Introduction
Only rarely do cartilage tumors involve the craniofacial bones. Hence, there is an increased risk of misdiagnosis since pathologists don’t expect these tumor types in bones of the head and neck. The following general overview includes two benign cartilaginous tumors, chondromyxoid fibroma and chondroblastoma, and two malignant cartilage tumors, conventional chondrosarcoma and mesenchymal chondrosarcoma, that occasionally occur in the craniofacial bones.
Chondroblastoma
General
Chondroblastoma of bone is an uncommon tumor, representing 1–2% of all bone tumors and almost 5% of benign bone tumors. It typically occurs at the ends of the long bones in skeletally immature persons. Chondroblastoma arising in the craniofacial bones is very rare, representing only 6.4% of all chondroblastomas.
Clinical Features
Patients range in age from 2 to 83 years, but most are in the second decade of life. There is a definite male predominance. Patients with chondroblastoma of the skull tend to be older than those with chondroblastoma of the long bones; the mean age of those with skull lesions is 44 years.
Approximately 60% of all chondroblastomas involve the long bones. The femur, usually the distal epiphysis, is the most common site. The proximal humerus and proximal tibia are also commonly involved. There are only a few reports of chondroblastoma arising in the craniofacial bones. Seventy percent of skull chondroblastomas involve the temporal bone. There are approximately 60 cases in the literature of chondroblastoma involving the temporal bone. The next most common site is the mandible. A few isolated cases in the parietal bone and “skull” have also been reported.
Patients with chondroblastomas generally present with localized pain. When the tumor is located in the temporal bone, the most frequent complaints include pain, swelling, a plugged sensation in the ear, and hearing loss.
Radiographic Features
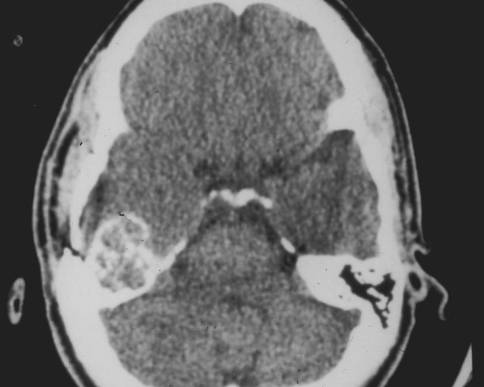
Radiographically, chondroblastomas in the extremities tend to appear as a round or oval lucency with a sclerotic rim. Calcification is rarely seen on plain radiographs but may be present on CT. Overall, they tend to have a benign radiographic appearance (Fig. 1).
Fig. 1.
CT scan of a chondroblastoma involving the temporal bone. The tumor forms a mineralized, expansile mass. There is no cortical destruction or soft tissue mass. The lesion has a benign radiographic appearance
Chondroblastomas involving the temporal bone are usually lucent, expansile lesions with sharp margins. The features are not suggestive of a specific diagnosis, but the overall appearance is that of a benign, aggressive process (Fig. 2).
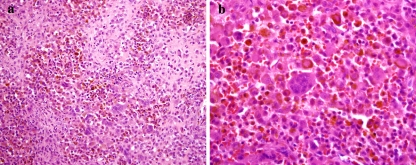
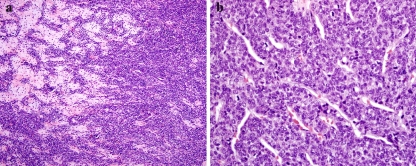
Fig. 2.
(a) Chondroblastoma of the temporal bone. The tumor contains pink nodules of chondroid differentiation surrounded by multinucleated giant cells and epithelioid tumor cells with brown pigment. (b) Higher magnification showing epithelioid cells with abundant eosinophilic cytoplasm containing brown hemosiderin
Histologic Features
Chondroblastomas contain a combination of mononuclear cells and chondroid matrix. The mononuclear cells have well-defined cytoplasmic boundaries and an oval-to-elongated nucleus that oftentimes has a characteristic longitudinal groove. Mitotic figures are usually present, but not numerous. Multinucleated giant cells are a common feature. Chondroid matrix can be focal or abundant, and oftentimes stains pink rather than blue. About 35% of chondroblastomas containing calcification, which oftentimes occurs in a lace-like pattern. Secondary aneurysmal bone cyst formation is present in up to one-third of tumors.
