Introduction
As odontogenic tumors may contain tissues similar to the ones present in the tooth germ, the diagnostic significance of immature odontogenic tissues in jaw specimens from children and adolescents is not always clear. They could represent neoplasia as well as normal components of the immature dentition. Drawing the line between both possibilities requires knowledge of the histological and radiological aspects of both developing teeth and odontogenic neoplasms. The purpose of this contribution is to provide some guidelines that may be helpful in this area. At first, relevant aspects of normal tooth development will be discussed. Thereafter, the histology of the odontogenic tumors that most closely resemble immature odontogenic tissues will be mentioned. Finally, features that allow the distinction between tooth germ components and odontogenic tumors will be discussed.
Tooth Development
Teeth develop from epithelial cells from the mucosal lining of the oral cavity and cranial neural crest derived ectomesenchymal cells. These latter cells originate at the border between ectoderm and neuroectoderm from a neural crest segment that extends from the caudal boundary of the hindbrain and neural tube to the midbrain and caudal forebrain. They give rise to most connective tissues in the craniofacial region including the bones of the calvarium, face and jaws. Also, they are responsible for the major part of the tissues that form the adult teeth and its adjacent structures.
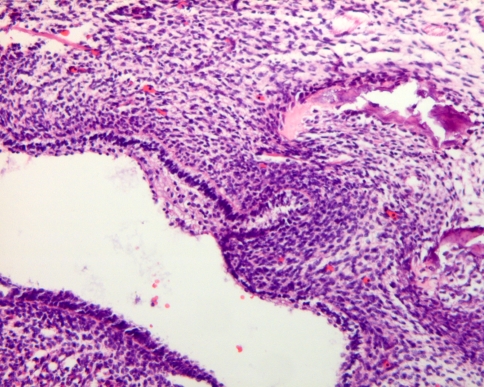
The development of the dentition starts with the formation of the dental lamina, a ridge of cells protruding from the oral epithelium into the underlying ectomesenchyme. In this epithelial ridge, focal bud-like thickenings originate at the sites of the future teeth. Together with the adjacent ectomesenchymal cells, they represent the earliest microscopically visible stage of the tooth germ that is already present in the 6 weeks old human fetus (Fig. 1).
Fig. 1.
Earliest signs of tooth development. Oral epithelial lining invaginating into underlying mesenchymal cell condensation
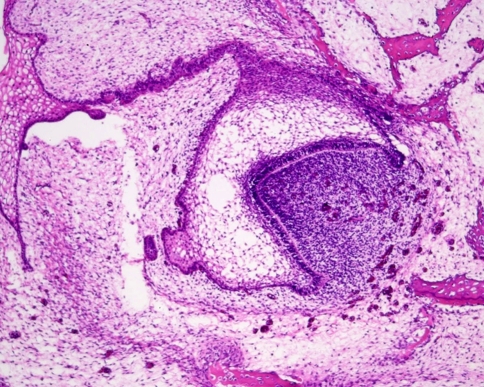
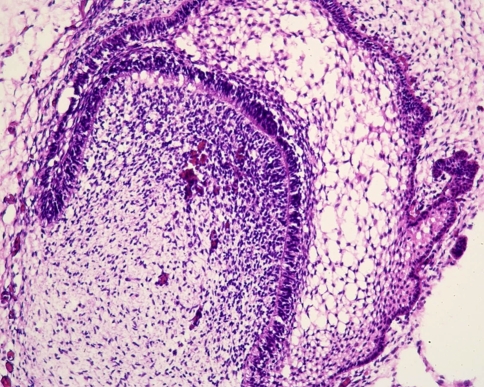
Subsequently, the epithelial bud transforms into a cap and from now on is called enamel organ. Due to the formation of a concavity along the inner surface, the cap transforms into a bell. Ectomesenchymal cells lying within this concavity form the dental papilla that will become the dental pulp, the soft tissue core of the teeth (Fig. 2). Other ectomesenchymal cells will invest the enamel organ. Together with ectomesenchymal cells at the base of the dental pulp, they form the dental follicle. In the bell-shaped enamel organ, three different components are discerned: the inner enamel epithelium facing the dental papilla, the outer enamel epithelium lying adjacent to the dental follicle, and the intervening loose stellate epithelium that is called the stellate reticulum (Fig. 3).
Fig. 2.
