Introduction
Adenocarcinomas of various types comprise 10% to 20% of all primary malignant neoplasms of the nasal cavity and paranasal sinuses [1].
Salivary Gland-Type Adenocarcinomas
Adenocarcinomas of salivary-gland type are uncommon constituting 5–10% of sinonasal adenocarcinomas. They are thought to originate from seromucus glands of the nasal cavity and paranasal sinuses as well as the surface epithelium. Histologically, these carcinomas are similar to those originating from major and minor salivary glands [2]. The most common type is adenoid cystic carcinoma (Fig. 1) usually occurring in the maxillary sinus and nasal cavity [3]. Long-term prognosis is poor and patients usually die due to local spread with no metastasis. Adenocarcinoma Not Otherwise Specified may exhibit many high-grade, poorly differentiated growth patterns, and should be considered as a diagnostic entity for many high-grade salivary gland-type adenocarcinomas of the sinonasal tract. These tumors have a poor prognosis [1, 4].
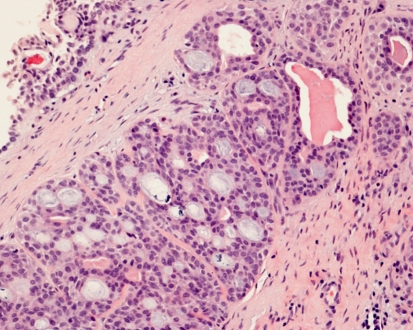
Fig. 1.
Adenoid cystic carcinoma of nasal cavity. Classic cribriform and cystic pattern with bland nuclear appearance. Nasal surface epithelium is at the top left corner. H-E-stain x250
Other types of salivary adenocarcinomas are rare in the sinonasal tract but cases of acinic cell carcinoma, mucoepidermoid carcinoma (Fig. 2) [5], epithelial-myoepithelial carcinoma, basal cell adenocarcinoma, salivary duct carcinoma, polymorphous low-grade adenocarcinoma (terminal tubulus adenocarcinoma) [6], and carcinoma-ex-pleomorphic adenoma have been reported. The possibility of specific types of salivary gland-type adenocarcinomas should be taken into account when considering the diagnosis of a sinonasal adenocarcinoma. Table 1 lists the occurrence of different types of salivary gland-type tumors in the sinonasal tract.
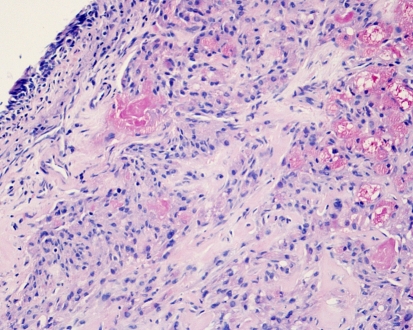
Fig. 2.
Low-grade mucoepidermoid carcinoma of nasal cavity. Islands of intermediate cells contain mucous cysts and few epidermoid cells. Nasal surface epithelium at left. H-E stain x250
Table 1.
Occurrence of sinonasal tumors of salivary gland type (modified from reference #5)
| Tumor type | Percentage (%) |
|---|---|
| High-grade adenocarcinoma not otherwise specified | 30 |
| Adenoid cystic carcinoma | 17 |
| Mucoepidermoid carcinoma | 5 |
| Low-grade salivary-type adenocarcinoma (various types including mucoepidermoid carcinoma) | 21 |
| Pleomorphic adenoma | 23 |
Non-Salivary Gland-Type Adenocarcinomas
The WHO classification of non-salivary gland-type adenocarcinomas [7] follows a classical distinction into intestinal-type adenocarcinoma and non-intestinal-type adenocarcinoma. WHO further subdivides the latter into low-grade and high-grade carcinoma [7]. Another approach first pools the various types of non-salivary gland-type sinonasal adenocarcinomas together and distinguishes them into six histological groups: low-grade, papillary, colonic, solid, mucinous, and mixed types [8]. In this presentation, the WHO classification of 2005 is used.
Intestinal-Type Sinonasal Adenocarcinoma
General
After adenoid cystic carcinoma, intestinal-type sinonasal adenocarcinoma is the second most common type of adenocarcinoma of the sinonasal tract. These carcinomas mimic the appearance intestinal adenomas and carcinomas, or exceptionally the normal glandular epithelium of the intestinal mucosa. The most commonly involved site is the ethmoid sinus (40%), followed by the nasal cavity (25%) and the maxillary antrum (20%). In advanced cases, local spread often occurs to the orbit, the pterygopalatine and infratemporal fossae, and into the cranial cavity.