Sometimes the mononuclear cells have an epithelioid appearance with vesicular nuclei and abundant pink cytoplasm. The cells are arranged in nests or ribbons and oftentimes contain brown granular pigment. These features are more commonly seen in chondroblastomas of the craniofacial bones. Chondroid matrix is generally present, but it may be subtle or absent in tumors with a prominent epithelioid component. A few additional findings described in chondroblastomas of the craniofacial bones include aneurysmal bone-cyst-like areas, necrosis, and permeation of tumor beyond the peripheral reactive rim of bone.
Immunohistochemical Features
The mononuclear cells usually show some immunoreactivity with S-100 protein. While the tumor cells may also be positive with cytokeratin and EMA, the staining pattern is not strong and diffuse.
Histologic Differential Diagnosis
The differential diagnosis includes giant cell tumor, chondromyxoid fibroma, and osteosarcoma. In the craniofacial bones tumors containing epithelioid cells with brown pigment, melanoma and giant cell reparative granuloma are also considerations.
The mononuclear cells in giant cell tumor lack the nuclear groove commonly seen in chondroblastoma and are also a bit smaller. Giant cell tumor also does not contain the chondroid matrix characteristic of chondroblastoma. Giant cell tumors of the jawbones and temporal bone are even more uncommon than chondroblastoma at these sites. Giant cell reparative granuloma occasionally occurs at the base of the skull, but not as a circumscribed lesion in the temporal bone. In addition, they do not contain chondroid matrix or a nodular arrangement of the mononuclear cells.
Chondromyxoid fibroma and chondroblastoma can show overlapping features. However, the epithelioid cells so commonly found in chondroblastomas of the temporal bones are not a feature of chondromyxoid fibroma.
Chondroblastomas with epithelioid cells and brown pigment can simulate metastatic melanoma. Both tumors will stain positive with S-100 protein. Cytologic atypia and prominent nucleoli are features of melanoma that are not seen in chondroblastoma.
Treatment
Curettage is the treatment of choice. Depending on the location and extent of the lesion, a wider margin may be necessary in order to remove the entire tumor. Reported recurrence rates vary from 6 to 15%.
Chondromyxoid Fibroma
General
Chondromyxoid fibroma is a rare benign cartilaginous tumor of bone that accounts for only 1.8% of all benign neoplasms of bone. It was first described by Lichtenstein and Jaffe in 1948. Because of its rarity and heterogeneous histologic appearance, this tumor causes diagnostic difficulty for pathologists (Fig. 3).
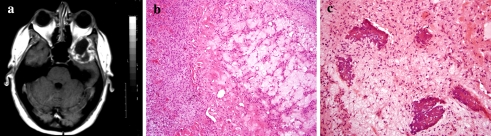
Fig. 3.
Chondromyxoid fibroma of the temporal bone. (a) MRI scan showing an expansile lesion in the temporal bone that is compressing the temporal lobe. (b) Histologically, the tumor contains hypocellular microlobules surrounded by a moderately cellular proliferation of spindle-shaped or stellate cells. (c) Focal plaque-like calcification in myxoid stroma
Clinical Features
Chondromyxoid fibroma has a predilection for patients in the second and third decades of life. The age range is quite broad, but up to 75% of patients are younger than 40 years of age. There seems to be a slight male predominance.
The long bones are the sites most frequently affected, particularly the tibia. Craniofacial localization represents 2–5% of cases. Most of the reported craniofacial cases have been located in the mandible and intracranial bones, particularly the base of skull and cranial vault. There are approximately 35 intracranial and 25 jaw bone chondromyxoid fibromas in the literature. A Mayo review of 278 cases of chondromyxoid fibroma included 15 tumors that involved the skull and facial bones. Of these, three lesions each (20%) were in the frontal bones, the sphenoid, and mandible. Two tumors (13.3) involved the occipital bone and one each (6.7%) the zygoma, the maxilla, the ethmoid, and the calvarium.
Patients with a chondromyxoid fibroma at any anatomic site usually present complaining of a painful swelling or enlargement. Occasionally the tumor is an incidental finding. The duration of symptoms ranges from weeks to years.
Radiographic Features
The radiographic findings of chondromyxoid fibroma almost always suggest a benign lesion. They typically have a lobulated outline with sharp margins, and the majority has a sclerotic rim. The cortex of the bone is usually thinned and expanded. In approximately 50% of cases, a portion of the cortex may be absent. Up to one-third of cases show radiographic evidence of soft tissue extension. The majority of tumors have purely lucent matrix. However, approximately 13% of tumors show some degree of slight to moderate mineralization. Radiographic features of gnathic lesions are variable, but nevertheless compatible with those of lesions in other locations.