Bell stage of tooth development. The epithelial part, the so-called enamel organ, is still connected with the oral epithelial lining by a thin epithelial strand, the dental lamina. The enamel organ embraces the entire mesenchymal cell condensation that is called the dental papilla
Fig. 3.
Higher magnification of the enamel organ to illustrate the columnar inner enamel epithelium facing the dental papilla and the cuboidal outer enamel epithelium facing the mesenchyme of the dental follicle. The stellate reticulum is interposed between both inner and outer enamel epithelium and recognizable as a loose network of epithelial cells connected with each other through long slender cytoplasmic extensions
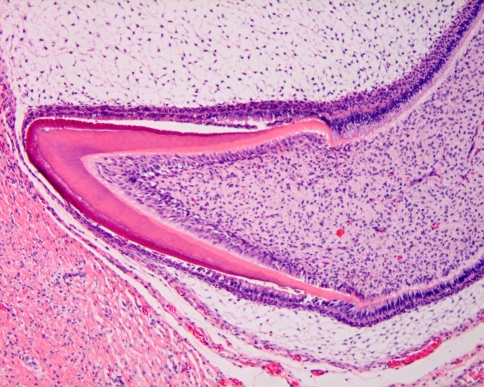
Prompted by reciprocal inductive events, inner enamel epithelium and adjacent dental papilla cells develop into enamel forming ameloblasts and dentin producing odontoblasts. The differentiation of dental papilla cells into odontoblasts requires a stimulus from the inner enamel epithelium whereas the terminal differentiation of inner enamel epithelium into enamel producing ameloblasts cannot occur without the presence of dentin. So, dentin production goes ahead of enamel production and this is recapitulated in some odontogenic lesions that may display dentin formation but no enamel production whereas the converse is never seen (Fig. 4).
Fig. 4.
During the bell stage, dental papilla cells lying adjacent to the inner enamel epithelium differentiate into long columnar odontoblasts lying down dentin. Subsequently, inner enamel epithelium differentiates into enamel matrix producing ameloblasts
When the formation of the tooth is completed, the enamel organ atrophies. The stellate reticulum disappears and inner and outer enamel epithelium form an epithelial covering of the tooth crown that remains present till the tooth erupts into the oral cavity, the so-called reduced enamel epithelium (Fig. 5). Fluid accumulation between enamel surface and this epithelial investment may give origin to cystic lesions.
Fig. 5.
During the later stages of tooth development, the stellate reticulum disappears and the enamel organ atrophies to form a thin continuous epithelial layer that invests the tooth crown (left side). The part of the enamel organ facing the root surface disintegrates into separate tiny epithelial nests that remain a life-long component of gingiva and periodontal ligament (right side)
Remnants of the dental lamina and the enamel organ can be found in the fibrous tissue of the gingiva, the so-called rests of Serres as well as in the periodontal ligament, the epithelial rests of Malassez. Sometimes, they are present in high numbers and also they may reach a considerable size.
Odontogenic Tumors Resembling Immature Odontogenic Tissues
Ameloblastomas closely resemble the epithelial part of the tooth germ. They consist of either anastomosing epithelial strands and fields or discrete epithelial islands. The former pattern is called the plexiform type, the other the follicular. The peripheral cells at the border with the adjacent fibrous stroma are columnar with nuclei usually in the apical half of the cell body away from the basement membrane. The cells lying more centrally are fusiform to polyhedral and loosely connected to each other through cytoplasmic extensions. At the periphery of the lesion, the tumour infiltrates into the adjacent cancellous bone. The lower cortical border of the mandible and the periosteal layer usually expand, but will not be perforated, the periosteum in particular forming a barrier.
Odontogenic myxomas consist of rather monotonous cells with multipolar or bipolar slender cytoplasmic extensions that lie in a myxoid stroma. Nuclei vary from round to fusiform in appearance. Binucleated cells and mitotic figures are present, but scarce. Occasionally, the lesion contains odontogenic epithelial rests. They are a fortuitous finding without any diagnostic or prognostic significance. The lesion spreads into the jaw bone without any encapsulation, thereby engulfing neighboring cancellous bone. Histologically, myxoma resembles the dental papilla.
Odontogenic fibroma consists of fibroblasts lying in a background of myxoid material intermingled with collagen fibers that may vary from delicate to coarse. Odontogenic epithelium, either scarce or abundant, may occur. All histologic features shown by odontogenic fibroma may also be displayed by the dental follicle.