The presenting symptoms include nasal obstruction and epistaxis. Most patients are male (male-female ratio is around 4:1) and there is a wide age range at presentation.
Nasal cavity adenocarcinoma of a non-salivary type was first recognized by Citelli and Calamida in 1903. Cell types and growth patterns of these tumors were characterized later by Masson and Martin in 1928, Ringertz in 1938, and Jarvi in 1944.
Major interest for the intestinal-type sinonasal adenocarcinoma was first stimulated by the discovery of its link to long-term exposure to hardwood dusts in British woodworkers [9]. Since then, the association of occupational exposure to hardwood (beech, oak) and leather dusts and intestinal-type sinonasal adenocarcinomas has been firmly established [10–12]. Also, occupational exposure to softwood dusts, textile dusts, paints, varnishes, lacquers, glues, and adhesives has been incriminated [13, 14]. Reportedly, exposure to softwood dusts (pine, fir) could also associate with sinonasal squamous cell carcinoma [15]. In woodworking industry the incidence of sinonasal carcinoma may be 1,000 times that of the population in general [16]. Approximately one in five cases of sinonasal intestinal-type adenocarcinoma has been traced to wood-dust exposure in woodworking industry. An average exposure time of 40–43 years has been reported [1]. Cases related to industrial wood-dust exposure show a strong predilection for the ethmoid sinus, while sporadic tumor cases are often seen in the maxillary antrum.
Histopathology
Histopathology of these adenocarcinomas recapitulates the range of appearances of neoplastic and normal large and small intestinal mucosa [17]. Classification of these tumors according to Barnes includes five patterns: colonic, papillary, solid, mucinous and mixed [1]. Alternatively, the classification of Kleinsasser and Schroeder comprises four types: papillary tubular cylinder cell of various grades, alveolar goblet type, signet-ring type, and transitional type [13].
