Abstract
Non-small cell neuroendocrine carcinomas (NSNECs) of the sinonasal tract are rare. Due to their rarity, the clinical and pathologic characteristics of these neoplasms are not adequately understood. We report two additional examples of NSNEC. The patients were male, 67- and 34-year-old. The first had a tumor involving left ethmoid sinus and nasal cavity and the second, a neoplasm involving nasopharynx, sphenoid sinus, with bilateral involvement of cavernous sinuses. Both tumors were composed of small to medium size cells showing round nuclei with finely dispersed chromatin and small to inconspicuous nucleoli. One case was characterized by variable size individual and confluent nests while the second demonstrated patternless sheets only. One case had punctate necrosis and a mitotic rate of 10–11/10 hpf whereas the second did not have necrosis and the mitotic rate was only 1–2/10 hpf. Both tumors were positive for keratins, CD56, and NSE. One case was positive for chromogranin and the second for synaptophysin. One patient is alive and free of disease 4 years after external beam radiotherapy. The second is alive with locally advanced disease 7 years after radiotherapy and chemotherapy. The literature suggests that NSNECs are a heterogenous group of neoplasms with a morphologic spectrum encompassing tumors resembling “atypical carcinoids”, neoplasms composed of large cells akin to large cell neuroendocrine carcinomas, and tumors with glandular and goblet cell differentiation reminiscent of “goblet cell carcinoids”. Other cases do not show specific features and are probably best regarded as “neuroendocrine carcinoma, NOS”. More studies are needed to better define the histopathologic spectrum of these lesions and to develop a clinically relevant classification.
Keywords: Nasal cavity, Paranasal sinuses, Neuroendocrine carcinoma, Small cell carcinoma, Carcinoid tumor, Olfactory neuroblastoma
Introduction
Neuroendocrine carcinomas of the sinonasal tract and nasopharynx are rare. The 2005 World Health Organization Classification of Head and Neck Tumors categorizes these neoplasms as typical carcinoid tumor, atypical carcinoid tumor, small cell carcinoma neuroendocrine type (SCNEC), and neuroendocrine carcinoma “not otherwise specified” [1]. The most common and best studied of these neoplasms is SCNEC [2, 3]; however, the pathological features of other types of sinonasal neuroendocrine carcinomas remain unclear and poorly defined [3–8]. To advance our understanding of sinonasal non-small cell neuroendocrine carcinoma (NSNEC), we describe the clinicopathologic features of two additional cases.
Material and Methods
Both surgical specimens were fixed in neutral buffered formalin, routinely processed, with tissue sections embedded in paraffin. The sections were cut at 4 μm thick and stained with hematoxylin and eosin. Immunohistochemical stains for pan-keratin (AE1:AE3, Dako, 1:500), epithelial membrane antigen (EMA, MC5, Ventana, prediluted), low molecular weight keratin (LMWK, Becton Dickinson, Cam 5.2, 1:50), high molecular keratin (HMWK, Ventana 34BE12 prediluted), chromogranin (Ventana, prediluted), synaptophysin (Ventana, prediluted), neuron specific enolase (NSE, Dako, 1:500), CD56 (123C3, Zymed, 1:400), S-100 (Ventana, polyclonal, prediluted), and HMB45 (Ventana, prediluted) were performed in an automated Ventana BenchMark® instrument (Tucson, AZ) with routine positive and negative controls.
Case Reports
Case 1
Patient 1 is a 67-year-old male smoker with a long-standing history of COPD. He presented at a community hospital with a large mass involving the ethmoid sinus and nasal cavity. A biopsy of the mass was interpreted as olfactory neuroblastoma (ONB). He was referred to our centre for treatment where after review of the initial biopsy, the tumor was reclassified as an intermediate grade sinonasal neuroendocrine carcinoma. The patient was treated with external beam radiotherapy. Initially he received 4,600 cGy with a boost of 2,400 cGy. The tumor underwent complete clinical and radiologic response and subsequent total body bone scans, head and neck CT-scans and MRIs and sinonasal endoscopies have shown no recurrent disease. The patient is alive and remains free of sinonasal disease, but has developed a biopsy-proven poorly differentiated squamous cell carcinoma of the lung. He is currently receiving radiation treatment for his lung carcinoma, 4 years after initial diagnosis and treatment of his sinonasal tumor.