Ameloblastic fibroma consists of soft tissues similar to those found in the epithelial as well as mesenchymal part of the immature tooth germ. The epithelial part of ameloblastic fibroma consists of branching and anastomosing epithelial strands that form knots of varying size. These knots have a peripheral rim of columnar cells that embraces a loosely arranged spindle-shaped epithelium. These epithelial strands lie in a myxoid cell-rich mesenchyme. The amount of epithelium may vary among cases and regionally within an individual case. There is no formation of dental hard tissues.
If there is any presence of dental hard tissues, the lesion is called ameloblastic fibro-odontoma, a lesion that combines a soft tissue component, similar to ameloblastic fibroma, with the presence of dentin and enamel. So, this lesion may mimic the tooth germ in which differentiation has progressed to the stage of dentin and enamel formation.
Immature Tooth Tissues Resembling Odontogenic Neoplasms
As already mentioned before, immature odontogenic tissues may closely mimic odontogenic tumours. Quite often, it may be very difficult to decide if the structures visible in the slide are indicating the presence of an odontogenic tumour or that they are just remnants of tissues connected with tooth development. Three different parts of the immature tooth are important in this respect: remnants of the dental lamina, the dental papilla and the dental follicle. All will be discussed in some detail. Before, it has to be emphasized that one should be very cautious in making a diagnosis of some kind of odontogenic tumour on tissue taken from a jaw area still containing developing teeth and not showing any clinical or radiographic sign of tumor being present. The ubiquitousness of immature odontogenic tissues in the jaw of children makes understandable that the chance of encountering immature odontogenic tissues in tissue samples from this area and at this age is rather high.
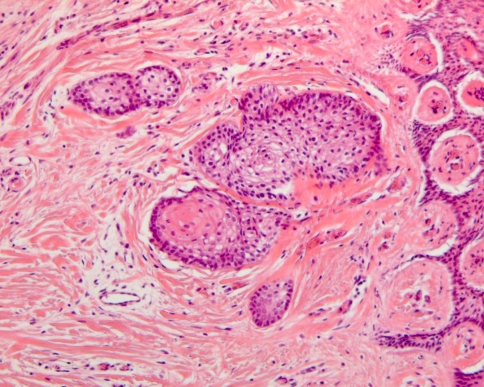
Odontogenic epithelial rests are remnants of the dental lamina. They can be found in the gingiva and when present in considerable size and number, they could cause confusion with ameloblastoma. However, they almost always lack a stellate reticulum center that is typical for an epithelial island in ameloblastoma. Moreover, they may show squamous metaplasia and dystrophic calcification and usually, the peripheral cells are not as columnar as in ameloblastoma whereas the nuclear polarization also is less pronounced (Fig. 6).
Fig. 6.
Dental lamina rests in the gingiva. When prominent with peripheral palisading and edema between the central cells, they may be confused with ameloblastoma, follicular type
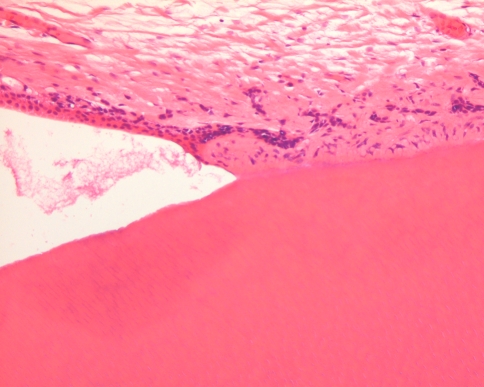
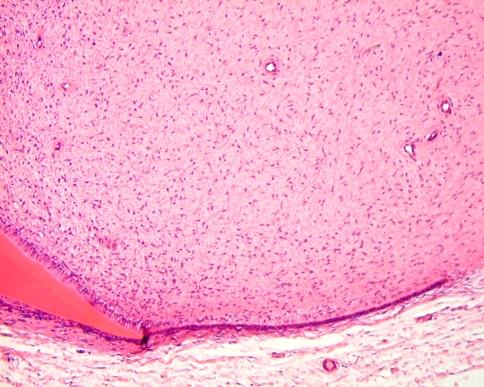
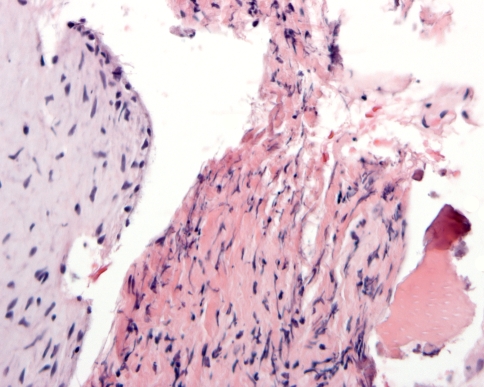
The dental papilla is the mesenchymal soft tissue part of the tooth germ. During development and maturation, it gradually transforms into the dental pulp. Concomitant with this transformation, it decreases in volume and changes its histomorphology from mainly myxoid to more fibrous. Due to this myxoid nature, the dental papilla can be mistaken for odontogenic myxoma with serious consequences for the patient. This diagnostic pitfall can be avoided by an enquiry into the source of the tissue. If there is evidence that it comes from the vicinity of a developing tooth, dental papilla is much more likely to be the appropriate diagnosis than odontogenic myxoma. Moreover, in case of dental papilla, the myxoid tissue quite often is accompanied by some strands of odontogenic epithelium representing remnants of the enamel organ and also, it may show a peripheral cell condensation indicating presence of odontoblasts (Figs. 7, 8). Finally, a shell of dentin adjacent to the myxoid tissue may be helpful in differentiating odontogenic myxoma from dental papilla (Fig. 9).