Reportedly, the most common forms of intestinal-type sinonasal adenocarcinoma resemble colonic adenocarcinoma. In the colonic type, a predominantly tubulo-glandular architecture with some papillary elements is seen. The glandular neoplastic cells of these tumors have palisaded hyperchromatic nuclei and a few goblet cells. In the papillary type, growth pattern is predominantly papillary with tubular elements (Fig. 3). In the solid type, tumor is poorly differentiated and the glandular architecture is gradually lost and replaced by a trabecular and solid proliferation. In analogy to colonic adenocarcinomas, some tumors exhibit predominantly mucinous features. In such cases, neoplastic glands tend to be distended with mucin, or clusters of tumor cells are found within pools of mucin that extend into connective tissue stroma (Fig. 4). Often, small numbers of signet-ring cells can be identified in these tumors. The mixed type is composed of two or more of the previous patterns in variable mixtures. In all histologic types, specialized cell types such as goblet cells, resorptive cells, Paneth cells, and argentaffin cells may be encountered.
Fig. 3.
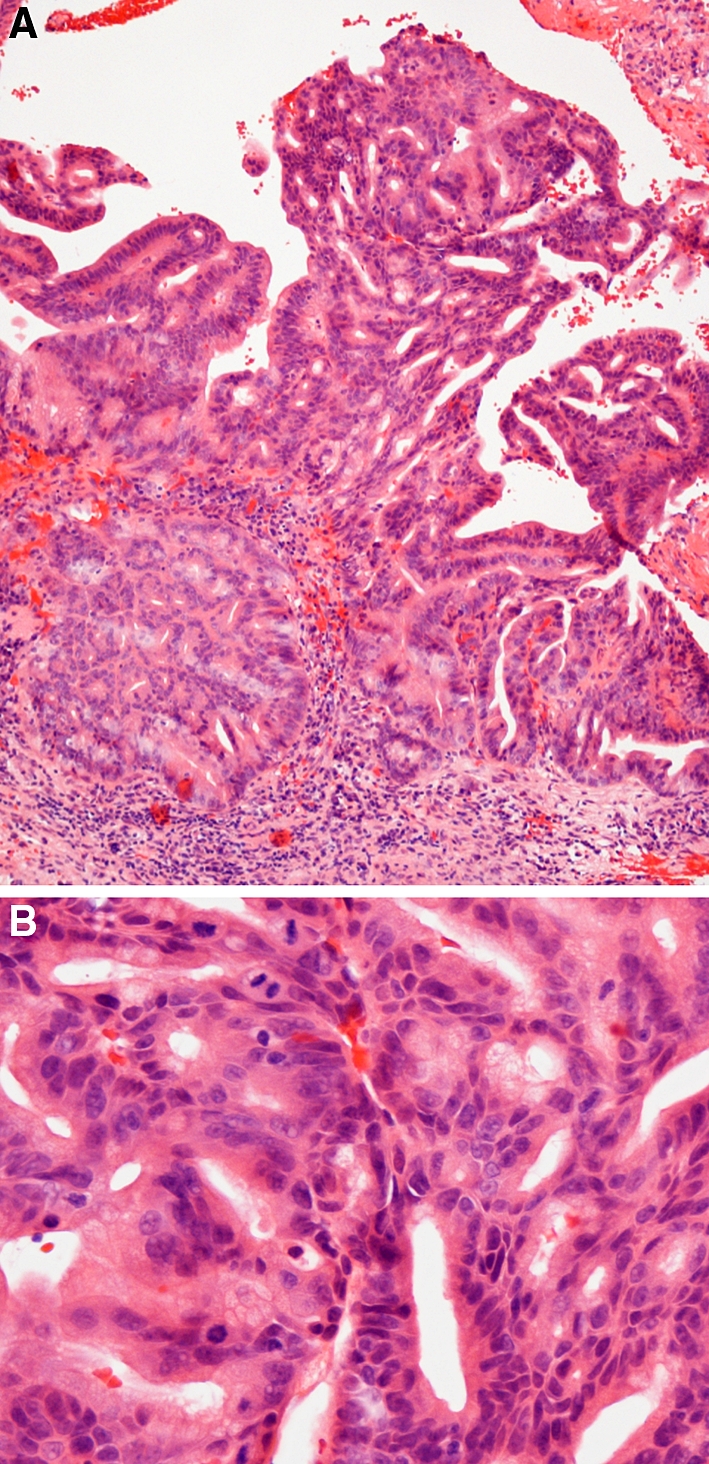
(A) Intestinal-type adenocarcinoma, papillary growth pattern. The papillary elements contain tubular and glandular structures of various types. Invasive growth to stroma is seen. H-E-stain x 250. (B) Tumor of Fig. 3A. The nuclei are often hyperchromatic and show some piling. Cells are cylindrical with some goblet-type cells. A number of mitotic figures are seen. H-E-stain x400
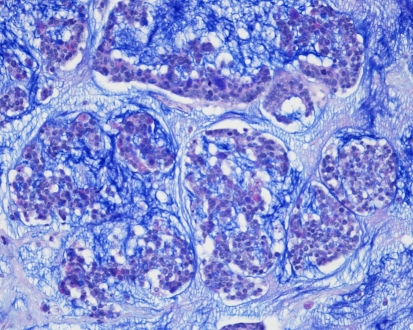
Fig. 4.
Intestinal-type adenocarcinoma, mucinous growth pattern. Alveolar pattern of groups of neoplastic cells containing goblet-type cells, and suspended in a pool of mucin. In another area of this tumor, poorly differentiated papillae of intestinal type containing goblet cells were seen. Alcian-Blue PAS-stain. x250
Exceptional well-differentiated intestinal-type sinonasal adenocarcinomas may closely resemble normal small intestinal mucosa (Fig. 5). Well-formed villi and a muscularis mucosae can be present. These lesions may look deceptively like benign heterotopias, but they are aggressive and invasive in nature [18].