Histologic Features
The histologic features of chondromyxoid fibroma involving the craniofacial bones does not differ from those seen at other anatomic sites. The classic histology is the presence of stellate or spindle-shaped cells in a myxoid background, arranged in lobules. A lobulated growth pattern is seen in approximately 85% of tumors. The lobules take on a macrolobulated or microlobulated pattern that can be appreciated at low magnification. They show a hypocellular center with condensation and hypercellularity towards the periphery. In the microlobular pattern, the lobulation is much less distinct.
The tumor cells within the lobules are either spindle- or stellate-shaped. They contain pink cytoplasm and are embedded in a slightly blue-staining matrix with variable amounts of myxoid change. The interlobular tissue is cellular and composed of oval or spindle-shaped cells. Multinucleated giant cells are present between the lobules in approximately 50% of tumors. Calcification is present in one-third of tumors. It is either granular or, more commonly, in the form of chunks and more common in lesions involving the skull, facial bones and ribs. Well-developed hyaline cartilage is focally present in only 19% of tumors. Approximately 18% of tumors show cytologic atypia reminiscent of the type seen in degenerative changes.
Immunohistochemical Features
Immunostains are not particularly helpful in the diagnosis of chondromyxoid fibroma. The chondroid areas tend to show variable staining for S-100 protein. The interlobular tissue typically shows some staining for muscle specific actin and smooth muscle actin.
Differential Diagnosis
The histologic differential includes chondrosarcoma, osteosarcoma, chondroblastoma and occasionally fibrous dysplasia. Chordoma is also in the differential of intracranial tumors.
Chondrosarcomas may have a lobulated growth pattern, myxoid matrix, and spindling of the nuclei. However, chondrosarcomas usually do not contain lobules with a hypocellular center and peripheral hypercellularity as one would expect in chondromyxoid fibroma. They cytologic features also differ. Chondromyxoid fibromas typically contain cells with small, oval- to spindle-shaped nuclei without atypia. The nuclei in chondrosarcomas are usually larger, hyperchromatic, and with some degree of atypia. When spindling is seen in chondrosarcoma, the tumor is of a higher grade and shows more extensive liquefactive change of the myxoid matrix. Most importantly, chondrosarcomas typically have a malignant radiographic appearance whereas chondromyxoid fibromas look benign.
Chondromyxoid fibromas can have features that overlap with chondroblastoma. However, this is more of a problem in the long bones since the epithelioid cells commonly seen in chondroblastomas of the craniofacial bones do not resemble the tumor cells of chondromyxoid fibroma.
The combination of spindled cells, matrix and occasional cytologic atypia seen in chondromyxoid fibroma sometimes brings osteosarcoma into the differential. In general, chondromyxoid fibroma does not show the nuclear anaplasia characteristic of osteosarcoma. The aggressive radiographic features of osteosarcoma also differ from those of chondromyxoid fibroma (Fig. 4).
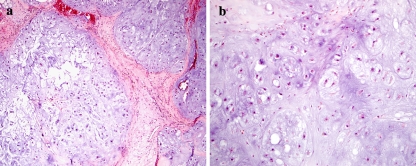
Fig. 4.
Grade 1 chondrosarcoma of the maxilla. (a) The tumor has a lobulated growth pattern with hypercellularity and myxoid change in the matrix. (b) There is slight to moderate cytologic atypia
In chondromyxoid fibromas of the skull base, chordoma is in the differential. Careful analysis of the cytologic features can be helpful in this situation. Chordomas contain epithelioid cells with fairly prominent nucleoli and vacuolated or eosinophilic cytoplasm. The tumor cells are arranged in nests and cords. They are also positive with cytokeratin markers.
Treatment
Chondromyxoid fibroma is treated by curettage or excision. A 12–25% recurrence rate has been reported. With local recurrence, soft tissue implantation may occur. Occasionally patients develop multiple local recurrences. Craniofacial tumors may have a higher rate of local recurrence due to difficulties with total removal of tumor in confined areas and greater risk of cosmetic and functional consequences.
Conventional Chondrosarcoma
Chondrosarcoma constitutes approximately 15% of all primary malignant bone tumors. Chondrosarcoma of the jaw and facial bones is extremely uncommon.
