fibro-osseous lesions of the cranio-facial skeleton. Osteosarcomas demonstrate greater cytologic atypia, atypical mitoses and a permeative growth pattern. The fibro-osseous lesions demonstrate uniform collagenous stroma that lacks the conspicuous dilated capillaries and hemorrhage typical of osteoblastoma and although focal osteoblastic rimming may be seen in these lesions (especially in ossifying fibroma) it is rarely as well developed as in osteoblastoma. Clinical and radiographic findings also aid in distinguishing between these entities.
Osteosarcoma
Osteosarcoma (OSA) is the most common primary malignant tumor of bone and is defined as a primary mesenchymal malignancy in which the neoplastic cells synthesize and secrete the organic components of bone matrix, which may or may not be mineralized. It classically involves the long bones of the appendicular skeleton. In the craniofacial area osteosarcoma most frequently affects the jaw bones, with approximately 6% of OSAs, or 0.07 cases per 100,000 population per year, arising in the mandible or maxilla [14].
Although gnathic osteosarcomas can affect all age groups, they tend to occur approximately a decade later than osteosarcomas of the long bones, with the majority of patients being older than 30 years of age [2, 14]. Males are affected more frequently than females [2, 7]. Some studies have shown a higher incidence of OSA in the mandible than in the maxilla [2, 14] whereas, other studies have shown the reverse [44]. The most common sites of involvement are the body of the mandible and the alveolar ridge or the antral area of the maxilla. The majority of tumors arise within the medullary cavity of the affected bone with rare examples developing on the bony surfaces [32, 47].
Clinical signs and symptoms depend on the location of the tumor, its size and rate of growth. Patients most frequently present with swelling and pain. Other symptoms include paresthesia, displacement or loosening of teeth, toothache, bleeding and nasal obstruction [7, 14, 19].
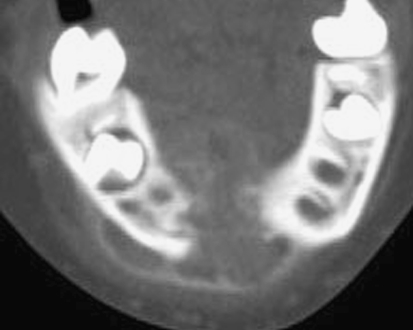
Radiographic studies show a poorly defined destructive lesion that can be sclerotic, lytic or mixed sclerotic and lytic, with or without soft tissue extension (Fig. 7). Early tumors may show a symmetrical widening of the periodontal membrane space about one or more teeth [2, 14].
Fig. 7.
Axial CT of mandible showing poorly defined destructive osteosarcoma of mandible. The neoplasm extends into the soft tissues and has a cloud-like pattern of mineralization
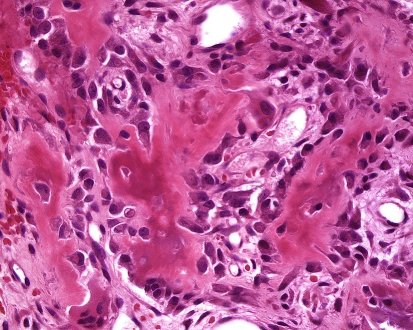
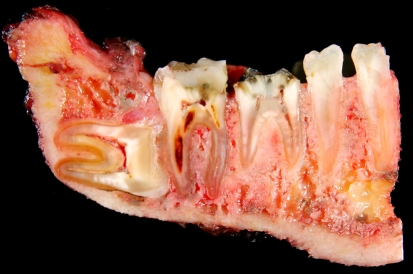
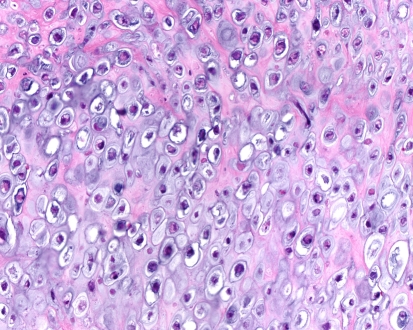
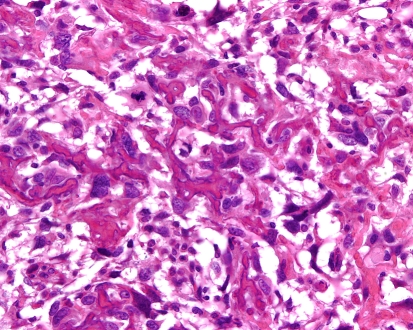
Grossly the tumors are gritty, tan-white and sometimes myxoid. They destroy the underlying bone with or without soft tissue extension (Fig. 8). All subtypes of osteosarcomas can occur in the jaw bones; however, up to half of them show chondroid differentiation (chondroblastic osteosarcoma) [44]. In chondroblastic OSA the tumor has a lobular architecture with central cartilaginous areas that are surrounded by hypercellular regions (Fig. 9), in which spindle or polygonal cells that demonstrate nuclear atypia and mitotic activity deposit neoplastic bone. In many chondroblastic osteosarcomas, bone