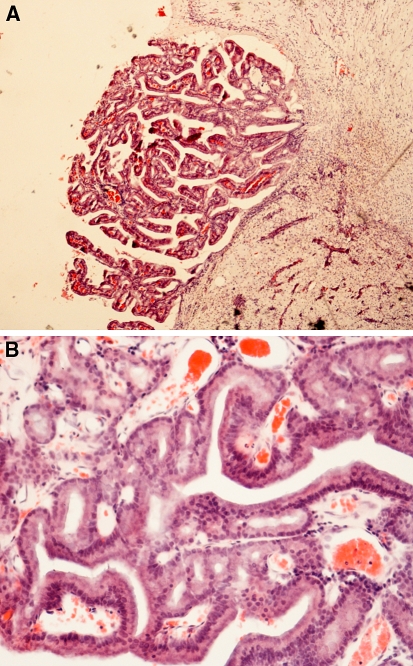
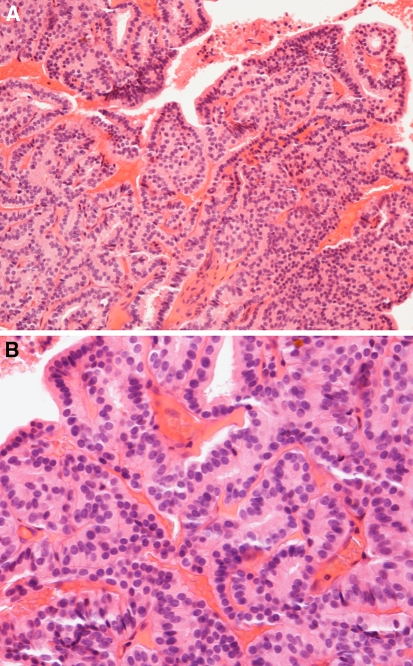
Fig. 5.
(A) Low-grade non-intestinal adenocarcinoma. A complex papillary growth pattern with tubular and glandular structures. A single layer of bland columnar cells line the papillae. H-E stain x160. (B) Another low-grade non-intestinal adenocarcinoma. A single layer of bland columnar cells line the papillae. No nuclear pleomorphism or mitoses are seen in papillae or tubules. H-E stain x400
Differential Diagnosis
Sinonasal adenocarcinomas with an appearance of conventional or mucinous colonic adenocarcinoma should be examined with a second thought of a possible metastatic lesion from the gastrointestinal tract. In accordance to colonic adenocarcinomas, intestinal-type sinonasal adenocarcinomas stain for CK20, CDX-2, MUC2, but in contrast to colonic adenocarcinomas also for CK7 [19, 20]. Intestinal-type sinonasal adenocarcinomas often stain for chromogranin while staining for CEA is variable [21]. However, so far the differentiation of primary sinonasal tumors of the intestinal type from the rare possibility of a metastatic lesion using immunohistochemical markers or morphologic features remains uncertain [19, 22]. Molecular genetic studies of intestinal-type sinonasal adenocarcinomas indicate frequent mutations in K-RAS [23] and TP53 [24, 25]. In cases with occupational dust exposure, intestinal-type sinonasal adenocarcinomas showed a deregulation of p16(INK4a) associated with that of TP53 or p14(ARF) [25]. The gatekeeper events of their early development (p16(INK4a) and TP53) seem to differ from colorectal adenocarcinomas (APC) [24].
When considering the differential diagnostic possibility of a metastatic lesion, one should remember that among metastatic lesions to the sinonasal tract, primary tumors in the gastrointestinal tract are unusual [1, 4].
However, if the microscopic appearance of a sinonasal carcinoma resembles that of a colorectal carcinoma, colonoscopy is a straightforward and recommended approach. The rare intestinal-type sinonasal adenocarcinomas that have an appearance reminiscent of normal intestinal mucosa and those resembling a villous adenoma are always nasal in origin, and should pose no differential diagnostic problems.
The differential diagnosis of intestinal-type sinonasal adenocarcinomas may include low-grade sinonasal adenocarcinoma [20, 22] or sometimes non-neoplastic papillary sinusitis with abundant mucinous material.
Treatment and Prognosis
The treatment of intestinal-type sinonasal adenocarcinoma is adequate surgical removal, and in advances cases this is combined with postoperative radiotherapy. Surgical procedures include lateral rhinotomy combined with partial maxillectomy and ethmoidectomy according to spread of the tumor. In advances cases, radical maxillectomy may be indicated. Chemotherapy seems to be of uncertain benefit.
Intestinal-type sinonasal adenocarcinomas behave as high-grade aggressive malignancies. In reviewed materials [1, 17], half of patients experienced local recurrence, and locoregional and/or distant metastasis was seen in 10–20% of cases. Up to two thirds of patients died of disease, usually due to local spread to vital structures. It was reported that cases related to industrial dust exposure had a 50% survival at 5 years, while sporadic tumors showed a 20–40% 5-year survival [1]. In another material, however, no survival difference between cases with occupational exposure and sporadic cases was found [17].