General
Chondrosarcoma arising in the jaw and facial bones accounts for only approximately 4% of all conventional chondrosarcomas in the Mayo series. It is difficult to assess the incidence of conventional chondrosarcoma of the head and neck in the literature since oftentimes it is not clear whether reported numbers include all histologic subtypes or only conventional chondrosarcoma. Nevertheless, the numbers vary from 4% to 15%.
Clinical Features
Chondrosarcoma is a disease of adulthood and old age. More than 60% of patients are in the fourth through sixth decades of life. This contrasts with the relatively young age of patients in larger series of craniofacial chondrosarcomas. Saito reported that 10 of 56 patients were under 17 years and Huvos reported that one-third of their patients were younger than 20 years. However, a series of chondrosarcomas of the head and neck in pediatric patients by Gadwal did not confirm an increased percentage of head and neck chondrosarcomas in pediatric patients.
The most common sites for chondrosarcoma of the head and neck have been variably reported as jawbones, paranasal sinuses, nasal cavity, and the maxilla. Of the 56 patients in the Mayo series, 41.1% were located in the nasal septum, ethmoid, and sphenoid, 25% in the maxillary sinus, 19.6% in the maxilla, 10.7% in the mandible and 3.6% in the tip of the nose.
Presenting symptoms vary depending on the site of origin of the tumor. Pain, swelling, and nasal obstruction are common complaints of patients with chondrosarcoma of the jaw and facial bones.
Radiographic Features
Chondrosarcomas of the jaws and facial bones are similar to lesions in the long bones, exhibiting soft tissue extension with partial calcification or an ill-defined osteolytic mass. Overall, the findings suggest a malignant process. However, since chondrosarcoma is rare at these sites, chondrosarcoma may or may not be on the radiographic differential diagnosis.
Histologic Features
The histologic features of chondrosarcoma involving the jaws and facial bones are the same as those that apply to any other site of origin. The tumors have a lobulated growth pattern, hypercellularity, and cytologic atypia. Occasionally there is spindling of the tumor cells, myxoid degeneration of the matrix, and calcification or ossification.
Histologic Differential
Since chondrosarcoma carries a better prognosis than osteosarcoma, the differential diagnosis between chondrosarcoma and osteosarcoma is probably the most important distinction to consider when dealing with a malignant cartilaginous tumor at this site. It is also one of the most difficult since chondroid differentiation in osteosarcoma of the jaw is more common than in other sites. When diagnosing chondrosarcoma, the tumor should be composed purely of hyaline cartilage. In chondroblastic osteosarcoma, there is spindling of the tumor cells toward the periphery of the lobules and the tumor cells surrounding the lobules are cytologically malignant. Lace-like osteoid is usually present between the spindle cells. Alternatively, bone formation can be seen at the center of the lobules. This differs from metaplastic bone rimming the periphery of tumor nodules that can be seen in chondrosarcoma.
Enchondroma should only be diagnosed if the lesion is a small incidental finding with no radiographic feature of aggressive behavior. Any clinically significant cartilaginous mass should be considered malignant.
Chondroid chordoma is also an important consideration in the differential diagnosis. In particular, chondrosarcomas with myxoid change can resemble chondroid chordoma. Careful attention paid to the cytologic features of the tumor cells can be helpful. In general, chondrosarcomas lack the epithelioid nuclei, nucleoli, esoinophilic cytoplasm, and vacuoles typically seen in chordoma. In addition, the tumor cells of chordoma are positive with keratin markers whereas chondrosarcomas are negative.
Treatment and Prognosis
Chondrosarcomas of the jaw and facial bones are most effectively treated by surgical removal. Radiation and chemotherapy are of little to no benefit. As with chondrosarcoma at any other site, a wide surgical margin is associated with a lower incidence of recurrence. However, this can be a challenging task in the more confined spaces surrounding the craniofacial bones. Recurrent tumor at these sites is associated with a poor prognosis. Overall in the Mayo series at 5, 10, and 15 years was 80.7, 65.3, and 56%, respectively. There was no statistically significant difference based on location, size, or histologic grade of the tumor. No distant metastases were found.