production may be minimal and in those cases it may be difficult to distinguish between an osteosarcoma and a chondrosarcoma. Conventional osteosarcoma arising in the head and neck region have the histologic appearance seen in other locations, being composed of malignant neoplastic cells and lace like deposition of bone (Fig. 10). On small biopsies it can sometimes be difficult to distinguish osteosarcoma from a fibro-osseous lesion. In those instances the presence of an infiltrative growth pattern can be helpful as it is seen in osteosarcoma, but, not in benign lesions. Serum alkaline phosphatase is often elevated in primary osteosarcomas although high levels generally suggest an osteosarcoma arising in Paget’s disease [19].
Fig. 8.
Resected osteosarcoma of mandible. The tumor replaces portions of the medullary cavity and infiltrates around the roots of several teeth
Fig. 9.
Cartilaginous component of high grade chondroblastic osteosarcoma
Fig. 10.
High grade osteosarcoma with malignant cells encased by coarse lace-like neoplastic bone
The treatment of choice is radical surgery with or without adjuvant chemotherapy or radiation therapy. In our institution all high-grade osteosarcomas, regardless of location are treated with adjuvant chemotherapy. The reported prognosis for osteosarcoma of the jawbones has been variable, and in some studies has been shown to be better than for those arising in the appendicular skeleton [7]. Other studies have not shown biologic advantage for osteosarcomas of the jaw bones [2]. The prognosis seems to be more favorable for mandibular osteosarcomas in comparison to those that arise in the maxilla. The site of origin within these bones also seems to affect prognosis, with maxillary antral tumors having the worst prognosis and tumors of the mandibular symphysis having the best prognosis [14]. This may be related to the length of time the tumor has been present and its resectability. Lastly, in some studies the chondroblastic variant has also been associated with a better prognosis than other histologic types of osteosarcoma [7].
Gnathic osteosarcomas should be separated from osteosarcomas involving extragnathic craniofacial bones, because the latter are generally high grade and clinically extensive and have a much worse prognosis than jawbone osteosarcomas. In the study by Nora et al. of extragnathic craniofacial osteosarcoma only 2 out of 21 patients survived for 5 years and only 1 patient was a long-term survivor [33]. A high percentage of these patients also had a history of a predisposing condition such as Paget’s disease of bone or prior radiation therapy.
Rare examples of gnathic well differentiated osteosarcomas have been reported. In the largest series of 9 cases, 5 involved the maxilla and 4 the mandible. The mean age was 43 (range 20–66) years. Radiographically they were mixed lytic and sclerotic; one tumor was a surface low grade osteosarcoma. The well differentiated osteosarcomas were composed of well developed osseous trabeculae surrounded by sparsely to moderately cellular fibroblastic tumor cells. There was mild nuclear atypia and rare mitotic figures. Chondroblastic differentiation was seen on the surface of 2 tumors. Follow up information (mean 15 months) revealed no local recurrence or metastases [9].