Case 2
Patient 2 is a 34-year-old male who presented with proptosis and extraocular muscle palsies. CT scans of the head and neck showed a mass involving the nasopharynx and sphenoid sinus with bilateral involvement of cavernous sinuses. The initial biopsy of the tumor was interpreted as ONB. The patient then underwent combined chemotherapy and external beam radiation, with no clinical or radiological response. The lack of response to treatment prompted his referral to our centre for additional therapeutic consideration. A secondary pathology review indicated that the tumor was a low grade neuroendocrine carcinoma. The patient could not receive additional radiation therapy and was deemed unresectable since the residual tumor was in close proximity to the optic chiasm and brainstem. The tumor is slow growing and has extended to the left nasal cavity. Currently, the patient is alive with stable disease 7 years after initial diagnosis.
Pathologic Findings
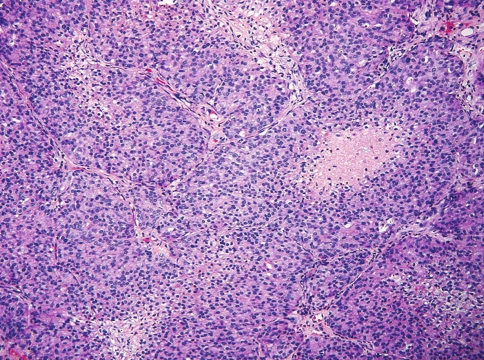
The tumor in case 1 was characterized by variable size individual and confluent nests of cells, surrounded by a delicate fibrovascular stroma (Fig. 1). The tumor cells had indistinct cell borders and exhibited moderate amounts of pale eosinophilic to amphophilic cytoplasm (Fig. 2). The nuclei were centrally or eccentrically placed and contained finely dispersed chromatin with a single small nucleolus. There was minimal cellular pleomorphism and only isolated foci of necrosis were present. Mitotic figures were easily identifiable with a rate of 10–11/10 hpf. No rosettes or fibrillary stroma were present.
Fig. 1.
Case 1. Neuroendocrine carcinoma demonstrating confluent cell nests and sheets with focal necrosis
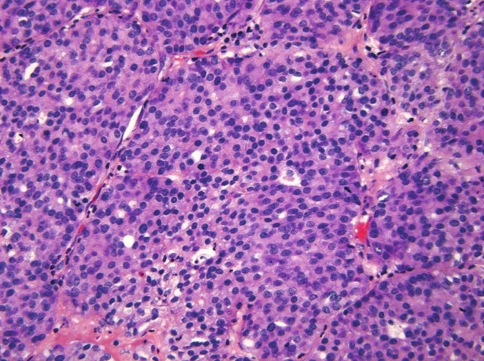
Fig. 2.
Case 1. The tumor cells are round and exhibit pale eosinophilic to amphophilic cytoplasm. The nuclei are round to oval and display thin nuclear membrane, finely dispersed chromatin and inconspicuous nucleoli. Two mitoses are present in this field
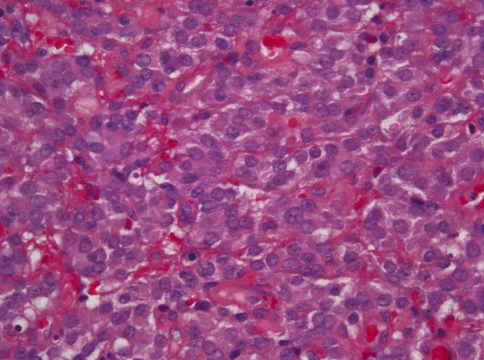
The tumor in case 2 did not show a distinctive architectural pattern and was characterized by sheets of small to medium sized tumor cells with moderate amounts of eosinophilic or clear cytoplasm and eccentrically placed nuclei (Fig. 3). The nuclei had finely dispersed chromatin with only occasional small nucleoli. The tumor cells did not show significant pleomorphism and mitotic activity was low with only 1–2/10 hpf. No tumor nests, rosettes, or fibrillary stroma were seen. The tumor was ulcerated and there was extensive hemorrhage, but no tumor necrosis was identified.
Fig. 3.