Mesenchymal Chondrosarcoma
General
Mesenchymal chondrosarcoma is a rare histologic subtype of chondrosarcoma. There are only 32 examples of mesenchymal chondrosarcoma in the Mayo Clinic files compared with 992 examples of conventional chondrosarcoma. Mesenchymal chondrosarcoma has a predilection for the facial skeleton, particularly the jaw. It has been reported as involving the jaws in 22–27% of cases in mesenchymal chondrosarcomas. In a recent National Cancer Database (NCDB) report on 400 chondrosarcomas of the head and neck, 8.8% were mesenchymal chondrosarcoma.
It is important to recognize mesenchymal chondrosarcoma as a distinct entity because it is associated with a more aggressive clinical course than conventional chondrosarcoma. Mesenchymal chondrosarcoma can occur as a primary intra-osseous or extra-osseous tumor. Approximately one third of tumors occur in soft tissues. These tumors have clinical and histologic features identical to those of the skeletal counterparts (Fig. 5).
Fig. 5.
(a) Mesenchymal chondrosarcoma arising in the maxilla. The tumor has a bimorphic appearance with multiple cartilaginous nodules surrounded by small blue cells. (b) Higher power magnification of a hemangiopericytomatous pattern in the small blue cell component
Clinical Features
Mesenchymal chondrosarcoma tends to affect children and young adults, although the age range can be broad. About half of patients in the Mayo series were in the second and third decades of life. The NCDB report on head and neck chondrosarcoma found that 61.8% of the 35 mesenchymal chondrosarcomas arose in patients younger than 30 years. This report also found mesenchymal chondrosarcomas to be more common in African-American and Hispanic patients.
Any part of the skeleton may be affected by mesenchymal chondrosarcoma. However, the jawbones have been reported as the most common site. In most studies, maxilla and mandible appear to be involved with comparable frequency.
The most common presenting symptom of head and neck mesenchymal chondrosarcoma is a swelling or mass that may or may not be painful. Additional symptoms may include sinusitis, nasal obstruction, change in vision, headaches, epistaxis, or signs of nerve disturbance.
Radiographic Features
The radiographic features of mesenchymal chondrosarcoma nearly always suggest a malignant neoplasm. The tumors typically show some form of calcification, reflecting the chondroid component of the tumor. Reports of mesenchymal chondrosarcoma involving the head and neck bones describe expansile lesions with either a radiopaque or radiolucent appearance. Invasion and destruction of the bone is commonly present.
Histologic Features
The histologic features of mesenchymal chondrosarcoma involving the head and neck bones are the same as those seen at any other site. The tumor consists of two distinct elements: well differentiated hyaline cartilage and a small, round cell undifferentiated malignancy. The proportion of the two elements is variable. The change from these two components is usually abrupt and distinct, but occasionally it is more gradual. The lobulated cartilage portion shows features of low-grade chondrosarcoma. It may also contain areas of calcification or ossification. The high grade small cell component is composed of round to oval shaped cells with hyperchromatic nuclei, inconspicuous nucleoli and scant cytoplasm. This part of the tumor frequently contains dilated and branched thin-walled vessels, giving rise to a hemangiopericytomatous pattern. The small round cells may also be arranged in sheets or have an alveolar pattern.
Immunohistochemical Features
The cartilaginous areas will stain for S-100 protein. The small cells are usually positive with CD99. They may also show focal immunoreactivity with desmin, MyoD1, and smooth muscle actin. The tumors are typically negative for myogenin, cytokeratins, and HMB-45.
Histologic Differential Diagnosis
An adequate amount of tissue is necessary for the correct diagnosis of mesenchymal chondrosarcoma. Needle biopsies can be misleading due to sampling error. The histologic differential diagnosis on a small amount of tissue varies depending upon whether both the cartilage and small cell components are represented in the sample. When the small cell area predominates, hemangiopericytoma and other small round blue cell tumors, such as Ewing sarcoma, rhabdomyosarcoma, lymphoma, carcinoma, and even melanoma are considerations in the differential diagnosis. Immunohistochemical stains may or may not be helpful in sorting through the differential. A careful search for cartilaginous differentiation may be the most helpful approach.
When the cartilaginous component predominates, conventional chondrosarcoma is in the differential diagnosis. Mesenchymal chondrosarcoma may be confused with small cell osteosarcoma or chondroblastic osteosarcoma, particularly if the cartilage has undergone focal ossification. However, small cell osteosarcoma does not contain cartilage and the stromal cells in chondroblastic osteosarcoma do not have a small round cell appearance.