Even rarer is the occurrence of an extraskeletal osteosarcoma arising in the head and neck region [18].
References
- 1.Bartlett JR. Intracranial neurological complications of frontal and ethmoidal osteomas. Br J Surg. 1971;58:607–13. doi: 10.1002/bjs.1800580818. [DOI] [PubMed] [Google Scholar]
- 2.Bertoni F, Dallera P, Bacchini P, et al. The Istituto Rizzoli-Beretta experience with osteosarcoma of the jaw. Cancer. 1991;68:1555–63. doi: 10.1002/1097-0142(19911001)68:7<1555::AID-CNCR2820680717>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 3.Bertoni F, Unni KK, Lucas DR, et al. Osteoblastoma with cartilaginous matrix. An unusual morphologic presentation in 18 cases. Am J Surg Pathol. 1993;17:69–74. doi: 10.1097/00000478-199301000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Boysen M. Osteomas of the paranasal sinuses. J Otolaryngol. 1978;7:366–70. [PubMed] [Google Scholar]
- 5.Byers PD. Solitary benign osteoblastic lesions of bone. Osteoid osteoma and benign osteoblastoma. Cancer. 1968;22:43–57. doi: 10.1002/1097-0142(196807)22:1<43::AID-CNCR2820220108>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 6.Cheung FM, Wu WC, Lam CK, et al. Diagnostic criteria for pseudomalignant osteoblastoma. Histopathology. 1997;31:196–200. doi: 10.1046/j.1365-2559.1997.5870820.x. [DOI] [PubMed] [Google Scholar]
- 7.Clark JL, Unni KK, Dahlin DC, et al. Osteosarcoma of the jaw. Cancer. 1983;51:2311–6. doi: 10.1002/1097-0142(19830615)51:12<2311::AID-CNCR2820511224>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 8.Della Rocca C, Huvos AG. Osteoblastoma: varied histological presentations with a benign clinical course. An analysis of 55 cases. Am J Surg Pathol. 1996;20:841–50. doi: 10.1097/00000478-199607000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Deshpande V, Nielsen GP, Rosenberg AE. Gnathic well differentiated osteosarcomas: A clinicopathologic study of 9 cases [abstract]. Modern Pathol 2007:13A
- 10.Dorfman HD, Weiss SW. Borderline osteoblastic tumors: problems in the differential diagnosis of aggressive osteoblastoma and low-grade osteosarcoma. Semin Diagn Pathol. 1984;1:215–34. [PubMed] [Google Scholar]
- 11.Earwaker J. Paranasal sinus osteomas: a review of 46 cases. Skeletal Radiol. 1993;22:417–23. doi: 10.1007/BF00538443. [DOI] [PubMed] [Google Scholar]
- 12.Fechner RE, Mills SE. Tumors of the bone, joints. Washington D.C: Armed Forces Institute of Pathology; 1993. [Google Scholar]
- 13.Fu YS, Perzin KH. Non-epithelial tumors of the nasal cavity, paranasal sinuses, and nasopharynx. A clinicopathologic study. II. Osseous and fibro-osseous lesions, including osteoma, fibrous dysplasia, ossifying fibroma, osteoblastoma, giant cell tumor, and osteosarcoma. Cancer. 1974;33:1289–305. doi: 10.1002/1097-0142(197405)33:5<1289::AID-CNCR2820330514>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 14.Garrington GE, Scofield HH, Cornyn J, et al. Osteosarcoma of the jaws. Analysis of 56 cases. Cancer. 1967;20:377–91. doi: 10.1002/1097-0142(1967)20:3<377::AID-CNCR2820200306>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 15.Gitelis S, Schajowicz F. Osteoid osteoma and osteoblastoma. Orthop Clin North Am. 1989;20:313–25. [PubMed] [Google Scholar]