Case 2. Neuroendocrine carcinoma composed of sheets with no distinctive architectural pattern. The individual tumor cells have eosinophilic cytoplasm with indistinct cytoplasmic borders. The nuclei are round and exhibit delicate nuclear membrane with finely dispersed chromatin and inconspicuous nucleoli
Both tumors were diffusely and strongly positive for keratins (AE1:AE3 and cam 5.2), CD56, and NSE. Case 1 was positive for chromogranin, but negative for synaptophysin. Conversely, case 2 was negative for chromogranin, but positive for synaptophysin. There was no S-100 staining of the tumor cells, nor was there staining of a sustentacular cell network in either case. Both tumors were negative for HMWK, EMA, CD45, and HMB45.
Discussion
The development of a clinically meaningful and pathologically reproducible classification of NSNECs has been precluded by their rarity and confusion with other neoplasms such as ONB [9]. In its 2005 Classification of Head and Neck Tumors, the WHO categorized these neoplasms as “typical carcinoid tumor”, “atypical carcinoid tumor”, and neuroendocrine carcinoma “not otherwise specified” [1]. This classification is partly modeled after the system devised for neuroendocrine neoplasms of lung, although does not include a category for large cell neuroendocrine carcinoma (LCNEC). The classification system of pulmonary neuroendocrine neoplasms is based on well defined pathologic criteria, which have been shown to be of clinical and prognostic value. Typical carcinoid tumors show no necrosis and are characterized by a mitotic rate of less than 2/10 hpf [10]. Atypical carcinoid tumors exhibit punctate areas of necrosis and a mitotic rate higher than 2/10 hpf but less than 11/10 hpf [10]. Lastly, large cell neuroendocrine carcinomas (LCNEC) are composed of large cells with coarse chromatin and prominent nucleoli with a mitotic rate of 11 or more per 10 high power fields [11].
The two cases reported herein were not easily classifiable using the WHO classification of sinonasal neuroendocrine carcinomas. Both cases were composed of small to intermediate size cells with round nuclei containing finely dispersed chromatin with conspicuous or small nucleoli. Case 1 can be classified as atypical carcinoid because of the nested architecture, focal punctate necrosis and a mitotic rate of 10–11/10 hpf. Although the mitotic activity in this case was high enough to consider a diagnosis of a LCNEC or SCNEC the cytologic features were not in keeping with either of these diagnoses. Case 2 showed no necrosis and had a mitotic rate of 1–2/10 hpf in keeping with a typical carcinoid tumor; nonetheless, the patternless sheets of tumor cells did not conform to the characteristic architecture of typical carcinoid tumors.
Critical analysis of published NSNECs suggests they are a morphologically heterogeneous group of neoplasms with certain similarities to carcinoid tumors although not easily classifiable using the WHO terminology (Table 1). Westerveld et al. [4] described a tumor composed of epithelial islands, glands and strands with hyperchromatic cells showing “polymorphic” nuclei and “occasional” mitotic activity. No necrosis was identified in the tumor. The authors referred to this tumor as “atypical carcinoid”. Siwerson and Kindblom [5] reported an example of “oncocytic carcinoid” in a 13-year-old girl. The tumor was composed of oncocytic cells arranged in trabeculae or nests with fibrovascular stroma. There was variable degree of cytologic atypia including multinucleated giant tumor cells with vesicular nuclei and prominent nucleoli. “Very few mitotic figures” were identified. Smith et al. [7] reported four neuroendocrine carcinomas, at least three of them composed of large cells with coarse chromatin and large nucleoli forming sheets, ribbons, and trabeculae. One additional case had prominent glandular differentiation with goblet cells. Necrosis and “increased” mitotic figures were present in three cases. Three cases appear to represent LCNEC and the tumor with glandular differentiation, a “goblet cell carcinoid”. Kameya et al. [8] also described four sinonasal neuroendocrine carcinomas. Their cases 1 and 2 were labeled “malignant carcinoid” or “malignant paraganglioma”, case 3 was diagnosed as “small cell carcinoma” or “poorly differentiated olfactory neuroblastoma”, and case 4 was designated “malignant carcinoid” or undifferentiated carcinoma”. The authors provided no specific mitotic counts other than “rare” and “numerous” [8]. Cases 1 and 2 could be regarded as “atypical carcinoid tumors”, but the classification of cases 3 and 4 is more problematic since case 3 may represent a SCNEC or a high grade ONB and case 4 a LCNEC. A case described by Esposito et al. [12] was labeled “large cell neuroendocrine carcinoma” although no mitotic activity or necrosis were observed. McCluggage et al. [6] described a sinonasal neuroendocrine carcinoma with exocrine and neuroendocrine differentiation or as the authors suggested, a “goblet cell carcinoid”, a neoplasm significantly different from “typical” or “atypical” carcinoids tumors. More recently, Rosenthal et al. [3] published a report of neuroendocrine tumors of the sinonasal tract and their patterns of failure according to histologic type. The series included 31 ONBs, 17 SCNECs, and 18 neuroendocrine carcinomas not otherwise specified. Pathologic features and criteria for diagnosis were not described.