Treatment and Prognosis
Wide surgical excision is the mainstay of treatment for mesenchymal chondrosarcoma. The role of chemotherapy and radiotherapy is uncertain. It is difficult to gather meaningful information on the effect of multimodality therapy since this is such a rare tumor. Nevertheless, mesenchymal chondrosarcoma is a high-grade sarcoma so patients are not infrequently given chemotherapy and/or radiotherapy in addition to surgery.
The clinical course of mesenchymal chondrosarcoma is variable. Patients typically develop recurrences from months to years after initial presentation and most will die of disease. The 5-year and 10-year survival rates for mesenchymal chondrosarcoma overall have been reported as ranging from 35–60% and 20–40%, respectively. Some reports have suggested that jaw lesions tend to have a better prognosis with 5-year and 10-year survival rates of 82% and 56%, respectively. However, a recent review of maxillary mesenchymal chondrosarcoma reported a less promising 59.1% 5-year survival. Another report on mesenchymal chondrosarcoma of the sinonasal tract found disease-free 5-year and 10-year survival rates of 64% and 55%, respectively, with an overall mean survival of 12.1 years.
Bibliography
- 1.Arlen M, Tollefsen HR, Huvos AG, et al. Chondrosarcoma of the head and neck. Am J Surg. 1970;120:456–60. doi: 10.1016/S0002-9610(70)80006-X. [DOI] [PubMed] [Google Scholar]
- 2.Baujat B, Attal P, Racy E, et al. Chondromyxoid fibroma of the nasal bone with extension into the frontal and ethmoidal sinuses: report of one case and a review of the literature. Am J Otolaryngol. 2001;22:150–3. doi: 10.1053/ajot.2001.22582. [DOI] [PubMed] [Google Scholar]
- 3.Bertoni F, Unni KK, Beabout JW, et al. Chondroblastoma of the skull and facial bones. Am J Clin Pathol. 1987;88:1–9. doi: 10.1093/ajcp/88.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Burkey BB, Hoffman HT, Baker SR, et al. Chondrosarcoma of the head and neck. Laryngoscope. 1990;100:1301–5. doi: 10.1288/00005537-199012000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Gadwal SR, Fanburg-Smith JC, Gannon FH, et al. Primary chondrosarcoma of the head and neck in pediatric patients. A clinicopathologic study of 14 cases with a review of the literature. Cancer. 2000;88:2181–8. doi: 10.1002/(SICI)1097-0142(20000501)88:9<2181::AID-CNCR26>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 6.Gaudet EG, Nuss DW, Johnson DH, Jr, et al. Chondroblastoma of the temporal bone involving the temporomandibular joint, mandibular condyle, and middle cranial fossa: case report and review of the literature. Cranio. 2004;22:160–8. doi: 10.1179/crn.2004.021. [DOI] [PubMed] [Google Scholar]
- 7.Hammad HM, Hammond HL, Kurago ZB, et al. Chondromyxoid fibroma of the jaws: case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:293–300. doi: 10.1016/S1079-2104(98)90011-7. [DOI] [PubMed] [Google Scholar]
- 8.Karkuzhali P, Chithralekha S, Muthuvel E, et al. Chondromyxoid fibroma of the parietal bone. Neuropathol. 2005;25:84–8. doi: 10.1111/j.1440-1789.2004.00576.x. [DOI] [PubMed] [Google Scholar]
- 9.Keel SB, Bhan AK, Liebsch NJ, et al. Chondromyxoid fibroma of the skull base: a tumor which may be confused with chordoma and chondrosarcoma. Report of three cases and review of the literature. Am J Surg Pathol. 1997;21:577–82. doi: 10.1097/00000478-199705000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Knott PD, Gannon FH, Thompson LDR. Mesenchymal chondrosarcoma of the sinonasal tract: a clinicopathological study of 13 cases with a review of the literature. Laryngoscope. 2003;113:783–90. doi: 10.1097/00005537-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi Y, Murakami R, Toba M, et al. Chondroblastoma of the temporal bone. Skeletal Radiol. 2001;30:714–8. doi: 10.1007/s002560100435. [DOI] [PubMed] [Google Scholar]