- 16.Greenspan A. Benign bone-forming lesions: osteoma, osteoid osteoma, and osteoblastoma. Clinical, imaging, pathologic, and differential considerations. Skeletal Radiol. 1993;22:485–500. doi: 10.1007/BF00209095. [DOI] [PubMed] [Google Scholar]
- 17.Haddad FS, Haddad GF, Zaatari G. Cranial osteomas: their classification and management. Report on a giant osteoma and review of the literature. Surg Neurol. 1997;48:143–7. doi: 10.1016/S0090-3019(96)00485-5. [DOI] [PubMed] [Google Scholar]
- 18.Hatano H, Morita T, Kobayashi H, et al. Extraskeletal osteosarcoma of the jaw. Skeletal Radiol. 2005;34:171–5. doi: 10.1007/s00256-004-0797-3. [DOI] [PubMed] [Google Scholar]
- 19.Huvos AG. Bone tumors, diagnosis, treatment and Prognosis. Philadelphia: W. B. Saunders Company; 1991. pp. 179–200. [Google Scholar]
- 20.Ida M, Kurabayashi T, Takahashi Y, et al. Osteoid osteoma in the mandible. Dentomaxillofac Radiol. 2002;31:385–7. doi: 10.1038/sj.dmfr.4600725. [DOI] [PubMed] [Google Scholar]
- 21.Jackson RP, Reckling FW, Mants FA. Osteoid osteoma and osteoblastoma. Similar histologic lesions with different natural histories. Clin Orthop. 1977;128:303–13. [PubMed] [Google Scholar]
- 22.Kaplan I, Calderon S, Buchner A. Peripheral osteoma of the mandible: a study of 10 new cases and analysis of the literature. J Oral Maxillofac Surg. 1994;52:467–70. doi: 10.1016/0278-2391(94)90342-5. [DOI] [PubMed] [Google Scholar]
- 23.Kirwan EO, Hutton PA, Pozo JL, et al. Osteoid osteoma and benign osteoblastoma of the spine. Clinical presentation and treatment. J Bone Joint Surg [Br] 1984;66:21–6. doi: 10.1302/0301-620X.66B1.6693472. [DOI] [PubMed] [Google Scholar]
- 24.Jones AC, Prihoda TJ, Kacher JE. Osteoblastoma of the maxilla and mandible: a report of 24 cases, review of the literature, and discussion of its relationship to osteoid osteoma of the jaws. Oral Surg Oral Med Oral Pathol Radiol Endod. 2006;102:639–50. doi: 10.1016/j.tripleo.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Lin YC, Commins DL, Fedenko AN, et al. A rare case of periosteal osteoblastoma located in the frontal cranial bone. Arch Pathol Lab Med. 2005;129:787–9. doi: 10.5858/2005-129-787-ARCOPO. [DOI] [PubMed] [Google Scholar]
- 26.Loizaga JM, Calvo M, Lopez Barea F, et al. Osteoblastoma and osteoid osteoma. Clinical and morphological features of 162 cases. Pathol Res Pract. 1993;189:33–41. doi: 10.1016/S0344-0338(11)80114-7. [DOI] [PubMed] [Google Scholar]
- 27.Lucas DR, Unni KK, McLeod RA, et al. Osteoblastoma: clinicopathologic study of 306 cases. Hum Pathol. 1994;25:117–34. doi: 10.1016/0046-8177(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 28.Marsh BW, Bonfiglio M, Brady LP, et al. Benign osteoblastoma: range of manifestations. J Bone Joint Surg [Am] 1975;57:1–9. [PubMed] [Google Scholar]
- 29.Mirra JM, Kendrick RA, Kendrick RE. Pseudomalignant osteoblastoma versus arrested osteosarcoma: a case report. Cancer. 1976;37:2005–14. doi: 10.1002/1097-0142(197604)37:4<2005::AID-CNCR2820370452>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 30.Mirra JM, Picci P, Gold RH. Bone tumors: clinical, radiologic and pathologic correlations. Philadelphia: Lea & Febiger; 1989. [Google Scholar]