Most patients have an advanced disease at presentation, and clinical staging is usually not informative of prognosis. However, the histologic subtype has prognostic significance for clinical behaviour [1, 13, 17]. The papillary subtype has been associated with an 80% survival at 5 years, while the mucinous and solid subtypes carry a poor prognosis. H-RAS mutation and certain immunophenotypes may also associate with behaviour [26].
Non-Intestinal-Type Sinonasal Adenocarcinomas
These adenocarcinomas apparently arise from the surface epithelium of the sinonasal tract. They do not share microscopic appearances with salivary gland-type sinonasal adenocarcinomas or intestinal-type sinonasal adenocarcinomas. According to WHO [17] they are divided into low- and high-grade types.
Non-salivary-type Low-grade Adenocarcinoma
General
Low-grade sinonasal adenocarcinomas occur in a wide age range with a mean age of 37–53 years at presentation. Most frequently involved sites are the ethmoid sinus, the nasal cavity, and the maxillary sinuses [4]. Association with occupational or environmental carcinogens has not been reported in these tumors.
Histopathology
Non-salivary-type (and non-intestinal-type) sinonasal low-grade adenocarcinomas are a distinctive group of tumors with mostly papillary and glandular appearance [7]. Other patterns include trabecular, cribriform, clear cell [27], and mucinous. In non-intestinal-type sinonasal adenocarcinomas, papillae and small glands are lined by a single layer of uniform cells (Fig. 5), or less frequently by a double layer containing also basal/myoepithelial cells. Neoplastic glands may have a back-to-back arrangement without intervening stroma. The glands may also contain well-formed papillae, be cystically dilated, and contain mucin. Nuclei tend to be round and uniform in size with inconspicuous nucleoli. Mitotic figures are rare, and no atypical mitoses and necrosis are seen. The appearance of these neoplasms is bland but the complexity of their pattern and local invasive growth indicate malignancy. A tubulopapillary variant (Fig. 6) that may simulate terminal tubulus adenocarcinoma of the nasal seromucus glands, has been discussed recently [28–30].
Fig. 6.
(A) Tubulopapillary low-grade (non-intestinal) adenocarcinoma. A papillary growth pattern with tubular and trabecular formations in the stroma. A single layer of cuboidal to columnar cells with round uniform nuclei, indistinct nucleoli and eosinophilic cytoplasm. (With permission from (26)). H-E-stain x250. (B) Tumor of Fig. 6A. A papillary growth pattern with tubular and trabecular elements in the stroma. A single layer of cuboidal to columnar cells with round uniform nuclei, indistinct nucleoli and eosinophilic cytoplasm. (With permission from (26)). H-E-stain x400
Tumors with similar appearances termed nasopharyngeal papillary adenocarcinomas may originate in the nasopharynx and may extend into the nasal cavity [31]. In these tumors, the papillae are arborized and have fibrovascular cores. The epithelial cells are columnar or pseudostratified. Moderate nuclear pleomorphism may be encountered but mitoses are infrequent [31].
Differential Diagnosis
The most important differential diagnosis of low-grade sinonasal adenocarcinoma is intestinal-type sinonasal adenocarcinoma. Low-grade sinonasal adenocarcinomas have been reported to lack intestine-specific immunophenotypic markers such as CDX-2, MUC-2, and CK20 [22]. In intestinal-type adenocarcinomas, the intestinal appearance and a distinct intestine-specific immunophenotype [22] facilitate distinction. Intestinal-type adenocarcinomas also show more cytologic pleomorphism, except in rare cases where the nasal neoplasm closely resembles normal intestinal mucosa. Low-grade sinonasal adenocarcinomas may sometimes be difficult to differentiate from oncocytic Schneiderian papillomas or metastatic papillary carcinoma of the thyroid [4].
Treatment and Prognosis
The treatment of low-grade sinonasal adenocarcinoma is complete surgical excision. These tumors are mostly localized at presentation, and the required surgery usually is not extensive. In cases of extensive disease or higher-grade lesions, radiotherapy may be utilized. Local recurrences have been reported in some cases, and death of disease has occurred due to local invasion [4]. However, low-grade sinonasal adenocarcinoma has a favourable overall prognosis.
References
- 1.Barnes L. Intestinal-type adenocarcinoma of the nasal cavity and paranasal sinuses. Am J Surg Pathol. 1986;10:192–202. doi: 10.1097/00000478-198603000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Eveson J. Salivary gland-type carcinomas. WHO Histological classification of tumors of the nasal cavity and paranasal sinuses. In: Barnes L, Eveson JW, Reichardt P, Sidransky D, editors. Pathology & genetics, head and neck tumors. Lyon: IARC Press; 2005. p. 24–5.