- 12.Koch BB, Karnell LH, Hoffman HT, et al. National cancer database report on chondrosarcoma of the head and neck. Head Neck. 2000;22:408–25. doi: 10.1002/1097-0347(200007)22:4<408::AID-HED15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 13.Kondoh T, Hamada Y, Kamei K, et al. Chondroblastoma of the mandibular condyle: report of a case. J Oral Maxillofac Surg. 2002;60:198–203. doi: 10.1053/joms.2002.29823. [DOI] [PubMed] [Google Scholar]
- 14.Kurt A-M, Unni KK, Sim FH, et al. Chondroblastoma of bone. Hum Pathol. 1989;20:965–76. doi: 10.1016/0046-8177(89)90268-2. [DOI] [PubMed] [Google Scholar]
- 15.Lustmann J, Gazit D, Ulmansky M, et al. Chondromyxoid fibroma of the jaws: clinicopathological study. J Oral Pathol. 1986;15:343–6. doi: 10.1111/j.1600-0714.1986.tb00637.x. [DOI] [PubMed] [Google Scholar]
- 16.Nakashima Y, Unni KK, Shives TC, et al. Mesenchymal chondrosarcoma of bone and soft tissue: a review of 111 cases. Cancer. 1986;57:2444–543. doi: 10.1002/1097-0142(19860615)57:12<2444::AID-CNCR2820571233>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 17.Rassehk CH, Nus DW, Silloo BK, et al. Chondrosarcoma of the nasal septum: Skull base imaging and the clinicopathologic correlation. Otolaryngol Heck Neck Surg. 1996;115:29–37. doi: 10.1016/S0194-5998(96)70132-8. [DOI] [PubMed] [Google Scholar]
- 18.Saito K, Unni KK, Wollan PC, et al. Chondrosarcoma of the jaw and facial bones. Cancer. 1995;76:1550–8. doi: 10.1002/1097-0142(19951101)76:9<1550::AID-CNCR2820760909>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi K, Sato K, Kanazawa H, et al. Mesenchymal chondromas of the jaw-Report of a case and review of 41 cases in the literature. Head Neck. 1983;15:459–64. doi: 10.1002/hed.2880150516. [DOI] [PubMed] [Google Scholar]
- 20.Thompson SH, Weathers DR, Vatral JJ. Chondromyxoid fibroma of the jaws. Head & Neck Surgery. 1982;4:330–4. doi: 10.1002/hed.2890040411. [DOI] [PubMed] [Google Scholar]
- 21.Tien N, Chaisuparat R, Fernandes R, et al. Mesenchymal chondrosarcoma of the maxilla: case report and literature review. J Oral Maxillofac Surg. 2007;65:1260–6. doi: 10.1016/j.joms.2005.11.074. [DOI] [PubMed] [Google Scholar]
- 22.Unni KK, Bridge J, Inwards C, Wold LE, Kindblom L-G. Tumors of the bones and joints (AFIP Atlas of tumor pathology series IV). 1st ed. Washington: American Registry of Pathology; 2005.
- 23.Vencio EF, Reeve CM, Unni KK, et al. Mesenchymal chondrosarcoma of the jaw bones. Cancer. 1998;82:2350–5. doi: 10.1002/(SICI)1097-0142(19980615)82:12<2350::AID-CNCR8>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 24.Vernon SE, Casiano RR. Sphenoid sinus chondromyxoid fibroma mimicking a mucocele. Am J Otolaryngol. 2006;27:406–8. doi: 10.1016/j.amjoto.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Wolf DA, Chaljub G, Maggio W, et al. Intracranial chondromyxoid fibroma. Report of a case and review of the literature. Arch Pathol Lab Med. 1997;121:626–30. [PubMed] [Google Scholar]
- 26.Wu CT, Inwards CY, O’Laughtlin S, et al. Chondromyxoid fibroma of bone: a clinicopathologic review of 278 cases. Hum Pathol. 1998;29:438–46. doi: 10.1016/S0046-8177(98)90058-2. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto S, Motoori K, Takano H, et al. Chondrosarcoma of the nasal septum. Skeletal Radiol. 2002;31:543–6. doi: 10.1007/s00256-002-0535-7. [DOI] [PubMed] [Google Scholar]
- 28.Zakkak TB, Flynn TR, Boguslaw B, et al. Mesenchymal chondrosarcoma of the mandible: case report and review of the literature. J Oral Maxillofac Surg. 1998;56:84–91. doi: 10.1016/S0278-2391(98)90922-3. [DOI] [PubMed] [Google Scholar]
- 29.Zillmer DA, Dortman HD. Chondromyxoid fibroma of the bone: 36 cases with clinical pathologic correlation. Hum Pathol. 1989;20:932–64. doi: 10.1016/0046-8177(89)90267-0. [DOI] [PubMed] [Google Scholar]