- 31.Moon KS, Jung S, Lee JH, et al. Benign osteoblastoma of the occipital bone: case report and literature review. Neuropathology. 2006;26:141–6. doi: 10.1111/j.1440-1789.2006.00643.x. [DOI] [PubMed] [Google Scholar]
- 32.Newland JR, Ayala AG. Parosteal osteosarcoma of the maxilla. Oral Surg Oral Med Oral Pathol. 1977;43:727–34. doi: 10.1016/0030-4220(77)90057-3. [DOI] [PubMed] [Google Scholar]
- 33.Nora FE, Unni KK, Pritchard DJ, Dahlin DC. Osteosarcoma of extragnathic craniofacial bones. Mayo Clin Proc. 1983;58(4):268–72. [PubMed] [Google Scholar]
- 34.O’Connell JX, Nanthakumar SS, Nielsen GP, et al. Osteoid osteoma: the uniquely innervated bone tumor. Mod Pathol. 1998;11:175–80. [PubMed] [Google Scholar]
- 35.Raskas DS, Graziano GP, Herzenberg JE, et al. Osteoid osteoma and osteoblastoma of the spine. J Spinal Disord. 1992;5:204–11. doi: 10.1097/00002517-199206000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Rawal YB, Angiero F, Allen CM, et al. Gnathic osteoblastoma: clinicopathologic review of seven cases with long-term follow-up. Oral Oncol. 2006;42:123–30. doi: 10.1016/j.oraloncology.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 37.Rosenthal DI, Hornicek FJ, Wolfe MW, et al. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment [see comments] J Bone Joint Surg Am. 1998;80:815–21. doi: 10.2106/00004623-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Schajowicz F. Bone-forming tumors. In: Tumors and tumorlike lesions of bone. Pathology, radiology, and treatment. New York: Springer-Verlag; 1994. p. 30–5.
- 39.Schajowicz F, Lemos C. Osteoid osteoma and osteoblastoma. Closely related entities of osteoblastic derivation. Acta Orthop Scand. 1970;41:272–91. doi: 10.3109/17453677008991514. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu N, Sakata K, Yamamoto I. Benign osteoblastoma of the temporal bone: case report and review of the literature. Surg Neurol. 2006;66:534–8. doi: 10.1016/j.surneu.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 41.Smith ME, Calcaterra TC. Frontal sinus osteoma. Ann Otol Rhinol Laryngol. 1989;98:896–900. doi: 10.1177/000348948909801111. [DOI] [PubMed] [Google Scholar]
- 42.Swanson KS, Guttu RL, Miller ME. Gigantic osteoma of the mandible: report of a case. J Oral Maxillofac Surg. 1992;50:635–8. doi: 10.1016/0278-2391(92)90449-A. [DOI] [PubMed] [Google Scholar]
- 43.Ugur HC, Torun F, Kanpolat Y. Petrous bone osteoblastoma invading the cavernous sinus. J Clin Neurosci. 2005;12:489–92. doi: 10.1016/j.jocn.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 44.Unni K. Dahlin’s bone tumors. General aspects of data on 11,087 cases. 5. Philadelphia: Lippincott-Raaven; 1996. [Google Scholar]
- 45.Whittet HB, Quiney RE. Middle turbinate osteoma; an unusual cause of nasal obstruction. J Laryngol Otol. 1988;102:359–61. doi: 10.1017/s0022215100104967. [DOI] [PubMed] [Google Scholar]
- 46.Wilkes SR, Trautmann JC, DeSanto LW, et al. Osteoma: an unusual cause of amaurosis fugax. Mayo Clin Proc. 1979;54:258–60. [PubMed] [Google Scholar]
- 47.Zarbo RJ, Regezi JA, Baker SR. Periosteal osteogenic sarcoma of the mandible. Oral Surg Oral Med Oral Pathol. 1984;57:643–7. doi: 10.1016/0030-4220(84)90287-1. [DOI] [PubMed] [Google Scholar]