Table 1.
Reported non-small cell neuroendocrine carcinomas of sinonasal tract
| Case | Original diagnosis | Architecture | Cytologic features | Mitotic rate | Necrosis | IHC | Follow-up |
|---|---|---|---|---|---|---|---|
| Westerveld et al. [4] | Atypical carcinoid | Epithelial islands, glands and strands | ND | Occasional | No | NSE+ | AWD, multiple bone metastases |
| Chr+ | |||||||
| CD57+ | |||||||
| CK− | |||||||
| EMA− | |||||||
| Siwerson [5] | Oncocytic carcinoid | Trabeculae, nests, oncocytic cells | Oval to polygonal large cells with variable pleomorphism | Very few | No | NSE+ | AND, 18 m |
| McCluggage et al. [6] | Sinonasal neuroendocrine carcinoma with amphicrine differentiation | Organoid arrangement, glandular differentiation | Nuclei with vesicular to coarse chromatin, signet ring cells | 2–5/per hpf | Yes | CEA+ | AND, 3 m |
| CK+ | |||||||
| Chr+ | |||||||
| NSE+ | |||||||
| PGP 9.5+ | |||||||
| Smith et al. [7] | |||||||
| Case #7 | High-grade NEC | Large cells? | Present | Present | CK+ | DOD, 14 m | |
| Case #8 | High-grade NEC | Large cells? | Present | Present | CK+ | AND, 31 m | |
| NSE+ | |||||||
| S100+ focal | |||||||
| Case #9 | High-grade NEC | Large cells? | Present | Present | CK+ | DOD, 41 m | |
| Syn+ | |||||||
| Chr− | |||||||
| Case #10 | Moderately differentiated NEC | “Bland nuclei” | No | No | NSE+ | AND, 108 m | |
| S100+ | |||||||
| Vimentin+ | |||||||
| Kameya et al. [8] | |||||||
| Case #1 | Atypical carcinoid or malignant paraganglioma | Nests with fibrovascular stroma | Inconspicuous nucleoli, stipple chromatin | Rare | No | NA | DOD, 120 m |
| Case #2 | Atypical carcinoid or malignant paraganglioma | Nests with fibrovascular stroma | Inconspicuous nucleoli with occasional prominent nucleoliStipple chromatin | Rare | No | NA | DOD, 72 m, multiple local recurrences and lymph node metastases |
| Case #3 | Small cell carcinoma or poorly differentiated olfactory neuroblastoma | Solid alveolar structures with fibrovascular stroma | High n/c ratio, hypochromatic nuclei, occasional multinucleated tumor cells | Numerous | ND | NA | Post-operative death |
| Case #4 | Undifferentiated carcinoma or malignant carcinoid | Solid nests with thin fibrous stroma | Round small or medium size nuclei, often with prominent nucleoli | Numerous | ND | NA | DOD, 2 m, cervical spine and lymph node metastases |
| Esposito et al. [12] | High-grade NEC | Sheets and vague nests | Large pleomorphic cells | None | ND | CK+ | AWD, 18 m |
| Chr+ | |||||||
| Syn+ | |||||||
| Current series | |||||||
| Case 1 | Solid nests and sheets | Small to medium size cells | 10–11/10 hpf | Focal | CK+ | AND, 48 m | |
| Chr+ | |||||||
| Syn− | |||||||
| Case 2 | Sheets | Small to medium size cells | 1–2/10 hpf | No | CK+ | AWD, 84 m | |
| Syn+ | |||||||
| Chr− | |||||||
NEC, neuroendocrine carcinoma; IHC, immunohistochemistry; hpf, high power fields; CK, keratin; EMA, epithelial membrane antigen; NSE, neuron specific enolase; Chr, chromogranin; Syn, synaptophysin; ND, not described; NA, not available; AND, alive no disease, AWD; alive with disease, DOD, dead of disease
NSNECs appear to be aggressive neoplasms regardless of cytologic grade. Fourteen cases are included in this review with follow-up periods ranging from 3 to 120 months. Five (36%) patients died of their disease 2, 14, 41, 72, and 120 months after diagnosis; three (21%) were alive with local disease (2) or metastases (1) with follow-up ranging from 18 to 84 months; and five (36%) were alive with no evidence of recurrent disease after 3, 18, 31, 48, and 108 months. One patient died of postoperative complications. Rosenthal et al. [3] reported a 5-year overall survival rate of 64.2% for NSNECs with 72.6% local control rate, 12.9% regional failure rate and 14.1% distant metastasis rate. The overall survival rate, local control rate, regional failure rate, and distant metastasis rate of patients affected by NSNECs were poorer than those with ONB but significantly better than for those with SCNEC [3].