Fig. 7.
Photomicrograph showing myxoid tissue of dental papilla bordered by a rim of dentin and a long slender epithelial extension of the enamel organ
Fig. 8.
Dental papilla mimicking odontogenic myxoma. The small strip of adjacent columnar epithelium indicates that the myxoid tissue represents dental papilla
Fig. 9.
Also presence of dentin (right side) recognizable by its tubular nature may be helpful in recognizing myxoid tissue (left side) as tooth germ component and not as odontogenic myxoma
In practice, this diagnostic pitfall especially applies to biopsies taken from elevations of the bottom of the maxillary sinus. These elevations may well represent the cranial part of developing maxillary tooth germs. If biopsied and submitted as tissue from a swelling in the maxillary sinus, an erroneous diagnosis of maxillary odontogenic myxoma is easily made.
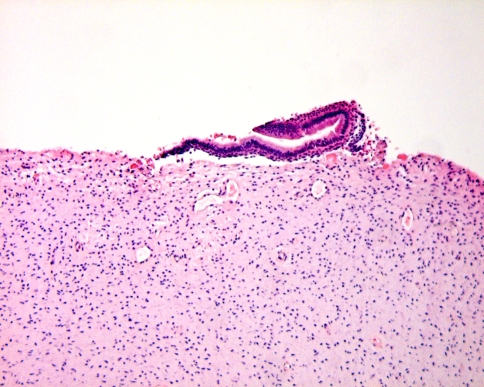
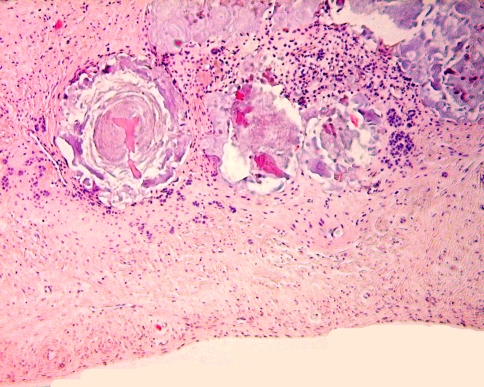
The dental follicle is the fibrous sac that surrounds the not yet erupted immature tooth. At radiographs, it can be seen as a radiolucent rim surrounding the tooth germ. Sometimes, this rim is larger than usually seen. This may lead to further examination including biopsy. As these dental follicles may consist of myxoid tissue, they may be mistaken for myxoma similar as for the dental pulp. However, they may also contain a lot of other tissue components derived from the odontogenic apparatus including cementum, bone, dystrophic calcifications and nests and strands of odontogenic epithelium. The arrangement of these tissues may simulate a variety of odontogenic tumours, either epithelial, mesenchymal, or mixed. Again it should be emphasized that these pictures encountered in tissues taken from the vicinity of a tooth germ should never been taken as evidence for neoplasia in the absence of clinical or radiologic signs suggesting tumour growth. They should be considered hamartomatous tissue alterations connected with tooth development (Fig. 10).
Fig. 10.