- 3.Wiseman SM, Popat SR, Rigual NR, et al. Adenoid cystic carcinoma of the paranasal sinuses or nasal cavity: a 40-year review of 35 cases. Ear Nose Throat J. 2002;81:510–7. [PubMed] [Google Scholar]
- 4.Heffner DK, Hyams VJ, Hauck KW, et al. Low-grade adenocarcinoma arising in nasal cavity and paranasal sinuses. Cancer. 1982;50:312–22. doi: 10.1002/1097-0142(19820715)50:2<312::AID-CNCR2820500225>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 5.Thomas GR, Regalado JJ, McClinton M. A rare case of mucoepidermoid carcinoma of the nasal cavity. Ear Nose Throat J. 2002;81:519–22. [PubMed] [Google Scholar]
- 6.Kleinsasser O. Terminal tubulus adenocarcinoma of the nasal seromucous glands: a specific entity. Arch Otorhinolaryngol. 1985;19:365–70. doi: 10.1007/BF00454353. [DOI] [PubMed] [Google Scholar]
- 7.Franchi A, Santucci M, Wenig BM. Adenocarcinoma. WHO Histological classification of tumors of the nasal cavity and paranasal sinuses. In: Barnes L, Eveson JW, Reichardt P, Sidransky D, editors. Pathology and genetics, head and neck tumors. Lyon: IARC Press; 2005. p. 20–3.
- 8.Zarbo RJ, Torres FX, Gomez J. Nasal cavity and paranasal sinuses. In: Pilch BZ, editors. Head and neck surgical pathology. Philadelphia: Lippincott Williams Wilkins; 2001. p. 80–156.
- 9.Acheson E, Hadfield E, Macbeth R. Carcinoma of the nasal cavity and accessory sinuses in woodworkers. Lancet 1967;311–2. [DOI] [PubMed]
- 10.Imbus HR, Dyson WL. A review of nasal cancer in furniture manufacturing and woodworking in North Carolina, the United States, and other countries. J Occup Med. 1987;29:734–40. [PubMed] [Google Scholar]
- 11.Ironside P, Matthews J. Adenocarcinoma of the nose and paranasal sinuses in woodworkers in the state of Victoria, Australia. Cancer. 1975;36:1115–21. doi: 10.1002/1097-0142(197509)36:3<1115::AID-CNCR2820360342>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 12.Leclerc A, Luce D, Demers PA, et al. Sinonasal cancer and occupation. Results from the reanalysis of twelve case-control studies. Am J Ind Med. 1997;31:153–65. doi: 10.1002/(SICI)1097-0274(199702)31:2<153::AID-AJIM4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Kleinsasser O, Schroeder HG. Adenocarcinomas of the inner nose after exposure to wood dust. Morphological findings and relationships between histopathology and clinical behavior in 79 cases. Arch Otorhinolaryngol. 1988;245:1–15. doi: 10.1007/BF00463541. [DOI] [PubMed] [Google Scholar]
- 14.Luce D, Gerin M, Leclerc A, et al. Sinonasal cancer and occupational exposure to formaldehyde and other substances. Int J Cancer. 1993;53:224–31. doi: 10.1002/ijc.2910530209. [DOI] [PubMed] [Google Scholar]
- 15.Jansing PJ, Chanda R, Gore C, et al. Profiles of occupational exposure in patients with wood dust-induced nasal carcinoma. Int J Occup Med Environ Health. 2003;16:329–35. [PubMed] [Google Scholar]
- 16.Klintenberg C, Olofsson J, Hellquist H, et al. Adenocarcinoma of the ethmoid sinuses: a review of 28 cases with special reference to wood dust exposure. Cancer. 1984;54:482–8. doi: 10.1002/1097-0142(19840801)54:3<482::AID-CNCR2820540317>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 17.Franchi A, Gallo O, Santucci M. Clinical relevance of the histological classification of sinonasal intestinal-type adenocarcinomas. Hum Pathol. 1999;30:1140–5. doi: 10.1016/S0046-8177(99)90029-1. [DOI] [PubMed] [Google Scholar]