The diagnosis of NSNEC requires increased diagnostic awareness and use of appropriate ancillary studies. NSNEC are malignant epithelial neoplasms composed of small, intermediate or large cells with immunohistochemical and/or ultrastructural evidence of diffuse neuroendocrine differentiation. The differential diagnosis of NSNEC comprises other sinonasal neuroendocrine neoplasms such as ONB, paraganglioma, and pituitary adenoma as well as non-neuroendocrine lesions such as sinonasal undifferentiated carcinoma (SNUC), basaloid squamous cell carcinoma, malignant melanoma, and intestinal-type sinonasal adenocarcinoma (ITAC). Due to morphologic and immunohistochemical similarities ONB and NSNEC are often mistaken for each other [13]. Our two cases were initially diagnosed as ONB. Most NSNEC and ONB are composed of individual and confluent cell nests of variable size; however, the tumor cells in ONB tend to be smaller with denser chromatin and often contain a neurofibrillary stroma not seen in NSNEC. The tumor cells in NSNEC are diffusely positive for pan-keratins and low-molecular weight keratin while their expression in ONB is focal or only present in areas with olfactory rosettes. The tumor nests in ONB are surrounded by sustentacular cells positive for S-100; not a characteristic finding of NSNEC. It could be said that NSNEC is a carcinoma with neuroendocrine differentiation whereas ONB is a neuroendocrine/neuroectodermal neoplasm with occasional epithelial differentiation. Paragangliomas are extremely rare in the sinonasal tract. Unlike NSNEC, paragangliomas lack diffuse expression of keratin and are surrounded by S-100 positive sustentacular cells. Ectopic or invasive pituitary adenoma should also be separated from NSNEC. The definitive diagnosis of pituitary adenoma resides in the demonstration of pituitary hormones by immunohistochemistry or ultrastructural studies. The distinction of NSNEC from basaloid squamous cell carcinoma and SNUC may be difficult in small biopsies, however demonstration of immunoreactivity for chromogranin and/or synaptophysin can separate the latter two malignancies from NSNEC. ITACs may show significant neuroendocrine differentiation [14]. There is no published experience assessing the expression of CDX-2 and keratin 20 in NSNEC with glandular differentiation [6, 7] therefore no definitive statement can be regarding the value of these markers of intestinal differentiation in the distinction between ITAC with neuroendocrine differentiation and NSNEC with glandular differentiation. It is interesting to note that the neuroendocrine carcinoma with goblet cell differentiation described by McCluggage et al. [6], occurred in a patient with long term exposure to wood dust. This case raises the possibility that goblet cell carcinoid/neuroendocrine carcinoma with glandular differentiation may be part of the spectrum of ITAC.
NSNECs represent a rare and heterogenous group of neoplasms with a morphologic spectrum ranging from tumors resembling “atypical carcinoid”, to others composed of large cells akin to LCNEC, and tumors with glandular and goblet cell differentiation reminiscent of “goblet cell carcinoid”. Other cases do not show specific features and are probably best regarded as “neuroendocrine carcinoma, NOS”. NSNECs appear to be less aggressive than SCNEC; however, more studies are needed to delineate the histopathologic spectrum of these lesions and to develop a relevant classification.