Dental follicle tissue may contain fragments of dentin covered with enamel matrix. These hamartomatous deposits should not be mistaken for odontoma. This latter diagnosis requires evidence either clinical or radiological of a space-occupying jaw lesion
Odontogenic hamartomatous lesions may also be found in the so-called opercula, the soft tissue barrier lying between the oral cavity and the crown of the erupting tooth. In case of delayed tooth eruption, the oral surgeon may excise this soft tissue mass that consists of both gingival fibrous tissue and the adjacent part of the underlying dental follicle for exposure of the tooth crown and thus facilitating subsequent eruption. Histologic examination of these opercula should be done with the same caveats as already mentioned.
Bibliography
- 1.Barnes L, Eveson JW, Reichart PA, et al., editors. World Health Organization classification of tumours. Pathology and genetics of tumours of the head and neck. Chapter 6. Odontogenic tumours. Lyon: IARC; 2005. pp 283–327.
- 2.Kim J, Ellis GL. Dental follicular tissue: misinterpretation as odontogenic tumors. J Oral Maxillofac Surg. 1993;51:762–7. doi: 10.1016/s0278-2391(10)80417-3. [DOI] [PubMed] [Google Scholar]
- 3.Massey D. Potential pitfalls in diagnostic oral pathology. A review for the general surgical pathologist. Adv Anat Pathol. 2005;12:332–49. doi: 10.1097/01.pap.0000194631.43254.00. [DOI] [PubMed] [Google Scholar]
- 4.Miletich I, Sharpe PT. Neural crest contribution to mammalian tooth formation. Birth Defects Research (Part C) 2004;72:200–12. doi: 10.1002/bdrc.20012. [DOI] [PubMed] [Google Scholar]
- 5.Müller H, Slootweg PJ. A peculiar finding in Le Fort I osteotomy. J Craniomaxillofac Surg. 1988;16:238–9. doi: 10.1016/s1010-5182(88)80054-4. [DOI] [PubMed] [Google Scholar]
- 6.Onishi T, Sakashita S, Ogawa T, et al. Histopathological characteristics of eruption mesenchymal calcified hamartoma: two case reports. J Oral Pathol Med. 2003;32:246–9. doi: 10.1034/j.1600-0714.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- 7.Philipsen HP, Thosaporn W, Reichart PA, et al. Odontogenic lesions in opercula of permanent molars delayed in eruption. J Oral Pathol Med. 1992;21:38–41. doi: 10.1111/j.1600-0714.1992.tb00967.x. [DOI] [PubMed] [Google Scholar]
- 8.Philipsen HP, Reichart PA, Praetorius F. Mixed odontogenic tumours and odontomas. Considerations on interrelationship. Review of the literature and presentation of 134 cases of odontomas. Oral Oncol. 1997;33:86–99. doi: 10.1016/S0964-1955(96)00067-X. [DOI] [PubMed] [Google Scholar]
- 9.Sharpe PT. Neural crest and tooth morphogenesis. Adv Dent Res. 2001;15:4–7. doi: 10.1177/08959374010150011001. [DOI] [PubMed] [Google Scholar]
- 10.Slootweg PJ. Mini-Symposium: Head and Neck Pathology. Odontogenic tumours—An update. Current Diagnostic Pathology. 2006;12:54–65. doi: 10.1016/j.cdip.2005.10.003. [DOI] [Google Scholar]
- 11.Slootweg PJ. Chapter 4. Maxillofacial skeleton and teeth. In: Cardesa A, Slootweg PJ, editors. Pathology of the head and neck. Berlin-Heidelberg: Springer; 2006. p. 115. [Google Scholar]
- 12.Suarez PA, Batsakis JG, El-Naggar AJ. Pathology consultation. Don’t confuse dental soft tissues with odontogenic tumors. Ann Otol Rhinol Laryngol. 1996;105:490–4. doi: 10.1177/000348949610500615. [DOI] [PubMed] [Google Scholar]
- 13.Thesleff I, Keränen S, Jernvall J. Enamel knots as signaling centers linking tooth morphogenesis and odontoblast differentiation. Adv Dent Res. 2001;15:14–8. doi: 10.1177/08959374010150010401. [DOI] [PubMed] [Google Scholar]
- 14.Tucker A. Sharpe P. The cutting edge of mammalian development. How the embryo makes teeth. Nat Rev Genet. 2004;5:499–508. doi: 10.1038/nrg1380. [DOI] [PubMed] [Google Scholar]