- 18.Mills SE, Fechner RE, Cantrell RW. Aggressive sinonasal lesion resembling normal intestinal mucosa. Am J Surg Pathol. 1982;6:803–9. doi: 10.1097/00000478-198212000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Franchi A, Massi D, Palomba A, et al. CDX-2, cytokeratin 7 and cytokeratin 20 immunohistochemical expression in the differential diagnosis of primary adenocarcinomas of the sinonasal tract. Virchows Arch. 2004;445:63–7. doi: 10.1007/s00428-004-1030-4. [DOI] [PubMed] [Google Scholar]
- 20.Resto VA, Krane JF, Faquin WC, et al. Immunohistochemical distinction of intestinal-type sinonasal adenocarcinoma from metastatic adenocarcinoma of intestinal origin. Ann Otol Rhinol Laryngol. 2006;115:59–64. doi: 10.1177/000348940611500109. [DOI] [PubMed] [Google Scholar]
- 21.Abecasis J, Viana G, Pissarra C, et al. Adenocarcinomas of the nasal cavity and paranasal sinuses: a clinicopathological and immunohistochemical study of 14 cases. Histopathology. 2004;45:254–9. doi: 10.1111/j.1365-2559.2004.01949.x. [DOI] [PubMed] [Google Scholar]
- 22.Cathro HP, Mills SE. Immunophenotypic differences between intestinal-type and low-grade papillary sinonasal adenocarcinomas. Am J Surg Pathol. 2004;28:1026–32. doi: 10.1097/01.pas.0000126856.09058.71. [DOI] [PubMed] [Google Scholar]
- 23.Saber AT, Nielsen LR, Dictor M, et al. K-ras mutations in sinonasal adenocarcinomas in patients occupationally exposed to wood or leather dust. Cancer Lett. 1998;126:59–65. doi: 10.1016/S0304-3835(97)00536-3. [DOI] [PubMed] [Google Scholar]
- 24.Frattini M, Perrone F, Suardi S, et al. Phenotype-genotype correlation: Challenge of intestinal-type adenocarcinoma of the nasal cavity and paranasal sinuses. Head Neck. 2006;28:909–15. doi: 10.1002/hed.20433. [DOI] [PubMed] [Google Scholar]
- 25.Perrone F, Oggionni M, Birindelli S, et al. TP53, p14ARF, p16INK4a, and H-ras gene molecular analysis in intestinal-type adenocarcinoma of the nasal cavity and paranasal sinuses. Int J Cancer. 2003;105:196–203. doi: 10.1002/ijc.11062. [DOI] [PubMed] [Google Scholar]
- 26.Perez P, Dominguez O, Gonzales S, et al. Ras gene mutations in ethmoid sinus adenocarcinoma: prognostic implications. Cancer. 1999;86:255–64. doi: 10.1002/(SICI)1097-0142(19990715)86:2<255::AID-CNCR9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 27.Zur KB, Brandwein M, Wang B, et al. Primary description of a new entity; renal cell-like carcinoma of the nasal cavity. Van Meegeren in the house of Vermeer. Arch Otolaryngol Head Neck Surg. 2002;128:441–7. doi: 10.1001/archotol.128.4.441. [DOI] [PubMed] [Google Scholar]
- 28.Franchi A, Palomba A, Massi D, Biancalani M, Sardi I, Gallo O, Santucci M. Low-grade salivary type tubulo-papillary adenocarcinoma of the sinonasal tract. Histopathology. 2006;48:869–86. doi: 10.1111/j.1365-2559.2006.02435.x. [DOI] [PubMed] [Google Scholar]
- 29.Luna MA. Sinonasal tubulopapillary low-grade adenocarcinoma: a specific diagnosis or just another seromucous adenocarcinoma ? Adv Anat Pathol. 2005;12:109–15. doi: 10.1097/01.pap.0000163961.18730.ba. [DOI] [PubMed] [Google Scholar]
- 30.Skalova A, Cardesa A, Leivo I, et al. Sinonasal tubulopapillary low grade adenocarcinoma. Histopathological, immunohistochemical and ultrastructural features of a poorly recognized entity. Virchows Arch. 2003;443:152–8. doi: 10.1007/s00428-003-0844-9. [DOI] [PubMed] [Google Scholar]
- 31.Wenig BM, Hyams VJ, Heffner DK. Nasopharyngeal adenocarcinoma: a clinicopathologic study of a low-grade carcinoma. Am J Surg Pathol. 1988;12:946–53. [PubMed] [Google Scholar]