References
- 1.Perez-Ordonez B. Neuroendocrine tumors. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization classification of tumours. Pathology and genetics. head and neck tumours. Lyon: AIRCPress; 2005. pp. 26–27. [Google Scholar]
- 2.Perez-Ordonez B, Caruana SM, Huvos AG, Shah JP. Small cell neuroendocrine carcinoma of the nasal cavity and paranasal sinuses. Hum Pathol. 1998;29:826–32. doi: 10.1016/S0046-8177(98)90452-X. [DOI] [PubMed] [Google Scholar]
- 3.Rosenthal DI, Barker JL, Jr, El Naggar AK, et al. Sinonasal malignancies with neuroendocrine differentiation: patterns of failure according to histologic phenotype. Cancer. 2004;101:2567–73. doi: 10.1002/cncr.20693. [DOI] [PubMed] [Google Scholar]
- 4.Westerveld GJ, Diest PJ, Nieuwkerk EB. Neuroendocrine carcinoma of the sphenoid sinus: a case report. Rhinology. 2001;39:52–4. [PubMed] [Google Scholar]
- 5.Siwersson U, Kindblom LG. Oncocytic carcinoid of the nasal cavity and carcinoid of the lung in a child. Pathol Res Pract. 1984;178:562–9. doi: 10.1016/S0344-0338(84)80089-8. [DOI] [PubMed] [Google Scholar]
- 6.McCluggage WG, Napier SS, Primrose WJ, Adair RA, Toner PG. Sinonasal neuroendocrine carcinoma exhibiting amphicrine differentiation. Histopathology. 1995;27:79–82. doi: 10.1111/j.1365-2559.1995.tb00296.x. [DOI] [PubMed] [Google Scholar]
- 7.Smith SR, Som P, Fahmy A, Lawson W, Sacks S, Brandwein M. A clinicopathological study of sinonasal neuroendocrine carcinoma and sinonasal undifferentiated carcinoma. Laryngoscope. 2000;110:1617–22. doi: 10.1097/00005537-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Kameya T, Shimosato Y, Adachi I, Abe K, Ebihara S, Ono I. Neuroendocrine carcinoma of the paranasal sinus. A morphological and endocrinological study. Cancer. 1980;45:330–9. doi: 10.1002/1097-0142(19800115)45:2<330::AID-CNCR2820450222>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 9.Silva EG, Butler JJ, Mackay B, Goepfert H. Neuroblastomas and neuroendocrine carcinomas of the nasal cavity. A proposed new classification. Cancer. 1982;50:2388–405. doi: 10.1002/1097-0142(19821201)50:11<2388::AID-CNCR2820501126>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 10.Beasley MB, Thunnissen FB, Hasleton PS, et al. Carcinoid tumor. In: Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC, et al., editors. World Health Organization classification of tumours. Pathology and genetics. Tumours of the lung, pleura, thymus and heart. Lyon: AIRCPress; 2004. pp. 59–62. [Google Scholar]
- 11.Brambilla E, Pugatch B, Geisinger KR, et al. Large cell carcinoma. In: Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC, et al., editors. World Health Organization classification of tumours. Pathology and genetics. Tumours of the lung, pleura, thymus, and heart. Lyon: AIRCPress; 2004. pp. 45–50. [Google Scholar]
- 12.Esposito F, Kelly DF, Vinters HV, DeSalles AA, Sercarz J, Gorgulhos AA. Primary sphenoid sinus neoplasms: a report of four cases with common clinical presentation treated with transsphenoidal surgery and adjuvant therapies. J Neurooncol. 2006;76:299–306. doi: 10.1007/s11060-005-7285-z. [DOI] [PubMed] [Google Scholar]
- 13.Cohen ZR, Marmor E, Fuller GN, Demonte F. Misdiagnosis of olfactory neuroblastoma. Neurosurg Focus. 2002;12:e3. doi: 10.3171/foc.2002.12.5.4. [DOI] [PubMed] [Google Scholar]
- 14.McKinney CD, Mills SE, Franquemont DW. Sinonasal intestinal-type adenocarcinoma: immunohistochemical profile and comparison with colonic adenocarcinoma. Mod Pathol. 1995;8:421–5. [PubMed] [Google Scholar]